Synthesis and Characterization of Cu2ZnSnS4 Thin Films Obtained by Combined Magnetron Sputtering and Pulsed Laser Deposition
Abstract
:1. Introduction
2. Materials and Methods
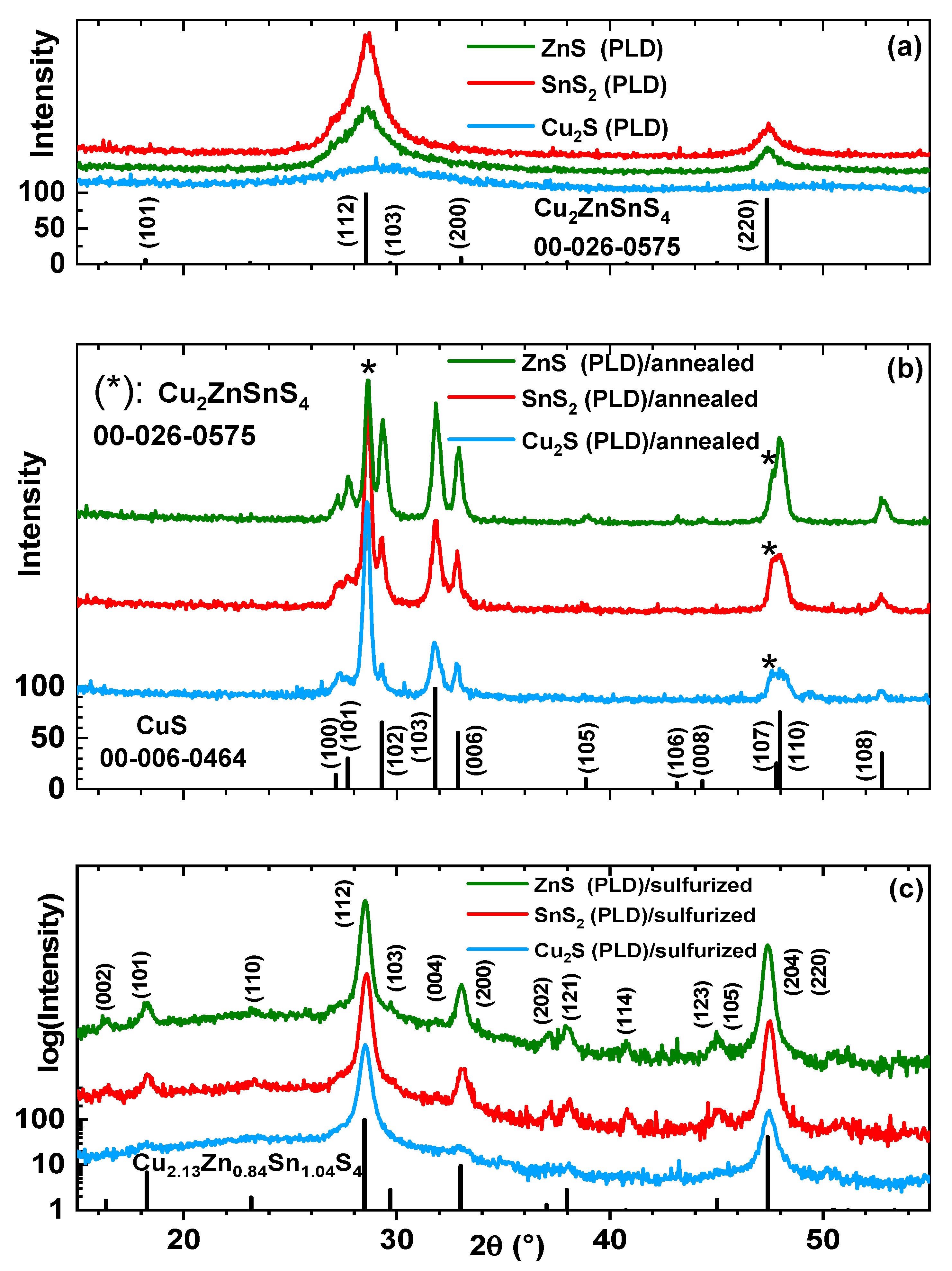
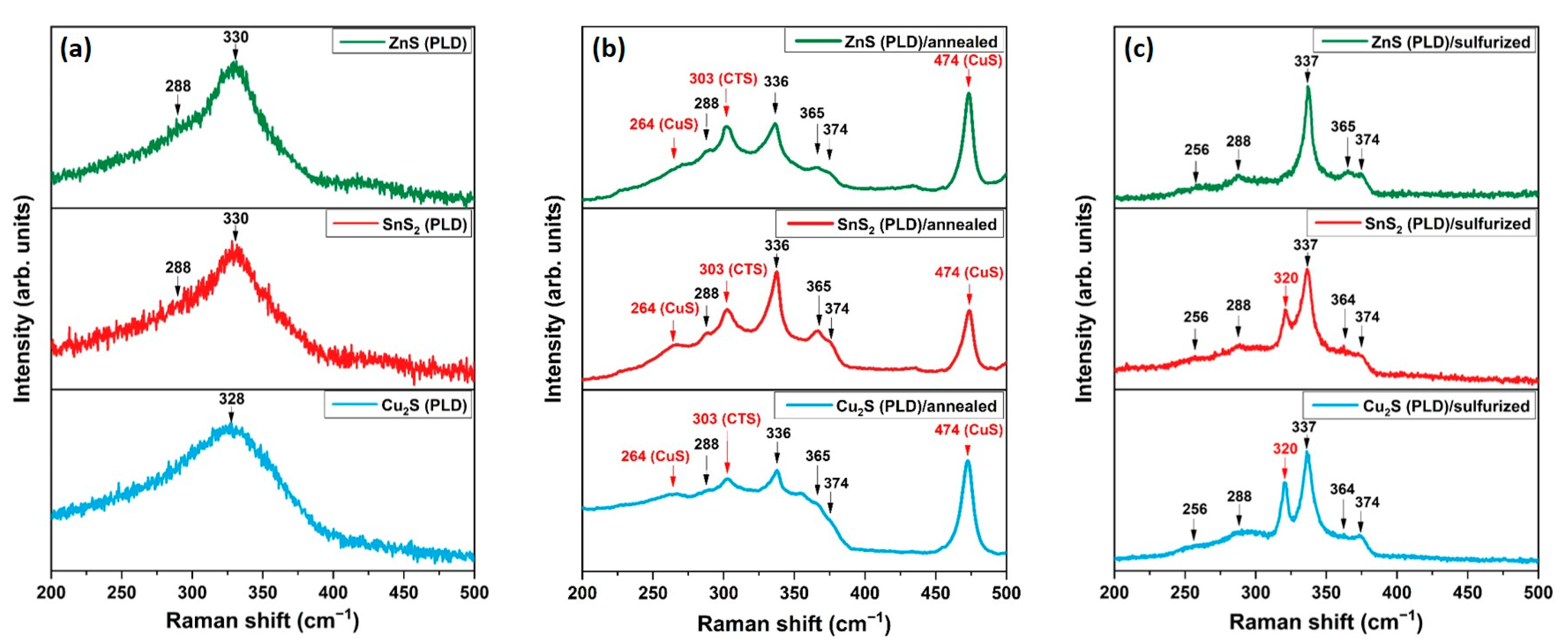
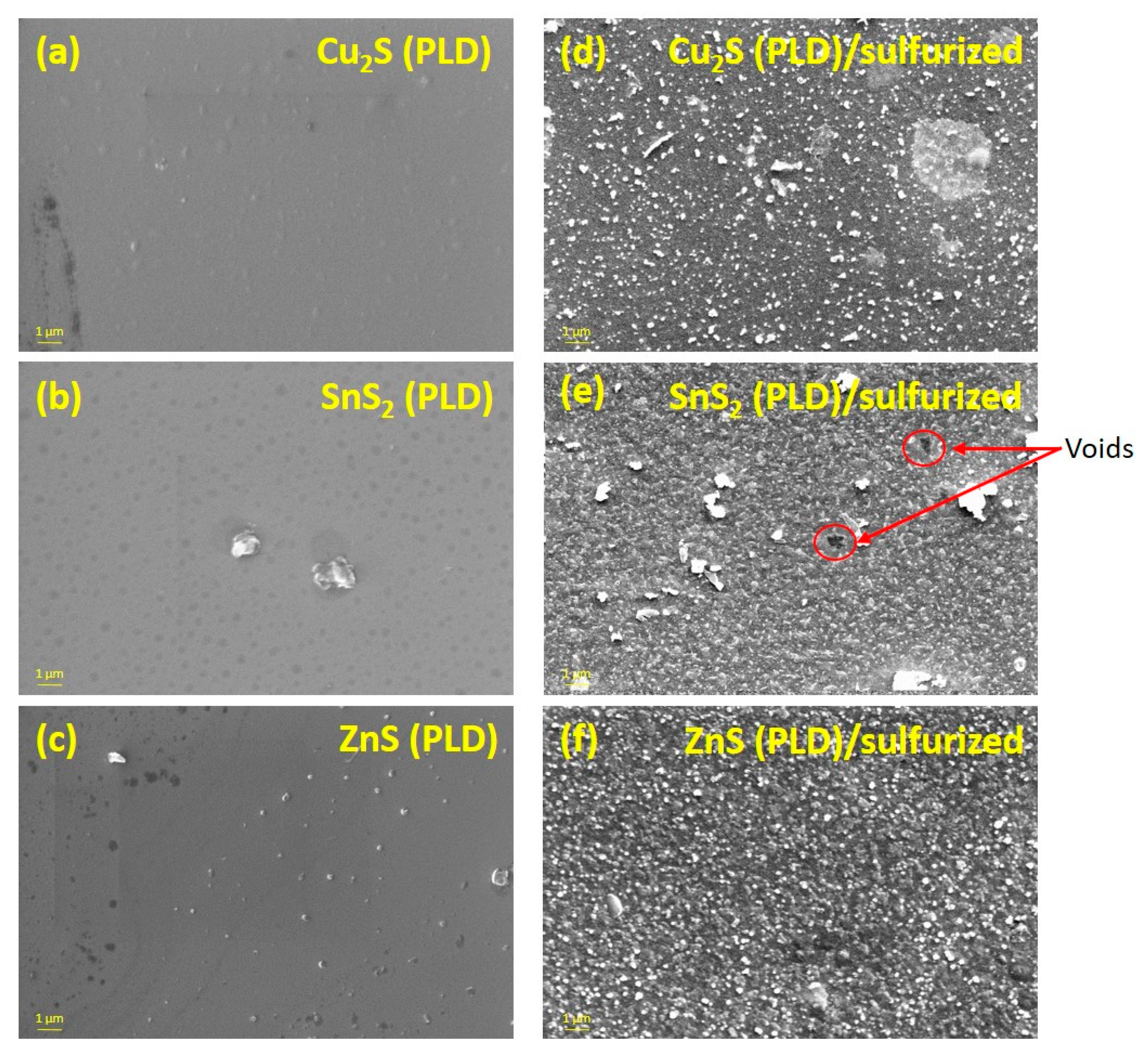
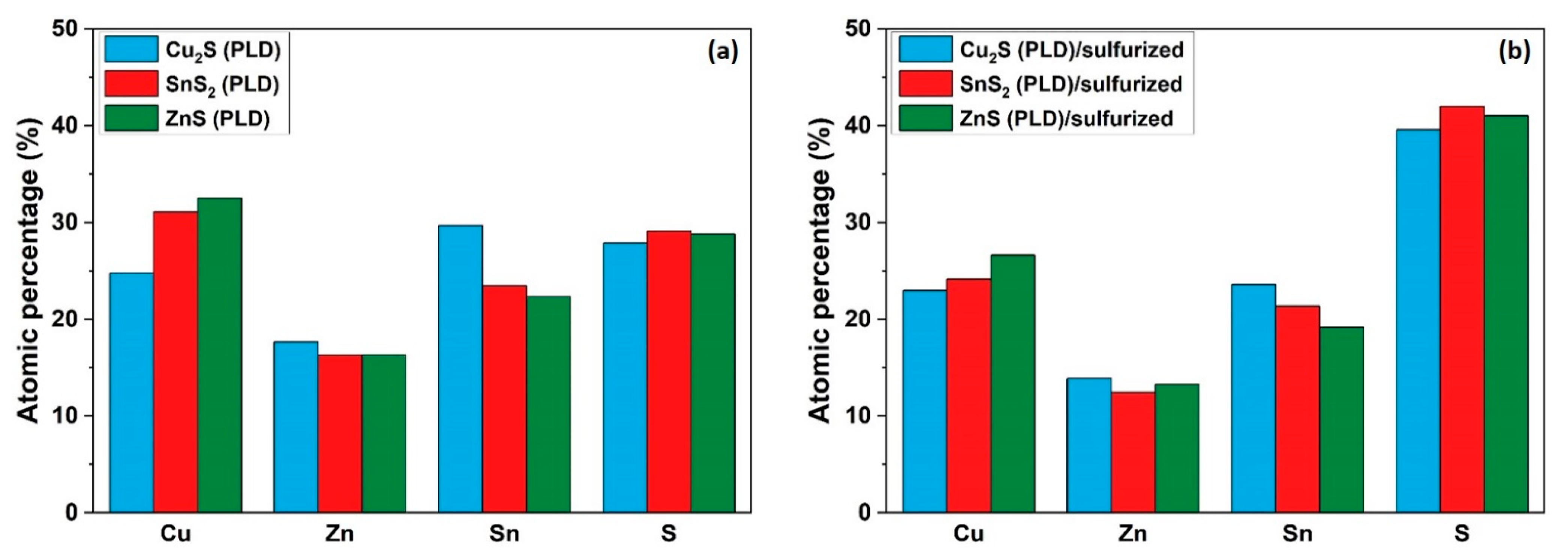
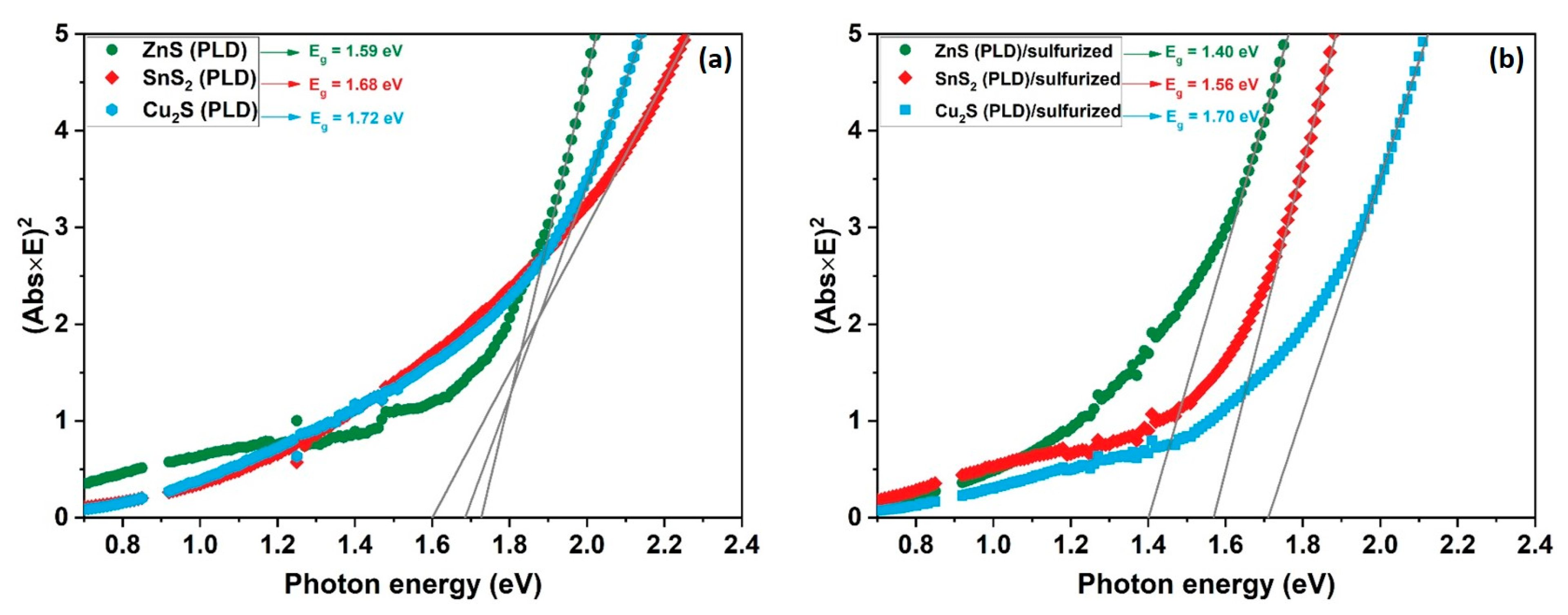
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmoum, H.; Boughrara, M.; Su’ait, M.S.; Li, G.; Chopra, S.; Wang, Q.; Kerouad, M. Understanding the Effect of the Carbon on the Photovoltaic Properties of the Cu2ZnSnS4. Mater. Chem. Phys. 2020, 251, 123065. [Google Scholar] [CrossRef]
- Ataollahi, N.; Bazerla, F.; Malerba, C.; Chiappini, A.; Ferrari, M.; Di Maggio, R.; Scardi, P. Synthesis and Post-Annealing of Cu2ZnSnS4 Absorber Layers Based on Oleylamine/1-Dodecanethiol. Materials 2019, 12, 3320. [Google Scholar] [CrossRef] [Green Version]
- Sharmin, A.; Bashar, M.S.; Sultana, M.; Al Mamun, S.M.M. Sputtered Single-Phase Kesterite Cu2ZnSnS4 (CZTS) Thin Film for Photovoltaic Applications: Post Annealing Parameter Optimization and Property Analysis. AIP Adv. 2020, 10, 015230. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Ji, X.; Li, M.; Lin, W.; Luo, X.; Zhang, H. A Review on Development Prospect of CZTS Based Thin Film Solar Cells. Int. J. Photoenergy 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Vanalakar, S.A.; Agawane, G.L.; Shin, S.W.; Suryawanshi, M.P.; Gurav, K.V.; Jeon, K.S.; Patil, P.S.; Jeong, C.W.; Kim, J.Y.; Kim, J.H. A Review on Pulsed Laser Deposited CZTS Thin Films for Solar Cell Applications. J. Alloys Compd. 2015, 619, 109–121. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Shen, T.; Yi, J.; Gan, G.; Huang, Q. Direct Current Magnetron Sputtered Cu2ZnSnS4 Thin Films Using a Ceramic Quaternary Target. J. Alloys Compd. 2017, 727, 1115–1125. [Google Scholar] [CrossRef]
- Whittles, T.J. Electronic Characterisation of Earth-Abundant Sulphides for Solar Photovoltaics; Springer Theses; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-91664-4. [Google Scholar] [CrossRef]
- Ogugua, S.N.; Ntwaeaborwa, O.M.; Swart, H.C. Latest Development on Pulsed Laser Deposited Thin Films for Advanced Luminescence Applications. Coatings 2020, 10, 1078. [Google Scholar] [CrossRef]
