Surface-Functionalized Separator for Stable and Reliable Lithium Metal Batteries: A Review
Abstract
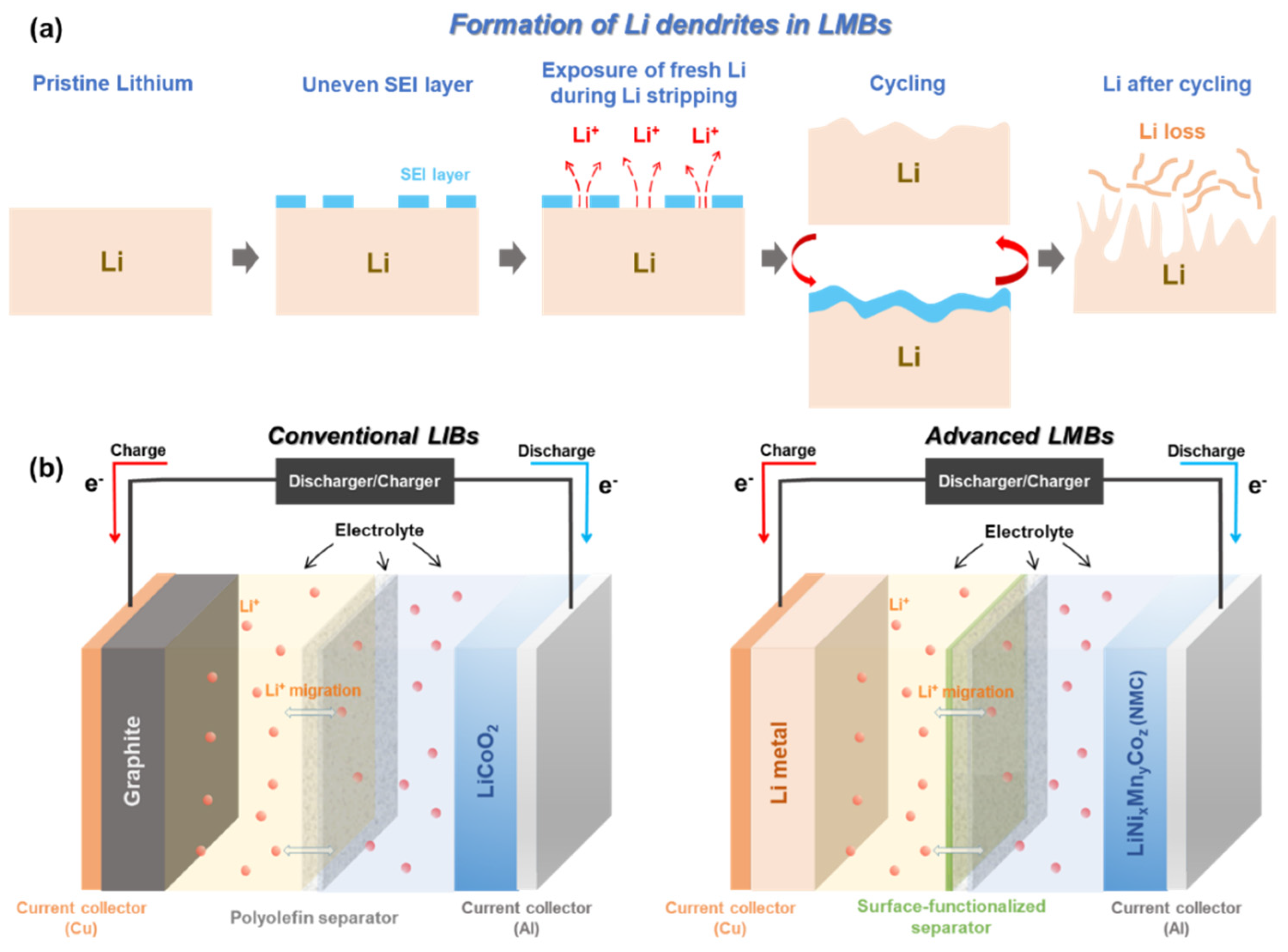
:1. Introduction
2. Conventional Polymer Separators for LIBs
2.1. General Characteristics of Separator for LIB and Requirements of Separator for LMBs
2.1.1. Porosity, Pore Size, and Thickness
2.1.2. Electrolyte Wettability
2.1.3. Thermal Properties
2.1.4. Mechanical/Electrochemical Stability
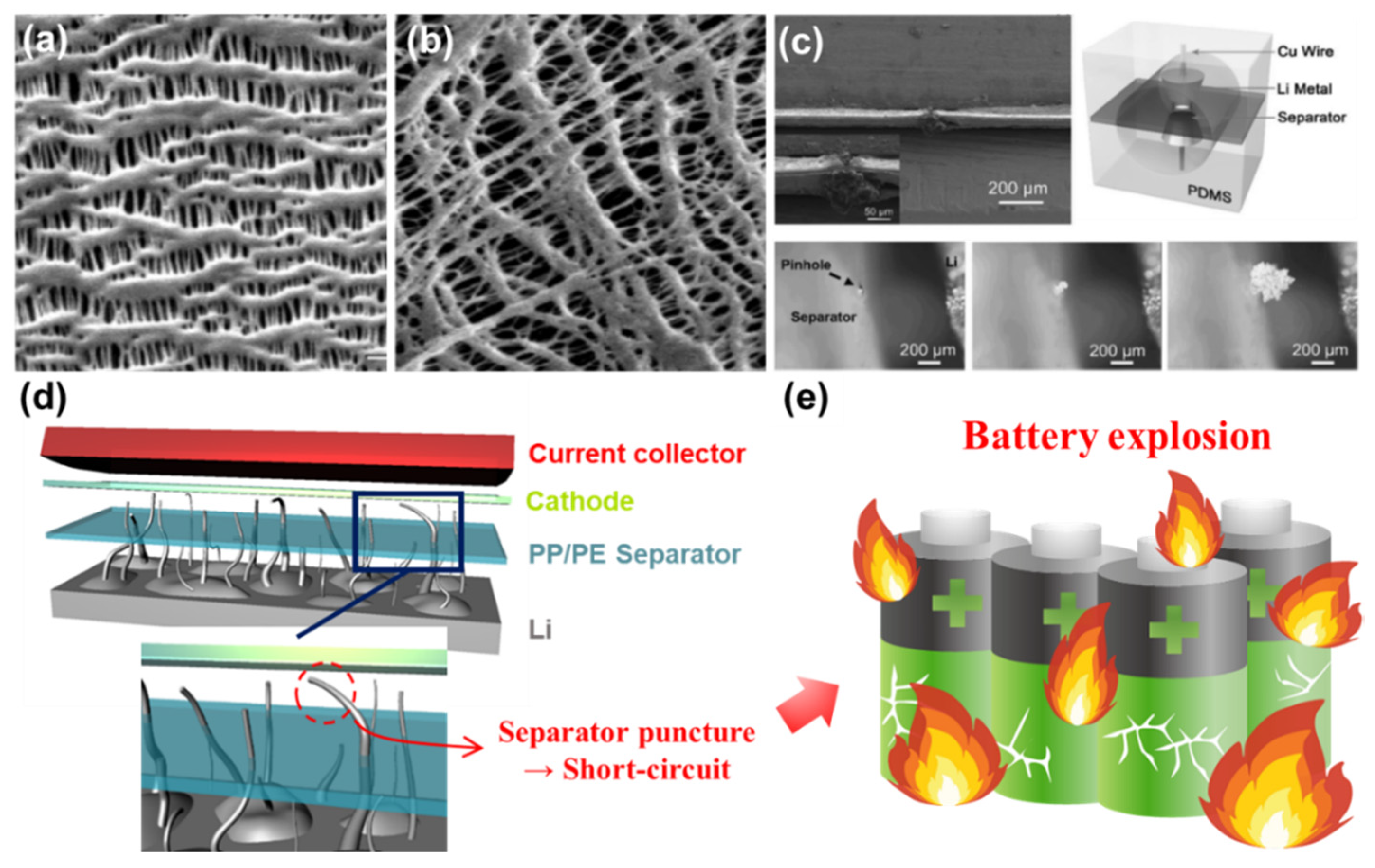
2.2. Technical and Inherent Issues to Be Resolved
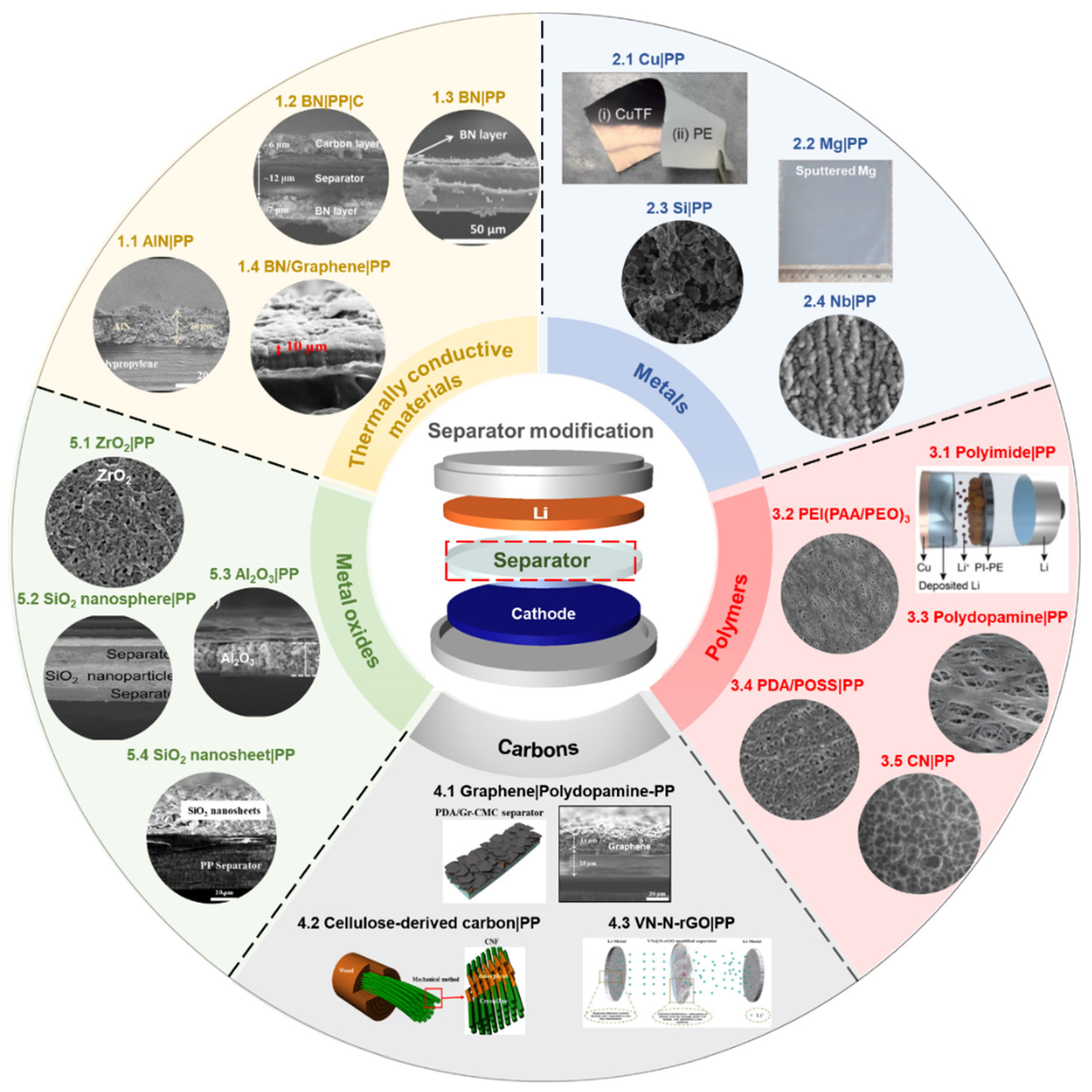
3. Approaches to Modify the Surface of Conventional Polymer Separators for LMBs
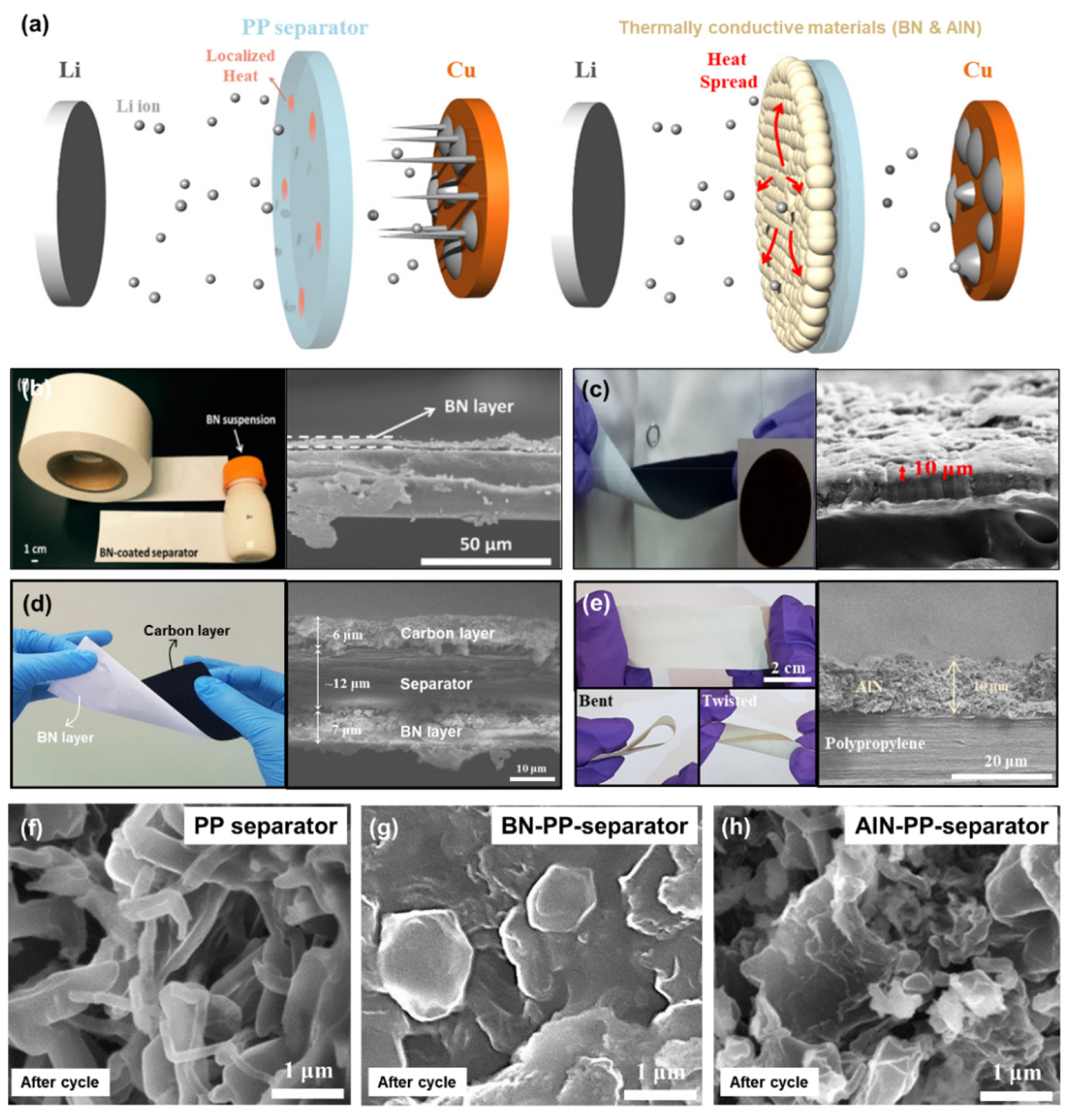
3.1. Surface Functionalization of Polymer Separator with Thermally Conductive Materials
3.2. Surface Functionalization of Polymer Separator with Metal Oxides
3.3. Surface Functionalization of Polymer Separator with Carbons
3.4. Surface Functionalization of Polymer Separator with Metals
3.5. Surface Functionalization of Polymer Separator with Polymers
3.6. Surface Functionalization of Polymer Separator with Other Materials (such as Solid-State Electrolytes)
4. Conclusions and Outlooks
Funding
Data Availability Statement
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Brandt, K. Historical development of secondary lithium batteries. Solid State Ion. 1994, 69, 173–183. [Google Scholar] [CrossRef]
- Scrosati, B. History of lithium batteries. J. Solid State Electr. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early lithium-battery development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Levin, K.; Fransen, T.; Schumer, C.; Davis, C. What Does” Net-Zero Emissions” Mean? 8 Common Questions, Answered. World Resources Institute. Available online: https://www.wri.org/insights/net-zero-ghg-emissions-questions-answered (accessed on 17 September 2019).
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing lithium metal batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O 2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, Y.; Chen, C.; Yang, J.; Gao, C.; Chen, Y.; Wu, J.; Shen, Y.; Zhang, W.; Li, S. Conductive MOF-modified separator for mitigating the shuttle effect of lithium–sulfur battery through a filtration method. ACS Appl. Mater. Interfaces 2019, 11, 11459–11465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, M.; Lv, W.; He, Y.-B.; Wang, C.; Yun, Q.; Li, B.; Kang, F.; Yang, Q.-H. Dense coating of Li4Ti5O12 and graphene mixture on the separator to produce long cycle life of lithium-sulfur battery. Nano Energy 2016, 30, 1–8. [Google Scholar] [CrossRef]
- Paolella, A.; Demers, H.; Chevallier, P.; Gagnon, C.; Girard, G.; Delaporte, N.; Zhu, W.; Vijh, A.; Guerfi, A.; Zaghib, K. A platinum nanolayer on lithium metal as an interfacial barrier to shuttle effect in Li-S batteries. J. Power Sources 2019, 427, 201–206. [Google Scholar] [CrossRef]
- Qian, X.; Jin, L.; Zhao, D.; Yang, X.; Wang, S.; Shen, X.; Rao, D.; Yao, S.; Zhou, Y.; Xi, X. Ketjen black-MnO composite coated separator for high performance rechargeable lithium-sulfur battery. Electrochim. Acta 2016, 192, 346–356. [Google Scholar] [CrossRef]
