Coconut Carbon Dots: Progressive Large-Scale Synthesis, Detailed Biological Activities and Smart Sensing Aptitudes towards Tyrosine
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Chemicals
2.2. Fabrication of Coconut-Derived C-Dots via Thermal Calcination
2.3. Characterizations
2.4. Quantum Yield
2.5. Physiological Activity of C-Dots
2.5.1. Time Studies
2.5.2. Influence of pH
2.5.3. Salt Effect Analysis
2.6. Biological Activity of C-Dots
2.6.1. Binding Mechanism
2.6.2. Hemolysis and Blood Clotting Index Value in the Presence of C-Dots
2.6.3. MTT Assay
2.6.4. ROS Detection Using DCFH-DA
2.7. Response Behavior of C-Dots towards Amino Acids
2.7.1. Sensing Procedure
2.7.2. Concentration-Dependent Study of Tyrosine
2.7.3. Calculation of Detection Limit and Quantitation Limit
2.7.4. Calculation of Binding Constant
2.7.5. Interference Studies
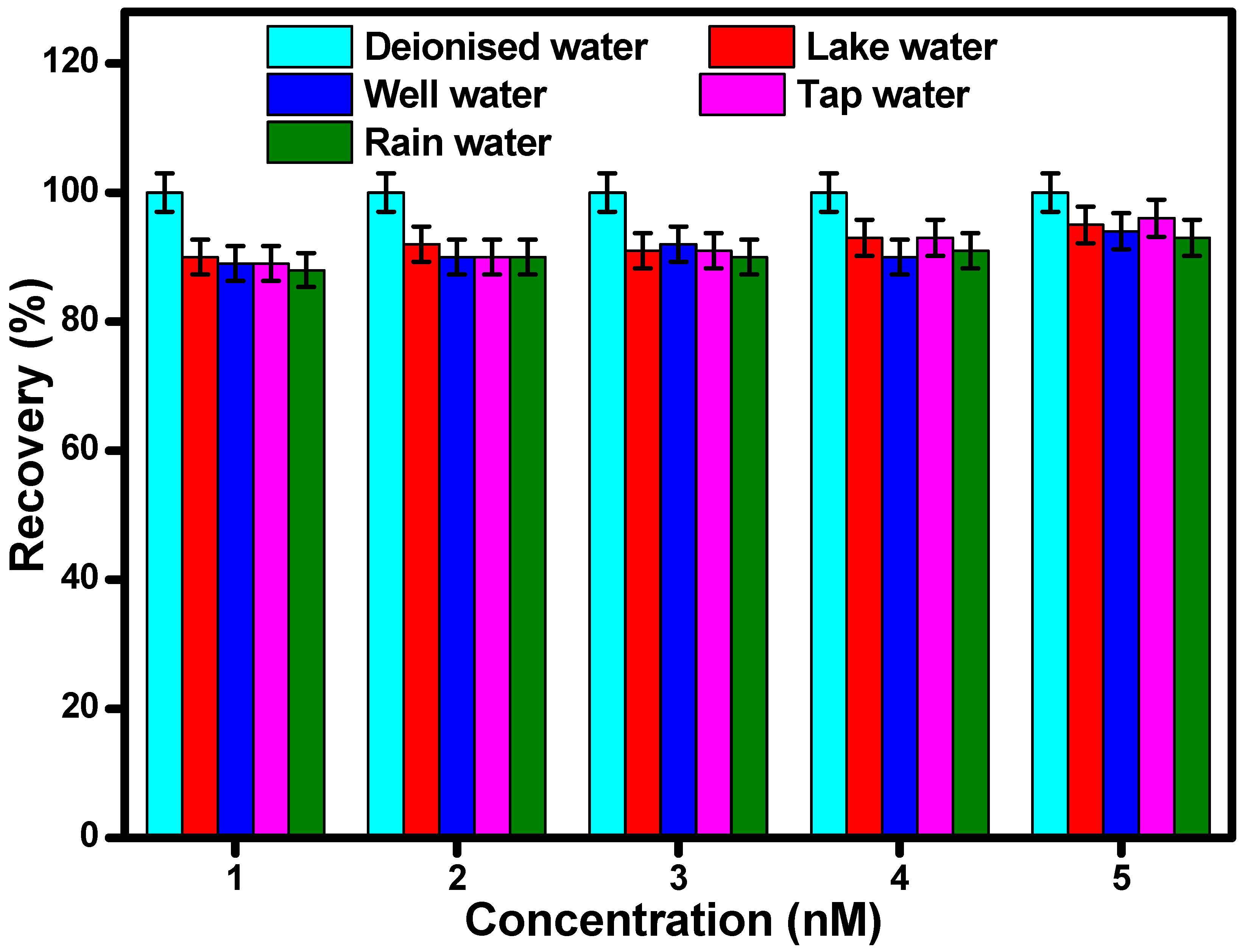
2.7.6. Analysis of Real Water Samples
3. Results and Discussion
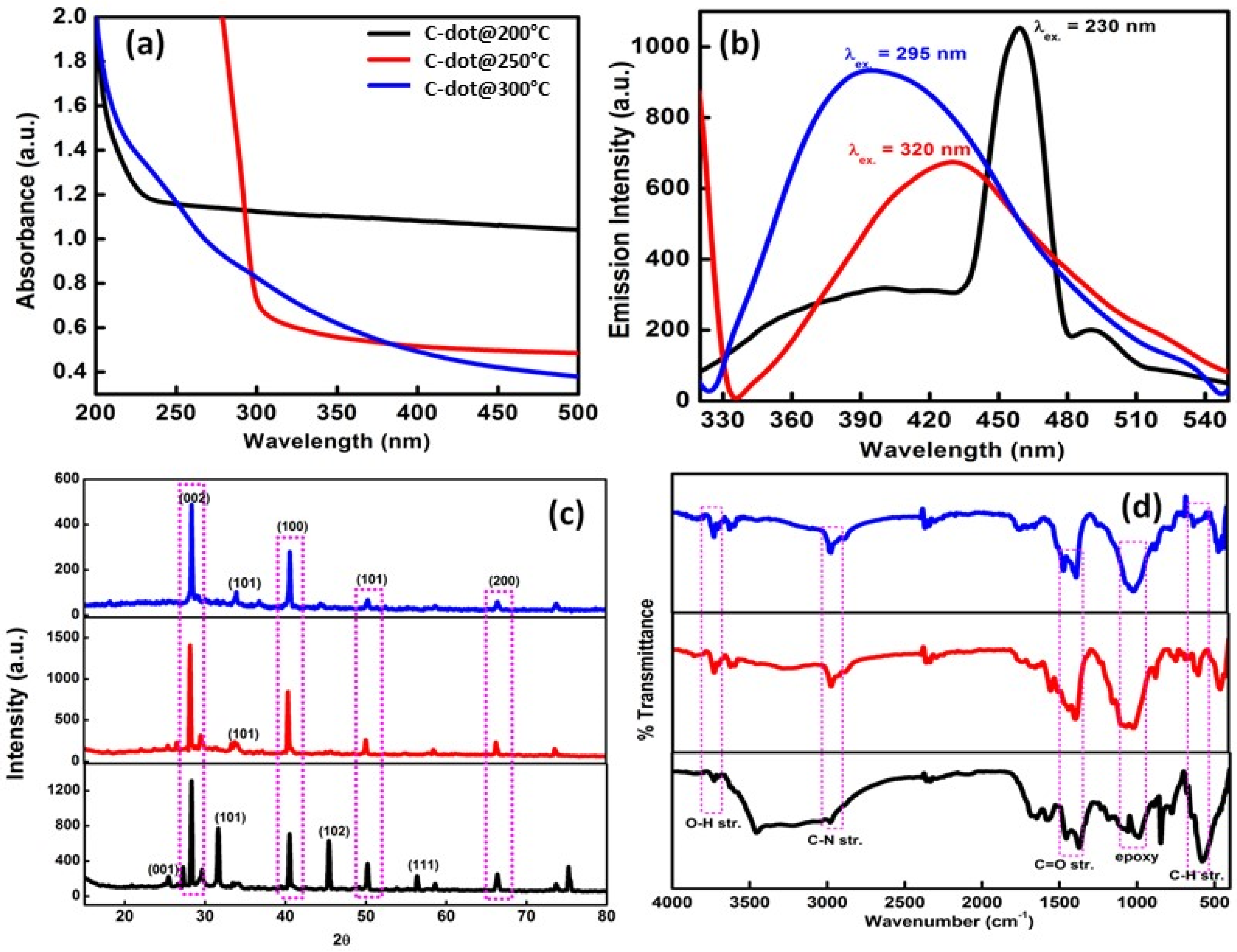
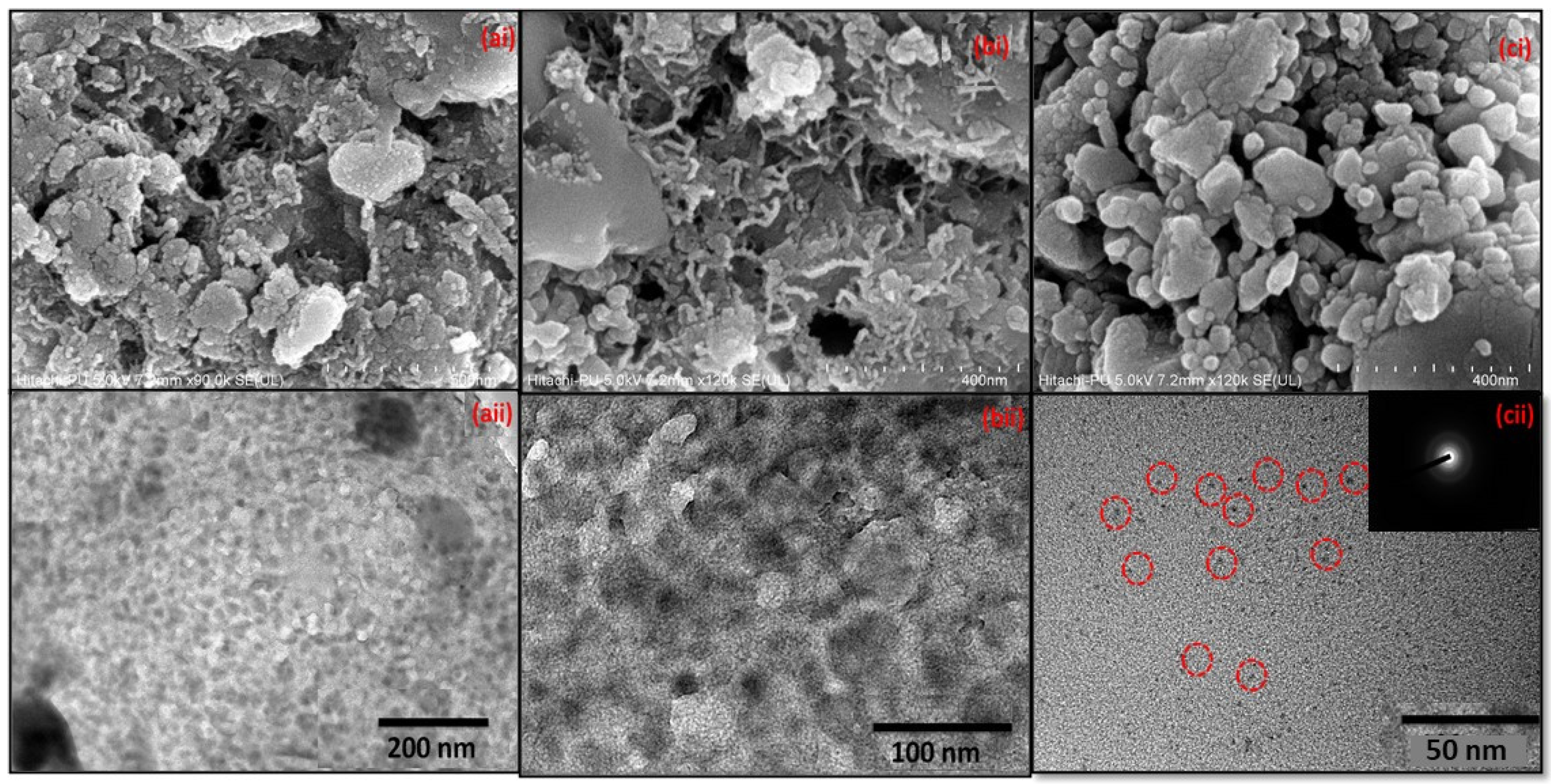
3.1. Characterization of C-Dots
3.1.1. Optical, Emission, Surface, Structural, and Morphological Characteristics of C-Dots
3.1.2. Thermal Properties of C-Dots
3.2. Physiological Activity of C-Dots
3.3. Biological Activity of C-Dots
3.3.1. Binding Mechanism
3.3.2. Hemolysis and Blood Clotting Index Value in the Presence of C-Dots
3.3.3. MTT Assay
3.3.4. ROS Detection Using DCFH-DA
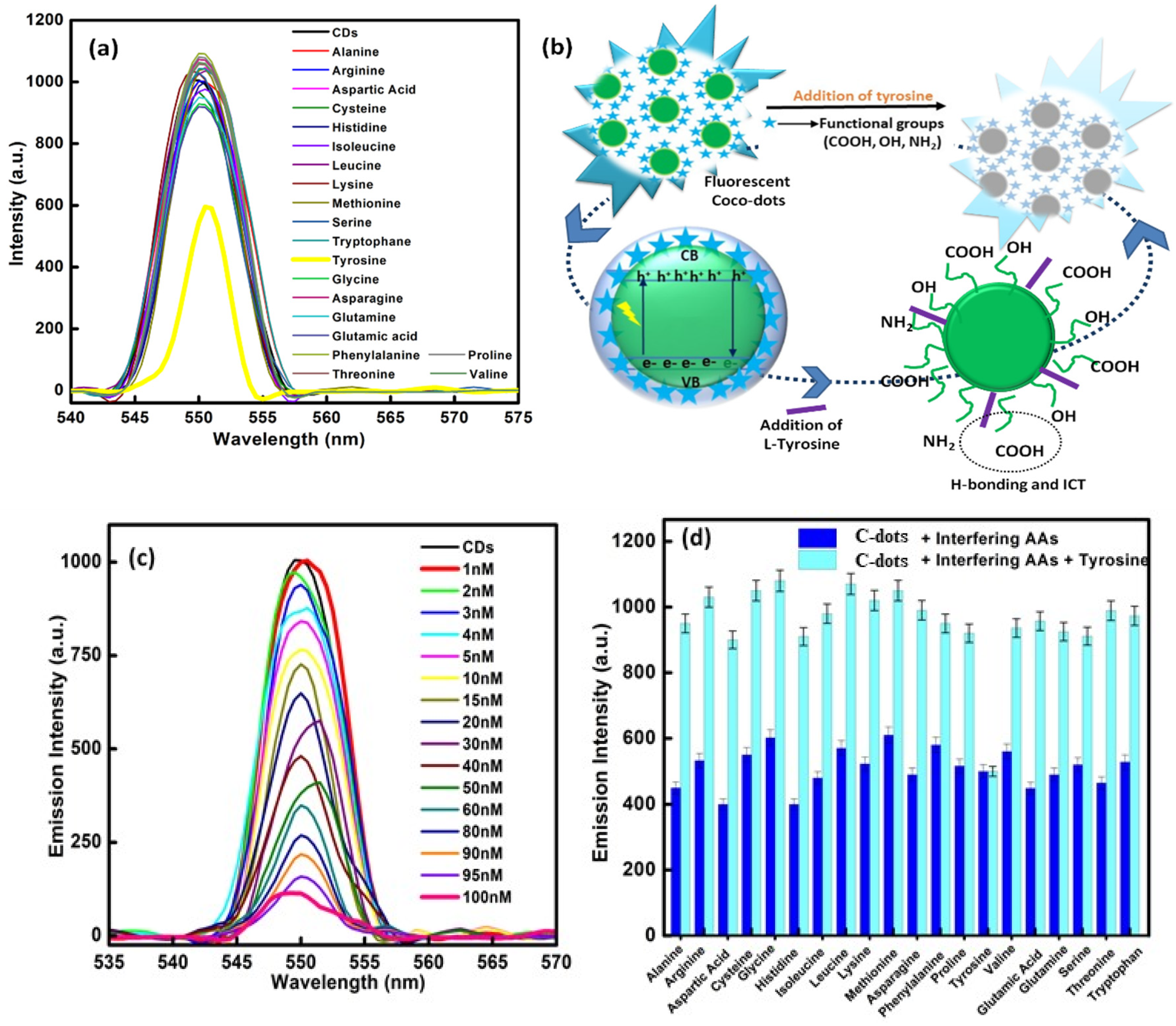
3.4. Response Behavior of C-Dots towards Amino Acids
Selectivity Study of C-Dots
3.5. Practical Application of the Developed Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ying, X.; Zhu, X.; Kang, A.; Li, X. Molecular imprinted electrospun chromogenic membrane for l-tyrosine specific recognition and visualized detection. Talanta 2019, 204, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, X.-Q.; Zhao, G.-Q.; Jiang, X.-Y.; Jiao, F.-P.; Yu, J.-G. A novel electrochemical chiral interface based on the synergistic effect of polysaccharides for the recognition of tyrosine enantiomers. Talanta 2019, 195, 628–637. [Google Scholar] [CrossRef]