- Rath, M.; Varadarajan, E.; Natarajan, V.; Ramachandra Rao, M.S. A Comparative Study on Macroscopic and Nanoscale Polarization Mapping on Large Area PLD Grown PZT Thin Films. Ceram. Int. 2018, 44, 8749–8755. [Google Scholar] [CrossRef]
- Oulad Elhmaidi, Z.; Abd-Lefdil, M.; El Khakani, M.A. Photoconversion Optimization of Pulsed-Laser-Deposited p-CZTS/n-Si-Nanowires Heterojunction-Based Photovoltaic Devices. Nanomaterials 2020, 10, 1393. [Google Scholar] [CrossRef]
- Oulad Elhmaidi, Z.; Pandiyan, R.; Abd-Lefdil, M.; El Khakani, M.A. Pulsed Laser Deposition of CZTS Thin Films, Their Thermal Annealing and Integration into n-Si/CZTS Photovoltaic Devices. In Proceedings of the 2016 International Renewable and Sustainable Energy Conference (IRSEC) 2016, Marrakech, Morocco, 14–17 November 2016; pp. 130–135. [Google Scholar] [CrossRef]
- Cazzaniga, A.; Crovetto, A.; Yan, C.; Sun, K.; Hao, X.; Ramis Estelrich, J.; Canulescu, S.; Stamate, E.; Pryds, N.; Hansen, O.; et al. Ultra-Thin Cu2ZnSnS4 Solar Cell by Pulsed Laser Deposition. Sol. Energy Mater. Sol. Cells 2017, 166, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Ettlinger, R.B.; Crovetto, A.; Canulescu, S.; Cazzaniga, A.; Ravnkilde, L.; Youngman, T.; Hansen, O.; Pryds, N.; Schou, J. Formation of Copper Tin Sulfide Films by Pulsed Laser Deposition at 248 and 355 Nm. Appl. Phys. A 2016, 122, 466. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, A.; Ettlinger, R.B.; Canulescu, S.; Schou, J.; Pryds, N. Nanosecond Laser Ablation and Deposition of Silver, Copper, Zinc and Tin. Appl. Phys. A 2014, 117, 89–92. [Google Scholar] [CrossRef]
- Noroozi, M.; Petruhins, A.; Greczynski, G.; Rosen, J.; Eklund, P. Structural and Mechanical Properties of Amorphous AlMgB14 Thin Films Deposited by DC Magnetron Sputtering on Si, Al2O3 and MgO Substrates. Appl. Phys. A 2020, 126, 133. [Google Scholar] [CrossRef] [Green Version]
- Olgar, M.A.; Klaer, J.; Mainz, R.; Levcenco, S.; Just, J.; Bacaksiz, E.; Unold, T. Effect of Precursor Stacking Order and Sulfurization Temperature on Compositional Homogeneity of CZTS Thin Films. Thin Solid Film. 2016, 615, 402–408. [Google Scholar] [CrossRef]
