Carbon Nanostructures, Nanolayers, and Their Composites
Abstract
1. Introduction
2. Carbon Nanostructures and Their Composites
2.1. Graphite
2.2. Diamond
2.3. Graphene
2.4. Carbon and Graphene Quantum Dots
2.5. Fullerenes
2.6. Carbon Nanotubes and Nanofibers
2.7. Carbon Nano-Onions
2.8. Globular Carbon Nanoparticles
2.9. Q-Carbon
2.10. Carbon Aerogel and Aeroraphite
2.11. Carbon-Carbon Materials
2.12. Carbon Materials Prepared from CO2
2.13. Diamond-Like Carbon (DLC) and PVD Technique
2.14. Hybrid Carbon Layers
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef]
- Skoda, M.; Dudek, I.; Jarosz, A.; Szukiewicz, D. Graphene: One Material, Many Possibilities-Application Difficulties in Biological Systems. J. Nanomater. 2014, 2014, 190. [Google Scholar] [CrossRef]
- Harris, P.J.F. New Perspectives on the Structure of Graphitic Carbons. Crit. Rev. Solid 2005, 30, 235–253. [Google Scholar] [CrossRef]
- Torres, L.E.F.; Roche, S.; Charlier, J.-C. Introduction to Carbon-Based Nanostructures, 2nd ed.; Cambridge University Press: Cambridge, UK, 2020; pp. 1–10. [Google Scholar]
- Khalaj, Z.; Monajjemi, M.; Diudea, M.V. Main Allotropes of Carbon: A Brief Review. In Sustainable Nanosystems Development, Properties, and Applications; Putz, M.V., Mirica, M.C., Eds.; IGI Global: Hershey, PA, USA, 2017; pp. 185–213. [Google Scholar]
- Slepicka, P.; Slepickova Kasalkova, N.; Siegel, J.; Kolska, Z.; Bacakova, L.; Svorcik, V. Nano-structured and functionalized surfaces for cytocompatibility improvement and bactericidal action. Biotechnol. Adv. 2015, 33, 1120–1129. [Google Scholar] [CrossRef]
- Grausova, L.; Vacik, J.; Vorlicek, V.; Svorcik, V.; Slepicka, P.; Bilkova, P.; Vandrovcova, M.; Lisa, V.; Bacakova, L. Fullerene C(60) films of continuous and micropatterned mophology as substrates for adhesion and growth of bone cells. Diam. Relat. Mater. 2009, 2009, 578–586. [Google Scholar] [CrossRef]
- Stankova, L.; Fraczek-Szczypta, A.; Blazewicz, M.; Filova, E.; Blazewicz, S.; Lisa, V.; Bacakova, L. Human osteoblast-like MG 63 cells on polysulfone modified with carbon nanotubes or carbon nanohorns. Carbon 2014, 67, 578–591. [Google Scholar] [CrossRef]
- Verdanova, M.; Rezek, B.; Broz, A.; Ukraintsev, E.; Babchenko, O.; Artemenko, A.; Izak, T.; Kromka, A.; Kalbac, M.; Hubalek Kalbacova, M. Nanocarbon allotropes-graphene and nanocrystalline diamond-promote cell proliferation. Small 2016, 12, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A. Carbon Materials and Nanotechnology, 1st ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 1–32. [Google Scholar]
- Falcao, E.H.; Wudl, F. Carbon allotropes: Beyond graphite and diamond. J. Chem. Technol. Biot. 2007, 82, 524–531. [Google Scholar] [CrossRef]
- Pisarciuc, C. Structure, Material Properties and Applications of Diamond-Like Materials. Nonconv. Technol. Rev. 2012, XVI, 13–18. [Google Scholar]
- Escudeiro, A.; Wimmer, M.A.; Polcar, T.; Cavaleiro, A. Tribological behavior of uncoated and DLC-coated CoCr and Ti-alloys in contact with UHMWPE and PEEK counterbodies. Tribology Int. 2015, 89, 97–104. [Google Scholar] [CrossRef]
- Mengesha, A.E.; Youan, B.B.C. Nanodiamonds for drug delivery systems. In Diamond-Based Materials for Biomedical Applications, 1st ed.; Narayan, R., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 186–205. [Google Scholar]
- Karczemska, A. Diamond materials for microfluidic devices. In Diamond-Based Materials for Biomedical Applications, 1st ed.; Narayan, R., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 256–271. [Google Scholar]
- Perez, G.; Maréchal, A.; Chicot, G.; Lefranc, P.; Jeannin, P.O.; Eon, D.; Rouger, N. Diamond semiconductor performances in power electronics applications. Diamond Rel. Mater. 2020, 110, 108154. [Google Scholar] [CrossRef]
- Narayan, J.; Bhaumik, A. Research Update: Direct conversion of amorphous carbon into diamond at ambient pressures and temperatures in air. APL Mater. 2015, 3, 100702-1–100702-11. [Google Scholar] [CrossRef]