- Hale, A.J.; Steege, E.T.; den Hertog, J. Recent advances in understanding the role of protein-tyrosine phosphatases in development and disease. Dev. Biol. 2017, 428, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Zhang, Z.-Y. Regulatory Mechanisms and Novel Therapeutic Targeting Strategies for Protein Tyrosine Phosphatases. Chem. Rev. 2018, 118, 1069–1091. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Zhao, M.; Liu, W.; Yu, S.; Niu, L.; Li, G.; Li, H.; Liu, W. Electrochemical sensor based on molecularly imprinted polymer/reduced graphene oxide composite for simultaneous determination of uric acid and tyrosine. J. Electroanal. Chem. 2018, 813, 75–82. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Ichinose, H.; Kobayashi, K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural Transm. 2019, 126, 397–409. [Google Scholar] [CrossRef]
- Aliu, E.; Kanungo, S.; Arnold, G.L. Amino acid disorders. Ann. Transl. Med. 2018, 6, 471. [Google Scholar] [CrossRef]
- Alsharhan, H.; Ficicioglu, C. Disorders of phenylalanine and tyrosine metabolism. Transl. Sci. Rare Dis. 2020, 5, 3–58. [Google Scholar] [CrossRef]
- Krishnan, R.G.; Saraswathyamma, B. Simultaneous Resolution and Electrochemical Quantification of Tyrosine and Tryptophan at a Poly (Diphenylamine) Modified Electrode. J. Electrochem. Soc. 2021, 168, 027509. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Yeh, Y.-C. Detection of tyrosine and monitoring tyrosinase activity using an enzyme cascade-triggered colorimetric reaction. RSC Adv. 2020, 10, 29745–29750. [Google Scholar] [CrossRef]
