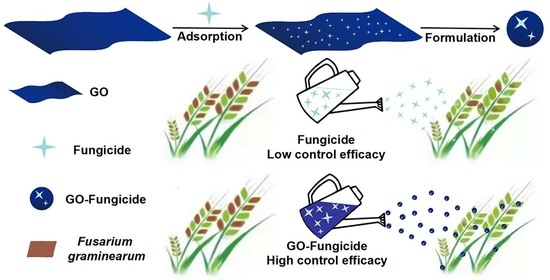
Synergistic Antifungal Activity of Graphene Oxide and Fungicides against Fusarium Head Blight In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of GO
2.3. Preparation of GO–Fungicide Nanocomposites
2.4. Fungus
2.5. Bioassay of Antifungal Activity of GO–Fungicide Nanocomposites In Vitro by Mycelial Growth Test
2.6. Bioassay of Antifungal Activity of GO–Fungicide Nanocomposites In Vitro by Spore Germination Test
2.7. Field Trials
2.8. Structural and Morphological Characterization by SEM and TEM
2.9. Data Analysis
3. Results and Discussion
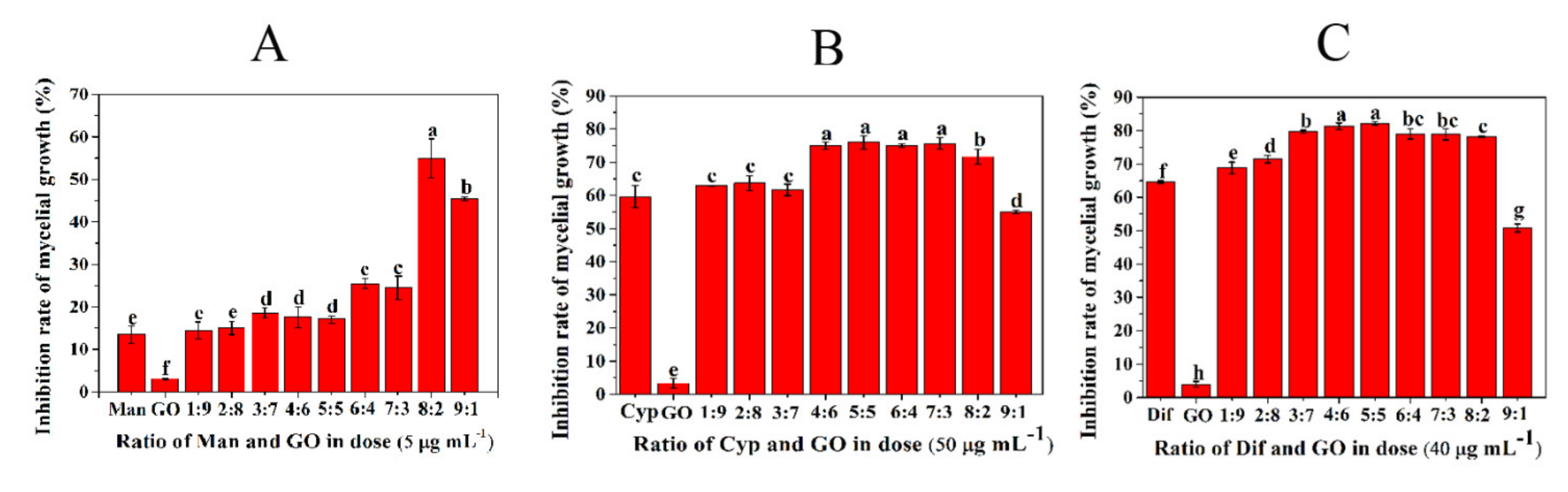
3.1. Screening of the Optimal Ratio of GO and Fungicides
3.2. Characterization of Formulated GO–Fungicides
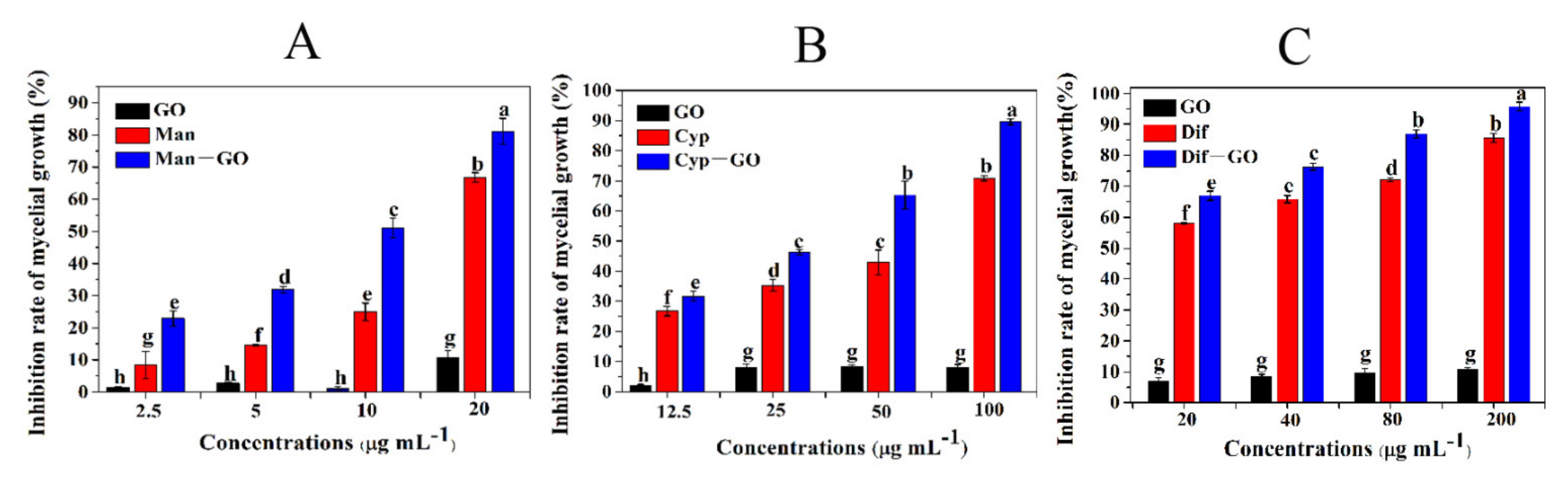
3.3. Synergistic Inhibitory Activity of GO–Fungicides on the Mycelial Growth of F. graminearum
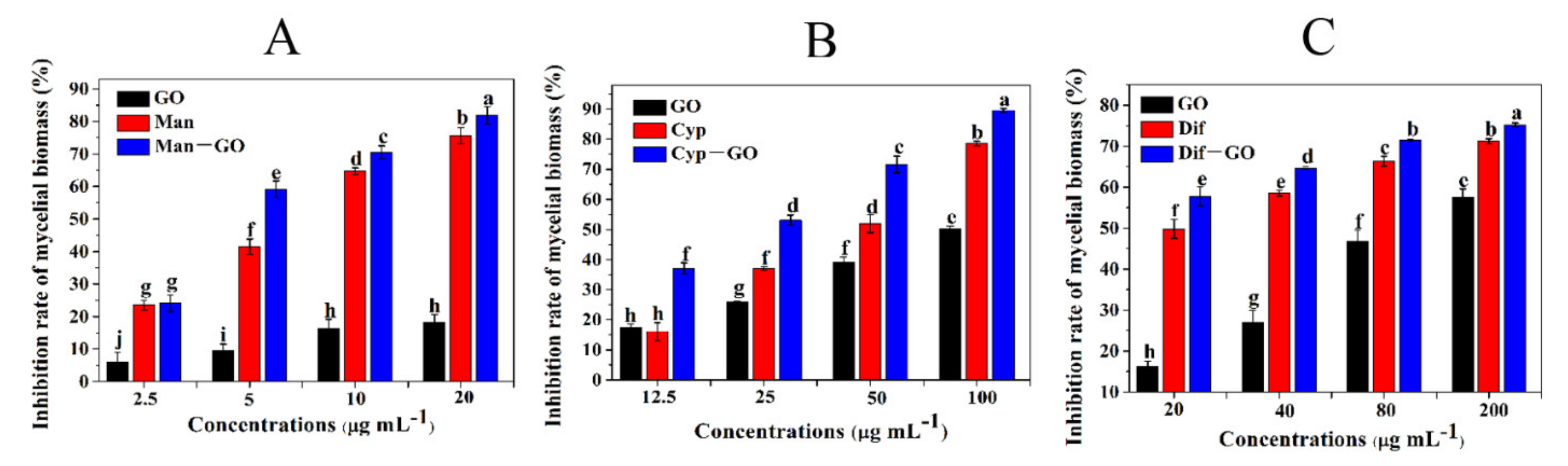
3.4. Synergistic Inhibitory Activities of GO–Fungicides on the Mycelial Biomass of F. graminearum
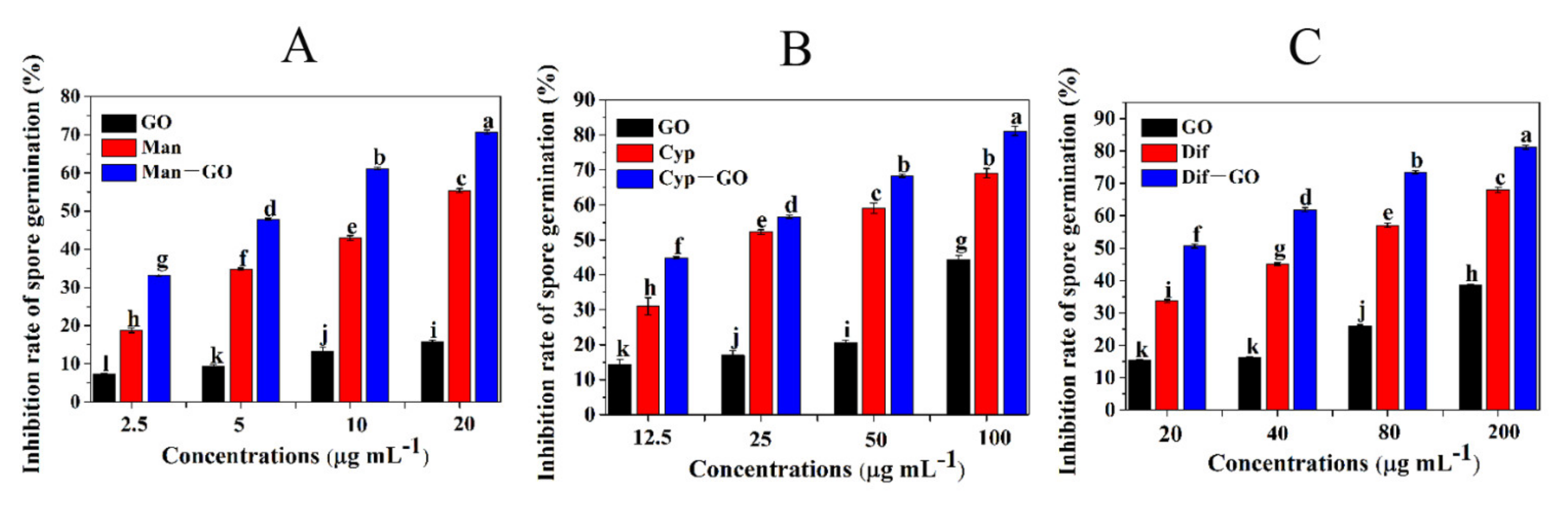
3.5. Synergistic Inhibitory Activity of GO–Fungicides on the Spore Germination of F. graminearum
3.6. Control Efficiencies of GO and GO–Fungicides on F. graminearum in the Field
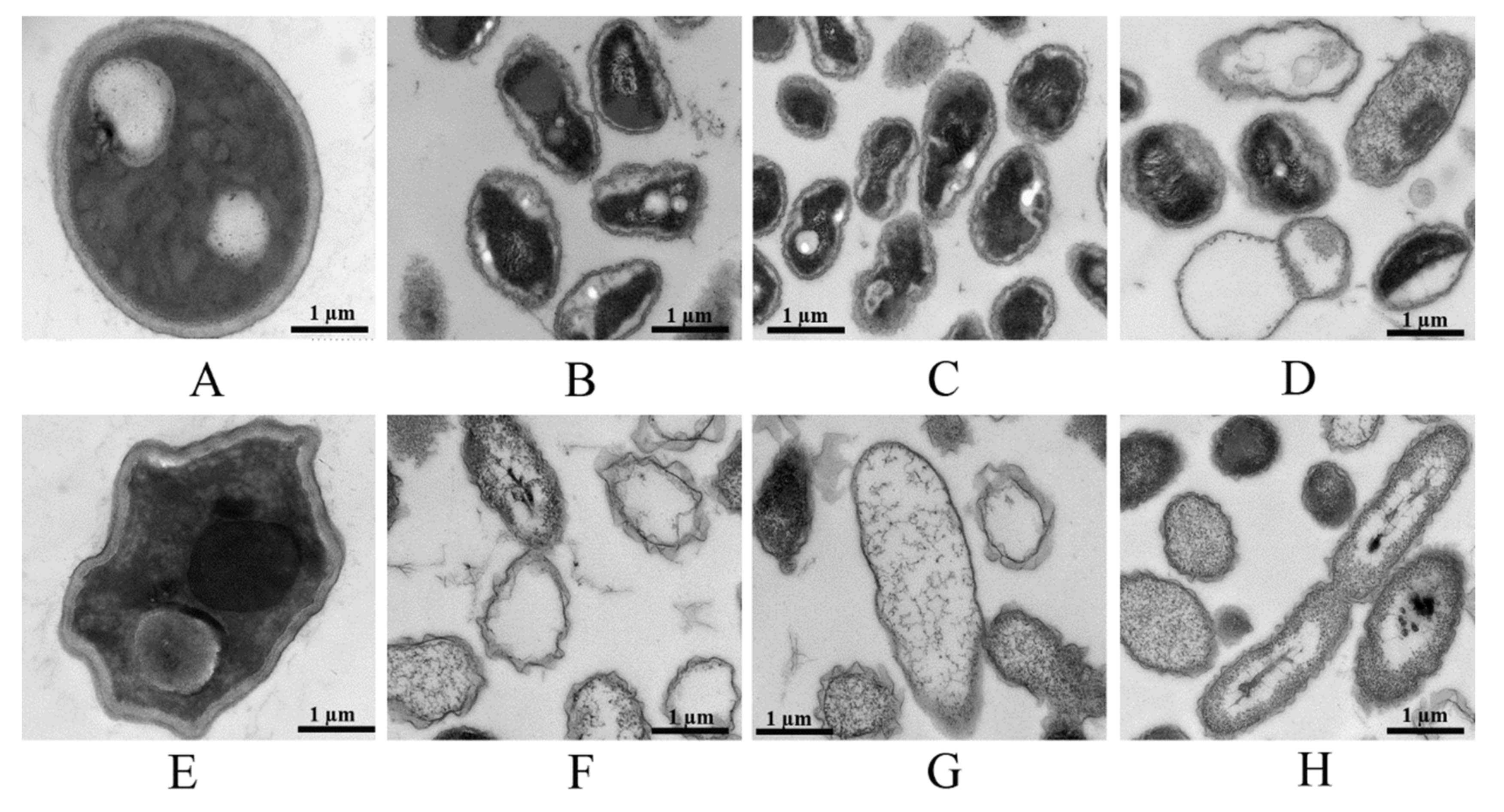
3.7. Structural Changes in the Morphology of F. graminearum Mycelia Induced by GO, Fungicides and GO–Fungicides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chong, Y.; Ge, C.C.; Fang, G.; Wu, R.F.; Zhang, H.; Chai, Z.F. Light–enhanced antibacterial activity of graphene oxide, mainly via accelerated electron transfer. Environ. Sci. Technol. 2017, 51, 10154–10161. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ciric, L.; Edirisinghe, M.J. Nanocomposites: Suitable alternatives as antimicrobial agents. Nanotechnology 2018, 29, 282001–282029. [Google Scholar] [CrossRef] [Green Version]