- Bhaumik, A.; Sachan, R.; Narayan, J. High-Temperature Superconductivity in Boron-Doped Q-Carbon. ACS Nano 2017, 11, 5351–5357. [Google Scholar] [CrossRef]
- Kopova, I.; Rezek, B.; Stehlik, S.; Ukraintsev, E.; Slepickova Kasalkova, N.; Slepicka, P.; Potocky, S.; Bacakova, L. Growth of Primary Human Osteoblasts on Plasma-Treated and Nanodiamond-Coated PTFE Polymer Foils. Phys. Status Solidi B 2018, 255, 1700595. [Google Scholar] [CrossRef]
- Naeayan, R.J.; Boehm, R.D.; Sumant, A.V. Introduction to medical applications of diamond particles and surfaces. In Diamond-Based Materials for Biomedical Applications, 1st ed.; Narayan, R., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; Volume 1, pp. 3–24. [Google Scholar]
- Catledge, S.A.; Thomas, V.; Vohra, Y.K. Nanostructured diamond coatings for orthopaedic applications. In Diamond-Based Materials for Biomedical Applications, 1st ed.; Narayan, R., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; Volume 5, pp. 105–150. [Google Scholar]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Weitz, T.R.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Kiew, S.F.; Kiew, L.F.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef]
- Pacakova, B.; Verhagen, T.; Bousa, M.; Hübner, U.; Vejpravova, J.; Kalbac, M.; Frank, O. Mastering the Wrinkling of Selfsupported Graphene. Sci. Rep. 2017, 7, 10003. [Google Scholar] [CrossRef]
- Akinwande, D.; Brennan, C.J.; Bunch, J.S.; Egberts, P.; Felts, J.R.; Gao, H.; Huang, R.; Kim, J.S.; Li, T.; Li, X.; et al. A review on mechanics and mechanical properties of 2D materials-Graphene and beyond. Extrem Mech. Lett. 2017, 13, 42–77. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, J.; Sim, D.H.; Tay, Y.Y.; Lu, Z.; Zhang, Y.; Sharma, Y.; Srinivasan, M.; Zhang, H.; Hng, H.H.; et al. Achieving high specific charge capacitances in Fe3O4/reduced graphene oxide nanocomposites. J. Mater. Chem. 2011, 21, 3422–3427. [Google Scholar] [CrossRef]
- Anju, M.; Renuka, N.K. Graphene-dye hybrid optical sensors. Nano-Struct. Nano-Objects 2019, 17, 194–217. [Google Scholar]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.G.; Ninan, N.; Muthoosamy, K.; Manickam, S. Graphene: A versatile platform for nanotheranostics and tissue engineering. Prog. Mater. Sci. 2018, 91, 24–69. [Google Scholar]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef]
- Pinto, A.M.; Goncalves, I.C.; Magalhaes, F.D. Graphene-based materials biocompatibility: A review. Colloid Surface B 2013, 111, 188–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Graphene: A versatile nanoplatform for biomedical applications. Nanoscale 2012, 4, 3833–3842. [Google Scholar] [CrossRef]
- Li, N.; Cheng, Y.; Song, Q.; Jiang, Z.; Tang, M.; Cheng, G. Graphene meets biology. Chin. Sci. Bull. 2014, 59, 1341–1354. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Paul, R.; Dai, L. Interfacial aspects of carbon composites. Compos. Interfaces 2018, 25, 539–605. [Google Scholar] [CrossRef]
- Du, X.; Zhou, H.; Sun, W.; Liu, H.-Y.; Zhou, G.; Zhou, H.; Mai, Y.-W. Graphene/epoxy interleaves for delamination toughening and monitoring of crack damage in carbon fibre/epoxy composite laminates. Compos. Sci. Technol. 2017, 140, 123–133. [Google Scholar] [CrossRef]
- Slepička, P.; Slepičková Kasálková, N.; Pinkner, A.; Sajdl, P.; Kolská, Z.; Švorčík, V. Plasma induced cytocompatibility of stabilized poly-L-lactic acid doped with graphene nanoplatelets. React. Funct. Polym. 2018, 131, 266–275. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Paul, R.; Gayen, R.N.; Biswas, S.; Venkataprasad Bhat, S.; Bhunia, R. Enhanced UV detection by transparent graphene oxide/ZnO composite thin films. RSC Adv. 2016, 6, 61661–61672. [Google Scholar] [CrossRef]
- Fajstavr, D.; Neznalova, K.; Svorcik, V.; Slepicka, P. LIPSS Structures Induced on Graphene-Polystyrene Composite. Materials 2019, 12, 3460. [Google Scholar] [CrossRef] [PubMed]
- Slepičková Kasálková, N.; Buřičová, L.; Slepička, P.; Kolská, Z.; Švorčík, V. Carbon nanolayers deposited on laser treated PLLA film. Chem. Listy 2015, 109, 879–884. [Google Scholar]