- Feng, D.-Q.; Liu, G.; Ma, G.; Nan, Z.; Wang, W. Phosphodiesters quaternary ammonium nanoparticles as label-free light scattering probe for turn-off detection of tyrosine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhou, Z.; Qin, D.; Huang, W.; Feng, J.; Tang, T.; Hu, G.; Li, L. Electrochemical Sensor Based on Electrospun Three-Dimensional Carbon Nanofibers to Determine Trace Levels of Cu(II). Sci. Adv. Mater. 2020, 12, 693–700. [Google Scholar] [CrossRef]
- Vyas, G.; Bhatt, S.; Si, M.K.; Jindani, S.; Suresh, E.; Ganguly, B.; Paul, P. Colorimetric dual sensor for Cu(II) and tyrosine and its application as paper strips for detection in water and human saliva as real samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118052. [Google Scholar] [CrossRef]
- Mahawong, S.; Dechtrirat, D.; Watcharin, W.; Wattanasin, P.; Muensit, N.; Chuenchom, L. Mesoporous Magnetic Carbon Adsorbents Prepared from Sugarcane Bagasse and Fe2+ and Fe3+ via Simultaneous Magnetization and Activation for Tetracycline Adsorption. Sci. Adv. Mater. 2020, 12, 161–172. [Google Scholar] [CrossRef]
- Zribi, R.; Maalej, R.; Messina, E.; Gillibert, R.; Donato, M.G.; Maragò, O.M.; Gucciardi, P.G.; Leonardi, S.G.; Neri, G. Exfoliated 2D-MoS2 nanosheets on carbon and gold screen printed electrodes for enzyme-free electrochemical sensing of tyrosine. Sens. Actuators B Chem. 2020, 303, 127229. [Google Scholar] [CrossRef]
- Chauhan, P.; Dogra, S.; Chaudhary, S.; Kumar, R. Usage of coconut coir for sustainable production of high-valued carbon dots with discriminatory sensing aptitude toward metal ions. Mater. Today Chem. 2020, 16, 100247. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Bose, M.; Mondal, S.; Das, A.K.; Das, N.C. Strongly blue-luminescent N-doped carbogenic dots as a tracer metal sensing probe in aqueous medium and its potential activity towards in situ Ag-nanoparticle synthesis. Sens. Actuators B Chem. 2017, 252, 735–746. [Google Scholar] [CrossRef]
