Improvement of Nanostructured Polythiophene Film Uniformity Using a Cruciform Electrode and Substrate Rotation in Atmospheric Pressure Plasma Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup for AP Plasma Polymerzation
2.2. Analysis and Characterization of the Nanostructured Polythiophene Film
3. Results and Discussion
3.1. Newly Designed AP Plasma Reactor with Cruciform Bare Electrode
3.2. Glow Discharge Behaviors during AP Plasma Polymerization
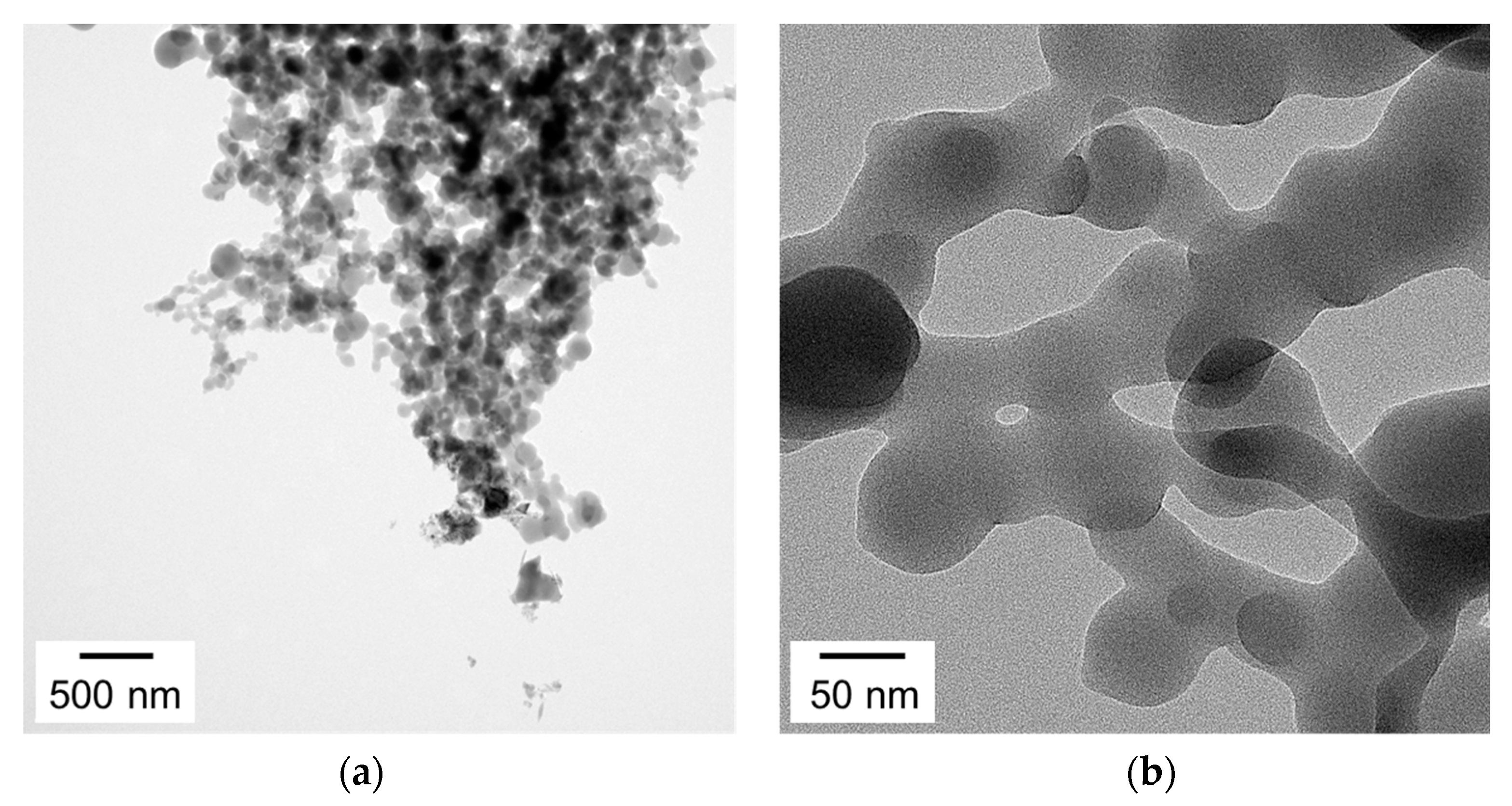
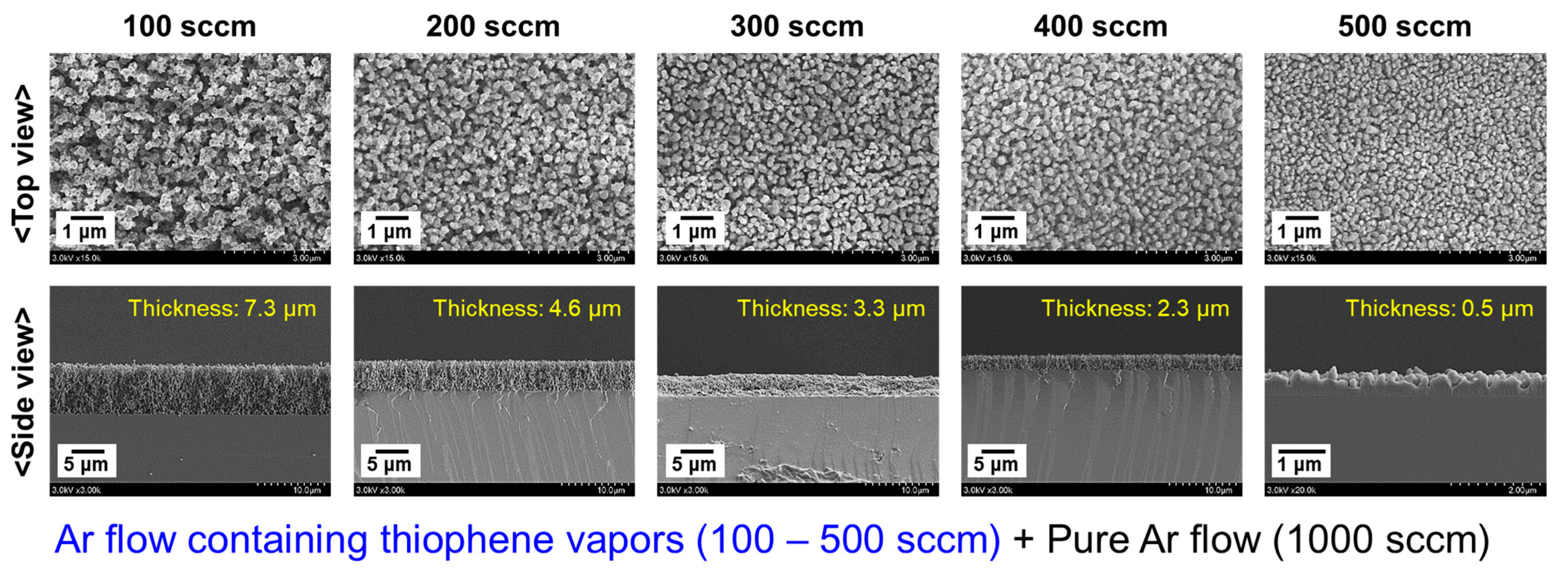
3.3. Observation of Uniformity of the PTh Film
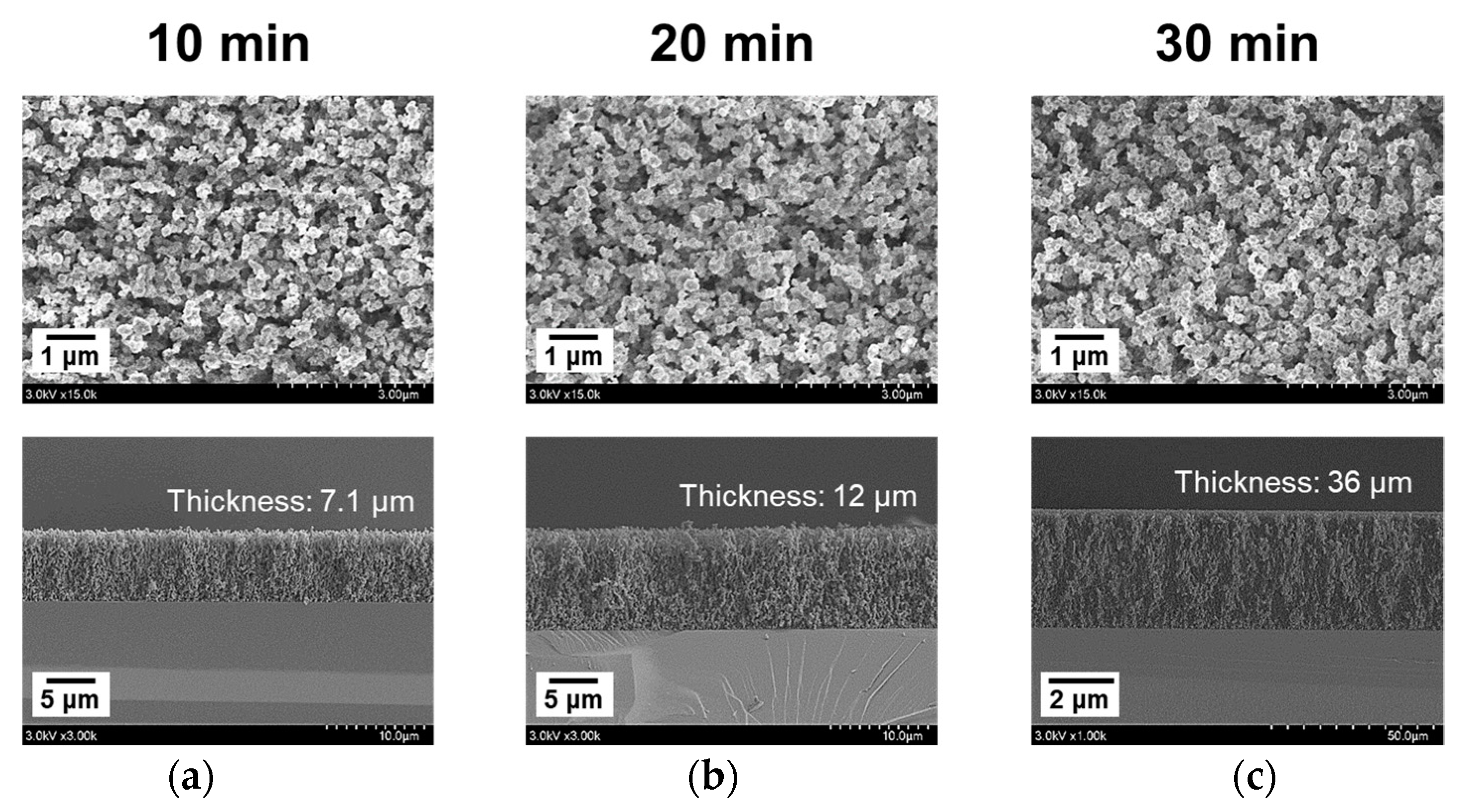
3.4. Substrate Rotation for Improvement of Uniformity of the PTh Film
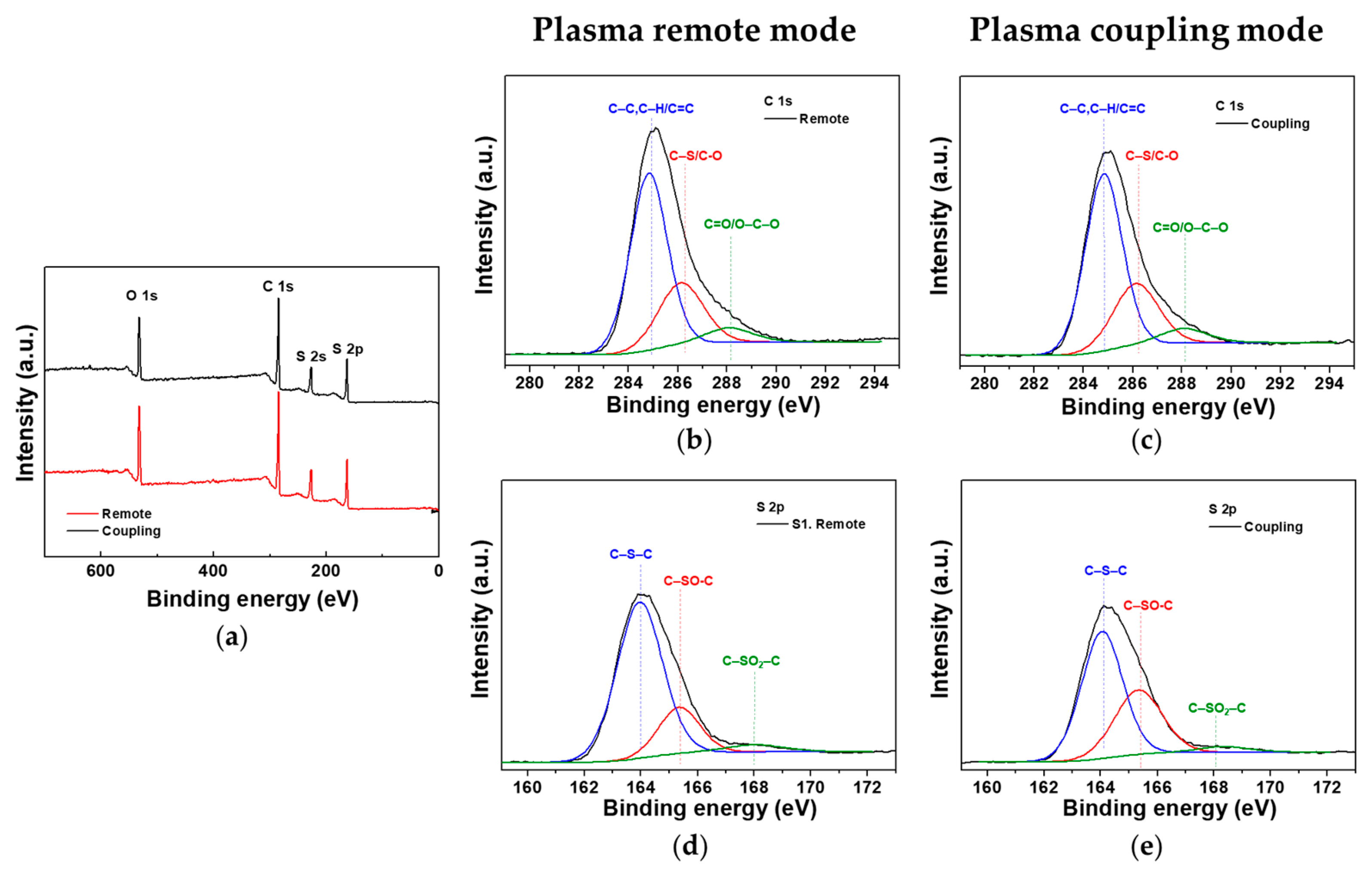
3.5. Morphological and Chemical Properties of the Nanostructured PTh Film
3.6. Comparison of Nanostructured PTh Films Synthesized in the Plasma Remote and Coupling Modes
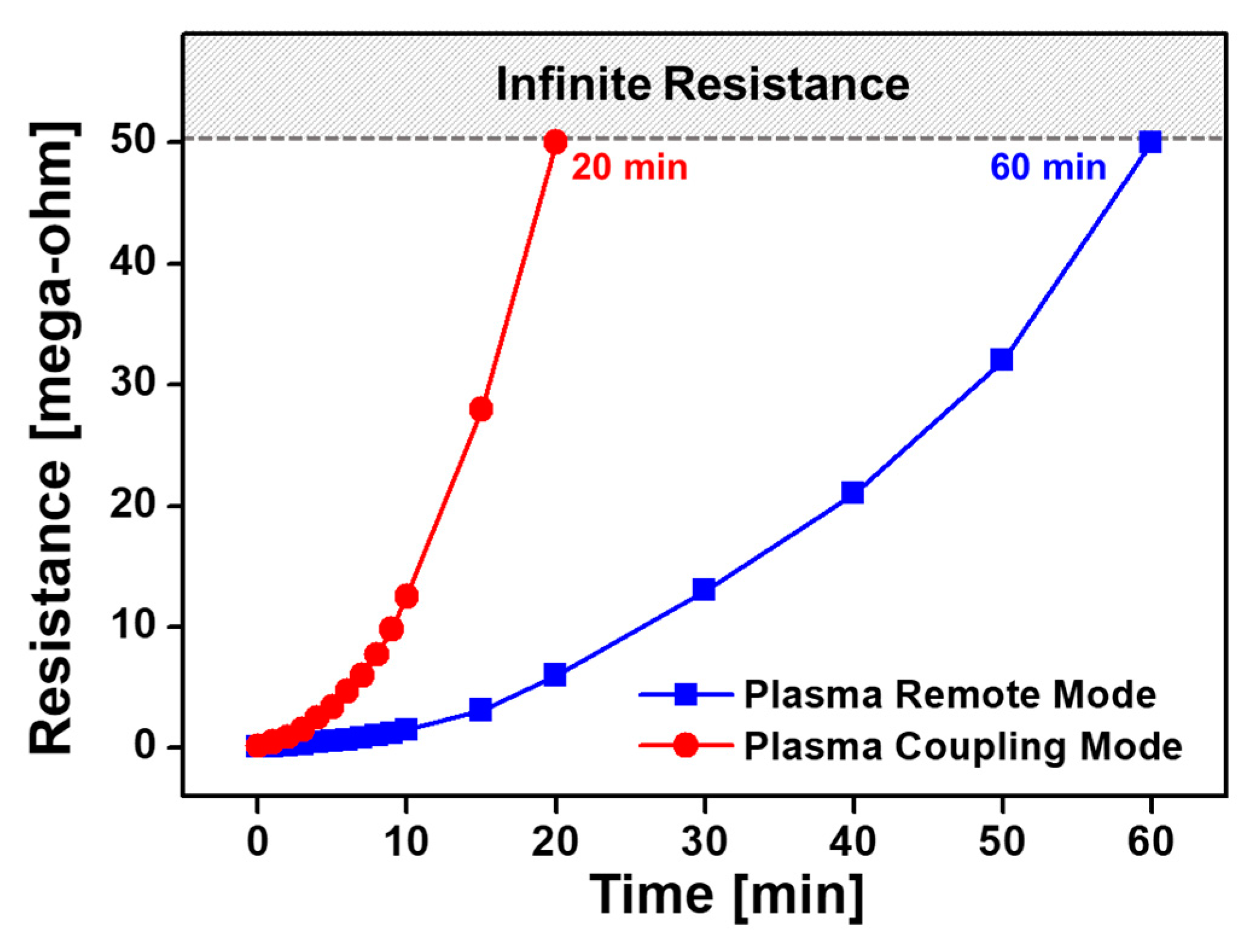
3.7. Electrical Properties of Nanostructured PTh Films Synthesized in the Plasma Remote and Coupling Modes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedrich, J. Mechanisms of plasma polymerization—Reviewed from a chemical point of view. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Bhatt, S.; Pulpytel, J.; Aref-Khonsari, F. Low and atmospheric plasma polymerisation of nanocoatings for bio-applications. Surf. Innov. 2015, 3, 63–83. [Google Scholar] [CrossRef]
- Michel, M.; Bour, J.; Petersen, J.; Arnoult, C.; Ettingshausen, F.; Roth, C.; Ruch, D. Atmospheric plasma deposition: A new pathway in the design of conducting polymer-based anodes for hydrogen. Fuel Cells 2010, 10, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Uygun, A.; Oksuz, L.; Yavuz, A.G.; Guleç, A.; Sen, S. Characteristics of nanocomposite films deposited by atmospheric pressure uniform RF glow plasma. Curr. Appl. Phys. 2011, 11, 250–254. [Google Scholar] [CrossRef]