- Slepička, P.; Neznalová, K.; Fajstavr, D.; Švorčík, V. Nanostructuring of honeycomb-like polystyrene with excimer laser. Prog. Org. Coat. 2020, 145, 105670. [Google Scholar] [CrossRef]
- Slepicka, P.; Siegel, J.; Lyutakov, O.; Slepickova Kasalkova, N.; Kolska, Z.; Bacakova, L.; Svorcik, V. Polymer nanostructures for bioapplications induced by laser treatment. Biotechnol. Adv. 2018, 36, 839–855. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Sajdl, P.; Veselý, M.; Švorčík, V. Surface analysis of ripple pattern on PS and PEN induced with ring-shaped mask due to KrF laser treatment. Surf. Interface Anal. 2017, 49, 25–33. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, C.; Li, R.; Mahdi Hamidinejad, S.; Park, C.B. Flexible, ultrathin, and high-efficiency electromagnetic shielding properties of poly (vinylidene fluoride)/carbon composite films. ACS Appl. Mater. Interfaces 2017, 9, 20873–20884. [Google Scholar] [CrossRef]
- Tuantranont, A. Nanomaterials for sensing applications: Introduction and perspectives. In Applications of Nanomaterials in Sensors and Diagnosis, 1st ed.; Tuantranont, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 14, p. 1. [Google Scholar]
- Das, S.; Wajid, A.S.; Shelburne, J.L.; Liao, Y.-C.; Green, M.J. Localized In situ Polymerization on Graphene Surfaces for Stabilized Graphene Dispersions. ACS Appl. Mater. Interfaces 2011, 3, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Wajid, A.S.; Das, S.; Irin, F.; Tanvir Ahmed, H.S.; Shelburne, J.L.; Parviz, D.; Fullerton, R.J.; Jankowski, A.F.; Hedden, R.C.; Green, M.J. Polymer-stabilized graphene dispersions at high concentrations in organic solvents for composite production. Carbon 2012, 50, 526–534. [Google Scholar] [CrossRef]
- Crevillen, A.G.; Escarpa, A.; García, C.D. (Eds.) Carbon-based nanomaterials in Analytical Chemistry. In Carbon-Based Nanomaterials in Analytical Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–36. [Google Scholar]
- Kim, K.; Abdala, A.A.; Macosko, C.W. Graphene/Polymer Nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Mohammad, N.S. Understanding quantum confinement in nanowires: Basics, applications and possible laws. J. Phys. Condens. Matter 2014, 26, 1–28. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Pan, D.; Guo, L.; Zhang, J.; Xi, C.; Xue, Q.; Huang, H.; Li, J.; Zhang, Z.; Yu, W.; Chen, Z.; et al. Cutting sp2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, C.; Zheng, X.; Gao, L.; Cui, Z.; Yang, H.; Guo, C.; Chi, H.; Li, C.M. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764–8766. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W.; et al. Surface Chemistry Routes to Modulate the Photoluminescence of Graphene Quantum Dots: From Fluorescence Mechanism to Up-Conversion Bioimaging Applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C 2018, 87, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.; Sofia da Rocha Freire Barros, A.S.C. Polysaccharides-Based Hybrids with Graphene. In Polysaccharide Based Hybrid Materials Metals and Metal Oxides, Graphene and Carbon Nanotubes, 1st ed.; Navard, P., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2018; pp. 69–93. [Google Scholar]
- Li, H.; Zhang, H. The isolated-pentagon rule and nice substructures infullerens. Ars Math. Contemp. 2018, 15, 487–497. [Google Scholar] [CrossRef]
- Hernández, E.; Ordejón, P.; Terrones, H. Fullerene growth and the role of nonclassical isomers. Phys. Rev. B 2001, 63, 193403.1–193403.4. [Google Scholar] [CrossRef]
- Fujine, K.; Ishida, T.; Aihara, J. Localization energies for graphite and fullerenes. Phys. Chem. Chem. Phys. 2001, 3, 3917–3919. [Google Scholar] [CrossRef]
- Mojica, M.; Alonso, J.A.; Méndez, F. Synthesis of fullerenes. J. Phys. Org. Chem. 2013, 26, 526–539. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Vacik, J.; Svorcik, V.; Slepicka, P.; Kasalkova, N.; Vorlicek, V.; Lavrentiev, V.; Vosecek, V.; Grausova, L.; Lisa, V.; et al. Fullerene C60 and hybrid C60/Ti films as substrates for adhesion and growth of bone cells. Phys. Status Solidi (a) 2008, 205, 2252–2261. [Google Scholar] [CrossRef]