- Jheng, B.-T.; Liu, P.-T.; Wu, M.-C. A Promising Sputtering Route for Dense Cu2ZnSnS4 Absorber Films and Their Photovoltaic Performance. Sol. Energy Mater. Sol. Cells 2014, 128, 275–282. [Google Scholar] [CrossRef]
- Xie, M.; Zhuang, D.; Zhao, M.; Zhuang, Z.; Ouyang, L.; Li, X.; Song, J. Preparation and Characterization of a Cu2ZnSnS4 Thin Films and Solar Cells Fabricated from Quaternary Cu-Zn-Sn-S Target. Int. J. Photoenergy 2013, 2013, 929454. [Google Scholar] [CrossRef]
- Olgar, M.A.; Klaer, J.; Mainz, R.; Ozyuzer, L.; Unold, T. Cu2ZnSnS4-Based Thin Films and Solar Cells by Rapid Thermal Annealing Processing. Thin Solid Film. 2017, 628, 1–6. [Google Scholar] [CrossRef]
- Behera, N.; Mohan, D.B. The Phase Optimization, Optical and Electrical Properties of Kesterite Cu2ZnSnS4 Thin Film Prepared by Single Target RF Magnetron Sputtering Technique for Solar Cell Application. Mater. Res. Express 2020, 6, 126457. [Google Scholar] [CrossRef]
- Rudisch, K.; Davydova, A.; Riekehr, L.; Adolfsson, J.; Quaglia Casal, L.; Platzer-Björkman, C.; Scragg, J. Prospects for Defect Engineering in Cu2ZnSnS4 Solar Absorber Films. J. Mater. Chem. A 2020, 8, 15864–15874. [Google Scholar] [CrossRef]
- Rondiya, S.; Rokade, A.; Jadhavar, A.; Nair, S.; Chaudhari, M.; Kulkarni, R.; Mayabadi, A.; Funde, A.; Pathan, H.; Jadkar, S. Effect of Calcination Temperature on the Properties of CZTS Absorber Layer Prepared by RF Sputtering for Solar Cell Applications. Mater. Renew. Sustain. Energy 2017, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Xie, J.; Mao, A. Effects of Bottom Layer Sputtering Pressures and Annealing Temperatures on the Microstructures, Electrical and Optical Properties of Mo Bilayer Films Deposited by RF/DC Magnetron Sputtering. Appl. Sci. 2019, 9, 1395. [Google Scholar] [CrossRef] [Green Version]
- Khalkar, A.; Lim, K.-S.; Yu, S.-M.; Patole, S.P.; Yoo, J.-B. Deposition of Cu2ZnSnS4 Thin Films by Magnetron Sputtering and Subsequent Sulphurization. Electron. Mater. Lett. 2014, 10, 43–49. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and Technology of Magnetron Sputtering Discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Alvarez, R.; Garcia-Valenzuela, A.; Lopez-Santos, C.; Ferrer, F.J.; Rico, V.; Guillen, E.; Alcon-Camas, M.; Escobar-Galindo, R.; Gonzalez-Elipe, A.R.; Palmero, A. High-Rate Deposition of Stoichiometric Compounds by Reactive Magnetron Sputtering at Oblique Angles. Plasma Process. Polym. 2016, 13, 960–964. [Google Scholar] [CrossRef] [Green Version]
