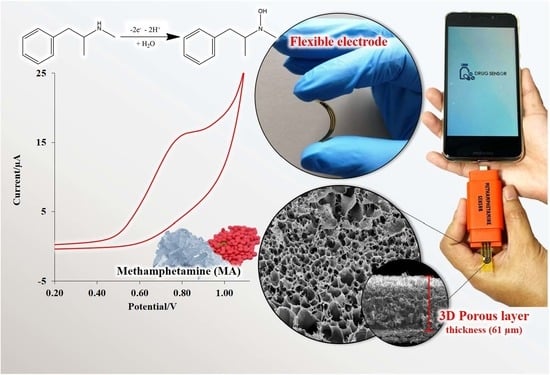
Electrochemical Sensor for Methamphetamine Detection Using Laser-Induced Porous Graphene Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
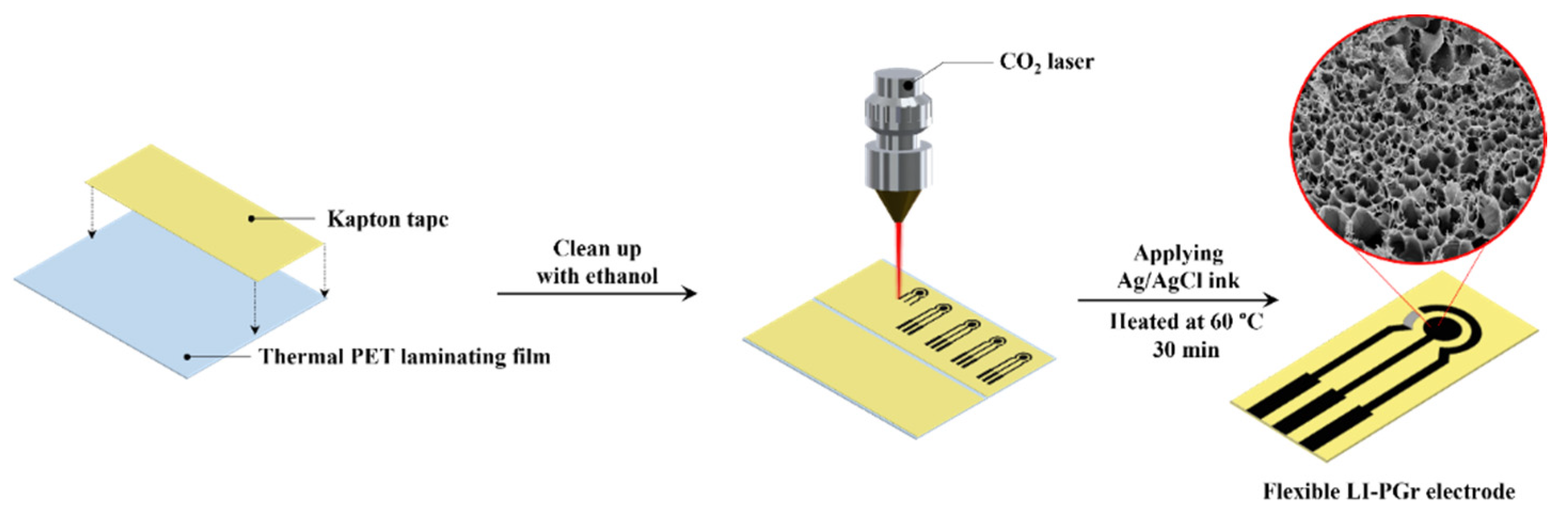
2.2. Preparation of Laser-Induced Porous Graphene (LI-PGr) Electrode
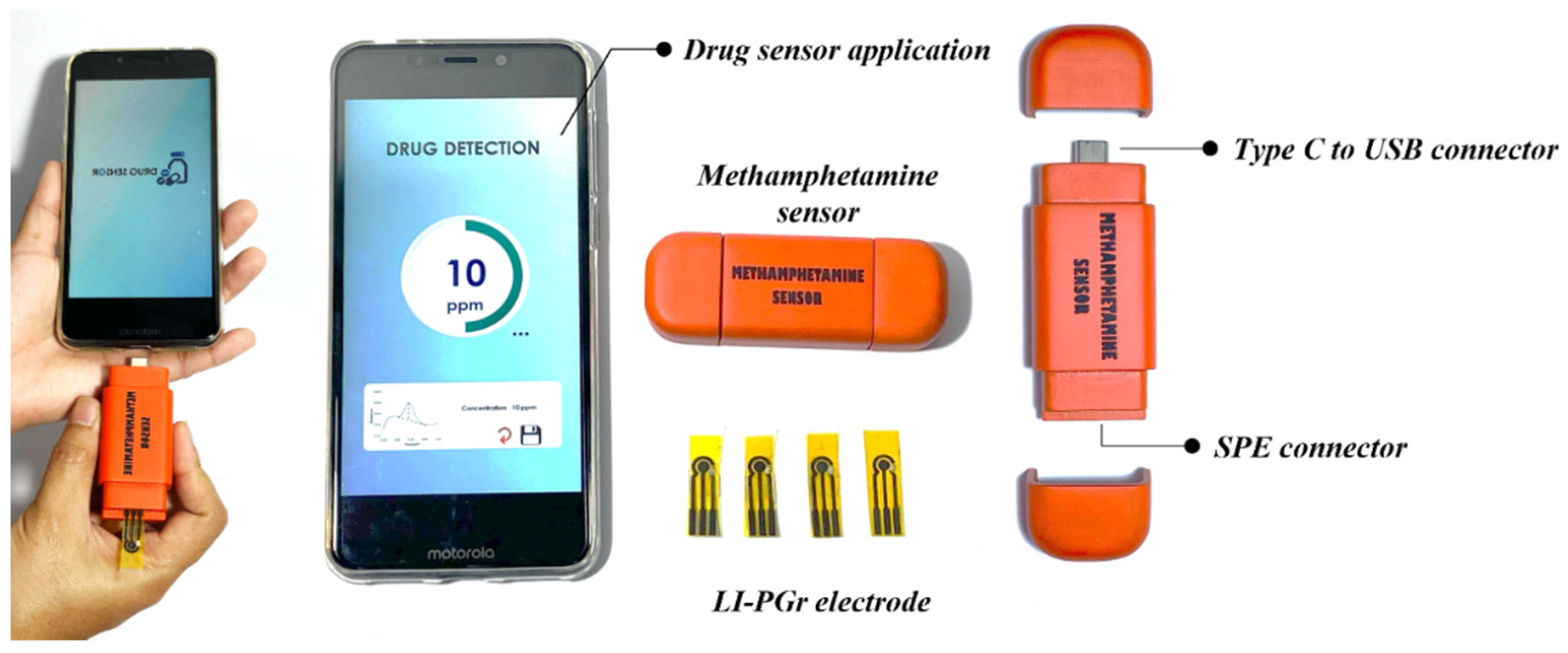
2.3. Electrochemical Measurement and Characterization
2.4. Sample Analysis
2.4.1. Surface Recovery Experiment
2.4.2. Saliva Sample
3. Results
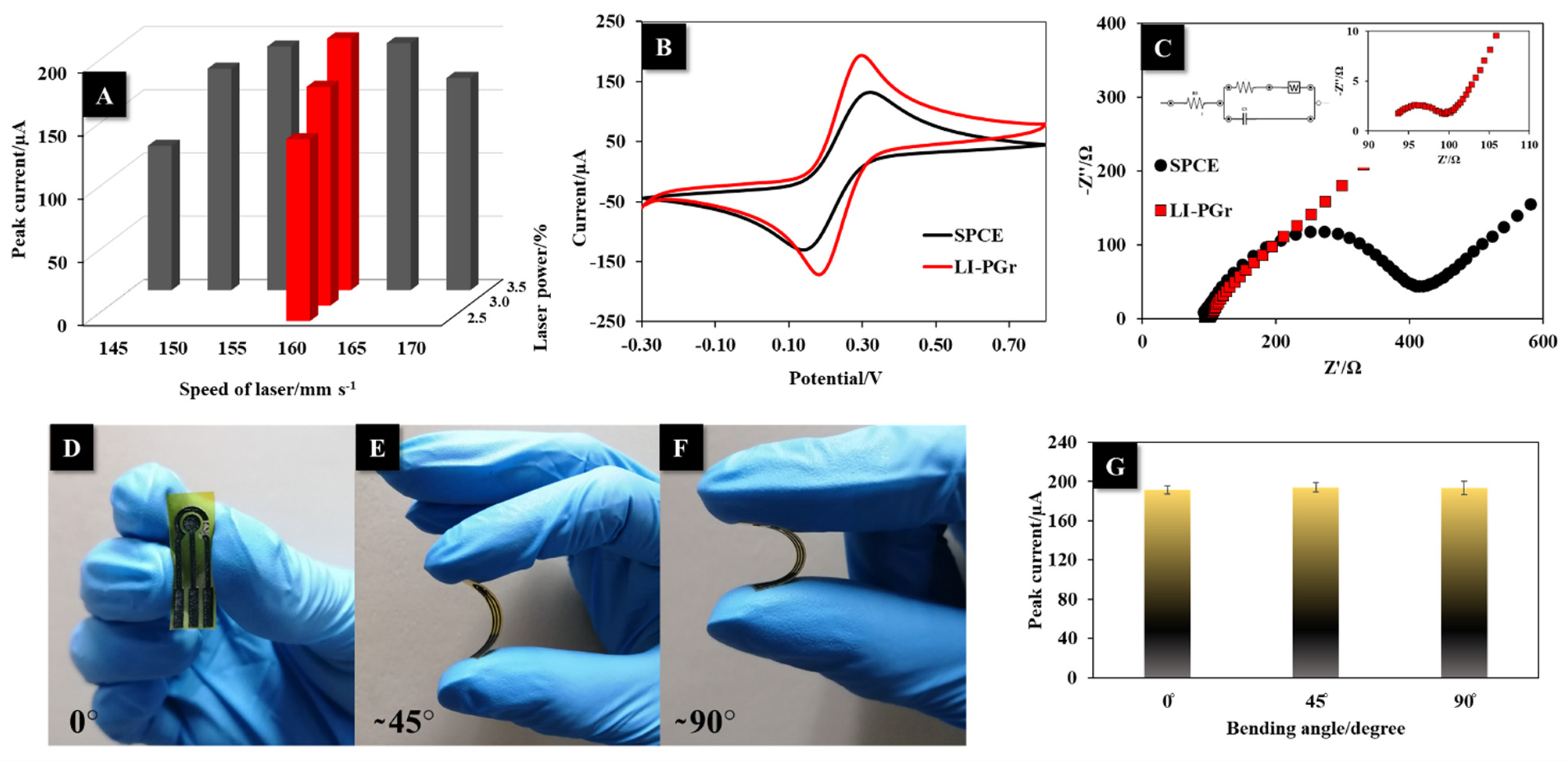
3.1. Optimization of LI-PGr Electrode Fabrication
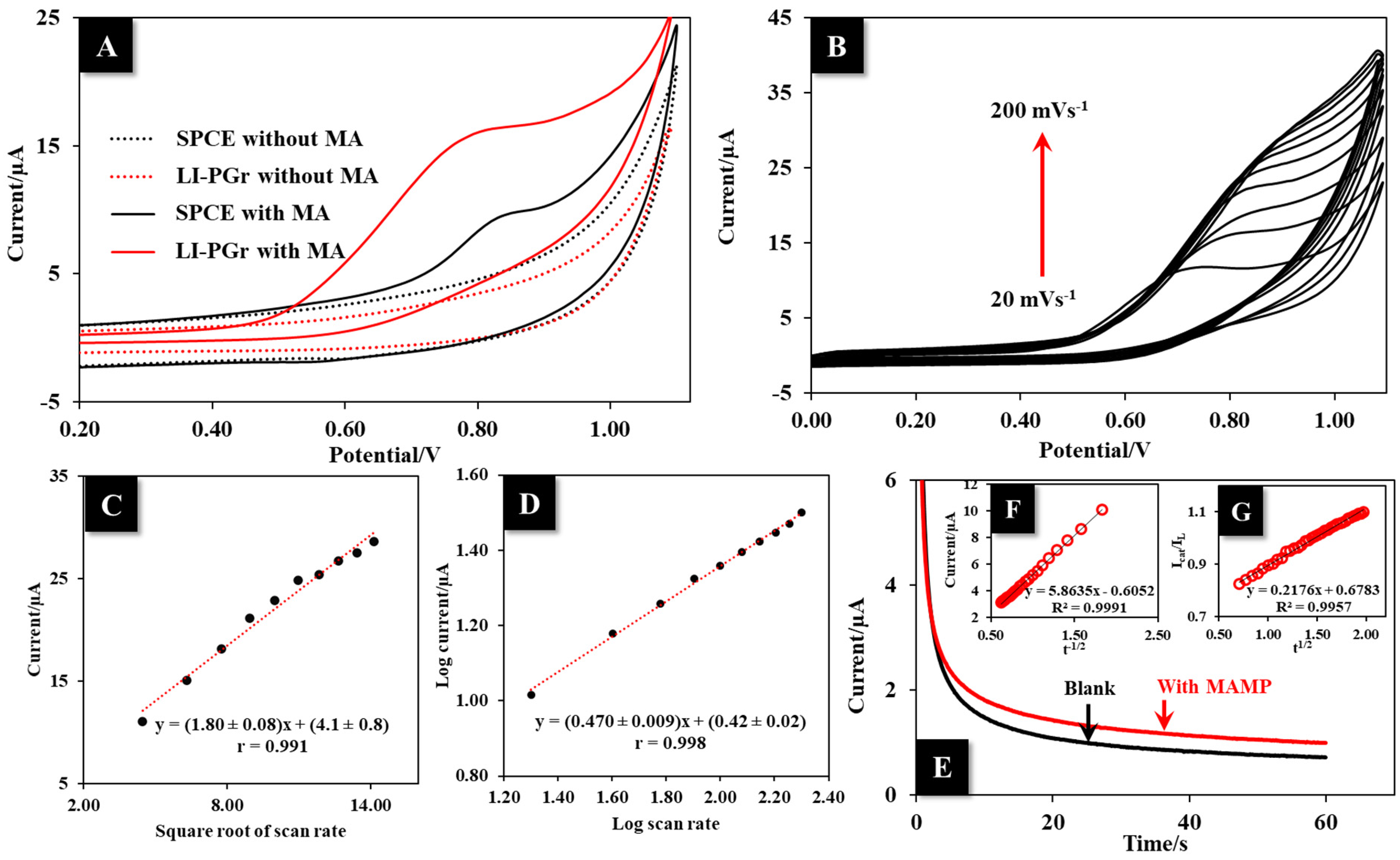
3.2. Electrochemical Characterization, Flexibility, and Stability Test of LI-PGr Electrode
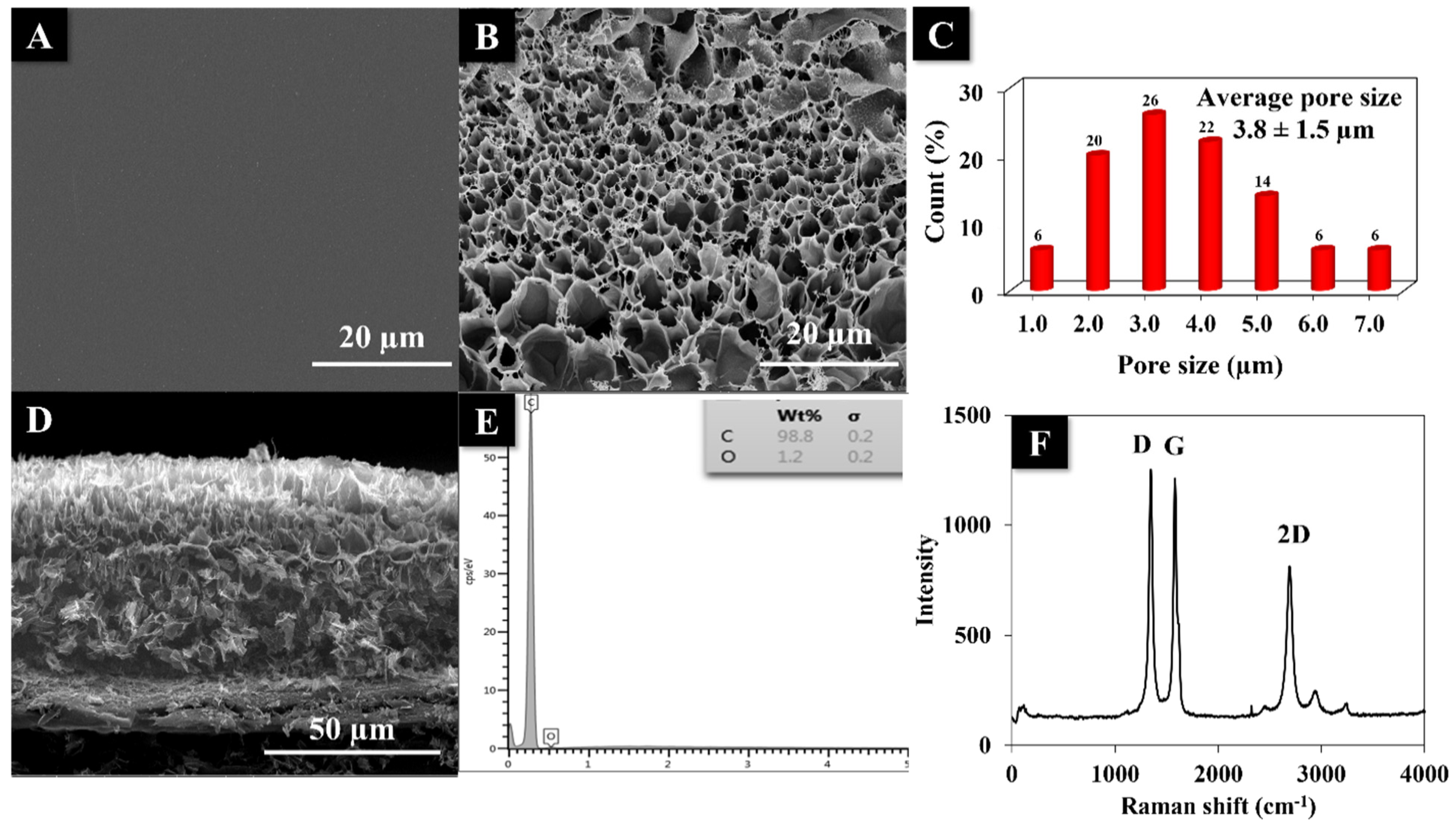
3.3. Physical Characterizations
3.4. Electrochemical Behavior of MA on LI-PGr Electrode
3.5. Effect of pH
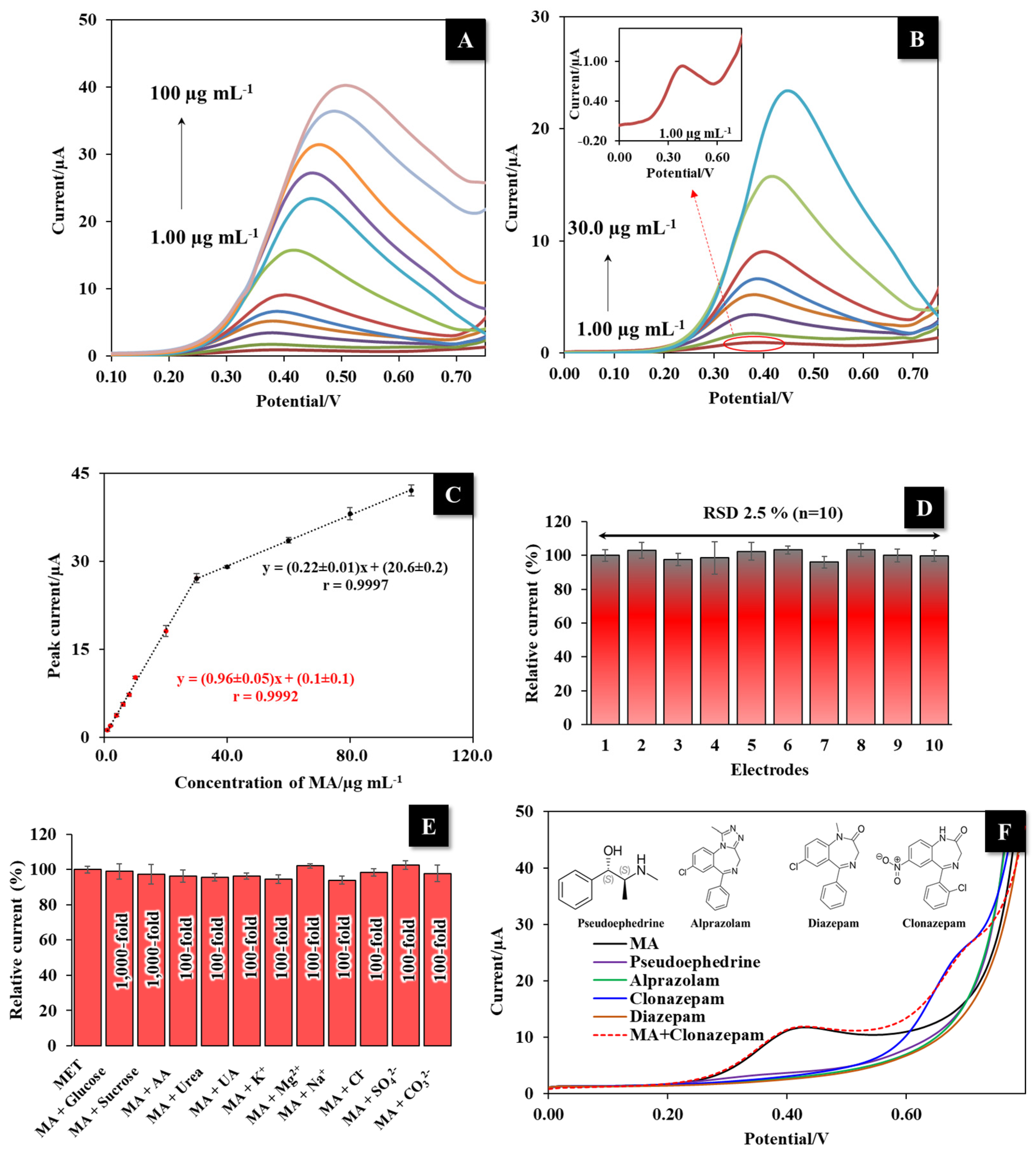
3.6. MA Detection using the LI-PGr Electrode
3.7. Reproducibility and Interference Study
3.8. Application with Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Office on Drugs and Crime. World Drug Report 2021; United Nations Publication Press: Vienn, Austria, 2021. [Google Scholar]
- National Institute on Drug Abuse. Methamphetamine; National Institute on Drug Abuse: North Bethesda, MD, USA, 2019. [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2016; United Nations Publication Press: New York, NY, USA, 2016. [Google Scholar]