- Paolella, A.; Laul, D.; Timoshevskii, V.; Zhu, W.; Marras, S.; Bertoni, G.; Wahba, A.S.; Girard, G.; Gagnon, C.; Rodrigue, L. The role of metal disulfide interlayer in Li–S batteries. J. Phys. Chem. C 2018, 122, 1014–1023. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, K.; Qian, J.; Guo, Y.; Song, X.; Pan, H.; Wang, D.; Li, X. Boosting lithium–sulfur battery performance by integrating a redox-active covalent organic framework in the separator. ACS Appl. Energy Mater. 2019, 2, 5793–5798. [Google Scholar] [CrossRef]
- Kim, J.H.; Fu, K.; Choi, J.; Sun, S.; Kim, J.; Hu, L.; Paik, U. Hydroxylated carbon nanotube enhanced sulfur cathodes for improved electrochemical performance of lithium–sulfur batteries. Chem. Commun. 2015, 51, 13682–13685. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Fu, K.; Choi, J.; Kil, K.; Kim, J.; Han, X.; Hu, L.; Paik, U. Encapsulation of S/SWNT with PANI web for enhanced rate and cycle performance in lithium sulfur batteries. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.J.H.; Kim, K.; Pol, V.G. Towards highly stable lithium sulfur batteries: Surface functionalization of carbon nanotube scaffolds. Carbon 2018, 131, 175–183. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, J.; Seo, J.; Kwon, J.; Paik, U. Two-dimensional Nafion nanoweb anion-shield for improved electrochemical performances of lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 11203–11206. [Google Scholar] [CrossRef]
- Kim, K.; Kim, P.J.; Youngblood, J.P.; Pol, V.G. Surface Functionalization of Carbon Architecture with Nano-MnO2 for Effective Polysulfide Confinement in Lithium–Sulfur Batteries. ChemsusChem. 2018, 11, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Albertus, P.; Anandan, V.; Ban, C.; Balsara, N.; Belharouak, I.; Buettner-Garrett, J.; Chen, Z.; Daniel, C.; Doeff, M.; Dudney, N.J. Challenges for and pathways toward Li-metal-based all-solid-state batteries. ACS Energy Lett. 2021, 6, 1399–1404. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nature Energy 2016, 1, 1–4. [Google Scholar] [CrossRef]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.-M.; Chen, Z. Practical challenges hindering the development of solid state Li ion batteries. J. Electrochem. Soc. 2017, 164, A1731. [Google Scholar] [CrossRef]
- Hu, Y.-S. Batteries: Getting solid. Nat. Energy 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Liu, D.-H.; Bai, Z.; Li, M.; Yu, A.; Luo, D.; Liu, W.; Yang, L.; Lu, J.; Amine, K.; Chen, Z. Developing high safety Li-metal anodes for future high-energy Li-metal batteries: Strategies and perspectives. Chem. Soc. Rev. 2020, 49, 5407–5445. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sakamoto, J. Correlating the interface resistance and surface adhesion of the Li metal-solid electrolyte interface. J. Power Sources 2018, 377, 7–11. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Wang, X.; Xu, M.; Li, W.; Lucht, B.L. LiFSI and LiDFBOP dual-salt electrolyte reinforces the solid electrolyte interphase on a lithium metal anode. ACS Appl. Mater. Interfaces 2020, 12, 33719–33728. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef]