- Benetti, D.; Nouar, R.; Nechache, R.; Pepin, H.; Sarkissian, A.; Rosei, F.; MacLeod, J.M. Combined Magnetron Sputtering and Pulsed Laser Deposition of TiO2 and BFCO Thin Films. Sci. Rep. 2017, 7, 2503. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Solano, R.E.; Arias-Cerón, J.S.; Ríos-Ramírez, J.J.; Ortega-López, M. Synthesis and Study of Structure and Phase Composition in Cu2–XS, SnxSy, ZnS, CuxSnSy and CuZnSnS Pellets. J. Mater. Sci. Mater. Electron. 2020, 31, 7519–7523. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Mkawi, E.M.; Al-Hartomy, O.; Bekyarova, E. Solvothermal Synthesis of Kesterite Cu2ZnSnS4 Nanocrystals: Influence of Glycine Complexing Agent Concentration on Properties. Ceram. Int. 2021, 47, 11568–11573. [Google Scholar] [CrossRef]
- Simandan, I.-D.; Sava, F.; Buruiana, A.-T.; Galca, A.-C.; Becherescu, N.; Burducea, I.; Mihai, C.; Velea, A. Influence of Deposition Method on the Structural and Optical Properties of Ge2Sb2Te5. Materials 2021, 14, 3663. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, J.; Wang, S.; Zhong, L.; Xiao, X. Forming an Ultrathin SnS Layer on Cu 2 ZnSnS 4 Surface to Achieve Highly Efficient Solar Cells with Zn(O,S) Buffer. Solar RRL 2020, 4, 2000010. [Google Scholar] [CrossRef]
- Tao, J.; Liu, J.; He, J.; Zhang, Z.; Jiang, J.; Sun, L.; Yang, P.; Chu, J. Synthesis and characterization of Cu2ZnSnS4 thin films by the sulfurization of co-electrodeposited Cu–Zn–Sn–S precursor layers for solar cell applications. RSC Adv. 2014, 4, 23977–23984. [Google Scholar] [CrossRef]
- Engberg, S.; Symonowicz, J.; Schou, J.; Canulescu, S.; Jensen, K.M.Ø. Characterization of Cu2ZnSnS4 Particles Obtained by the Hot-Injection Method. ACS Omega 2020, 5, 10501–10509. [Google Scholar] [CrossRef]
