A Scalable Solution Route to Porous Networks of Nanostructured Black Tungsten
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
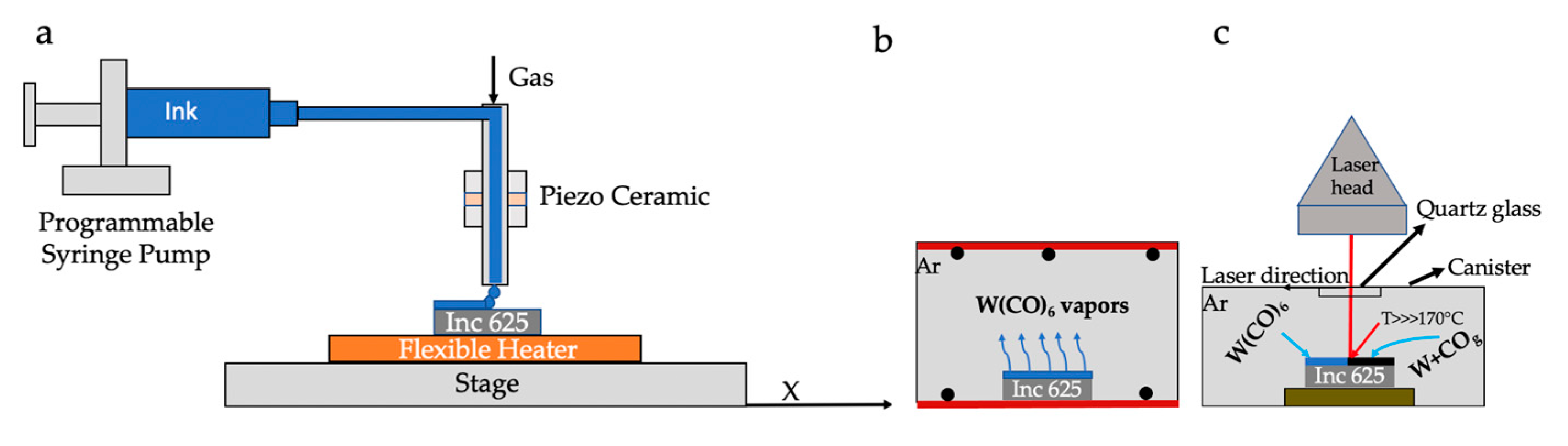
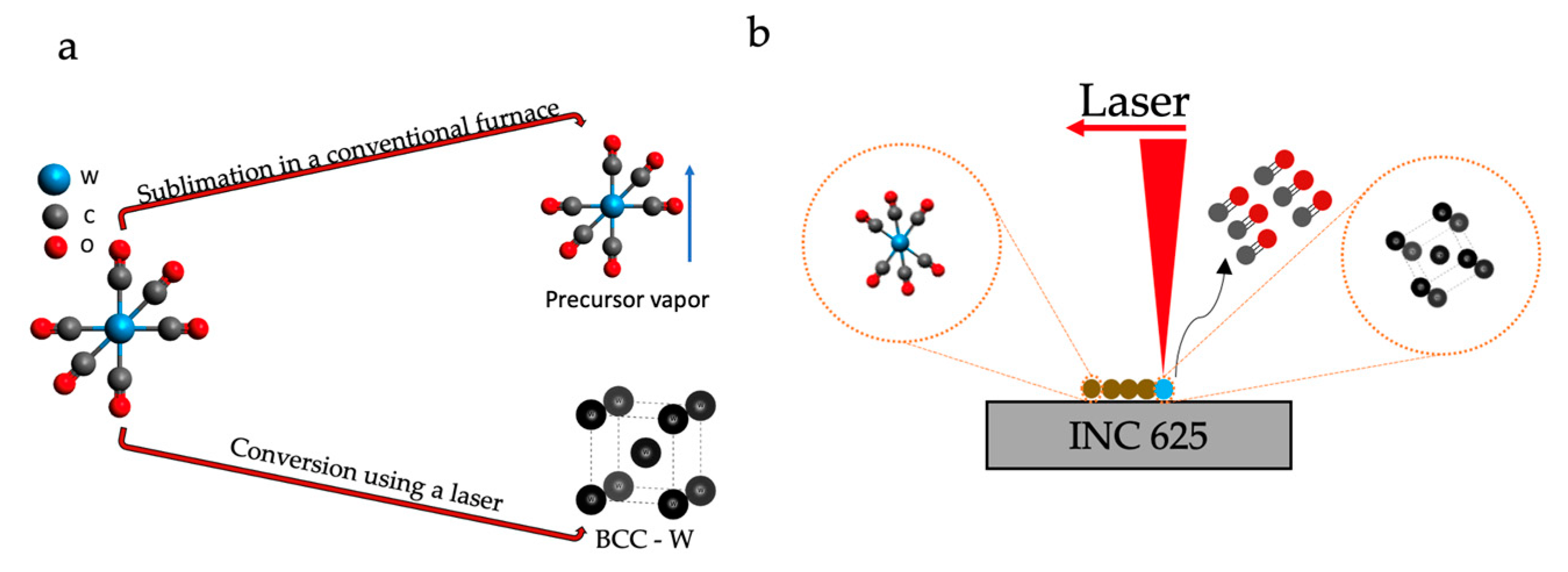
2.2. Deposition of Precursor and Laser-Assisted Conversion
2.3. Characterization Methods
3. Results and Discussion
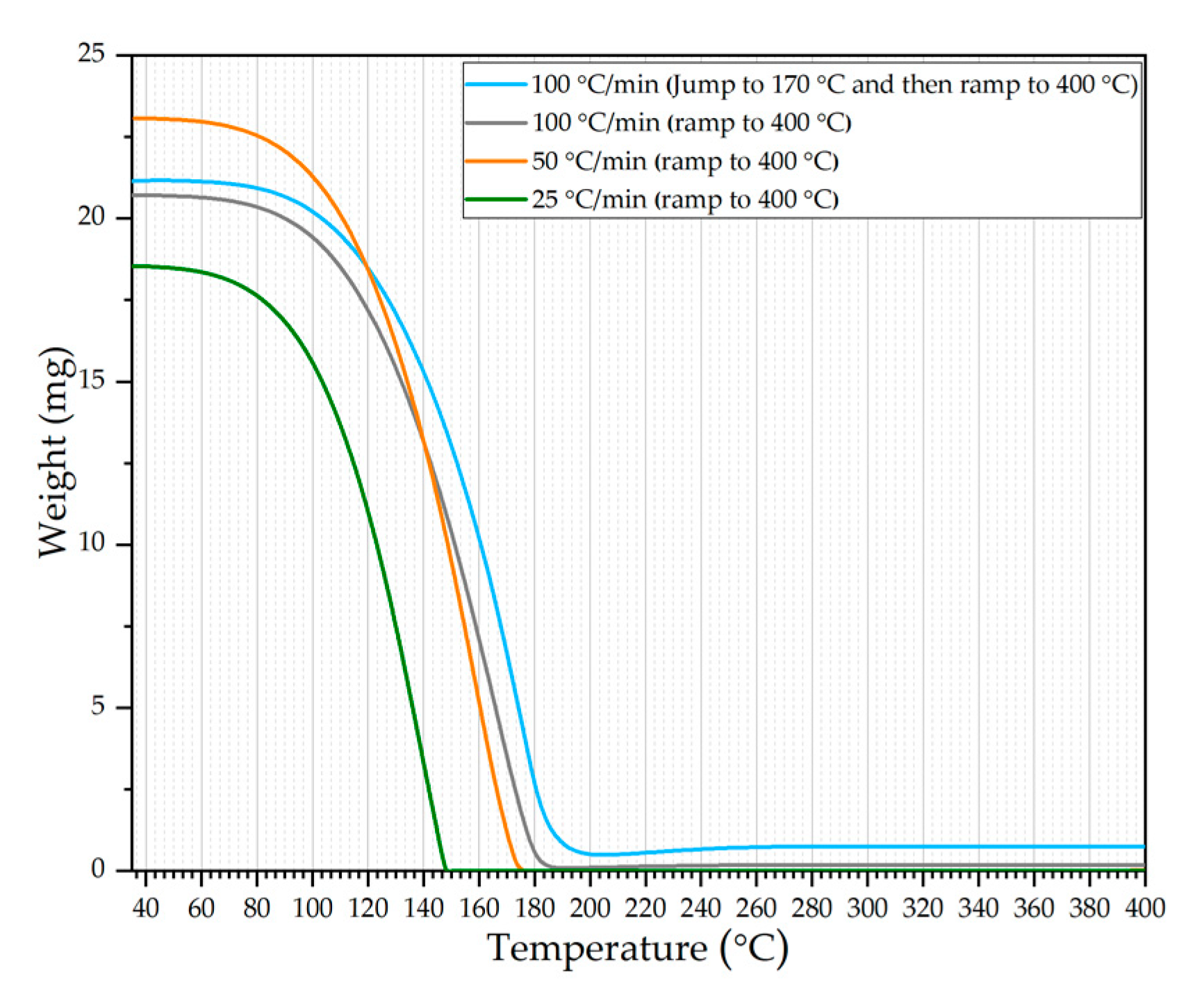
3.1. Thermogravimetric Analysis (TGA)
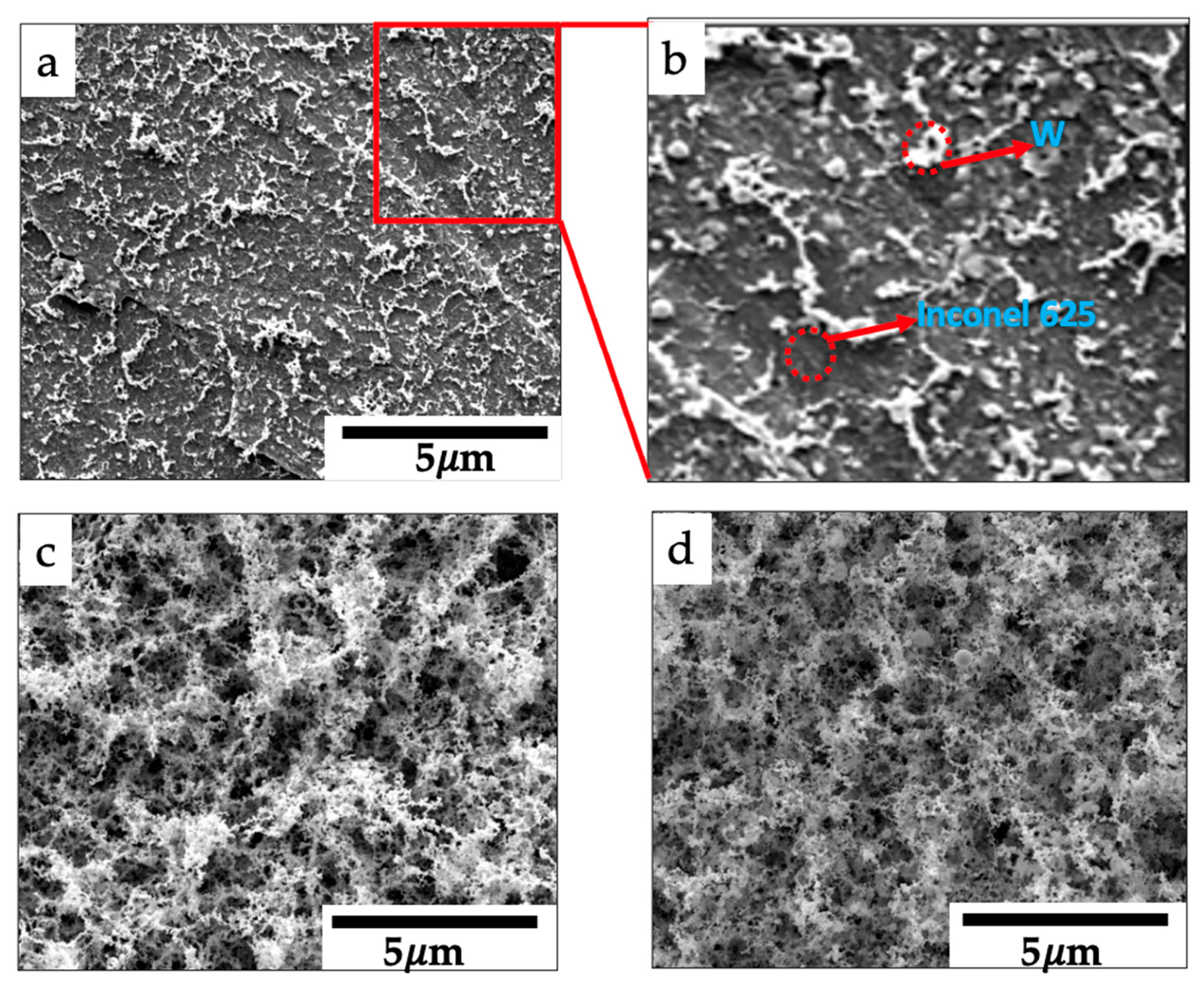
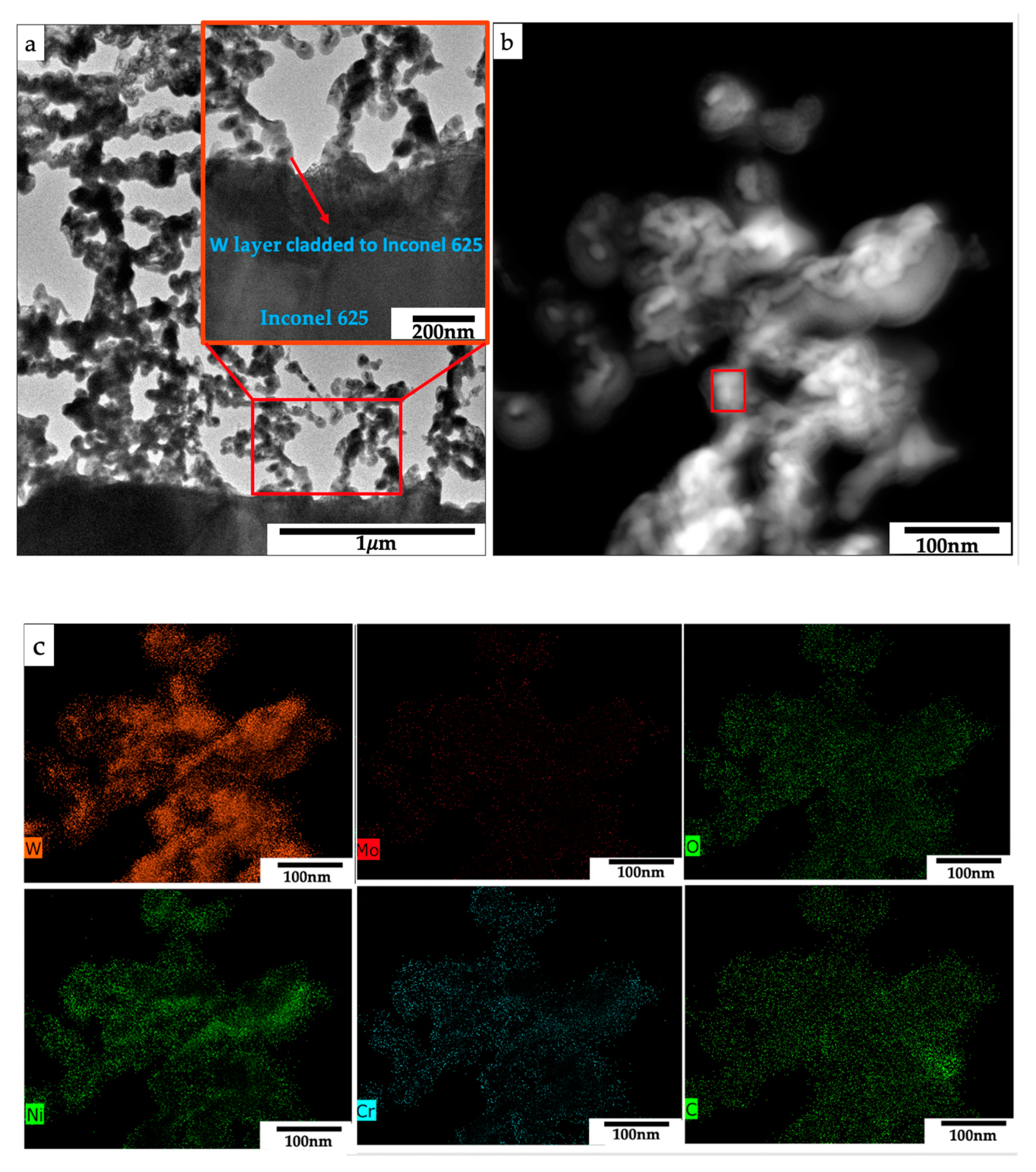
3.2. Crystal Structure, Morphology, and Composition
3.3. UV-VIS-NIR Reflectance and Solar Absorbance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.H.; Guo, Z.M.; Geng, Q.F.; Ma, P.J.; Liu, G. Enhanced absorptance of surface-textured tungsten thin film for solar absorber. Surf. Eng. 2016, 32, 840–845. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, P.; Akhtar, F. Aluminium matrix tungsten aluminide and tungsten reinforced composites by solid-state diffusion mechanism. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K.; Lamb, H.H. Tungsten chemical vapor deposition using tungsten hexacarbonyl: Microstructure of as-deposited and annealed films. Thin Solid Films 2000, 370, 114–121. [Google Scholar] [CrossRef]
- Sibin, K.P.; John, S.; Barshilia, H.C. Control of thermal emittance of stainless steel using sputtered tungsten thin films for solar thermal power applications. Sol. Energy Mater. Sol. Cells 2015, 133, 1–7. [Google Scholar] [CrossRef]
- Wang, W.; Wen, H.; Ling, S.; Li, Z.; Su, J.; Wang, C. Self-doped W-WO: X nanocermet multilayer films fabricated by single tungsten target reactive sputtering for selective solar absorption. J. Mater. Chem. A 2018, 6, 15690–15700. [Google Scholar] [CrossRef]
- Han, X.; He, K.; He, Z.; Zhang, Z. Tungsten-based highly selective solar absorber using simple nanodisk array. Opt. Express 2017, 25, A1072. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Yin, Q.; Chen, L.; Wang, Z.; Chen, X. Perfect spectrally selective solar absorber with dielectric filled fishnet tungsten grating for solar energy harvesting. Sol. Energy Mater. Sol. Cells 2020, 215, 110664. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Proietti Zaccaria, R.; Krahne, R.; Shih, W.C.; Garoli, D. Nanoporous metals: From plasmonic properties to applications in enhanced spectroscopy and photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Gupta, M.C. Spectral selective surfaces for concentrated solar power receivers by laser sintering of tungsten micro and nano particles. Sol. Energy Mater. Sol. Cells 2013, 117, 489–493. [Google Scholar] [CrossRef]
- Gesheva, K.A.; Gogova, D.S.; Stoyanov, G. Black tungsten selective optical coatings for photothermal solar energy conversion. J. Phys. III 1992, 2, 1453–1459. [Google Scholar] [CrossRef]
- Salama, E.; Eissa, M.M.; Tageldin, A.S. Distinct properties of tungsten austenitic stainless alloy as a potential nuclear engineering material. Nucl. Eng. Technol. 2019, 51, 784–791. [Google Scholar] [CrossRef]