- Markovic, Z.M.; Matijaševic, D.M.; Pavlovic, V.B.; Jovanovic, S.P.; Holclajtner–Antunovic, I.D.; Špitalsky, Z.; Mičušik, M.; Dramićanin, M.D.; Milivojevic, D.D.; Nikšic, M.P.; et al. Antibacterial potential of electrochemically exfoliated graphene sheets. J.Colloid Interf. Sci. 2017, 500, 30–43. [Google Scholar] [CrossRef]
- Matharu, R.K.; Tabish, T.A.; Trakoolwilaiwan, T.; Mansfield, J.; Edirisinghe, M. Microstructure and antibacterial efficacy of graphene oxide nanocomposite fibres. J. Colloid Interf. Sci. 2020, 571, 239–252. [Google Scholar] [CrossRef]
- Farhan, N.; Erum, Z.; Ekram, Y.D.; Munazza, G.; Syed, N.; Tahir Soomro, M. Superior antibacterial activity of reduced graphene oxide upon decoration with iron oxide nanorods. J. Environ. Eng. 2020, 8, 104424–104432. [Google Scholar]
- Wang, X.P.; Liu, X.Q.; Han, H.Y. Evaluation of antibacterial effects of carbon nanomaterials against copper–resistant ralstonia solanacearum. Colloid. Surface. B 2013, 103, 136–142. [Google Scholar] [CrossRef]
- Chen, J.N.; Wang, X.P.; Han, H.Y. A new function of graphene oxide emerges: Inactivating phytopathogenic bacterium xanthomonas oryzae pv. oryzae. J. Nanopart. Res. 2013, 15, 1658–1671. [Google Scholar] [CrossRef]
- Chen, J.N.; Peng, H.; Wang, X.P.; Shao, F.; Han, H.Y. Graphene oxide exhibits broad–spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Liu, X.Q.; Chen, J.N.; Han, H.Y.; Yuan, Z.D. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Kim, J.; Le, K.D.; Yu, N.H.; Kim, J.I.; Lee, C.W. Structure and antifungal activity of pelgipeptins from paenibacillus elgiiagainst phytopathogenic fungi. Pestic. Biochem. Phys. 2020, 163, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Nazanin, Z.N.; Jessica, K. Effects of host plant resistance and fungicide application on phoma stem canker, growth parameters and yield of winter oilseed rape. Crop Prot. 2018, 112, 313–321. [Google Scholar]