- Kawase, T.; Tanaka, K.; Seirai, Y.; Shiono, N.; Oda, M. Complexation of Carbon Nanorings with Fullerenes: Supramolecular Dynamics and Structural Tuning for a Fullerene Sensor. Angew. Chem. Int. Ed. 2003, 42, 5597–5600. [Google Scholar] [CrossRef]
- Levi, N.; Hantgan, R.R.; Lively, M.O.; Carroll, D.L.; Prasad, G.L. C60-Fullerenes: Detection of intracellular photoluminescence and lack of cytotoxic effects. J. Nanobiotechnol. 2006, 4, 14. [Google Scholar] [CrossRef]
- Yamakoshi, Y.; Umezawa, N.; Ryu, A.; Arakane, K.; Miyata, N.; Goda, Y.; Masumizu, T.; Nagano, T. Active Oxygen Species Generated from Photoexcited Fullerene (C60) as Potential Medicines: O2-• versus 1O2. J. Am. Chem. Soc. 2003, 125, 12803–12809. [Google Scholar] [CrossRef]
- Tang, Y.J.; Ashcroft, J.M.; Chen, D.; Min, G.; Kim, C.-H.; Murkhejee, B.; Larabell, C.; Keasling, J.D.; Chen, F.F. Charge-asociated effects of fullerene derivatives on microbial structural integrity and central metabolism. Nano Lett. 2007, 7, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.E.; Choi, H.J.; Hwang, J.Y.; Bae, D.H. Strengthening behavior of carbon/metal nanocomposites. Sci. Rep. 2015, 6, 16114. [Google Scholar] [CrossRef]
- Tjong, S.C. Recent progress in the development and properties of novel metal matrix nanocomposites reinforced both carbon nanotubes and graphene nanosheets. Mater. Sci. Eng. R 2013, 74, 281–350. [Google Scholar] [CrossRef]
- Robles-Hernandez, F.C.; Calderon, H.A. Nanostructured metal composites reinforced with fullerenes. JOM 2010, 62, 63–68. [Google Scholar] [CrossRef]
- Shpilevsky, E.M.; Penyazkov, O.G.; Filatov, S.A.; Shilagardi, G.; Tuvshintur, P.; Timur-Bator, D.; Ulam-Orgikh, D. Modification of materials by carbon nanoparticles. Solid State Phenom. 2018, 271, 70–75. [Google Scholar] [CrossRef]
- Shpilevsky, E.M.; Filatov, S.A.; Shilagardi, G.; Ulam-Orgikh, D.; Tuvshintur, P.; Otgonbaatar, M. Properties of Metal-Fullerene Composites. Solid State Phenom. 2018, 288, 124–129. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Pikhitsa, P.V.; Jiang, H.; Brown, D.P.; Krasheninnikov, A.V.; Anisimov, A.S.; Queipo, P.; Moisala, A.; Gonzalez, D.; Lientschnig, G.; et al. A novel hybrid carbon material. Nat. Nanotechnol. 2007, 2, 156–161. [Google Scholar] [CrossRef]
- Savi, P.; Giorcelli, M.; Quaranta, S. Multi-Walled Carbon Nanotubes Composites for Microwave Absorbing Applications. Appl. Sci. 2019, 9, 851. [Google Scholar] [CrossRef]
- Scarselli, M.; Castrucci, P.; De Crescenzi, M. Electronic and optoelectronic nano-devices based on carbon nanotubes. J. Phys. Condens. Matter 2012, 24, 313202. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.N.; Ratna Prabha, C. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Gately, R.D. Filling of carbon nanotubes and nanofibres. Beilstein J. Nanotechnol. 2015, 6, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Malá, Z.; Rimpelová, S.; Slepičková Kasálková, N.; Švorčík, V. Plasma treatment of the surface of poly(hydroxybutyrate) foil and non-woven fabric and assessment of the biological properties. React. Funct. Polym. 2015, 95, 71–79. [Google Scholar] [CrossRef]
- Slepičková Kasálková, N.; Váchová, K.; Slepička, P.; Švorčík, V. Surface Plasma Modification and Characterization of Poly(ethylene-alt-tetrafluoroethylene). Chem. Listy 2016, 110, 279–283. [Google Scholar]
- Kolská, Z.; Řezníčková, A.; Nagyová, M.; Slepičková Kasálková, N.; Sajdl, P.; Slepička, P.; Švorčík, V. Plasma activated polymers grafted with cysteamine improving surfaces cytocompatibility. Polym. Degrad. Stab. 2014, 101, 1–9. [Google Scholar] [CrossRef]
- Slepičková Kasálková, N.; Slepička, P.; Bačáková, L.; Sajd, P.; Švorčík, V. Biocompatibility of plasma nanostructured biopolymers. Nucl. Instrum. Methods B 2013, 307, 642–646. [Google Scholar] [CrossRef]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef]