- Harry, K.J.; Hallinan, D.T.; Parkinson, D.Y.; MacDowell, A.A.; Balsara, N.P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 2014, 13, 69–73. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Wu, H.; Xie, Z.; Wang, Y.; Zhang, P.; Sun, L.; Lu, C.; Ma, Z. A constitutive model coupling irradiation with two-phase lithiation for lithium-ion battery electrodes. Philos. Mag. 2019, 99, 992–1013. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Jiang, W.; Zou, Y.; Lei, W.; Ma, Z. A coupled electrochemical–thermal–mechanical model for spiral-wound Li-ion batteries. J. Mater. Sci 2018, 53, 10987–11001. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, H.; Wang, Y.; Pan, Y.; Lu, C. An electrochemical-irradiated plasticity model for metallic electrodes in lithium-ion batteries. Int. J. Plast. 2017, 88, 188–203. [Google Scholar] [CrossRef]
- Kozen, A.C.; Lin, C.-F.; Pearse, A.J.; Schroeder, M.A.; Han, X.; Hu, L.; Lee, S.-B.; Rubloff, G.W.; Noked, M. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 2015, 9, 5884–5892. [Google Scholar] [CrossRef]
- Kazyak, E.; Wood, K.N.; Dasgupta, N.P. Improved cycle life and stability of lithium metal anodes through ultrathin atomic layer deposition surface treatments. Chem. Mater. 2015, 27, 6457–6462. [Google Scholar] [CrossRef]
- Kozen, A.C.; Lin, C.-F.; Zhao, O.; Lee, S.B.; Rubloff, G.W.; Noked, M. Stabilization of lithium metal anodes by hybrid artificial solid electrolyte interphase. Chem. Mater. 2017, 29, 6298–6307. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, A.-Y.; Liu, G.; Woo, J.-Y.; Kim, H.; Lee, J.K. Li4SiO4-based artificial passivation thin film for improving interfacial stability of Li metal anodes. ACS Appl. Mater. Interfaces 2018, 10, 8692–8701. [Google Scholar] [CrossRef]
- Chen, L.; Connell, J.G.; Nie, A.; Huang, Z.; Zavadil, K.R.; Klavetter, K.C.; Yuan, Y.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Libera, J.A. Lithium metal protected by atomic layer deposition metal oxide for high performance anodes. J. Mater. Chem. A 2017, 5, 12297–12309. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, Q.; Li, W.; Wu, B.; Jia, W.; Wang, Y.; Li, J.; Li, H. Long lifespan lithium metal anodes enabled by Al2O3 sputter coating. Energy Storage Mater. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Y.; Engelhard, M.H.; Li, Q.; Zhang, J.-G.; Xu, W. Guided lithium metal deposition and improved lithium coulombic efficiency through synergistic effects of LiAsF6 and cyclic carbonate additives. ACS Energy Lett. 2017, 3, 14–19. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Sun, Z.; Fang, R.; Wang, D.-W.; He, K.; Cheng, H.-M.; Li, F. Reliable liquid electrolytes for lithium metal batteries. Energy Storage Mater. 2020, 30, 113–129. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.-P.; Phan, T.N.; Bertin, D.; Gigmes, D.; Devaux, D. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Han, F.; Yue, J.; Zhu, X.; Wang, C. Suppressing Li dendrite formation in Li2S-P2S5 solid electrolyte by LiI incorporation. Adv. Energy Mater. 2018, 8, 1703644. [Google Scholar] [CrossRef]
- Zheng, J.; Engelhard, M.H.; Mei, D.; Jiao, S.; Polzin, B.J.; Zhang, J.-G.; Xu, W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.; Xie, D.; Fu, X.; Ma, X.; Li, Y.; Liu, Y.; Lin, Z.; Yang, C.; Liu, M. Suppressing dendrite growth by a functional electrolyte additive for robust Li metal anodes. Energy Storage Mater. 2019, 23, 701–706. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, G.; Liu, Z.; Li, X.; Wang, X.; Chen, R.; Wu, F.; Wang, Z.; Chen, L. Competitive Solvation Enhanced Stability of Lithium Metal Anode in Dual-Salt Electrolyte. Nano Lett. 2021, 21, 3310–3317. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Li, M.; Xia, D.; Holoubek, J.; Deng, Z.; Yu, M.; Tian, J.; Shan, Z.; Ong, S.P. A long-lasting dual-function electrolyte additive for stable lithium metal batteries. Nano Energy 2020, 75, 104889. [Google Scholar] [CrossRef]