- Martyny, J.W.; Arbuckle, S.L.; McCammon, C.S.; Esswein, E.J.; Erb, N.; Van Dyke, M.V. Chemical concentrations and contamination associated with clandestine methamphetamine laboratories. J. Chem. Health Saf. 2007, 14, 40–52. [Google Scholar] [CrossRef]
- Abdullah, A.F.L.; Miskelly, G.M. Residues at Clandestine Methamphetamine Laboratories and Their Health Effects. Health Environ. 2010, 1, 21–29. [Google Scholar]
- Kuhn, E.J.; Walker, G.S.; Whiley, H.; Wright, J.; Ross, K.E. Household contamination with methamphetamine: Knowledge and uncertainties. Int. J. Environ. Res. Public Health 2019, 16, 4676. [Google Scholar] [CrossRef] [Green Version]
- United Nations International Drug Control Programme. Rapid Testing Methods of Drugs of Abuse: Manual for Use by National Law Enforcement and Narcotics Laboratory Personnel; United Nations Publications: New York, NY, USA, 1994; Volume 13. [Google Scholar]
- Peng, Y.; Gautam, L.; Hall, S.W. The detection of drugs of abuse and pharmaceuticals in drinking water using solid-phase extraction and liquid chromatography-mass spectrometry. Chemosphere 2019, 223, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Saar-Reismaa, P.; Erme, E.; Vaher, M.; Kulp, M.; Kaljurand, M.; Mazina-Šinkar, J. In Situ Determination of Illegal Drugs in Oral Fluid by Portable Capillary Electrophoresis with Deep UV Excited Fluorescence Detection. Anal. Chem. 2018, 90, 6253–6258. [Google Scholar] [CrossRef]