- Wang, X.P.; Xie, H.C.; Wang, Z.Y.; He, K.L.; Jing, D.P. Graphene oxide as a multifunctional synergist of insecticides against lepidopteran insect. Environ. Sci. Nano 2019, 6, 75. [Google Scholar] [CrossRef]
- Takemoto, J.Y.; Wegulo, S.N.; Yuen, G.Y.; Stevens, J.A.; Miller, G.W. Suppression of wheat fusarium head blight by novel amphiphilic aminoglycoside fungicide k20. Funagl Biol–UK 2018, 122, 465–470. [Google Scholar] [CrossRef]
- Yerkovich, N.; Cantoro, R.; Palazzini, J.M.; Torres, A.; Chulze, S.N. Fusarium head blight in argentina: Pathogen aggressiveness, triazole tolerance and biocontrol–cultivar combined strategy to reduce disease and deoxynivalenol in wheat. Crop Prot. 2020, 137, 105300. [Google Scholar] [CrossRef]
- Cendoya, E.; Nichea, M.J.; Monge, M.; Zachetti, V.; Chiacchiera, S.M.; Ramirez, M.L. Effect of fungicides commonly used for fusarium head blight management on growth and fumonisin production by fusarium proliferatum. Rev. Argent. Microbiol. 2021, 53, 64–74. [Google Scholar]
- Seifi, T.; Kamali, A.R. Anti–pathogenic activity of graphene nanomaterials: A review. Colloids Surface B 2021, 199, 111509. [Google Scholar] [CrossRef]
- Li, X.; Ke, M.; Zhang, M.; Peijnenburg, W.J.G.M.; Fan, X.; Xu, J.; Zhang, Z.; Lu, T.; Fu, Z.; Qian, H. The interactive effects of diclofop–methyl and silver nanoparticles on arabidopsis thaliana: Growth, photosynthesis and antioxidant system. Environ. Pollut. 2018, 232, 212–219. [Google Scholar] [CrossRef]
- Cui, B.; Feng, L.; Wang, C.; Yang, D.; Yu, M.; Zeng, Z.; Wang, Y.; Sun, C.; Zhao, X.; Cui, H. Stability and biological activity evaluation of chlorantraniliprole solid nanodispersions prepared by high pressure homogenization. PLoS ONE 2016, 11, e0160877. [Google Scholar]