- Paul, B.K.; Lee, K.; He, Y.; Ghayoor, M.; Chang, C.H.; Pasebani, S. Oxide dispersion strengthened 304 L stainless steel produced by ink jetting and laser powder bed fusion. CIRP Ann. 2020, 69, 193–196. [Google Scholar] [CrossRef]
- Raghu, T.; Sundaresan, R.; Ramakrishnan, P.; Rama Mohan, T.R. Synthesis of nanocrystalline copper-tungsten alloys by mechanical alloying. Mater. Sci. Eng. A 2001, 304, 438–441. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, H.; Gao, B.; Wang, X.; Hu, Y.; Shi, Z. Rapid consolidation of ultrafine grained W-30 wt.% Cu composites by field assisted sintering from the sol-gel prepared nanopowders. J. Alloys Compd. 2017, 724, 155–162. [Google Scholar] [CrossRef]
- Okamoto, H.; Akira, I. Tungsten metal film formed by spin-coating amorphous peroxopolytungstic acid. Appl. Phys. Lett. 1989, 55, 1923–1925. [Google Scholar]
- Wilkinson, N.J.; Smith, M.A.A.; Kay, R.W.; Harris, R.A. A review of aerosol jet printing—A non-traditional hybrid process for micro-manufacturing. Int. J. Adv. Manuf. Technol. 2019. [Google Scholar] [CrossRef]