- Das, P.; Moorthy, M.; Arumugam, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Shahraki, H.S.; Ahmad, A.; Bushra, R. Green carbon dots with multifaceted applications-Waste to wealth strategy. FlatChem 2021, 31, 100310. [Google Scholar] [CrossRef]
- Wang, G.; Mamat, X.; Li, Y.; Hu, X.; Wang, P.; Xin, X.; Hu, G. Highly Sensitive Electrochemical Sensor for the Detection of Chloramphenicol Based on Biomass Derived Porous Carbon. Sci. Adv. Mater. 2020, 12, 376–382. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Shi, P.; Xiao, Y.; Shi, Y.; Ding, L.; Xu, W. Synthesis of Fluorescent Carbon Quantum Dots from Tobacco Wastewater for the Detection of Ferric (III) Ions. Sci. Adv. Mater. 2020, 12, 966–972. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumari, M.; Chauhan, P.; Chaudhary, G.R. Upcycling of plastic waste into fluorescent carbon dots: An environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag. 2021, 120, 675–686. [Google Scholar] [CrossRef]
- Kumari, M.; Chaudhary, S. Modulating the physicochemical and biological properties of carbon dots synthesized from plastic waste for effective sensing of E. coli. Colloid Surf. B 2020, 196, 111333. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Chaudhary, S.; Kumar, R. Biogenic approach for fabricating biocompatible carbon dots and their application in colorimetric and fluorometric sensing of lead ion. J. Clean. Prod. 2021, 279, 123639. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Zhang, K.; Jiang, H.; Ji, T.; Li, J.; Liu, J.; Han, W. Loofah Activated Carbon Sodium Alginate Hydrogel Microspheres with High Efficiency Cyclic Adsorption for Antibiotic Contaminants. J. Nanoelectron. Optoelectron. 2020, 15, 219–225. [Google Scholar] [CrossRef]
- Sangubotla, R.; Kim, J. Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Mater. Sci. Eng. C 2021, 122, 111916. [Google Scholar] [CrossRef]