- Havryliuk, Y.; Valakh, M.Y.; Dzhagan, V.; Greshchuk, O.; Yukhymchuk, V.; Raevskaya, A.; Stroyuk, O.; Selyshchev, O.; Gaponik, N.; Zahn, D.R.T. Raman characterization of Cu2ZnSnS4 nanocrystals: Phonon confinement effect and formation of CuxS phases. RSC Adv. 2018, 8, 30736. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Xu, J.; Yang, Y. Effect of Sulfur Powder Mass on the Formation of MoS2 Interface Layer between Cu2ZnSnS4 Thin Film and Mo Foil. Superlattices Microstruct. 2020, 147, 106724. [Google Scholar] [CrossRef]
- Azmi, S.; Moujib, A.; Layachi, O.A.; Matei, E.; Galca, A.C.; Zaki, M.Y.; Secu, M.; Rusu, M.I.; Grigorescu, C.E.A.; Khoumri, E.M. Towards Phase Pure Kesterite Cu2ZnSnS4 Absorber Layers Growth via Single Step Free Sulfurization Electrodeposition under a Fix Applied Potential on Mo Substrate. J. Alloys Compd. 2020, 842, 155821. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Fairbrother, A.; Fontane, X.; Jawhari, T.; Izquierdo-Roca, V.; Saucedo, E.; Perez-Rodriguez, A. Multiwavelength excitation Raman scattering study of polycrystalline kesterite Cu2ZnSnS4 thin films. Appl. Phys. Lett. 2014, 104, 021901. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, J.J.; Joshi, U.S. Fabrication of high quality Cu2SnS3 thin film solar cell with 1.12% power conversion efficiency obtain by low cost environment friendly sol-gel technique. Mater. Res. Express 2018, 5, 036203. [Google Scholar] [CrossRef]
- Kumar, M.; Dubey, A.; Adhikari, N.; Venkatesan, S.; Qiao, Q. Strategic Review of Secondary Phases, Defects and Defect-Complexes in Kesterite CZTS–Se Solar Cells. Energy Environ. Sci. 2015, 8, 3134–3159. [Google Scholar] [CrossRef]
- Tan, J.M.R.; Lee, H.; Pedireddy, S.; Baikie, T.; Ling, X.Y.; Wong, L.H. Understanding the Synthetic Paterhway of a Single-Phase Quaternary Semiconductor Using Surface-Enhanced Raman Scattering: A case of Wurtzite Cu2ZnSnS4 Nanoparticles. J. Am. Chem. Soc. 2014, 136, 6684–6692. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Brandl, M.; Distaso, M.; Herre, P.; Spiecker, E.; Hock, R.; Peukert, W. A comprehensive study on the mechanism behind formation and depletion of Cu2ZnSnS4 (CZTS) phases. Cryst. Eng. Comm. 2015, 17, 6972–6984. [Google Scholar] [CrossRef] [Green Version]