- Du, Z.; Wang, C.; Tai, X.; Wang, G.; Liu, X. Optimization and characterization of biocompatible oil–in–water nanoemulsion for pesticide delivery. ACS Sustain.Chem. Eng. 2016, 4, 983–991. [Google Scholar] [CrossRef]
- Stephen, N.W.; Stephen, B.P.; John, H.N.; William, W.B.; Heather, H. Management of fusarium head blight of wheat and barley. Crop Prot. 2015, 73, 100–107. [Google Scholar]
- Zou, F.M.; Zhou, H.J.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled surface–mediated antibacterial activity of graphene oxide nanosheets. ACS Appl. Mater. Inter. 2017, 9, 1343–1351. [Google Scholar] [CrossRef]
- Wang, X.P.; Xie, H.C.; Wang, Z.Y.; He, K.L. Graphene oxide as a pesticide delivery vector for enhancing acaricidal activity against spider mites. Colloid. Surface B. 2019, 173, 632–638. [Google Scholar] [CrossRef]
- Shao, J.J.; Pei, Z.J.; Jing, H.J.; Wang, L.; Jiang, C.Y.; Du, X.J.; Jiang, C.; Lou, Z.X.; Wang, H.X. Antifungal activity of myriocin against fusarium graminearum and its inhibitory effect on deoxynivalenol production in wheat grains. Physiol. Mol. Plant P. 2021, 114, 101635. [Google Scholar] [CrossRef]
- Duan, Y.B.; Lu, F.; Zhou, Z.H.; Zhao, H.H.; Zhang, J.; Mao, Y.S.; Li, M.X.; Wang, J.X.; Zhou, M.G. Quinone outside inhibitors affect don biosynthesis, mitochondrial structure and toxisome formation in fusarium graminearum. J. Hazard. Mater. 2020, 398, 122908. [Google Scholar] [CrossRef]
- Xu, Y.B.; Li, H.P.; Zhang, J.B.; Song, B.; Chen, F.F.; Duan, X.J.; Xu, H.Q.; Liao, Y.C. Disruption of the chitin synthase gene CHS1 from fusarium asiaticum results in an altered structure of cell walls and reduced virulence. Fungal. Genet. Biol. 2010, 47, 205–215. [Google Scholar] [CrossRef]
- Stack, R.W.; Mcmullen, M.P. A visual scale to estimate severity of fusarium head blight in wheat. Ndsu Ext. Serv. 1998, PP–1095. [Google Scholar]