- Li, M.; Han, K.; Gao, Y.; Teng, Z.; Li, J.; Wang, M. Calcium-Assisted Regulation of the Structures of Porous Carbons at the Micro-Nano Scale. J. Nanoelectron. Optoelectron. 2020, 15, 284–290. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Agarwal, T.; Maity, P.; Ghosh, S.; Choudhary, S.; Gangopadhyay, S.; Maiti, T.K.; Dhara, S.; Banerjee, S.; et al. Heteroatom doped blue luminescent carbon dots as a nano-probe for targeted cell labeling and anticancer drug delivery vehicle. Mater. Chem. Phys. 2019, 237, 121860. [Google Scholar] [CrossRef]
- Kocaman, S. Synthesis and cationic dye biosorption properties of a novel low-cost adsorbent: Coconut waste modified with acrylic and polyacrylic acids. Int. J. Phytoremediation 2020, 22, 551–566. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Escobar-Villanueva, A.G.; Martínez-Gutiérrez, H.; González-Ortega, O. Interfacial photothermal water evaporator based on nanoporous microwave-expanded graphite and coconut waste fibers@recycled polystyrene as substrate. Int. J. Energy Res. 2020, 44, 10878–10893. [Google Scholar] [CrossRef]
- Kusumawati, N.; Muslim, S. Exploration and Standarization of Coconut Fiber Waste Utilization in Batik Dyeing Process. IOP Conf. Ser. Earth Environ. Sci. 2021, 709, 012034. [Google Scholar] [CrossRef]
- Jerzak, W.; Kuźnia, M. Examination of inorganic gaseous species and condensed phases during coconut husk combustion based on thermodynamic equilibrium predictions. Renew. Energy 2021, 167, 497–507. [Google Scholar] [CrossRef]
- Rahim, A.R.A.; Mohsin, H.M.; Thanabalan, M.; Rabat, N.E.; Saman, N.; Mat, H.; Johari, K. Effective carbonaceous desiccated coconut waste adsorbent for application of heavy metal uptakes by adsorption: Equilibrium, kinetic and thermodynamics analysis. Biomass Bioenergy 2020, 142, 105805. [Google Scholar] [CrossRef]