- Isik, M.; Gullu, H.H.; Terlemezoglu, M.; Bayrakli Surucu, O.; Parlak, M.; Gasanly, N.M. Investigation of Band Gap Energy Versus Temperature for SnS2 thin films grown by RF-Magnetron Sputtering. Phys. B 2020, 591, 412264. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Boero, F.; Litvinchuk, A.P.; Delsante, S.; Borzone, G.; Perez-Rodriguez, A.; Izquierdo-Roca, V. Structural Polymorphism in “Kesterite” Cu2ZnSnS4: Raman Spectroscopy and First-Principles Calculations Analysis. Inorg. Chem. 2017, 56, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Guc, M.; Levcenko, S.; Bodnar, I.V.; Izquierdo-Roca, V.; Fontane, X.; Volkova, L.V.; Arushanov, E.; Perez-Rodriguez, A. Polarized Raman Scattering Study of Kesterite Type Cu2ZnSnS4 Single Crystals. Sci. Rep. 2016, 6, 19414. [Google Scholar] [CrossRef] [Green Version]
- Sava, F.; Diagne, O.; Galca, A.-C.; Simandan, I.-D.; Matei, E.; Burdusel, M.; Becherescu, N.; Becherescu, V.; Mihai, C.; Velea, A. Secondary Crystalline Phases Influence on Optical Properties in Off-Stoichiometric Cu2S–ZnS–SnS2 Thin Films. Materials 2020, 13, 4624. [Google Scholar] [CrossRef]
- Chen, R.; Fan, J.; Li, H.; Liu, C.; Mai, Y. Efficiency Enhancement of Cu2ZnSnS4 Solar Cells via Surface Treatment Engineering. R Soc. Open Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, G.; Cai, H.; Chen, B.; Yao, L.; Yang, M.; Chen, S.; Huang, Z. The Effects of SnS2 Secondary Phases on Cu2ZnSnS4 Solar Cells: A Promising Mechanical Exfoliation Method for Its Removal. J. Mater. Chem. A 2018, 6, 2995–3004. [Google Scholar] [CrossRef]
- Zaki, M.Y.; Nouneh, K.; Ebn Touhami, M.; Belakhmima, R.A.; Galca, A.C.; Pintilie, L.; Enculescu, M.; Baibarac, M.; Taibi, M. Effect of Mixing Complexing Agents on the Properties of Electrodeposited CZTS Thin Films. Opt. Mater. 2018, 83, 252–256. [Google Scholar] [CrossRef]
- Wang, H.; Yasin, A.; Quitoriano, N.J.; Demopoulos, G.P. Aqueous-based Binary Sulfide Nanoparticle Inks for Cu2ZnSnS4 Thin Films Stabilized with Tin(IV) Chalcogenide Complexes. Nanomaterials 2019, 9, 1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khouja, O.; Galca, A.C.; Nouneh, K.; Zaki, M.Y.; Ebn Touhami, M.; Taibi, M.; Matei, E.; Negrila, C.C.; Enculescu, M.; Pintilie, L. Structural, Morphological and Optical Properties of Cu–Fe–Sn–S Thin Films Prepared by Electrodeposition at Fixed Applied Potential. Thin Solid Film. 2021, 721, 138547. [Google Scholar] [CrossRef]