- Ali, M.S.; French, T.A.; Hastings, G.W.; Rae, T.; Rushton, N.; Ross, E.R.S.; Wynn-Jones, C.H. Carbon fibre composite bone plates. J. Bone Jt. Surg. 1990, 72, 586–591. [Google Scholar] [CrossRef]
- Pimberton, D.J.; McKibbin, B.; Savage, R.; Tayton, K.; Stuart, D. Carbon-Fibre Reinforced Plates for Problem Fractures. J. Bone Jt. Surg. 1992, 74, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Dikbas, I.; Tanalp, J. An Overview of Clinical Studies on Fiber Post Systems. Sci. World J. 2013, 2013, 171380. [Google Scholar] [CrossRef]
- Huang, Y.; Miao, Y.; Ji, S.; Tjiu, W.; Liu, T. Electrospun Carbon Nanofibers Decorated with Ag–Pt Bimetallic Nanoparticles for Selective Detection of Dopamine. ACS Appl. Mater. Interfaces 2014, 6, 12449–12456. [Google Scholar] [CrossRef]
- Tavangarian, F.; Li, Y. Carbon nanostructures as nerve scaffolds for repairing large gaps in severed nerves. Ceram. Int. 2012, 38, 6075–6090. [Google Scholar] [CrossRef]
- Petersen, R. Carbon Fiber Biocompatibility for Implants. Fibers 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Callister, W.D. Materials Science and Engineering, 4th ed.; John Wiley & Sons: New York, NY, USA, 1997; p. 2. [Google Scholar]
- Chawla, K.K. Composite Materials, 2nd ed.; Springer: New York, NY, USA, 1998; pp. 252–277. [Google Scholar]
- Berglund, L.A. Thermoplastic Resins. In Handbook of Composites, 2nd ed.; Peters, S.T., Ed.; Chapman and Hall: New York, NY, USA, 1998; p. 122. [Google Scholar]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Asaro, L.; Villanueva, S.; Alvarez, V.; Manfredi, L.B.; Rodríguez, E.S. Fire performance of composites made from carbon/ phenolic prepregs with nanoclays. J. Compos. Mater. 2017, 51, 3515–3524. [Google Scholar] [CrossRef]
- Chen, X.; Xia, J.; Peng, J.; Li, W.; Xie, S. Carbon-nanotube metal-matrix composites prepared by electroless plating. Compos. Sci. Technol. 2000, 60, 301–306. [Google Scholar] [CrossRef]
- Chung, D.D.L. Carbon Fiber Composites; Butterworth-Heinemann: Newton, MA, USA, 1994; Volume 7, pp. 125–144. [Google Scholar]
- Rawal, S.P. Metal-matrix composites for space applications. JOM 2001, 53, 14–17. [Google Scholar] [CrossRef]
- Harris, P.J.F. Carbon nanotube composites. Int. Mater. Rev. 2004, 49, 31–43. [Google Scholar] [CrossRef]
- Gullapalli, S.; Wong, M.S. Nanotechnology: A guide to nano-objects. Chem. Eng. Prog. 2011, 107, 28–32. [Google Scholar]
- Maa, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Xiea, X.-L.; Maia, Y.-W.; Zhou, X.-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R-Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Abolhasani, M.M.; Polisetti, B.; Naebe, M. Periodical patterning of a fully tailored nanocarbon on CNT for fabrication of thermoplastic composites. Compos. Part A 2018, 107, 304–314. [Google Scholar] [CrossRef]
- Seyhan, A.T.; Tanoglu, M.; Schulte, K. Mode I and mode II fracture toughness of E-glass non-crimp fabric/carbon nanotube (CNT) modified polymer based composites. Eng. Fract. Mech. 2008, 75, 5151–5516. [Google Scholar] [CrossRef]
- Roy, S.; Petrova, R.S.; Mitra, S. Effect of carbon nanotube (CNT) functionalization in epoxy-CNT composites. Nanotechnol. Rev. 2018, 7, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, R.; Pourazizi, R. Influence of CNT functionalization on the interphase region between CNT and polymer. Comput. Mater. Sci. 2015, 96, 573–578. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Balaji, K.V.; Yadav, R.; Zabihi, O.; Ahmadi, M.; Adetunji, P.; Naebe, M. Balancing the toughness and strength in polypropylene composites. Compos. Part B 2021, 223, 109121. [Google Scholar] [CrossRef]
- Ahmadabadi, V.G.; Shirvanimoghaddam, K.; Kerr, R.; Showkath, N.; Naebe, M. Structure-rate performance relationship in Si nanoparticles-carbon nanofiber composite as flexible anode for lithium-ion batteries. Electrochim. Acta 2020, 330, 135232. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorg. Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Aref, A.R.; Chen, S.-W.; Rajagopalan, R.; Randall, C. Bimodal porous carbon cathode and prelithiated coalesced carbon onion anode for ultrahigh power energy efficient lithium ion capacitors. Carbon 2019, 152, 89–97. [Google Scholar] [CrossRef]