- Sulaiman, S.A.; Roslan, R.; Inayat, M.; Naz, M.Y. Effect of blending ratio and catalyst loading on co-gasification of wood chips and coconut waste. J. Energy Inst. 2018, 91, 779–785. [Google Scholar] [CrossRef]
- Kocaman, S.; Karaman, M.; Gursoy, M.; Ahmetli, G. Chemical and plasma surface modification of lignocellulose coconut waste for the preparation of advanced biobased composite materials. Carbohydr. Polym. 2017, 159, 48–57. [Google Scholar] [CrossRef]
- Sulaiman, S.; Ruslan, N.I.F. A heterogeneous catalyst from a mixture of coconut waste and eggshells for biodiesel production. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 154–159. [Google Scholar] [CrossRef]
- Choi, H.; Choi, C.; Kwon, S.H.; Park, Y.; Choi, W. Characteristics of Carbon Nanowalls According to the Indium Tin Oxide Interlayer Thickness. Sci. Adv. Mater. 2020, 12, 1261–1264. [Google Scholar] [CrossRef]
- Bhasin, A.K.K.; Chauhan, P.; Chaudhary, S. A novel sulfur-incorporated naphthoquinone as a selective “turn-on” fluorescence chemical sensor for rapid detection of Ba2+ ion in semi-aqueous medium. Sens. Actuators B Chem. 2019, 294, 116–122. [Google Scholar] [CrossRef]
- Tian, Y.-F.; Liao, R.-J. Recombination and Electrochemical Performance of Iodine and Mesoporous Carbon. J. Nanoelectron. Optoelectron. 2020, 15, 1089–1093. [Google Scholar]
- Lawson-Wood, K.; Upstone, S.; Evans, K. Determination of Relative Fluorescence Quantum Yields using the FL6500 Fluorescence Spectrometer. Fluoresc. Spectrosc. 2018, 4, 1–5. [Google Scholar]
- Chen, F. Raymond, Fluorescence quantum yields of tryptophan and tyrosine. Anal. Lett. 1967, 1, 35–42. [Google Scholar] [CrossRef]
- Sonthanasamy, R.S.A.; Sulaiman, N.M.N.; Tan, L.L.; Lazim, A.M. Comprehensive spectroscopic studies of synergism between Gadong starch based carbon dots and bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 218, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fang, J.; Cheng, Y.; Zheng, J.; Zhang, J.; Chen, T.; Ruan, B.H. Biomolecular interaction analysis for carbon nanotubes and for biocompatibility prediction. Anal. Biochem. 2016, 505, 1–7. [Google Scholar] [CrossRef]