- Malerba, C.; Biccari, F.; Azanza Ricardo, C.L.; Valentini, M.; Chierchia, R.; Müller, M.; Santoni, A.; Esposito, E.; Mangiapane, P.; Scardi, P.; et al. CZTS Stoichiometry Effects on the Band Gap Energy. J. Alloys Compd. 2014, 582, 528–534. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Oyaizu, W.; Kawase, M.; Kuzuya, T. Synthesis of Highly Non-Stoichiometric Cu2ZnSnS4 Nanoparticles with Tunable Bandgaps. J. Nanopart Res. 2017, 19, 9. [Google Scholar] [CrossRef]
- Elhmaidi, Z.O.; Pandiyan, R.; Abd-Lefdil, M.; Saucedo, E.; El Khakani, M.A. In-Situ Tuning of the Zinc Content of Pulsed-Laser-Deposited CZTS Films and Its Effect on the Photoconversion Efficiency of p-CZTS/n-Si Heterojunction Photovoltaic Devices. Appl. Surf. Sci. 2020, 507, 145003. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Mkawi, E.M.; Ibrahim, K.; Farrukh, M.A. Effect of Sulfurization Time on the Properties of Copper Zinc Tin Sulfide Thin Films Grown by Electrochemical Deposition. Sci. Rep. 2016, 6, 32431. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fu, E.; Wang, Y.; Zhang, C. Fabrication of Cu2ZnSnS4 (CZTS) Nanoparticle Inks for Growth of CZTS Films for Solar Cells. Nanomaterials 2019, 9, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, V.; Clemens, B. Effect of composition on high efficiency CZTSSe devices fabricated using co-sputtering of compound targets. In Proceedings of the 2012 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 002990–002992. [Google Scholar] [CrossRef]
- Hlaing Oo, W.; Johnson, J.; Bhatia, A.; Lund, E.-A.; Nowell, M.-M.; Scarpulla, M.-A. Grain Size and Texture of Cu2ZnSnS4 Thin Films Synthesized by Cosputtering Binary Sulfides and Annealing: Effects of Processing Conditions and Sodium. J. Electron. Mater. 2011, 40, 2214. [Google Scholar] [CrossRef]
- Bao, W.; Ichimura, M. Influence of Secondary Phases in Kesterite-Cu2ZnSnS4 Absorber Material Based on the First Principles Calculation. Int. J. Photoenergy 2015, 2015, 592079. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-S.; Chen, S.-Y.; Huang, C.-S.; Lee, M.-Y.; Kuo, H.-C. Investigation of Zn/Sn ratio for improving the material quality of CZTS thin films with the reduction of Cu2-xS secondary phase. J. Alloys Compd. 2020, 853, 157237. [Google Scholar] [CrossRef]