- Hou, S.; Chung, D.-H.; Lin, T. High-yield synthesis of carbon nano-onions in counterflow diffusion flames. Carbon 2009, 47, 938–947. [Google Scholar] [CrossRef]
- Chung, D.-H.; Lin, T.-H.; Hou, S.S. Flame synthesis of carbon nano-onions enhanced by acoustic modulation. Nanotechnology 2010, 21, 435604. [Google Scholar] [CrossRef]
- Chen, X.H.; Deng, F.M.; Wang, J.X.; Yang, H.S.; Wu, G.T.; Zhang, X.B.; Peng, J.C.; Li, W.Z. New method of carbon onion growth by radio-frequency plasma-enhanced chemical vapor deposition. Chem. Phys. Lett. 2001, 336, 201–204. [Google Scholar] [CrossRef]
- Camisasca, A.; Giordaniac, S. Carbon nano-onions in biomedical applications: Promising theranostic agents. Inorg. Chim. Acta 2017, 468, 67–76. [Google Scholar] [CrossRef]
- Bobrowska, D.M.; Brzezinski, K.; Plonska-Brzezinska, M.E. PEGylated Carbon Nano-onions Composite as a Carrier of Polyphenolic Compounds: A Promising System for Medical Applications and Biological Sensors. Colloid Interface Sci. Commun. 2017, 21, 6–9. [Google Scholar] [CrossRef]
- Krokosz, A.; Lichota, A.; Nowak, K.E.; Grebowski, J. Carbon nanoparticles as possible radioprotectors in biological systems. Radiat. Phys. Chem. 2016, 128, 143–150. [Google Scholar] [CrossRef]
- Xu, B.; Yue, S.; Qiao, N.; Chu, M.; Wei, G. Easy preparation of nitrogen-doped porous carbon nanospheres and their application in supercapacitors. Mater. Lett. 2014, 131, 49–52. [Google Scholar] [CrossRef]
- Choi, C.H.; Park, S.H.; Chung, M.W.; Woo, S.I. Easy and controlled synthesis of nitrogen-doped carbon. Carbon 2013, 55, 98–107. [Google Scholar] [CrossRef]
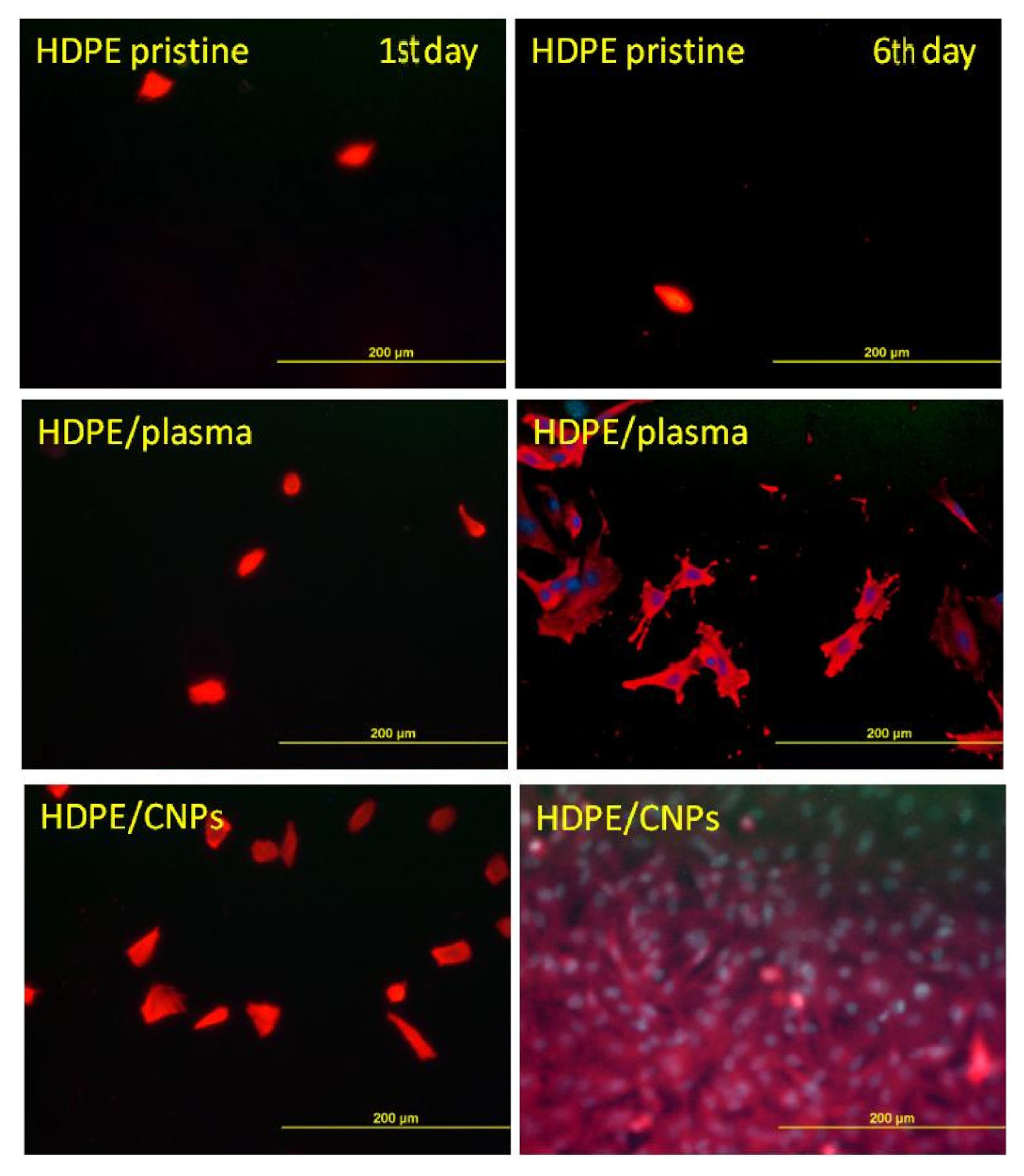
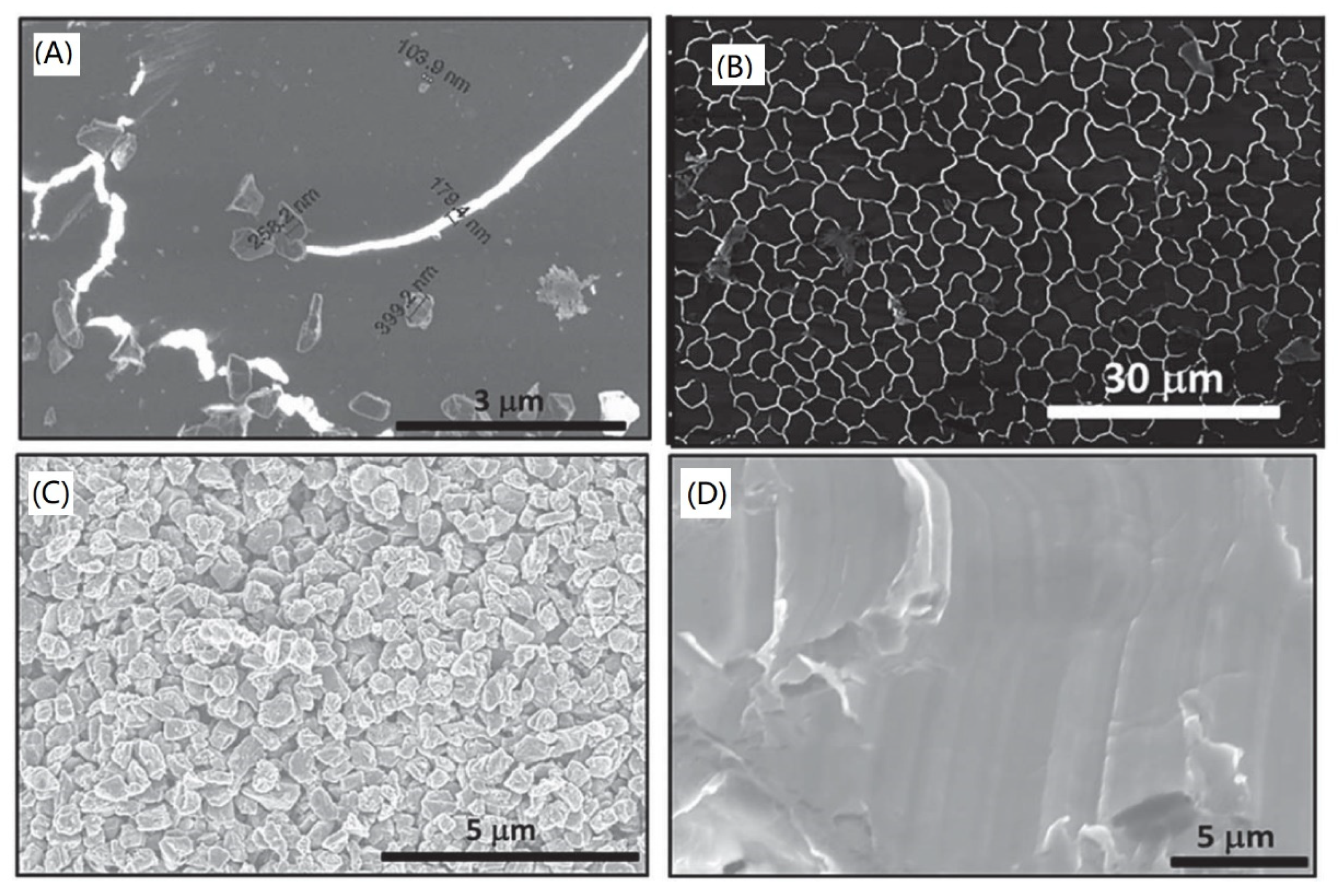
- Švorčík, V.; Makajová, Z.; Slepičková Kasálková, N.; Kolská, Z.; Žáková, P.; Karpíšková, J.; Stibor, I.; Slepička, P. Cytocompatibility of polymers grafted by activated carbon nano-particles. Carbon 2014, 69, 361–371. [Google Scholar] [CrossRef]
- Žáková, P.; Slepičková Kasálková, N.; Kolská, Z.; Leitner, J.; Karpíšková, J.; Stibor, I.; Slepička, P.; Švorčík, V. Cytocompatibility of amine functionalized carbon nanoparticles grafted on polyethylene. Mater. Sci. Eng. C 2016, 60, 294–401. [Google Scholar] [CrossRef] [PubMed]
- Žáková, P.; Slepičková Kasálková, N.; Slepička, P.; Kolská, Z.; Karpíšková, J.; Stibor, I.; Švorčík, V. Cytocompatibility of polyethylene grafted with triethylenetetramine functionalized carbon nanoparticles. Appl. Surf. Sci. 2017, 422, 809–816. [Google Scholar] [CrossRef]
- Haque, A.; Narayan, J. Electron field emission from Q-carbon. Diam. Relat. Mater. 2018, 86, 71–78. [Google Scholar] [CrossRef]
- Yoshinaka, H.; Inubushi, S.; Wakita, T.; Yokoya, T.; Muraoka, Y. Formation of Q-carbon by adjusting sp3 content in diamond-like carbon films and laser energy density of pulsed laser annealing. Carbon 2020, 167, 504–511. [Google Scholar] [CrossRef]
- Narayan, J.; Bhaumik, A. Q-carbon discovery and formation of single-crystal diamond nano- and microneedles and thin films. Mater. Res. Lett. 2016, 4, 118–126. [Google Scholar] [CrossRef][Green Version]
- Sachan, R.; Bhaumik, A.; Pant, P.; Prater, J.; Narayan, J. Diamond film growth by HFCVD on Q-carbon seeded substrate. Carbon 2019, 141, 182–189. [Google Scholar] [CrossRef]
- Narayan, J.; Gupta, S.; Bhaumik, A.; Sachan, R.; Cellini, F.; Riedo, E. Q-carbon harder than diamond. MRS Commun. 2018, 8, 428–436. [Google Scholar] [CrossRef]
- Narayan, J.; Bhaumik, A. Novel phase of carbon, ferromagnetism, and conversion into diamond. J. Appl. Phys. 2015, 118, 215303-1–215303-12. [Google Scholar] [CrossRef]
- Narayan, J.; Bhaumik, A.; Gupta, S.; Haque, A.; Sachan, R. Progress in Q-carbon and related materials with extraordinary properties. Mater. Res. Lett. 2018, 6, 353–364. [Google Scholar] [CrossRef]