- Sun, Y.B.; Chen, Y.; Liu, T.; Wang, Y.Y.; Wang, Y.; Han, L.R.; Ma, Z.Q.; Feng, J.T. Evaluating the efficacy of osthole and matrine for control of sorghum purple spot. J. Plant Dis. Protect. 2021. [Google Scholar] [CrossRef]
- Yang, X.P.; Zhang, H.Q. Synergistic interaction of tea saponin with mancozeb against Pestalotiopsis theae. Crop Prot. 2012, 40, 126–131. [Google Scholar] [CrossRef]
- Zhao, R.T.; Kong, W.; Sun, M.X.; Yang, Y.; Liu, W.Y.; Lv, M.; Song, S.P.; Wang, L.H.; Song, H.B.; Hao, R.Z. Highly stable graphene–based nanocomposite (go– pei– ag) with broad–spectrum, long–term antimicrobial activity and antibiofilm effects. ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef] [PubMed]
- Lazar, P.; Karlický, F.; Jurečka, P.; Kocman, M.; Otyepková, E.; Šafářová, K.; Otyepka, M. Adsorption of small organic molecules on graphene. J. Am. Chem. Soc. 2013, 135, 6372–6377. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first–principles study. Phy. Rev. B 2009, 77, 860–862. [Google Scholar]
- Harris, S.D. Morphogenesis in germinating Fusarium graminearum macroconidia. Mycologia 2005, 97, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.B.; Xiao, X.M.; Li, T.; Chen, W.W.; Wang, J.X.; Fraaije, B.A.; Zhou, M.G. Impact of epoxiconazole on Fusarium head blight control, grain yield and deoxynivalenol accumulation in wheat. Pestic. Biochem. Phys. 2018, 152, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. Acs Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical determinants of multiwalled carbon nanotube bacterial cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef]