- Francis, C.F.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Hung, L.; Chen, C. Materials science and engineering: R: Reports. Mater. Sci. Eng. 2002, 39, 143–222. [Google Scholar] [CrossRef]
- Nie, M.; Lucht, B.L. Role of lithium salt on solid electrolyte interface (SEI) formation and structure in lithium ion batteries. J. Electrochem. Soc. 2014, 161, A1001. [Google Scholar] [CrossRef]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef] [Green Version]
- Delaporte, N.; Perea, A.; Paolella, A.; Dubé, J.; Vigeant, M.-J.; Demers, H.; Clément, D.; Zhu, W.; Gariépy, V.; Zaghib, K. Alumina-flame retardant separators toward safe high voltage Li-Ion batteries. J. Power Sources 2021, 506, 230189. [Google Scholar] [CrossRef]
- Luo, X.; Lu, X.; Chen, X.; Chen, Y.; Song, C.; Yu, C.; Wang, N.; Su, D.; Wang, C.; Gao, X. A robust flame retardant fluorinated polyimide nanofiber separator for high-temperature lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 14788–14798. [Google Scholar] [CrossRef]
- Wu, N.; Wang, J.; Liao, C.; Han, L.; Song, L.; Hu, Y.; Mu, X.; Kan, Y. A flame retardant separator modified by MOFs-derived hybrid for safe and efficient Li-S batteries. J. Energy Chem. 2022, 64, 372–384. [Google Scholar] [CrossRef]
- Kim, P.J.H.; Pol, V.G. Surface functionalization of a conventional polypropylene separator with an aluminum nitride layer toward ultrastable and hsigh-rate lithium metal anodes. ACS Appl. Mater. Interfaces 2019, 11, 3917–3924. [Google Scholar] [CrossRef]
- Kim, P.J.H.; Seo, J.; Fu, K.; Choi, J.; Liu, Z.; Kwon, J.; Hu, L.; Paik, U. Synergistic protective effect of a BN-carbon separator for highly stable lithium sulfur batteries. NPG Asia Mater. 2017, 9, e375. [Google Scholar] [CrossRef]
- Kim, P.J.; Fontecha, H.D.; Kim, K.; Pol, V.G. Toward High-Performance Lithium-Sulfur Batteries: Upcycling of LDPE Plastic into Sulfonated Carbon Scaffold via Microwave-Promoted 66. ACS Appl. Mater. Interfaces 2018, 10, 14827–14834. [Google Scholar] [CrossRef]
- Kim, P.J.; Pol, V.G. High performance lithium metal batteries enabled by surface tailoring of polypropylene separator with a polydopamine/graphene layer. Adv. Energy Mater. 2018, 8, 1802665. [Google Scholar] [CrossRef]
- Kim, P.J.; Kim, K.; Pol, V.G. Uniform metal-ion flux through interface-modified membrane for highly stable metal batteries. Electrochim. Acta 2018, 283, 517–527. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Lee, H.R.; Yan, K.; Li, Y.; Shi, F.; Huang, W.; Pei, A.; Chen, G.; Subbaraman, R. Engineering stable interfaces for three-dimensional lithium metal anodes. Sci. Adv. 2018, 4, eaat5168. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Zhou, L.; Fu, K.; Yang, Z.; Wan, J.; Manno, M.; Yao, Y.; Zhu, H.; Yang, B.; Hu, L. A Thermally Conductive Separator for Stable Li Metal Anodes. Nano Lett. 2015, 15, 6149–6154. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, H.-S.; Ren, X.; Yu, L.; Engelhard, M.H.; Han, K.S.; Lee, J.; Kim, H.-T.; Xiao, J.; Liu, J. Detrimental effects of chemical crossover from the lithium anode to cathode in rechargeable lithium metal batteries. ACS Energy Lett. 2018, 3, 2921–2930. [Google Scholar] [CrossRef]