- Bhaumik, A.; Nori, S.; Sachan, R.; Gupta, S.; Kumar, D.; Majumdar, A.K.; Narayan, J. Room-Temperature Ferromagnetism and Extraordinary Hall Effect in Nanostructured Q-Carbon: Implications for Potential Spintronic Devices. ACS Appl. Nano Mater. 2018, 1, 807–819. [Google Scholar] [CrossRef]
- Sachan, R.; Gupta, S.; Narayan, J. Nonequilibrium Structural Evolution of Q-Carbon and Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 1330–1338. [Google Scholar] [CrossRef]
- Bhaumik, A.; Sachan, R.; Narayan, J. A novel high-temperature carbon-based superconductor: B-doped Q-carbon. J. Appl. Phys. 2017, 122, 045301-2–045301-15. [Google Scholar] [CrossRef]
- Haque, A.; Pant, P.; Narayan, J. Large-area diamond thin film on Q-carbon coated crystalline sapphire by HFCVD. J. Cryst. Growth 2018, 504, 17–25. [Google Scholar] [CrossRef]
- Gupta, S.; Sachan, R.; Bhaumik, A.; Narayan, J. Enhanced mechanical properties of Q-carbon nanocomposites by nanosecond pulsed laser annealing. Nanotechnology 2018, 29, 45LT02. [Google Scholar] [CrossRef]
- Joshi, P.; Gupta, S.; Haque, A.; Narayan, J. Fabrication of ultrahard Q-carbon nanocoatings on AISI 304 and 316 stainless steels and subsequent formation of high-quality diamond films. Diam. Relat. Mater. 2020, 104, 107742. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jung, J.C.; Park, S.; Seo, J.G.; Baeck, S.-H.; Yoon, J.R.; Yi, J.; Song, I.K. Effect of preparation method on electrochemical property of Mn-doped carbon aerogel for supercapacitor. Curr. Appl. Phys. 2011, 11, 1–5. [Google Scholar] [CrossRef]
- Alex, A.S.; Lekshmi, M.S.A.; Sekkar, V.; John, B.; Gouri, C.; Ilangovan, S.A. Microporous carbon aerogel prepared through ambient pressure drying route as anode material for lithium ion cells. Polym. Adv. Technol. 2017, 28, 1945–1950. [Google Scholar] [CrossRef]
- Hanzawa, Y.; Hatori, H.; Yoshizawa, N.; Yamada, Y. Structural changes in carbon aerogels with high temperaturetreatment. Carbon 2002, 40, 575–581. [Google Scholar] [CrossRef]
- Lai, F.; Huang, Y.; Zuo, L.; Gu, H.; Miao, Y.-E.; Liu, T. Electrospun nanofiber-supported carbon aerogel as a versatile platform toward asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 15861–15869. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Wang, J.; Shen, J.; Deng, Z.S.; Lai, Z.Q.; Zhou, B.; Attia, S.M.; Chen, L.Y. The investigation of the adsorption character. Nanostruct. Mater. 1999, 11, 375–381. [Google Scholar] [CrossRef]
- Sun, W.; Du, A.; Gao, G.; Shen, J.; Wu, G. Graphene-templated carbon aerogels combining with ultra-high electrical conductivity and ultra-low thermal conductivity. Microporous Mesoporous Mater. 2017, 253, 71–79. [Google Scholar] [CrossRef]
- Song, P.; Cui, J.; Di, J.; Liu, D.; Xu, M.; Tang, B.; Zeng, Q.; Xiong, J.; Wang, C.; He, Q.; et al. Carbon Microtube Aerogel Derived from Kapok Fiber: An Efficient and Recyclable Sorbent for Oils and Organic Solvents. ACS Nano 2020, 14, 595–602. [Google Scholar] [CrossRef]
- Mecklenburg, M.; Schuchardt, A.; Mishra, J.K.; Kaps, S.; Adelung, R.; Lotnyk, A.; Kienle, L.; Schulte, K. Aerographite: Ultra Lightweight, Flexible Nanowall, Carbon Microtube Material with Outstanding Mechanical Performance. Adv. Mater. 2012, 24, 3486–3490. [Google Scholar] [CrossRef]
- Ashrafiyan, O.; Saremi, M.; Pakseresht, A.; Ghasali, E. Oxidation-Protective Coatings for Carbon-Carbon Composites. In Production, Properties, and Applications of High Temperature Coatings; Pakseresht, A.H., Ed.; IGI Global: Hershey, PA, USA, 2018; pp. 429–446. [Google Scholar]
- Zeng, Y.; Xiong, X.; Guo, S.; Zhang, W.-Z. SiC/SiC–YAG–YSZ oxidation protective coatings for carbon/carbon composites. Corros. Sci. 2013, 70, 68–73. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Su, F.; Chen, C.-M.; Liu, F.; Wu, Z.-S.; Ma, Y. Recent advances in carbon nanostructures prepared from carbon dioxide for high-performance supercapacitors. J. Energy Chem. 2021, 54, 352–367. [Google Scholar] [CrossRef]