- Muda, M.S.; Kamari, A.; Bakar, S.A.; Yusoff, S.N.M.; Fatimah, I.; Phillip, E.; Din, S.M. Chitosan–graphene oxide nanocomposites as water–solubilising agents for rotenone pesticide. J. Mol. Liq. 2020, 318, 114066. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, B.; Bindra, P.; Panneerselvam, P.; Dwivedi, N.; Senapati, A.; Adholeya, A.; Shanmugam, V. Triple–Smart Eco–Friendly Chili Anthracnose Control AgroNanocarrier. ACS Appl. Mater. Interfaces 2021, 13, 9143–9155. [Google Scholar] [CrossRef]
- Shang, J.; Guo, Y.N.; He, D.L.; Qu, W.; Tang, Y.N.; Zhou, L.; Zhu, R.L. A novel graphene oxide–dicationic ionic liquid composite for Cr(VI) adsorption from aqueous solutions. J. Hazard. Marer. 2021, 416, 125706. [Google Scholar] [CrossRef]
- Hammerschlag, R.S.; Sisler, H.D. Benomyl and methyl–2–benzimidazolecarbamate (MBC): Biochemical, cytological and chemical aspects of toxicity to ustilago maydis and saccharomyces cerevesiae. Pestic. Biochem. Phys. 1973, 3, 42–54. [Google Scholar] [CrossRef]
- Vesentini, D.; Steward, D.; Singh, A.P.; Ball, R.; Daniel, G.; Franich, R. Chitosan–mediated changes in cell wall composition, morphology and ultrastructure in two wood–inhabiting fungi. Mycol. Res. 2007, 111, 875–890. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Riley, J.; Koerner, T. Effects of terconazole and other azole antifungal agents on the sterol and carbohydrate composition of candida albicans. Diagn. Micr. Infec. Dis. 1990, 13, 31–35. [Google Scholar] [CrossRef]
- Gündel, S.D.S.; Reis, T.D.; Copetti, P.M.; Favarin, F.R.; Sagrillo, M.R.; Silva, A.S.D.; Segat, J.C.; Baretta, D.; Ourique, A.F. Evaluation of cytotoxicity, genotoxicity and ecotoxicity of nanoemulsions containing mancozeb and eugenol. Ecotox. Environ. Safe 2019, 169, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, X.S.; Zhang, Z.K.; Ye, H.C.; Liu, Y.Q.; Yang, G.Z.; Chen, C.; Chen, M.; Yan, C.; Wang, L.Y.; et al. Fungicidal activities of camptothecin semisynthetic derivatives against colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Bio. Tec. 2019, 147, 139–147. [Google Scholar] [CrossRef]