- Belanger, R.L.; Commarieu, B.; Paolella, A.; Daigle, J.-C.; Bessette, S.; Vijh, A.; Claverie, J.P.; Zaghib, K. Diffusion control of organic cathode materials in lithium metal battery. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shestakov, A.; Yarmolenko, O.; Ignatova, A.; Mumyatov, A.; Stevenson, K.; Troshin, P. Structural origins of capacity fading in lithium-polyimide batteries. J. Mater. Chem. A 2017, 5, 6532–6537. [Google Scholar] [CrossRef] [Green Version]
- Arora, P.; Zhang, Z.J. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electr. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Jeon, D.H. Wettability in electrodes and its impact on the performance of lithium-ion batteries. Energy Storage Mater. 2019, 18, 139–147. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Li, J.; Zhong, Q.; Yao, Y.; Bi, S.; Zhou, T.; Guo, X.; Wu, M.; Feng, T.; Xiang, R. Electrochemical performance and thermal stability of the electrospun PTFE nanofiber separator for lithium-ion batteries. J. Appl. Poly. Sci. 2018, 135, 46508. [Google Scholar] [CrossRef]
- Boateng, B.; Zhang, X.; Zhen, C.; Chen, D.; Han, Y.; Feng, C.; Chen, N.; He, W. Recent advances in separator engineering for effective dendrite suppression of Li-metal anodes. Nano Sel. 2021, 2, 993–1010. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energ. Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Sahraei, E.; Campbell, J.; Wierzbicki, T. Modeling and short circuit detection of 18650 Li-ion cells under mechanical abuse conditions. J. Power Sources 2012, 220, 360–372. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Wang, Y.; Pu, Y.; Ma, Z.; Pan, Y.; Sun, C.Q. Interfacial adhesion energy of lithium-ion battery electrodes. Extrem. Mech. Lett. 2016, 9, 226–236. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Z.; Wang, Y.; Lu, C. Failure prediction of high-capacity electrode materials in lithium-ion batteries. J. Electrochem. Soc. 2016, 163, A1157. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Xie, Z.; Wang, Y.; Zhang, P.; Pan, Y.; Zhou, Y.; Lu, C. Failure modes of hollow core–shell structural active materials during the lithiation–delithiation process. J. Power Sources 2015, 290, 114–122. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Lei, W.; Zou, Y.; Jiang, W.; Ma, Z.; Lu, C. Enhancement Effects of Co Doping on Interfacial Properties of Sn Electrode− Collector: A First-Principles Study. ACS Appl. Mater. Interfaces 2019, 11, 24648–24658. [Google Scholar] [CrossRef]
- Liu, K.; Zhuo, D.; Lee, H.W.; Liu, W.; Lin, D.; Lu, Y.; Cui, Y. Extending the life of lithium-based rechargeable batteries by reaction of lithium dendrites with a novel silica nanoparticle sandwiched separator. Adv. Mater. 2017, 29, 1603987. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [Green Version]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for lithium-ion batteries: A review on the production processes and recent developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Bai, P.; Guo, J.; Wang, M.; Kushima, A.; Su, L.; Li, J.; Brushett, F.R.; Bazant, M.Z. Interactions between lithium growths and nanoporous ceramic separators. Joule 2018, 2, 2434–2449. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, C.-Y.; Wang, C.-C.; Chen, K.-H.; Mou, C.-Y.; Wu, H.-L. Advanced nanoporous separators for stable lithium metal electrodeposition at ultra-high current densities in liquid electrolytes. J. Mater. Chem. A 2020, 8, 5095–5104. [Google Scholar] [CrossRef]