- Barnes, A.J.; Kim, I.; Schepers, R.; Moolchan, E.T.; Wilson, L.; Cooper, G.; Reid, C.; Hand, C.; Huestis, M.A. Sensitivity, specificity, and efficiency in detecting opiates in oral fluid with the Cozart® Opiate Microplate EIA and GC-MS following controlled codeine administration. J. Anal. Toxicol. 2003, 27, 402–406. [Google Scholar] [CrossRef] [Green Version]
- Mahato, K.; Wang, J. Electrochemical sensors: From the bench to the skin. Sens. Actuators B Chem. 2021, 344, 130178. [Google Scholar] [CrossRef]
- da Silva, E.T.S.G.; Souto, D.E.P.; Barragan, J.T.C.; de F. Giarola, J.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical Biosensors in Point-of-Care Devices: Recent Advances and Future Trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Rauf, S.; Beduk, T.; Durmus, C.; Aljedaibi, A.; Timur, S.; Alshareef, H.N.; Amine, A.; Wolfbeis, O.S.; Salama, K.N. Electrochemical sensors and biosensors using laser-derived graphene: A comprehensive review. Biosens. Bioelectron. 2020, 168, 112565. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S.; Guo, D.; Yu, P.; Li, D.; Ye, J.; Mao, L. Comparative studies on electrochemical activity of graphene nanosheets and carbon nanotubes. Electrochem. Commun. 2009, 11, 1892–1895. [Google Scholar] [CrossRef]
- Soleh, A.; Saisahas, K.; Promsuwan, K.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. N-Doped Graphene Nanoplatelets for Direct Capsaicin Detection in Chili Pepper Samples. ACS Appl. Nano Mater. 2020, 3, 10094–10104. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kachatong, N.; Limbut, W. Simple flow injection system for non-enzymatic glucose sensing based on an electrode modified with palladium nanoparticles-graphene nanoplatelets/mullti-walled carbon nanotubes. Electrochim. Acta 2019, 320, 134621. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Nitrite amperometric sensor for gunshot residue screening. Electrochim. Acta 2020, 331, 135309. [Google Scholar] [CrossRef]
- Torrarit, K.; Promsuwan, K.; Soleh, A.; Saisahas, K.; Thiagchanya, A.; Phonchai, A.; Limbut, W. Adsorptive Anodic Stripping Voltammetric Determination of Atropine in Urine Sample. J. Electrochem. Soc. 2021, 168, 037512. [Google Scholar] [CrossRef]
- Soleh, A.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Novel electrochemical sensor using a dual-working electrode system for the simultaneous determination of glucose, uric acid and dopamine. Microchem. J. 2020, 153, 104379. [Google Scholar] [CrossRef]
- Saichanapan, J.; Promsuwan, K.; Saisahas, K.; Soleh, A.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. Voltammetric Determination of Tramadol Using a Hierarchical Graphene Oxide Nanoplatelets Modified Electrode. J. Electrochem. Soc. 2021, 168, 117512. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, H.-M. The global growth of graphene. Nat. Nanotechnol. 2014, 9, 726–730. [Google Scholar] [CrossRef]