- Jiang, J.H.; Chen, L.Z.; Wu, S.G.; Lv, L.; Liu, X.J.; Wang, Q.; Zhao, X.P. Effects of difenoconazole on hepatotoxicity, lipid metabolism and gut microbiota in zebrafish (danio rerio). Environ.Pollut. 2020, 265, 114844. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Liu, C.X.; Li, H.Q.; Zhang, H.T.; Ma, R.J.; Zhang, Q.W.; Yang, F.; Liao, Y.C.; Yuan, W.Y.; Chen, F.F. Metabonomics-Assisted label-free quantitative proteomics and transcriptome analysis reveals novel insights into antifungal effect of graphene oxide for controlling Fusarium graminearum. Environ. Sci. Nano 2019, 6, 3401–3421. [Google Scholar] [CrossRef]
- Bossche, H.V.; Marichal, P.; Gorrens, J.; Coene, M.C. Biochemical basis for the activity and selectivity of oral antifungal drugs. Br. J. Clin. Pract. Suppl. 1990, 71, 41–46. [Google Scholar]


| Treatment | Concentrations (µg mL−1) |
|---|---|
| Control | acetone, tween 80 and water solution (AT) (1:1:98, v/v) |
| Man/Man–GO | 2.5, 5, 10, 20 |
| Cyp/Cyp–GO Dif/Dif–GO | 12.5, 25, 50, 100 20, 40, 80, 200 |
| Treatment | Slope ± SE a | EC50 (μg mL−1) (95% CL) b | SR c |
|---|---|---|---|
| Man G | 2.69 ± 0.40 | 15.70(9.52~25.89) | — |
| Man–GO G | 3.40 ±0.28 | 7.80(5.98~10.17) | 2.01 |
| Cyp G | 2.52 ±0.75 | 40.65(24.75~63.19) | — |
| Cyp–GO G | 2.05+0.70 | 27.05(19.50~38.76) | 1.50 |
| Dif G | 4.37±0.15 | 5.80(3.57~9.43) | — |
| Dif–GO G | 4.69±0.21 | 2.33(0.95~5.72) | 2.49 |
| Man B | 3.66 ± 0.16 | 6.61 (5.61~7.80) | — |
| Man–GO B | 3.79 ± 0.31 | 4.09 (2.67~6.06) | 1.61 |
| Cyp B | 1.94 ± 0.25 | 40.94 (35.99~46.56) | — |
| Cyp–GO B | 2.69 ± 0.24 | 21.03 (17.99~24.60) | 1.95 |
| Dif B Dif–GO B | 4.29 ± 0.13 4.58 ± 0.11 | 17.83 (12.53~25.37) 7.12 (4.02–12.59) | — 2.50 |
| Man S | 3.75 ± 0.14 | 14.20 (11.10~18.16) | — |
| Man–GO S | 4.16 ± 0.06 | 5.90 (5.39~6.46) | 2.40 |