- Gould, R.D.; Kasap, S.; Ray, A.K. Thin Films. In Springer Handbook of Electronic and Photonic Materials, 2nd ed.; Kasap, S., Capper, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 659–711. [Google Scholar]
- Shah, S.I.; Jaffari, G.H.; Yassitepe, E.; Ali, B. Evaporation: Processes, Bulk Microstructures, and Mechanical Properties. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 135–252. [Google Scholar]
- Rossnagel, S.M. Thin film deposition with physical vapor deposition and related technologies. J. Vac. Sci. Technol. A 2003, 21, S74–S87. [Google Scholar] [CrossRef]
- Lišková, J.; Slepičková Kasálková, N.; Slepička, P.; Švorčík, V.; Bačáková, L. Heat-treated carbon coatings on poly (L-lactide) foils for tissue engineering. Mater. Sci. Eng. C 2019, 100, 117–128. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Donley, M.S. Preparation of amorphous diamond-like carbon by pulsed laser deposition: A critical review. Surf. Coat. Technol. 1996, 82, 199–213. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.-R. Biomedical Applications of Diamond-Like Carbon Coatings: A Review. J. Biomed. Mater. Res. B 2007, 83, 72–84. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Lifshitz, Y. Hydrogen-flee amorphous carbon films: Correlation between growth conditions and properties. Diam. Relat. Mater. 1996, 5, 388–400. [Google Scholar] [CrossRef]
- Robertson, J. Hard amorphous (diamond-like) carbons. Prog. Solid State Chem. 1991, 21, 199–333. [Google Scholar] [CrossRef]
- Lettington, A.H. Application of diamond-like carbon thin films. Carbon 1998, 36, 555–560. [Google Scholar] [CrossRef]
- Ohgoe, Y.; Hirakuri, K.K.; Saitoh, H.; Nakahigashi, T.; Ohtake, N.; Hirata, A.; Kanda, K.; Hiratsuka, M.; Fukui, Y. Classification of DLC films in terms of biological response. Surf. Coat. Technol. 2012, 207, 350–354. [Google Scholar] [CrossRef]
- Hubáček, T.; Siegel, J.; Khalili, R.; Slepičková Kasálková, N.; Švorčík, V. Carbon coatings on polymers and their biocompatibility. Appl. Surf. Sci. 2013, 275, 43–48. [Google Scholar] [CrossRef]
- Lifshitz, Y. Diamond-like carbon—Present status. Diam. Relat. Mater. 1999, 8, 1659–1676. [Google Scholar] [CrossRef]
- Fryčková, O.; Hubáček, T.; Slepička, P.; Švorčík, V. Characterization and cytocompatibility of carbon films. J. Nanosci. 2012, 12, 6724–6730. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Roth, H.; Ehrhardt, H.; Silva, S.R.P.; Robertson, J.; Samlenski, R.; Brenn, R. Tetrahedral amorphous carbon films prepared by magnetron sputtering and dc ion plating. J. Appl. Phys. 1996, 79, 1416–1422. [Google Scholar] [CrossRef]
- Schelz, S.; Richmond, T.; Kania, P.; Oelhafen, P.; Güntherodt, H.J. Electronic and atomic structure of evaporated carbon films. Surf. Sci. 1996, 359, 227–236. [Google Scholar] [CrossRef]
- Chester, D.W.; Klemic, J.F.; Stern, E.; Sigwortha, F.J.; Klemic, K.G. Holey carbon micro-arrays for transmission electron microscopy: A microcontact printing approach. Ultramicroscopy 2007, 107, 685–691. [Google Scholar] [CrossRef]
- Slepička, P.; Hurtuková, K.; Fajstavr, D.; Slepičková Kasálková, N.; Lyutakov, O.; Švorčík, V. Carbon-gold nanocomposite induced by unique high energy laser single-shot annealing. Mater. Lett. 2021, 301, 130256. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slepičková Kasálková, N.; Slepička, P.; Švorčík, V. Carbon Nanostructures, Nanolayers, and Their Composites. Nanomaterials 2021, 11, 2368. https://doi.org/10.3390/nano11092368
Slepičková Kasálková N, Slepička P, Švorčík V. Carbon Nanostructures, Nanolayers, and Their Composites. Nanomaterials. 2021; 11(9):2368. https://doi.org/10.3390/nano11092368
Chicago/Turabian StyleSlepičková Kasálková, Nikola, Petr Slepička, and Václav Švorčík. 2021. "Carbon Nanostructures, Nanolayers, and Their Composites" Nanomaterials 11, no. 9: 2368. https://doi.org/10.3390/nano11092368
APA StyleSlepičková Kasálková, N., Slepička, P., & Švorčík, V. (2021). Carbon Nanostructures, Nanolayers, and Their Composites. Nanomaterials, 11(9), 2368. https://doi.org/10.3390/nano11092368