- Sahiner, N. Single step poly(l-Lysine) microgel synthesis, characterization and biocompatibility tests. Polymer 2017, 121, 46–54. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture Using Human and Other Tumor Cell Lines. Am. Assoc. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Yun, K. Dual sensing of tetracycline and l-Lysine using green synthesized carbon dots from Nigella sativa seeds. Dye. Pigment. 2020, 182, 108640. [Google Scholar] [CrossRef]
- Cheng, R.; Yu, C.; Zhen, Z.; Tang, S.; Ou, S. Understanding the selective-sensing mechanism of lysine by fluorescent nanosensors based on graphene quantum dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118732. [Google Scholar] [CrossRef] [PubMed]
- Zaib, M.; Akhtar, A.; Maqsood, F.; Shahzadi, T. Green Synthesis of Carbon Dots and Their Application as Photocatalyst in Dye Degradation Studies. Arab. J. Sci. Eng. 2021, 46, 437–446. [Google Scholar] [CrossRef]
- Devi, P.; Kaur, G.; Thakur, A.; Kaur, N.; Grewal, A.; Kumar, P. Waste derivitized blue luminescent carbon quantum dots for selenite sensing in water. Talanta 2017, 170, 49–55. [Google Scholar] [CrossRef]
- Park, S.J.; Park, J.Y.; Chung, J.W.; Yang, H.K.; Moon, B.K.; Yi, S.S. Color tunable carbon quantum dots from wasted paper by different solvents for anti-counterfeiting and fluorescent flexible film. Chem. Eng. J. 2020, 383, 123200. [Google Scholar] [CrossRef]
- Devi, S.; Gupta, R.K.; Paul, A.K.; Tyagi, S. Waste carbon paper derivatized Carbon Quantum Dots/(3-Aminopropyl)triethoxysilane based fluorescent probe for trinitrotoluene detection. Mater. Res. Express 2018, 6, 025605. [Google Scholar] [CrossRef]