- Saichanapan, J.; Promsuwan, K.; Limbut, W. Adsorption and determination of sibutramine in illegal slimming product using porous graphene ink-modified electrode. Talanta 2020, 212, 120788. [Google Scholar] [CrossRef] [PubMed]
- Promsuwan, K.; Soleh, A.; Saisahas, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Guo, C.; Li, C.M.; Limbut, W. Discrimination of dopamine by an electrode modified with negatively charged manganese dioxide nanoparticles decorated on a poly(3,4 ethylenedioxythiophene)/reduced graphene oxide composite. J. Colloid Interface Sci. 2021, 597, 314–324. [Google Scholar] [CrossRef]
- Saisahas, K.; Soleh, A.; Promsuwan, K.; Phonchai, A.; Mohamed Sadiq, N.S.; Teoh, W.K.; Chang, K.H.; Lim Abdullah, A.F.; Limbut, W. A portable electrochemical sensor for detection of the veterinary drug xylazine in beverage samples. J. Pharm. Biomed. Anal. 2021, 198, 113958. [Google Scholar] [CrossRef]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N.; et al. Electronic Confinement and Coherence in Patterned Epitaxial Graphene. Science 2006, 312, 1191. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [Green Version]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Vashisth, A.; Kowalik, M.; Gerringer, J.C.; Ashraf, C.; van Duin, A.C.T.; Green, M.J. ReaxFF Simulations of Laser-Induced Graphene (LIG) Formation for Multifunctional Polymer Nanocomposites. ACS Appl. Nano Mater. 2020, 3, 1881–1890. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, S.; Haylock, B.; Kaur, J.; Tanner, P.; Thiel, D.; Sang, R.; Cole, I.S.; Li, X.; Lobino, M.; et al. Tuning the sub-processes in laser reduction of graphene oxide by adjusting the power and scanning speed of laser. Carbon 2019, 141, 83–91. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene: From discovery to translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef] [PubMed]
- Mongay, C.; Cerdà, V. A Britton-Robinson buffer of known ionic strength. Ann. Chim. 1974, 64, 409–412. [Google Scholar]
- Abdullah, A.F.L.; Miskelly, G.M. Recoveries of trace pseudoephedrine and methamphetamine residues from impermeable household surfaces: Implications for sampling methods used during remediation of clandestine methamphetamine laboratories. Talanta 2010, 81, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zeng, Z.; Li, Q.; Liu, Y.; Chu, W.; Maiyalagan, T.; Mao, S. Ultrasensitive detection of disinfection byproduct trichloroacetamide in drinking water with Ag nanoprism@MoS2 heterostructure-based electrochemical sensor. Sens. Actuators B Chem. 2021, 332, 129526. [Google Scholar] [CrossRef]
- Mendes, L.F.; de Siervo, A.; de Araujo, W.R.; Paixão, T.R.L.C. Reagentless fabrication of a porous graphene-like electrochemical device from phenolic paper using laser-scribing. Carbon 2020, 159, 110–118. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bard, A.J.; Faulkner, L.R. Fundamentals and Applications. In Electrochemical Methods, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Galus, Z. Fundamentals of Electrochemical Analysis; Ellis Horwood: New York, NY, USA, 1976; pp. 273–275. [Google Scholar]