- Chen, K.S.; Liao, S.C.; Lin, S.W.; Tsao, S.H.; Ting, T.H.; Inagaki, N.; Wu, H.M.; Chen, W.Y. The film deposition via atmospheric pressure plasma from ethanol and He mixing gases. Surf. Coat. Technol. 2013, 231, 408–411. [Google Scholar] [CrossRef]
- Phillips, J.; Luhrs, C.C.; Richard, M. Review: Engineering particles using the aerosol-through-plasma method. IEEE Trans. Plasma Sci. 2009, 37, 726–739. [Google Scholar] [CrossRef] [Green Version]
- Penkov, O.V.; Khadem, M.; Lim, W.-S.; Kim, D.-E. A review of recent applications of atmospheric pressure plasma jets for materials processing. J. Coat. Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Becker, K.H.; Schoenbach, K.H.; Eden, J.G. Microplasmas and applications. J. Phys. D Appl. Phys. 2006, 39, R55–R70. [Google Scholar] [CrossRef]
- Khelifa, F.; Ershov, S.; Habibi, Y.; Snyders, R.; Dubois, P. Free-radical-induced grafting from plasma polymer surfaces. Chem. Rev. 2016, 116, 3975–4005. [Google Scholar] [CrossRef] [PubMed]
- Akishev, Y.; Grushin, M.; Karalnik, V.; Kochetov, I.; Napartovich, A.; Trushkin, N. Generation of atmospheric pressure non-thermal plasma by diffusive and constricted discharges in air and nitrogen at the rest and flow. J. Phys. Conf. Ser. 2010, 257, 012014. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef] [PubMed]
- Haertel, B.; von Woedtke, T.; Weltmann, K.-D.; Lindequist, U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol. Ther. 2014, 22, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Jang, H.J.; Jung, E.Y.; Parsons, T.; Tae, H.-S.; Park, C.-S. A review of plasma synthesis methods for polymer films and nanoparticles under atmospheric pressure conditions. Polymers 2021, 13, 2267. [Google Scholar] [CrossRef]