| Cyp S | 3.45 ± 0.18 | 30.19 (22.97~39.67) | — |
| Cyp–GO S | 3.63 ± 0.06 | 17.16 (15.30~19.24) | 1.76 |
| Dif S | 3.44 ± 0.50 | 56.03 (51.05~61.47) | — |
| Dif–GO S | 3.89 ± 0.07 | 18.04 (14.41~22.58) | 3.10 |
| Treatment (250 µg mL−1) | Disease Incidence (%) (7 d) | Disease Severity (%) (7 d) | Control Efficacy (%) |
|---|---|---|---|
| CK | 81 ± 3.41 a | 50.15 ± 1.21 a | — |
| GO | 45 ± 1.91 b | 33.42 ± 0.88 b | 33.59 a |
| Man | 43 ± 3.41 b | 28.45 ± 0.90 c | 43.26 b |
| Man–GO | 21 ± 2.52 d | 19.50 ± 0.93 e | 61.19 d |
| Cyp | 37 ± 3.00 bc | 19.98 ± 0.56 e | 60.17 d |
| Cyp–GO | 19 ± 1.91 d | 12.41 ± 0.36 f | 75.26 e |
| Dif | 32 ± 1.63 c | 32.74 ± 0.58 b | 34.72 a |
| Dif–GO | 19 ± 1.91 d | 24.58 ± 0.94 d | 50.99 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Peng, F.; Cheng, C.; Chen, L.; Shi, X.; Gao, X.; Li, J. Synergistic Antifungal Activity of Graphene Oxide and Fungicides against Fusarium Head Blight In Vitro and In Vivo. Nanomaterials 2021, 11, 2393. https://doi.org/10.3390/nano11092393
Wang X, Peng F, Cheng C, Chen L, Shi X, Gao X, Li J. Synergistic Antifungal Activity of Graphene Oxide and Fungicides against Fusarium Head Blight In Vitro and In Vivo. Nanomaterials. 2021; 11(9):2393. https://doi.org/10.3390/nano11092393
Chicago/Turabian StyleWang, Xiuping, Fei Peng, Caihong Cheng, Lina Chen, Xuejuan Shi, Xiaoduo Gao, and Jun Li. 2021. "Synergistic Antifungal Activity of Graphene Oxide and Fungicides against Fusarium Head Blight In Vitro and In Vivo" Nanomaterials 11, no. 9: 2393. https://doi.org/10.3390/nano11092393
APA StyleWang, X., Peng, F., Cheng, C., Chen, L., Shi, X., Gao, X., & Li, J. (2021). Synergistic Antifungal Activity of Graphene Oxide and Fungicides against Fusarium Head Blight In Vitro and In Vivo. Nanomaterials, 11(9), 2393. https://doi.org/10.3390/nano11092393