- Gao, C.; Hong, B.; Sun, K.; Fan, H.; Zhang, K.; Zhang, Z.; Lai, Y. Self-Suppression of Lithium Dendrite with Aluminum Nitride Nanoflake Additive in 3D Carbon Paper for Lithium Metal Batteries. Energy Technol. 2020, 8, 1901463. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Zhang, Y.; Yang, Z.; Gao, T.; Kirsch, D.; Liu, B.; Song, J.; Yang, B.; Hu, L. 3D printed separator for the thermal management of high-performance Li metal anodes. Energy Storage Mater. 2018, 12, 197–203. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, T.-W.; Yin, Y.-C.; Zhu, Z.-X.; Lu, L.-L.; Ma, C.; Zhou, F.; Yao, H.-B. Chemically exfoliated boron nitride nanosheets form robust interfacial layers for stable solid-state Li metal batteries. Chem. Commun. 2019, 55, 7703–7706. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.R.; Kim, P.J.; Kim, K.; Qi, Z.; Wang, H.; Pol, V.G. Engineered heat dissipation and current distribution boron nitride-graphene layer coated on polypropylene separator for high performance lithium metal battery. J. Colloid Interface Sci. 2021, 583, 362–370. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, A.; Li, N.; Li, S.; Zangiabadi, A.; Huang, W.; Li, A.C.; Jin, T.; Song, Q.; Xu, W. Stabilizing solid electrolyte-anode interface in Li-metal batteries by boron nitride-based nanocomposite coating. Joule 2019, 3, 1510–1522. [Google Scholar] [CrossRef]
- Lee, H.; Ren, X.; Niu, C.; Yu, L.; Engelhard, M.H.; Cho, I.; Ryou, M.H.; Jin, H.S.; Kim, H.T.; Liu, J. Suppressing lithium dendrite growth by metallic coating on a separator. Adv. Funct. Mater. 2017, 27, 1704391. [Google Scholar] [CrossRef]
- Din, M.M.U.; Murugan, R. Metal coated polypropylene separator with enhanced surface wettability for high capacity lithium metal batteries. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Liu, Y.; Xiong, S.; Wang, J.; Jiao, X.; Li, S.; Zhang, C.; Song, Z.; Song, J. Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Storage Mater. 2019, 19, 24–30. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Zhao, R.; Qi, X.; Li, K.; Sun, Q.; Ma, M.; Qie, L.; Huang, Y. A “dendrite-eating” separator for high-areal-capacity lithium-metal batteries. Energy Storage Mater. 2020, 31, 181–186. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, K.; Shen, F.; Han, X. Dendrite-Suppressing Separator with High Thermal Stability Modified by Beaded-Chain-Like Polyimide Coating for a Li Metal Anode. Energy Fuels 2021, 35, 8417–8422. [Google Scholar] [CrossRef]
- Ryou, M.H.; Lee, D.J.; Lee, J.N.; Lee, Y.M.; Park, J.K.; Choi, J.W. Excellent cycle life of lithium-metal anodes in lithium-ion batteries with mussel-inspired polydopamine-coated separators. Adv. Energy Mater. 2012, 2, 645–650. [Google Scholar] [CrossRef]
- Shen, L.; Liu, X.; Dong, J.; Zhang, Y.; Xu, C.; Lai, C.; Zhang, S. Functional lithiophilic polymer modified separator for dendrite-free and pulverization-free lithium metal batteries. J. Energy Chem. 2021, 52, 262–268. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Zhou, H.; Wang, Z.; Li, R.; Zhu, J.; Qiu, Z.; Zhao, Y.; Zhang, M.; Yuan, S. Polyethylene separators modified by ultrathin hybrid films enhancing lithium ion transport performance and Li-metal anode stability. Electrochim. Acta 2018, 259, 386–394. [Google Scholar] [CrossRef]