- Jalaber, V.; Del Frari, D.; De Winter, J.; Mehennaoui, K.; Planchon, S.; Choquet, P.; Detrembleur, C.; Moreno-Couranjou, M. Atmospheric aerosol assisted pulsed plasma polymerization: An environmentally friendly technique for tunable catechol-bearing thin films. Front. Chem. 2019, 7, 183. [Google Scholar] [CrossRef]
- Petersen, J.; Becker, C.; Fouquet, T.; Addiego, F.; Toniazzo, V.; Dinia, A.; Ruch, D. Nano-ordered thin films achieved by soft atmospheric plasma polymerization. RSC Adv. 2013, 3, 4416–4424. [Google Scholar] [CrossRef]
- Sajeeb, U.S.; Mathai, C.J.; Sarvanan, S.; Ashokan, R.R.; Venkatachalam, S.; Anantharaman, M.R. On the optical and electrical properties of rf and a.c. plasma polymerized aniline thin films. Bull. Mater. Sci. 2006, 29, 159–163. [Google Scholar]
- Park, C.-S.; Kim, D.H.; Shin, B.J.; Tae, H.-S. Synthesis and characterization of nanofibrous polyaniline thin film prepared by novel atmospheric pressure plasma polymerization technique. Materials 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.-S.; Kim, D.H.; Shin, B.J.; Kim, D.; Lee, H.-K.; Tae, H.-S. Conductive polymer synthesis with single-crystallinity via a novel plasma polymerization technique for gas sensor applications. Materials 2016, 9, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Park, C.-S.; Kim, W.H.; Shin, B.J.; Hong, J.G.; Park, T.S.; Seo, J.H.; Tae, H.-S. Influences of guide-tube and bluff-body on advanced atmospheric pressure plasma source for single-crystalline polymer nanoparticle synthesis at low temperature. Phys. Plasmas 2017, 24, 23506. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.J.; Park, C.-S.; Jung, E.Y.; Bae, G.T.; Shin, B.J.; Tae, H.-S. Synthesis and properties of thiophene and aniline copolymer using atmospheric pressure plasma jets copolymerization technique. Polymers 2020, 12, 2225. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Iqbal, S.; Jang, H.J.; Jung, E.Y.; Bae, G.T.; Park, C.-S.; Shin, B.J.; Tae, H.-S. Transparent polyaniline thin film synthesized using a low-voltage-driven atmospheric pressure plasma reactor. Materials 2021, 14, 1278. [Google Scholar] [CrossRef]