- Kala, D.; Sharma, T.K.; Gupta, S.; Nagraik, R.; Verma, V.; Thakur, A.; Kaushal, A. AuNPs/CNF-modified DNA biosensor for early and quick detection of O. tsutsugamushi in patients suffering from scrub typhus. 3 Biotech 2020, 10, 446. [Google Scholar] [CrossRef]
- Abbas, M.N.; Saeed, A.A.; Ali, M.B.; Errachid, A.; Zine, N.; Baraket, A.; Singh, B. Biosensor for the oxidative stress biomarker glutathione based on SAM of cobalt phthalocyanine on a thioctic acid modified gold electrode. J. Solid State Electrochem. 2019, 23, 1129–1144. [Google Scholar] [CrossRef]
- AOAC. Guidelines for Standard Method Performance Requirements; AOAC: Rockville, MD, USA, 2016; pp. 1–16. [Google Scholar]
| Common Household Surface | Spiked (µg/100 cm3) | Found (µg mL−1) (n = 3) | % Recovery (n = 3) |
|---|---|---|---|
| Glass | 0 | N.D. | - |
| 5 | 5.6 ± 1.2 | 98.4 ± 0.3 | |
| 10 | 9.0 ± 0.5 | 90 ± 5 | |
| Stainless steel | 0 | N.D. | - |
| 5 | 4.3 ± 0.3 | 86 ± 6 | |
| 10 | 8.41 ± 0.09 | 84.1 ± 0.9 | |
| Plastic | 0 | N.D. | - |
| 5 | 4.4 ± 0.2 | 87 ± 3 | |
| 10 | 8.6 ± 0.3 | 86 ± 3 |
| Saliva Sample | Spiked (µg mL−1) | Found (µg mL−1) (n = 3) | % Recovery (n = 3) |
|---|---|---|---|
| S1 | 0 | N.D. | - |
| S2 | 5 | 4.2 ± 0.2 | 84 ± 4 |
| S3 | 10 | 10.4 ± 0.5 | 104 ± 5 |
| S4 | 15 | 16 ± 2 | 104 ± 8 |
| S5 | 20 | 19.9 ± 0.9 | 99 ± 4 |