- Jin, R.; Fu, L.; Zhou, H.; Wang, Z.; Qiu, Z.; Shi, L.; Zhu, J.; Yuan, S. High Li+ ionic flux separator enhancing cycling stability of lithium metal anode. ACS. Sustain. Chem. Eng. 2018, 6, 2961–2968. [Google Scholar] [CrossRef]
- Yang, Q.; Cui, M.; Hu, J.; Chu, F.; Zheng, Y.; Liu, J.; Li, C. Ultrathin Defective C–N Coating to Enable Nanostructured Li Plating for Li Metal Batteries. ACS Nano 2020, 14, 1866–1878. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shin, D.O.; Kim, K.M.; Oh, J.; Kim, J.; Kang, S.H.; Lee, M.J.; Lee, Y.-G. Graphene oxide induced surface modification for functional separators in lithium secondary batteries. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, Y.; Yu, B.; Wang, B.; Wang, X.; Zhang, W.; Yang, D.; He, J. Lithiophilic 3D VN@ N-rGO as a Multifunctional Interlayer for Dendrite-Free and Ultrastable Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 20125–20136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Xin, L.; Liu, Y.; Yang, F.; Stach, E.A.; Xie, J. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nat. Energy 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Na, W.; Koh, K.H.; Lee, A.S.; Cho, S.; Ok, B.; Hwang, S.-W.; Lee, J.H.; Koo, C.M. Binder-less chemical grafting of SiO2 nanoparticles onto polyethylene separators for lithium-ion batteries. J. Membr. Sci. 2019, 573, 621–627. [Google Scholar] [CrossRef]
- Kang, S.M.; Ryou, M.-H.; Choi, J.W.; Lee, H. Mussel-and diatom-inspired silica coating on separators yields improved power and safety in Li-ion batteries. Chem. Mater. 2012, 24, 3481–3485. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Q.; Zhou, H.; Qi, L. Binder-Free TiO2-Coated Polypropylene Separators for Advanced Lithium-Ion Batteries. Energy Technol. 2020, 8, 2000228. [Google Scholar] [CrossRef]
- Chi, M.; Shi, L.; Wang, Z.; Zhu, J.; Mao, X.; Zhao, Y.; Zhang, M.; Sun, L.; Yuan, S. Excellent rate capability and cycle life of Li metal batteries with ZrO2/POSS multilayer-assembled PE separators. Nano Energy 2016, 28, 1–11. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Wang, Z.; Shi, L.; Zhao, Y.; Yuan, S. Dual-Scale Al2O3 Particles Coating for High-Performance Separator and Lithium Metal Anode. Energy Technol. 2020, 8, 1901429. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Y.; Wang, J.; Zhang, Y.; Liao, C.; Mu, X.; Sheng, H.; Kan, Y.; Song, L.; Hu, Y. Enhanced electrochemical and safety performance of lithium metal batteries enabled by the atom layer deposition on PVDF-HFP separator. ACS Appl. Energy Mater. 2019, 2, 4167–4174. [Google Scholar] [CrossRef]
- Jeon, H.; Jin, S.Y.; Park, W.H.; Lee, H.; Kim, H.-T.; Ryou, M.-H.; Lee, Y.M. Plasma-assisted water-based Al2O3 ceramic coating for polyethylene-based microporous separators for lithium metal secondary batteries. Electrochim. Acta 2016, 212, 649–656. [Google Scholar] [CrossRef]
- Jung, B.; Lee, B.; Jeong, Y.-C.; Lee, J.; Yang, S.R.; Kim, H.; Park, M. Thermally stable non-aqueous ceramic-coated separators with enhanced nail penetration performance. J. Power Sources 2019, 427, 271–282. [Google Scholar] [CrossRef]