- Kim, J.Y.; Iqbal, S.; Jang, H.J.; Jung, E.Y.; Bae, G.T.; Park, C.-S.; Tae, H.-S. In-situ iodine doping characteristics of conductive polyaniline film polymerized by low-voltage-driven atmospheric pressure plasma. Polymers 2021, 13, 418. [Google Scholar] [CrossRef]
- Van Vrekhem, S.; Morent, R.; Geyter, N.D. Deposition of a PMMA coating with an atmospheric pressure plasma jet. J. Coat. Technol. Res. 2018, 15, 679–690. [Google Scholar] [CrossRef]
- Srisonphan, S.; Ruangwong, K.; Thammaniphit, C. Localized electric field enhanced streamer cold plasma interaction on biological curved surfaces and its shadow effect. Plasma Chem. Plasma Process. 2020, 40, 1253–1265. [Google Scholar] [CrossRef]
- Li, J.; Lei, B.; Wang, J.; Xu, B.; Ran, S.; Wang, Y.; Zhang, T.; Tang, J.; Zhao, W.; Duan, Y. Atmospheric diffuse plasma jet formation from positive-pseudo-streamer and negative pulseless glow discharges. Commun. Phys. 2021, 4, 64. [Google Scholar] [CrossRef]
- Vanraes, P.; Nikiforov, A.; Bogaerts, A.; Leys, C. Study of an AC dielectric barrier single micro-discharge filament over a water film. Sci. Rep. 2018, 8, 10919. [Google Scholar] [CrossRef] [Green Version]
- Teslaru, T.; Topala, I.; Dobromir, M.; Pohoata, V.; Curecheriu, L.; Dumitrascu, N. Polythiophene films obtained by polymerization under atmospheric pressure plasma conditions. Mater. Chem. Phys. 2016, 169, 120–127. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, L.; Tu, J.; Jiao, S. Electrochemically assembling of polythiophene film in ionic liquids(ILs) microemulsions and its application in an electrochemical capacitor. Electrochim. Acta 2014, 120, 122–127. [Google Scholar] [CrossRef]