- Hong, W.T.; Park, J.Y.; Chung, J.W.; Yang, H.K.; Je, J.Y. Anti-counterfeiting application of fluorescent carbon dots derived from wasted coffee grounds. Optik 2021, 241, 166449. [Google Scholar] [CrossRef]
- Liang, Y.-M.; Yang, H.; Zhou, B.; Chen, Y.; Yang, M.; Wei, K.-S.; Yan, X.-F.; Kang, C. Waste tobacco leaves derived carbon dots for tetracycline detection: Improving quantitative accuracy with the aid of chemometric model. Anal. Chim. Acta 2021, 339269. [Google Scholar] [CrossRef]
- Zulfajri, M.; Kao, Y.-T.; Huang, G.G. Retrieve of residual waste of carbon dots derived from straw mushroom as a hydrochar for the removal of organic dyes from aqueous solutions. Sustain. Chem. Pharm. 2021, 22, 100469. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, P.; Mundekkad, D.; Mukherjee, A.; Chaudhary, S.; Umar, A.; Baskoutas, S. Coconut Carbon Dots: Progressive Large-Scale Synthesis, Detailed Biological Activities and Smart Sensing Aptitudes towards Tyrosine. Nanomaterials 2022, 12, 162. https://doi.org/10.3390/nano12010162
Chauhan P, Mundekkad D, Mukherjee A, Chaudhary S, Umar A, Baskoutas S. Coconut Carbon Dots: Progressive Large-Scale Synthesis, Detailed Biological Activities and Smart Sensing Aptitudes towards Tyrosine. Nanomaterials. 2022; 12(1):162. https://doi.org/10.3390/nano12010162
Chicago/Turabian StyleChauhan, Pooja, Deepa Mundekkad, Amitava Mukherjee, Savita Chaudhary, Ahmad Umar, and Sotirios Baskoutas. 2022. "Coconut Carbon Dots: Progressive Large-Scale Synthesis, Detailed Biological Activities and Smart Sensing Aptitudes towards Tyrosine" Nanomaterials 12, no. 1: 162. https://doi.org/10.3390/nano12010162
APA StyleChauhan, P., Mundekkad, D., Mukherjee, A., Chaudhary, S., Umar, A., & Baskoutas, S. (2022). Coconut Carbon Dots: Progressive Large-Scale Synthesis, Detailed Biological Activities and Smart Sensing Aptitudes towards Tyrosine. Nanomaterials, 12(1), 162. https://doi.org/10.3390/nano12010162