- Muhunthan, N.; Singh, O.P.; Singh, S.; Singh, V.N. Growth of CZTS Thin Films by Cosputtering of Metal Targets and Sulfurization in H2S. Int. J. Photoenergy 2013, 2013, 752012. [Google Scholar] [CrossRef] [Green Version]
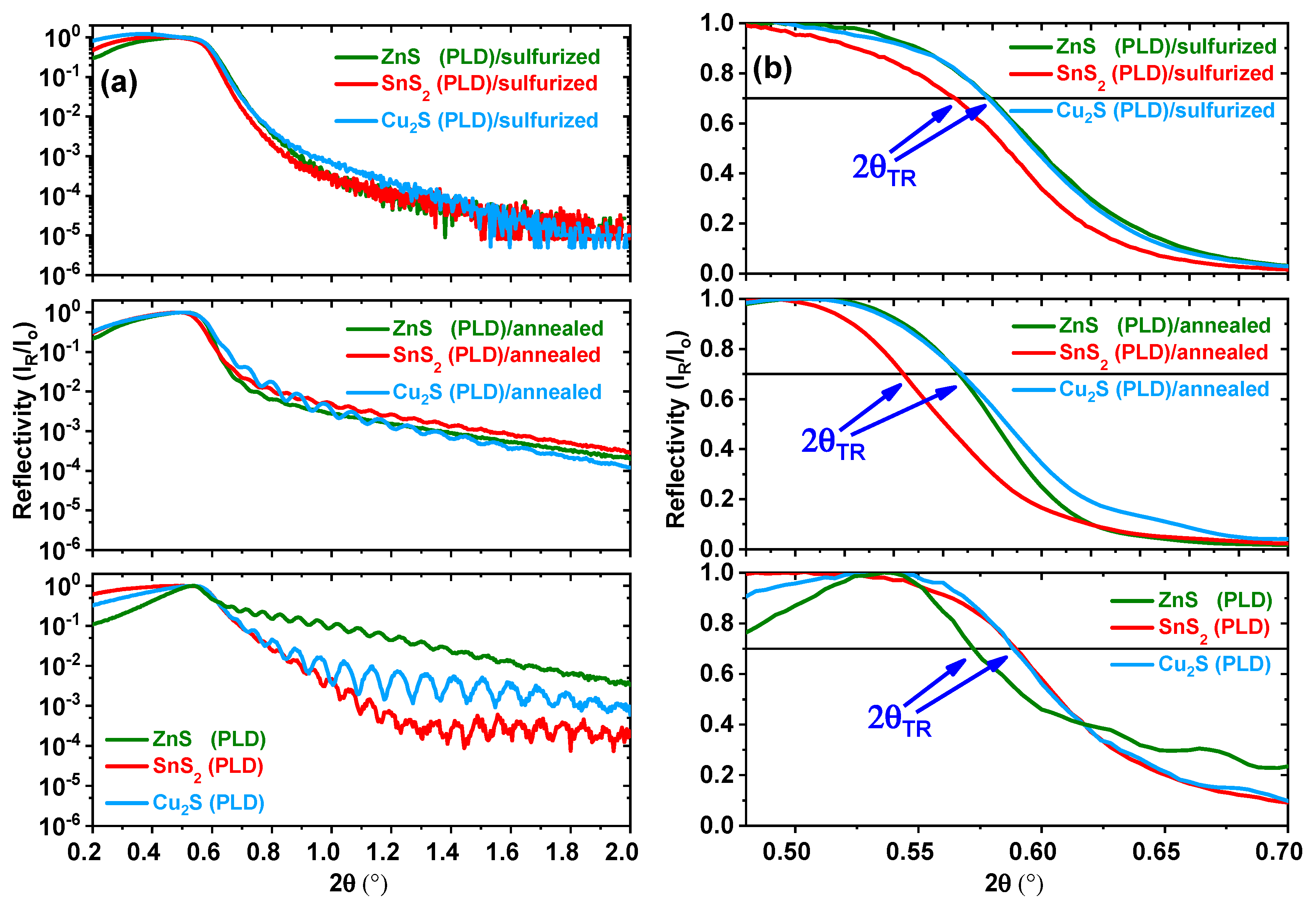
| 2θTR; Δρm/ρo | hm (nm); Δhm/ho | |||||
|---|---|---|---|---|---|---|
| Sample | As-Deposited | Annealed @ 500 °C | Sulfurized @ 500 °C | As-Deposited | Annealed @ 500 °C | Sulfurized @ 500 °C |
| ZnS (PLD) | 0.572° | 0.566°; −2.1% | 0.579; +2.5% | 145 | 142; −2.0% | - |
| SnS2 (PLD) | 0.589° | 0.544°; −14.7% | 0.565; +8.0% | 147 | 126; −14.3% | - |
| Cu2S (PLD) | 0.588° | 0.567°; −7.0% | 0.578; +3.4% | 108 | 97; −10.2% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaki, M.-Y.; Sava, F.; Buruiana, A.-T.; Simandan, I.-D.; Becherescu, N.; Galca, A.-C.; Mihai, C.; Velea, A. Synthesis and Characterization of Cu2ZnSnS4 Thin Films Obtained by Combined Magnetron Sputtering and Pulsed Laser Deposition. Nanomaterials 2021, 11, 2403. https://doi.org/10.3390/nano11092403
Zaki M-Y, Sava F, Buruiana A-T, Simandan I-D, Becherescu N, Galca A-C, Mihai C, Velea A. Synthesis and Characterization of Cu2ZnSnS4 Thin Films Obtained by Combined Magnetron Sputtering and Pulsed Laser Deposition. Nanomaterials. 2021; 11(9):2403. https://doi.org/10.3390/nano11092403
Chicago/Turabian StyleZaki, Mohamed-Yassine, Florinel Sava, Angel-Theodor Buruiana, Iosif-Daniel Simandan, Nicu Becherescu, Aurelian-Catalin Galca, Claudia Mihai, and Alin Velea. 2021. "Synthesis and Characterization of Cu2ZnSnS4 Thin Films Obtained by Combined Magnetron Sputtering and Pulsed Laser Deposition" Nanomaterials 11, no. 9: 2403. https://doi.org/10.3390/nano11092403
APA StyleZaki, M.-Y., Sava, F., Buruiana, A.-T., Simandan, I.-D., Becherescu, N., Galca, A.-C., Mihai, C., & Velea, A. (2021). Synthesis and Characterization of Cu2ZnSnS4 Thin Films Obtained by Combined Magnetron Sputtering and Pulsed Laser Deposition. Nanomaterials, 11(9), 2403. https://doi.org/10.3390/nano11092403