- Navale, S.T.; Mane, A.T.; Khuspe, G.D.; Chougule, M.A.; Patil, V.B. Room temperature NO2 sensing properties of polythiophene films. Synth. Met. 2014, 195, 228–233. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.-S.; Jung, E.Y.; Kum, D.S.; Kim, J.Y.; Kim, D.; Bae, G.T.; Cho, B.-G.; Shin, B.J.; Lee, D.H.; et al. Experimental study on atmospheric pressure plasma polymerized conducting polymer under coupling and remote conditions. Mol. Cryst. Liq. Cryst. 2018, 663, 108–114. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.-S.; Jung, E.Y.; Shin, B.J.; Kim, J.Y.; Bae, G.T.; Jang, H.J.; Cho, B.-G.; Tae, H.-S. Effects of iodine dopant on atmospheric pressure plasma polymerized pyrrole in remote and coupling methods. Mol. Cryst. Liq. Cryst. 2018, 677, 135–142. [Google Scholar] [CrossRef]
- Ji, H.; Wang, T.; Liu, Y.; Lu, C.; Yang, G.; Ding, W.; Hou, W. A novel approach for sulfur-doped hierarchically porous carbon with excellent capacitance for electrochemical energy storage. Chem. Commun. 2016, 52, 12725–12728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, W.; Li, Y.; Gai, L.; Zhou, J.; Ma, W. Multishell structured magnetic nanocomposites carrying a copolymer of pyrrole-thiophene for highly selective Au(III) recovery. J. Mater. Chem. A 2016, 4, 19060–19069. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Nash, J.; Macdonald, T.J.; Jasieniak, M.; Griesser, H.J.; Nann, T. Photo-doping of plasma-deposited polyaniline (PAni). RSC Adv. 2016, 6, 70691–70699. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Xu, X. A simple strategy to enhance electrical conductivity of nanotube-conjugate polymer composites via io-dine-doping. RSC Adv. 2015, 5, 78104–78108. [Google Scholar] [CrossRef]