| S6 | 25 | 24.3 ± 0.7 | 97 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saisahas, K.; Soleh, A.; Somsiri, S.; Senglan, P.; Promsuwan, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Lee, K.; Chang, K.H.; et al. Electrochemical Sensor for Methamphetamine Detection Using Laser-Induced Porous Graphene Electrode. Nanomaterials 2022, 12, 73. https://doi.org/10.3390/nano12010073
Saisahas K, Soleh A, Somsiri S, Senglan P, Promsuwan K, Saichanapan J, Kanatharana P, Thavarungkul P, Lee K, Chang KH, et al. Electrochemical Sensor for Methamphetamine Detection Using Laser-Induced Porous Graphene Electrode. Nanomaterials. 2022; 12(1):73. https://doi.org/10.3390/nano12010073
Chicago/Turabian StyleSaisahas, Kasrin, Asamee Soleh, Sunita Somsiri, Patthamaporn Senglan, Kiattisak Promsuwan, Jenjira Saichanapan, Proespichaya Kanatharana, Panote Thavarungkul, Khai Lee, Kah Haw Chang, and et al. 2022. "Electrochemical Sensor for Methamphetamine Detection Using Laser-Induced Porous Graphene Electrode" Nanomaterials 12, no. 1: 73. https://doi.org/10.3390/nano12010073
APA StyleSaisahas, K., Soleh, A., Somsiri, S., Senglan, P., Promsuwan, K., Saichanapan, J., Kanatharana, P., Thavarungkul, P., Lee, K., Chang, K. H., Abdullah, A. F. L., Tayayuth, K., & Limbut, W. (2022). Electrochemical Sensor for Methamphetamine Detection Using Laser-Induced Porous Graphene Electrode. Nanomaterials, 12(1), 73. https://doi.org/10.3390/nano12010073