- Tonneau, D.; Auvert, G.; Pauleau, Y. Laser-induced decomposition of metal carbonyls for chemical vapor deposition of microstructures. J. Phys. Colloq. 1989, 50, C5-647–C5-656. [Google Scholar] [CrossRef][Green Version]
- Gogova, D.; Gesheva, K.; Kakanakova-Georgieva, A.; Surtchev, M. Investigation of the structure of tungsten oxide films obtained by chemical vapor deposition. Eur. Phys. J. Appl. Phys. 2000, 167–174. [Google Scholar] [CrossRef]
- Sowdari, D.; Majumdar, P. Finite element analysis of laser irradiated metal heating and melting processes. Opt. Laser Technol. 2010, 42, 855–865. [Google Scholar] [CrossRef]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-specific enhancement of the carbon-carbon dioxide (boudouard) reaction. J. Phys. Chem. C 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Yuan, P.; Gu, D.; Dai, D. Particulate migration behavior and its mechanism during selective laser melting of TiC reinforced Al matrix nanocomposites. Mater. Des. 2015, 82, 46–55. [Google Scholar] [CrossRef]
- ASTM International. G173-03(2012) Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Ho, C.K. Advances in central receivers for concentrating solar applications. Sol. Energy 2017, 152, 38–56. [Google Scholar] [CrossRef]


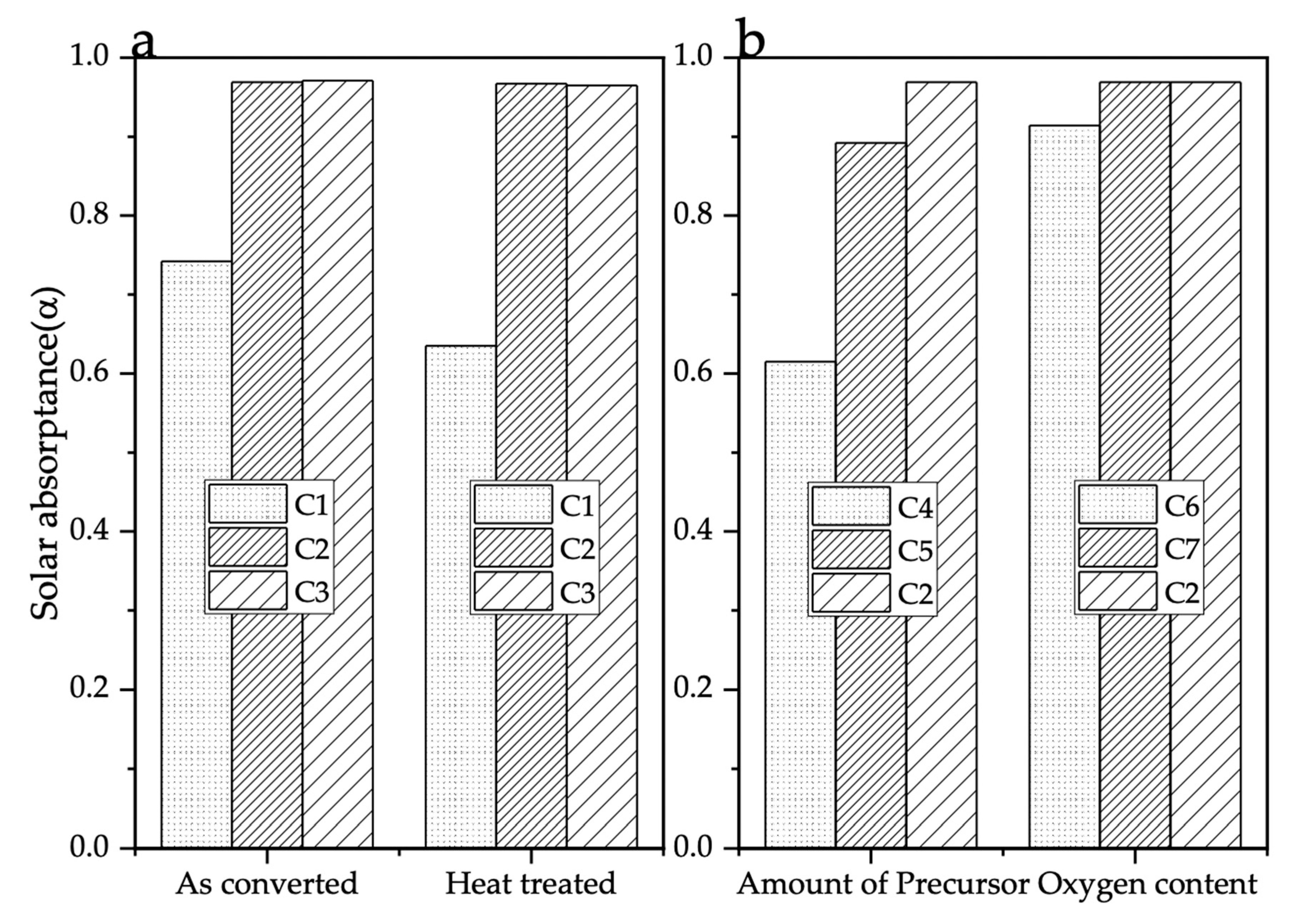
| Sample | Laser Power (W) | Scan Speed (mm/s) | Atmosphere | Amount of Precursor Ink Deposited (mL) |
|---|---|---|---|---|
| C1 | 60 | 1000 | Ar | 1.0 |
| C2 | 200 | 1000 | Ar | 1.0 |
| C3 | 400 | 1000 | Ar | 1.0 |
| C4 | 200 | 1000 | Ar | 0.1 |
| C5 | 200 | 1000 | Ar | 0.35 |
| C6 | 200 | 1000 | 21 vol% O2 | 1.0 |
| C7 | 200 | 1000 | 1.8 vol% O2/Ar | 1.0 |
| Elements | W | Ni | Cr | Mo | Fe | Ta | Nb | Si | O | C | Mn | Al | Co | Ti | Pt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass% | 52.2 | 7.33 | 3.08 | 2.18 | 1.76 | 7.95 | 2.28 | 2.96 | 4.30 | 6.66 | 1.16 | 1.45 | 1.45 | 0.63 | 4.55 |
| At% | 35.2 | 10.11 | 4.92 | 2.31 | 2.55 | 5.51 | 2.57 | 6.12 | 9.67 | 9.02 | 1.70 | 4.06 | 2.04 | 1.09 | 3.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doddapaneni, V.V.K.; Lee, K.; Colbert, T.T.; Mirzababaei, S.; Paul, B.K.; Pasebani, S.; Chang, C.-H. A Scalable Solution Route to Porous Networks of Nanostructured Black Tungsten. Nanomaterials 2021, 11, 2304. https://doi.org/10.3390/nano11092304
Doddapaneni VVK, Lee K, Colbert TT, Mirzababaei S, Paul BK, Pasebani S, Chang C-H. A Scalable Solution Route to Porous Networks of Nanostructured Black Tungsten. Nanomaterials. 2021; 11(9):2304. https://doi.org/10.3390/nano11092304
Chicago/Turabian StyleDoddapaneni, V. Vinay K., Kijoon Lee, Tyler T. Colbert, Saereh Mirzababaei, Brian K. Paul, Somayeh Pasebani, and Chih-Hung Chang. 2021. "A Scalable Solution Route to Porous Networks of Nanostructured Black Tungsten" Nanomaterials 11, no. 9: 2304. https://doi.org/10.3390/nano11092304
APA StyleDoddapaneni, V. V. K., Lee, K., Colbert, T. T., Mirzababaei, S., Paul, B. K., Pasebani, S., & Chang, C.-H. (2021). A Scalable Solution Route to Porous Networks of Nanostructured Black Tungsten. Nanomaterials, 11(9), 2304. https://doi.org/10.3390/nano11092304