- Pron, A.; Rannou, P. Processible conjugated polymers: From organic semiconductors to organic metals and superconductors. Prog. Polym. Sci. 2002, 27, 135–190. [Google Scholar] [CrossRef]
- Mathai, C.J.; Saravanan, S.; Anatharaman, M.R.; Venkitachalam, S.; Jayalekshmi, S. Effect of iodine doping on the bandgap of plasma polymerized aniline thin films. J. Phys. D Appl. Phys. 2002, 35, 2206–2210. [Google Scholar] [CrossRef]
- Wang, J.; Neoh, K.G.; Kang, E.T. Comparative study of chemically synthesized and plasma polymerized pyrrole and thio-phene thin films. Thin Solid Films 2004, 446, 205–217. [Google Scholar] [CrossRef]
- Silverstein, M.S.; Visoly-Fisher, I. Plasma polymerized thiophene: Molecular structure and electrical properties. Polymer 2002, 43, 11–20. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jang, H.-J.; Jung, E.; Bae, G.; Lee, S.; Park, C.-S.; Shin, B.; Tae, H.-S. Improvement of the uniformity and electrical properties of polyaniline nanocomposite film by addition of auxiliary gases during atmospheric pressure plasma polymerization. Nanomaterials 2021, 11, 2315. [Google Scholar] [CrossRef]
- Park, C.-S.; Jung, E.Y.; Kim, D.H.; Kim, D.Y.; Lee, H.-K.; Shin, B.J.; Lee, D.H.; Tae, H.-S. Atmospheric pressure plasma polymerization synthesis and characterization of polyaniline films doped with and without iodine. Materials 2017, 10, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.-S.; Kim, D.Y.; Kim, D.H.; Lee, H.-K.; Shin, B.J.; Tae, H.-S. Humidity-independent conducting polyaniline films syn-thesized using advanced atmospheric pressure plasma polymerization with in-situ iodine doping. Appl. Phys. Lett. 2017, 110, 33502. [Google Scholar] [CrossRef] [Green Version]
- Ameen, S.; Song, M.; Kim, D.-G.; Im, Y.-B.; Seo, H.-K.; Kim, Y.S.; Shin, H.-S. Iodine doped polyaniline thin film for hetero-structure devices via PECVD technique: Morphological, structural, and electrical properties. Macromol. Res. 2012, 20, 30–36. [Google Scholar] [CrossRef]
- Mohammed, M.K.A.; Al-Mousoi, A.K.; Khalaf, H.A. Deposition of multi-layer graphene (MLG) film on glass slide by flame synthesis technique. Optik 2016, 127, 9848–9852. [Google Scholar] [CrossRef]
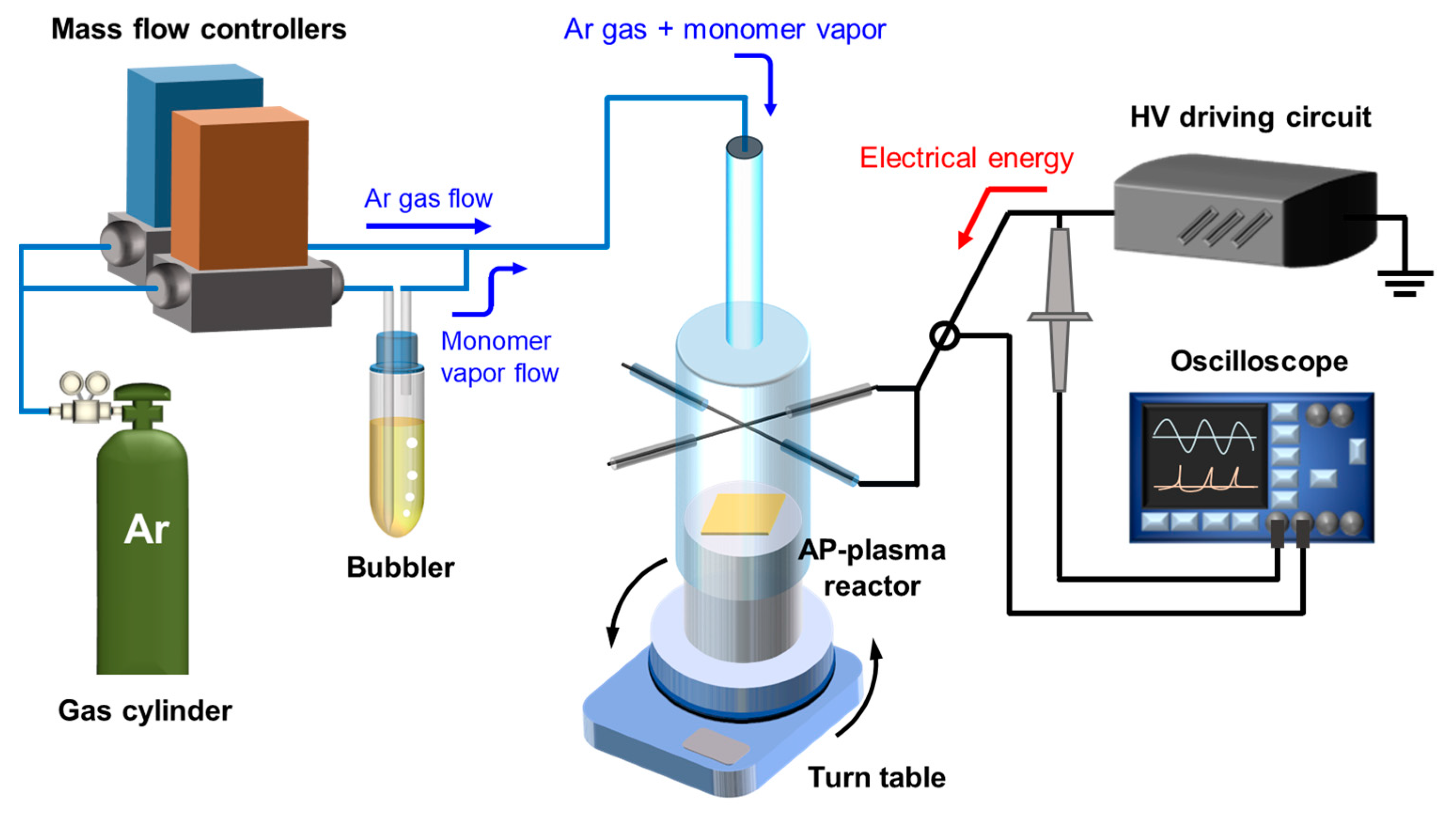
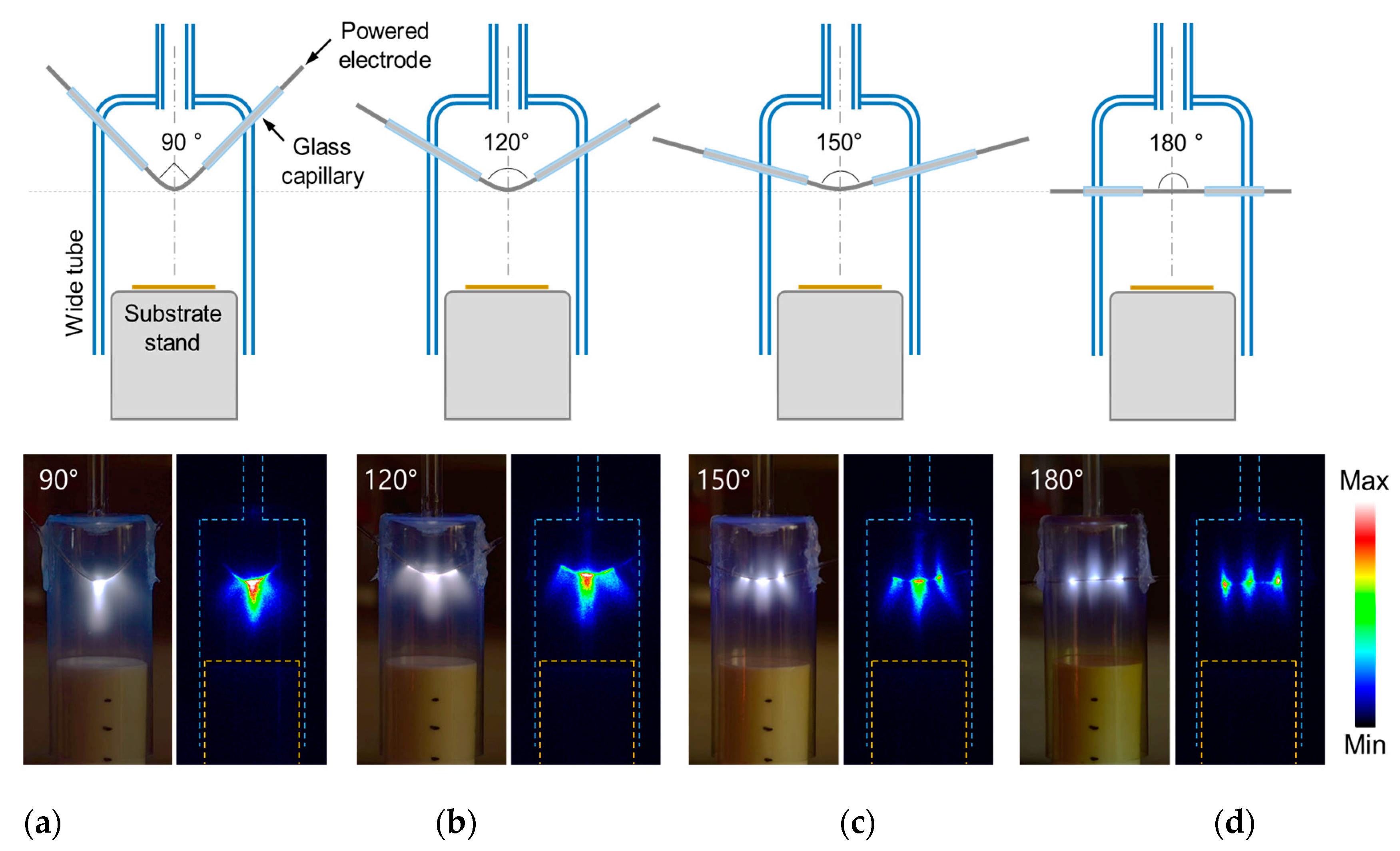
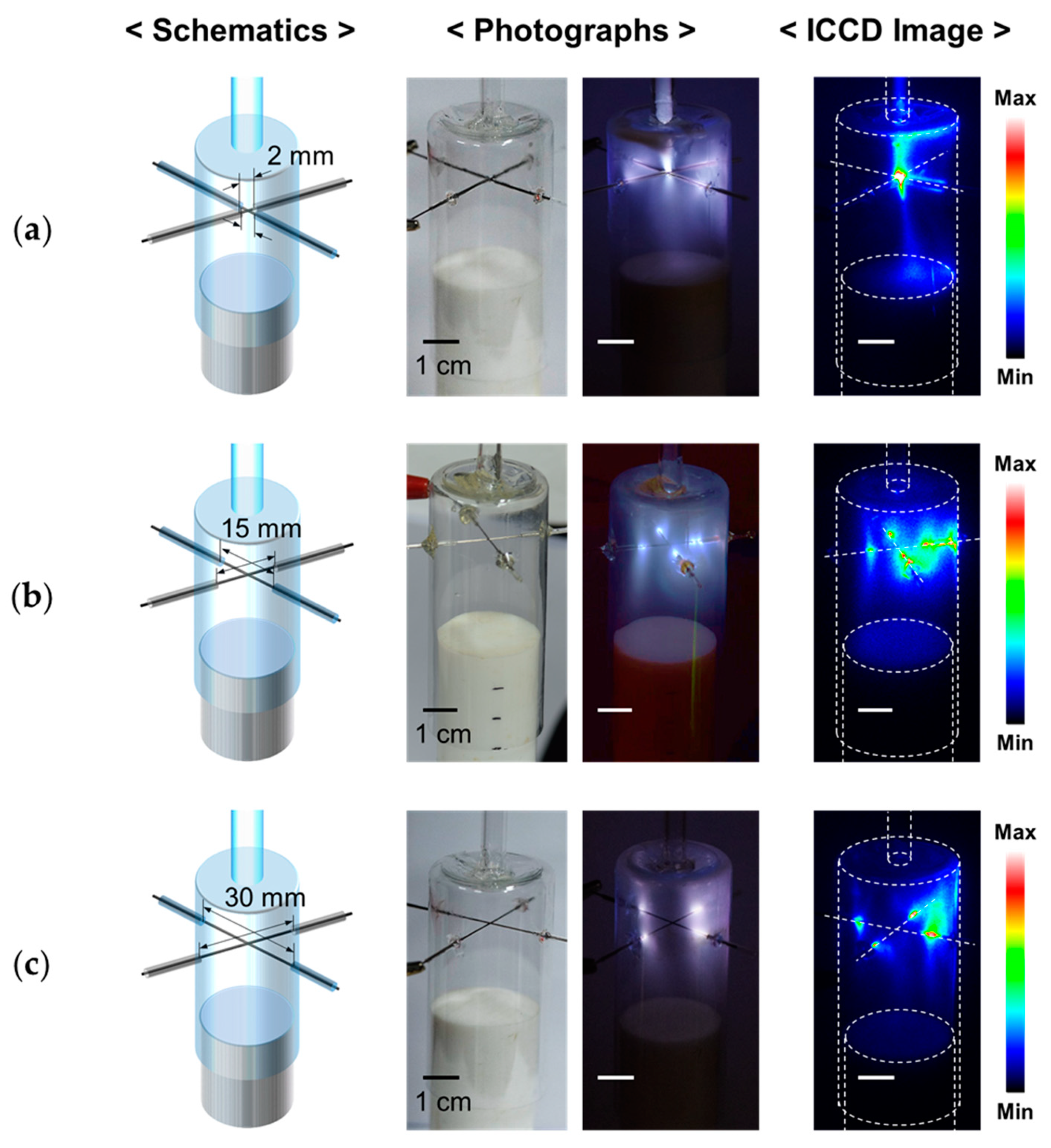

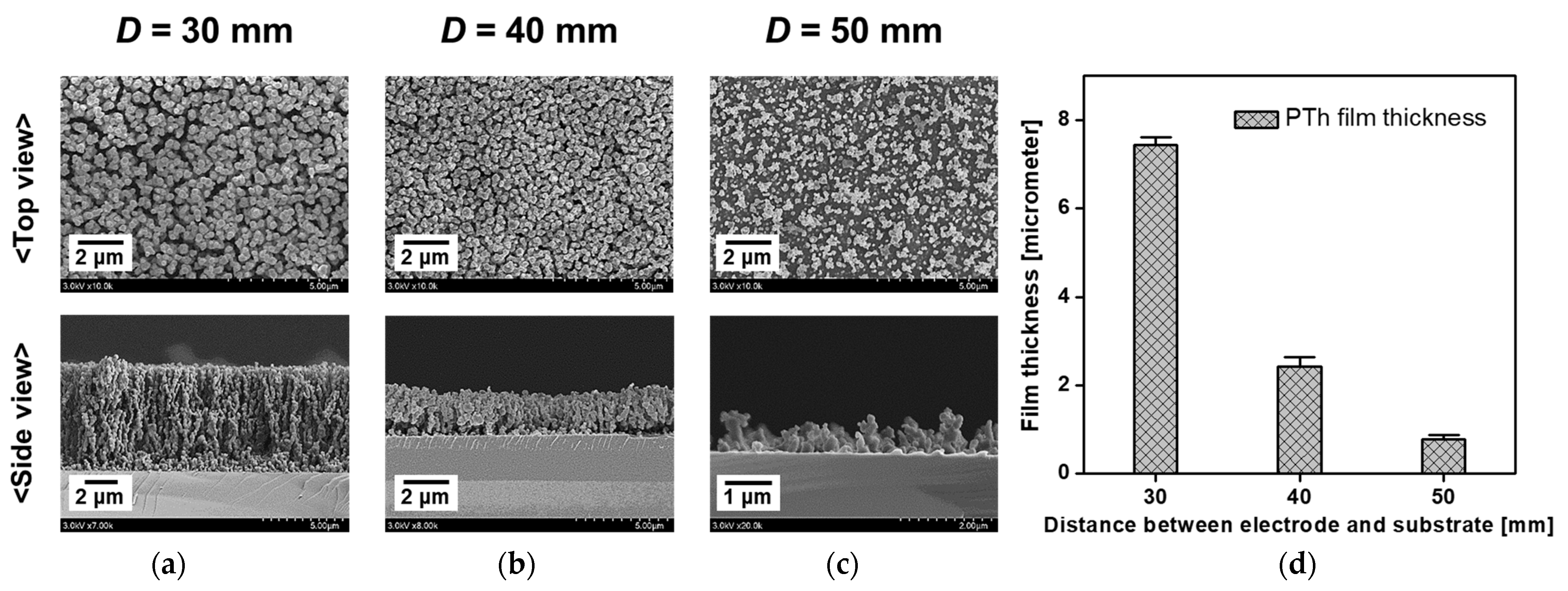


| Experimental Conditions | AP Plasma Reactor | |
|---|---|---|
| Device Configuration | Powered electrode shape | Cruciform |
| Electrode material | Tungsten wire | |
| Inner diameter of wide tube | 34 mm | |
| Diameter of substrate stand | 30 mm | |
| Distance between electrode and substrate | 30 mm | |
| Driving Conditions | Voltage waveform | Sinusoidal |
| Plasma initiation voltage (Vp) | 4 kV | |
| Plasma driving voltage (Vp) | 10 kV | |
| Driving frequency | 28 kHz | |
| Averaged power 1 | 4.40 W | |
| Gas Conditions | Discharge and monomer carrier gas | Ar (HP grade: 99.999%) |
| Gas flow rate for AP plasma discharge | 1000 sccm | |
| Gas flow rate for thiophene monomer vapor | 100 sccm | |
| Polymerization process time | 10 min | |
| Parametric Studies | |||||
|---|---|---|---|---|---|
| Conditions | Variables | ||||
| Length of the bare-wire part of the electrode | 2, 15, 30 mm | 15 mm | 15 mm | 15 mm | 15 mm |
| Distance between electrode and substrate | 30 mm | 30, 40, 50 mm | 30 mm | 30 mm | 20 mm |
| Ar flow rate for AP plasma discharge | 1000 sccm | 1000 sccm | 1000 sccm | 1000 sccm | 1000 sccm |
| Ar flow rate for thiophene monomer vapor | 100 sccm | 100 sccm | 100–500 sccm | 100 sccm | 100 sccm |
| Polymerization process time | 10 min | 10 min | 10 min | 10, 20, 30 min | 10 min |
| Results (Figures) | 3 and 4 | 5 and 6 | 8 | 9 | 10–12 |
| Conditions | C (%) | O (%) | S (%) | C/S |
|---|---|---|---|---|
| Plasma remote mode | 65.4 | 18.5 | 16.1 | 4.1 |
| Plasma coupling mode | 66.6 | 17.0 | 16.5 | 4.0 |
| Peak Assignment | Binding Energy (eV) | Composition (%) | ||
|---|---|---|---|---|
| Remote Mode | Coupling Mode | |||
| C 1s | C–C, C–H, C=C | 284.9 | 62.6 | 65.3 |
| C–S, C–O | 286.2 | 31.3 | 28.1 | |
| C=O, O–C–O | 288.1 | 6.1 | 6.6 | |
| S 2p | C–S–C ➀ | 164.0 | 75.2 | 60.8 |
| C–SO–C ➁ | 165.3 | 20.3 | 35.7 | |
| C–SO2–C ➂ | 168.2 | 4.5 | 3.5 | |
| (➁ + ➂)/➀ | - | 0.33 | 0.64 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Jang, H.J.; Bae, G.T.; Park, C.-S.; Jung, E.Y.; Tae, H.-S. Improvement of Nanostructured Polythiophene Film Uniformity Using a Cruciform Electrode and Substrate Rotation in Atmospheric Pressure Plasma Polymerization. Nanomaterials 2022, 12, 32. https://doi.org/10.3390/nano12010032
Kim JY, Jang HJ, Bae GT, Park C-S, Jung EY, Tae H-S. Improvement of Nanostructured Polythiophene Film Uniformity Using a Cruciform Electrode and Substrate Rotation in Atmospheric Pressure Plasma Polymerization. Nanomaterials. 2022; 12(1):32. https://doi.org/10.3390/nano12010032
Chicago/Turabian StyleKim, Jae Young, Hyo Jun Jang, Gyu Tae Bae, Choon-Sang Park, Eun Young Jung, and Heung-Sik Tae. 2022. "Improvement of Nanostructured Polythiophene Film Uniformity Using a Cruciform Electrode and Substrate Rotation in Atmospheric Pressure Plasma Polymerization" Nanomaterials 12, no. 1: 32. https://doi.org/10.3390/nano12010032
APA StyleKim, J. Y., Jang, H. J., Bae, G. T., Park, C.-S., Jung, E. Y., & Tae, H.-S. (2022). Improvement of Nanostructured Polythiophene Film Uniformity Using a Cruciform Electrode and Substrate Rotation in Atmospheric Pressure Plasma Polymerization. Nanomaterials, 12(1), 32. https://doi.org/10.3390/nano12010032