- Mao, Y.; Sun, W.; Qiao, Y.; Liu, X.; Xu, C.; Fang, L.; Hou, W.; Wang, Z.; Sun, K. A high strength hybrid separator with fast ionic conductor for dendrite-free lithium metal batteries. Chem. Eng. J. 2021, 416, 129119. [Google Scholar] [CrossRef]
- Zhao, C.-Z.; Chen, P.-Y.; Zhang, R.; Chen, X.; Li, B.-Q.; Zhang, X.-Q.; Cheng, X.-B.; Zhang, Q. An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 2018, 4, eaat3446. [Google Scholar] [CrossRef] [Green Version]
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366. [Google Scholar] [CrossRef]
- Yan, J.; Liu, F.; Hu, Z.; Gao, J.; Zhou, W.; Huo, H.; Zhou, J.; Li, L. Realizing dendrite-free lithium deposition with a composite separator. Nano Lett. 2020, 20, 3798–3807. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-H.; Zhang, M.; Lu, P.-X.; Wan, Y.-L.; Chen, Q.-L.; Niu, H.-Y.; Yu, Z.-W. A multifunctional separator with Mg (OH) 2 nanoflake coatings for safe lithium-metal batteries. J. Energy Chem. 2021, 52, 75–83. [Google Scholar] [CrossRef]
- Kim, P.J.; Kim, K.; Pol, V.G. A comparative study of cellulose derived structured carbons on the electrochemical behavior of lithium metal-based batteries. Energy Storage Mater. 2019, 19, 179–185. [Google Scholar] [CrossRef]
- Lu, J.G.; Chang, P.; Fan, Z. Quasi-one-dimensional metal oxide materials—Synthesis, properties and applications. Mater. Sci. Eng. R Rep. 2006, 52, 49–91. [Google Scholar] [CrossRef]
- Yang, C.; Tong, H.; Luo, C.; Yuan, S.; Chen, G.; Yang, Y. Boehmite particle coating modified microporous polyethylene membrane: A promising separator for lithium ion batteries. J. Power Sources 2017, 348, 80–86. [Google Scholar] [CrossRef]
- Nestler, T.; Schmid, R.; Münchgesang, W.; Bazhenov, V.; Schilm, J.; Leisegang, T.; Meyer, D.C. Separators-technology review: Ceramic based separators for secondary batteries. AIP Conf. Proc. 2014, 1597, 155–184. [Google Scholar]
- Kim, K.J.; Kwon, H.K.; Park, M.-S.; Yim, T.; Yu, J.-S.; Kim, Y.-J. Ceramic composite separators coated with moisturized ZrO2 nanoparticles for improving the electrochemical performance and thermal stability of lithium ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 9337–9343. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.-Y. Current status and future research directions of separator membranes for lithium-ion rechargeable batteries. Membr. J. 2016, 26, 337–350. [Google Scholar] [CrossRef]
- Xiao, J.; Han, J.; Zhang, C.; Ling, G.; Kang, F.; Yang, Q.H. Dimensionality, Function and Performance of Carbon Materials in Energy Storage Devices. Adv. Energy Mater. 2021, 2100775. [Google Scholar] [CrossRef]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Zhang, R.; Cheng, X.B.; Zhao, C.Z.; Peng, H.J.; Shi, J.L.; Huang, J.Q.; Wang, J.; Wei, F.; Zhang, Q. Conductive nanostructured scaffolds render low local current density to inhibit lithium dendrite growth. Adv. Mater. 2016, 28, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Mori, T.; Nishida, T.; Fukunaka, Y.; Rosso, M. Li dendrite growth and Li+ ionic mass transfer phenomenon. J. Electroanal. Chem. 2011, 661, 84–89. [Google Scholar] [CrossRef]
- Paquin, R.A. Properties of metals. Handb. Opt. 1995, 2, 35–49. [Google Scholar]
- Lopez, J.; Mackanic, D.G.; Cui, Y.; Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 2019, 4, 312–330. [Google Scholar] [CrossRef] [Green Version]
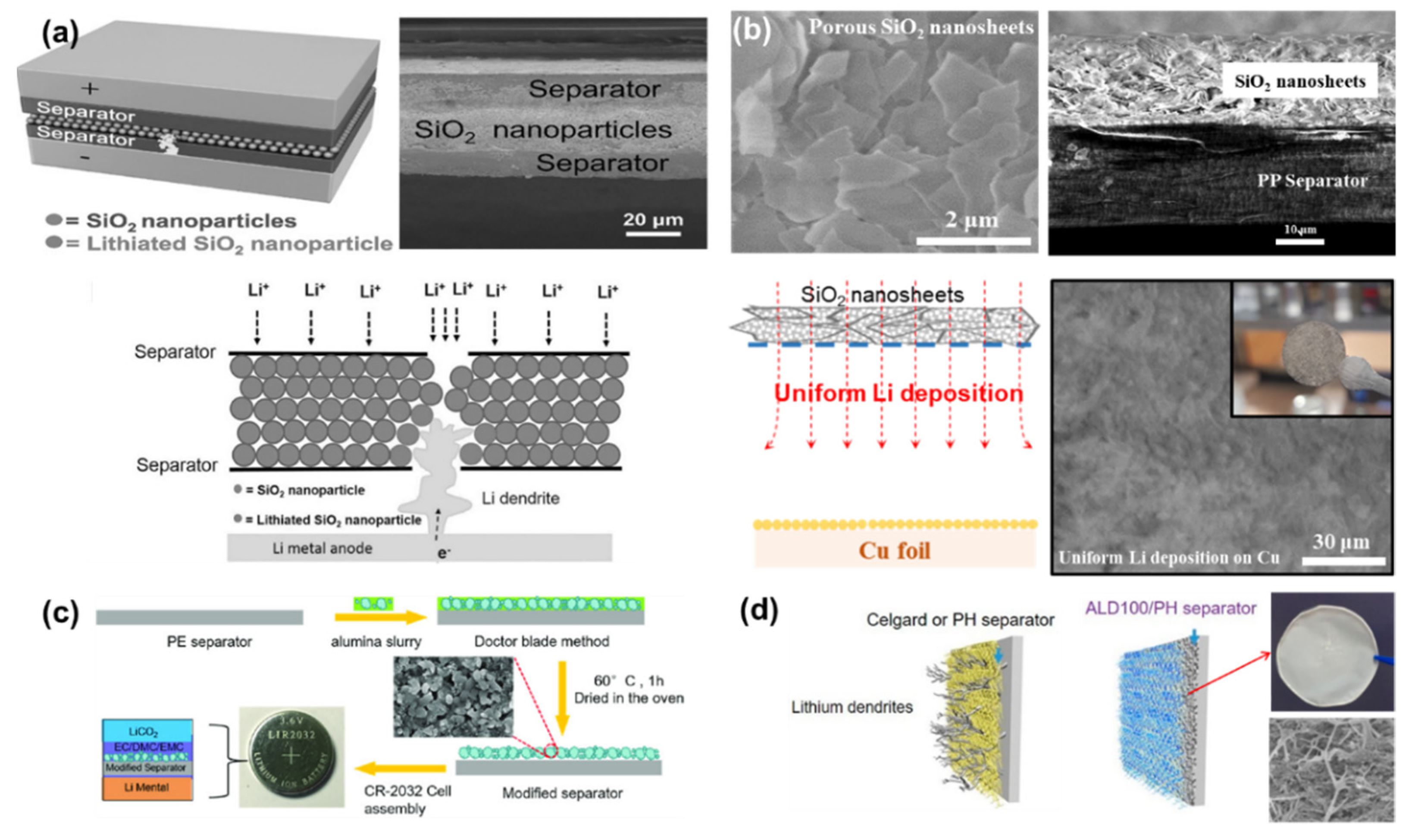
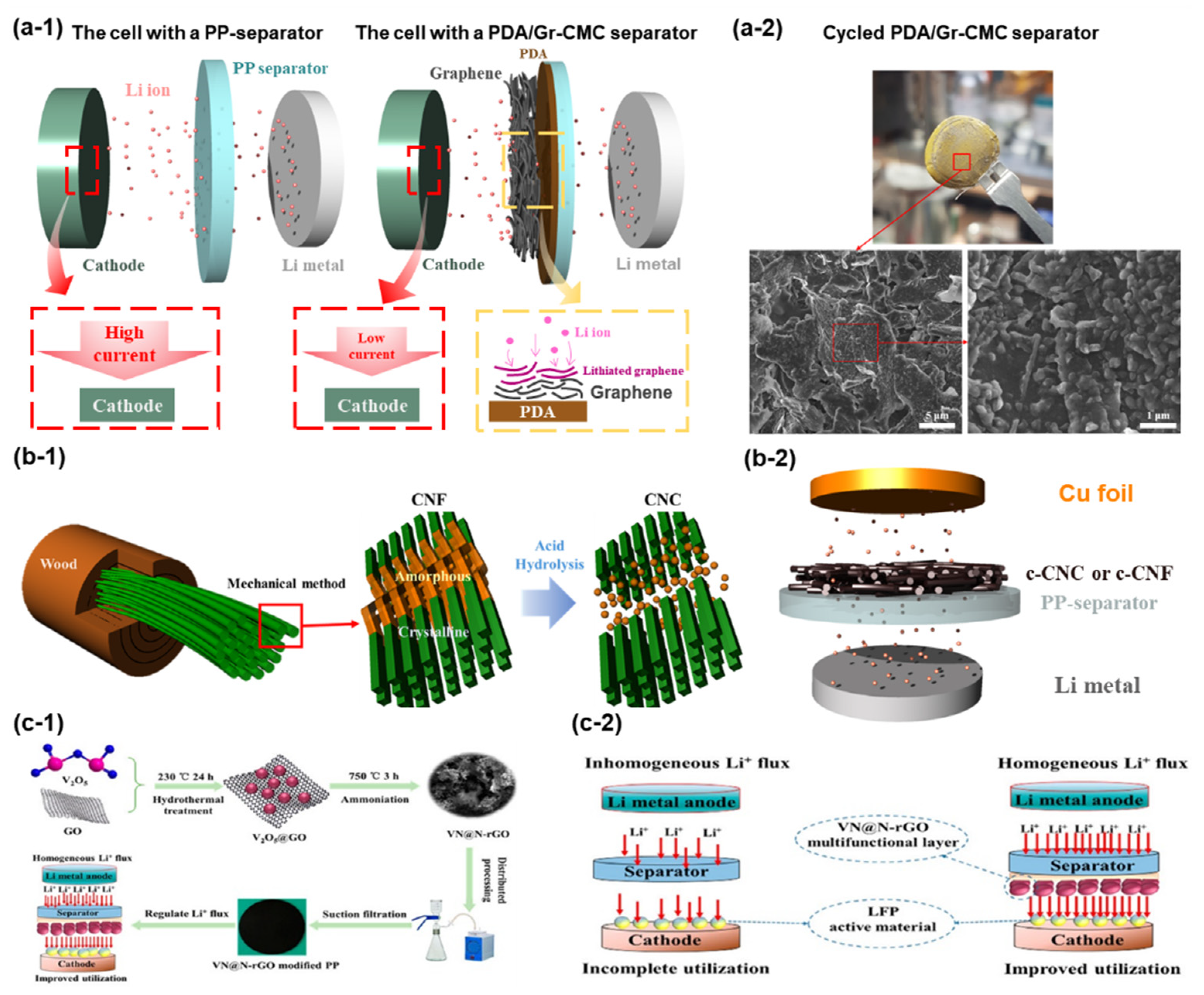
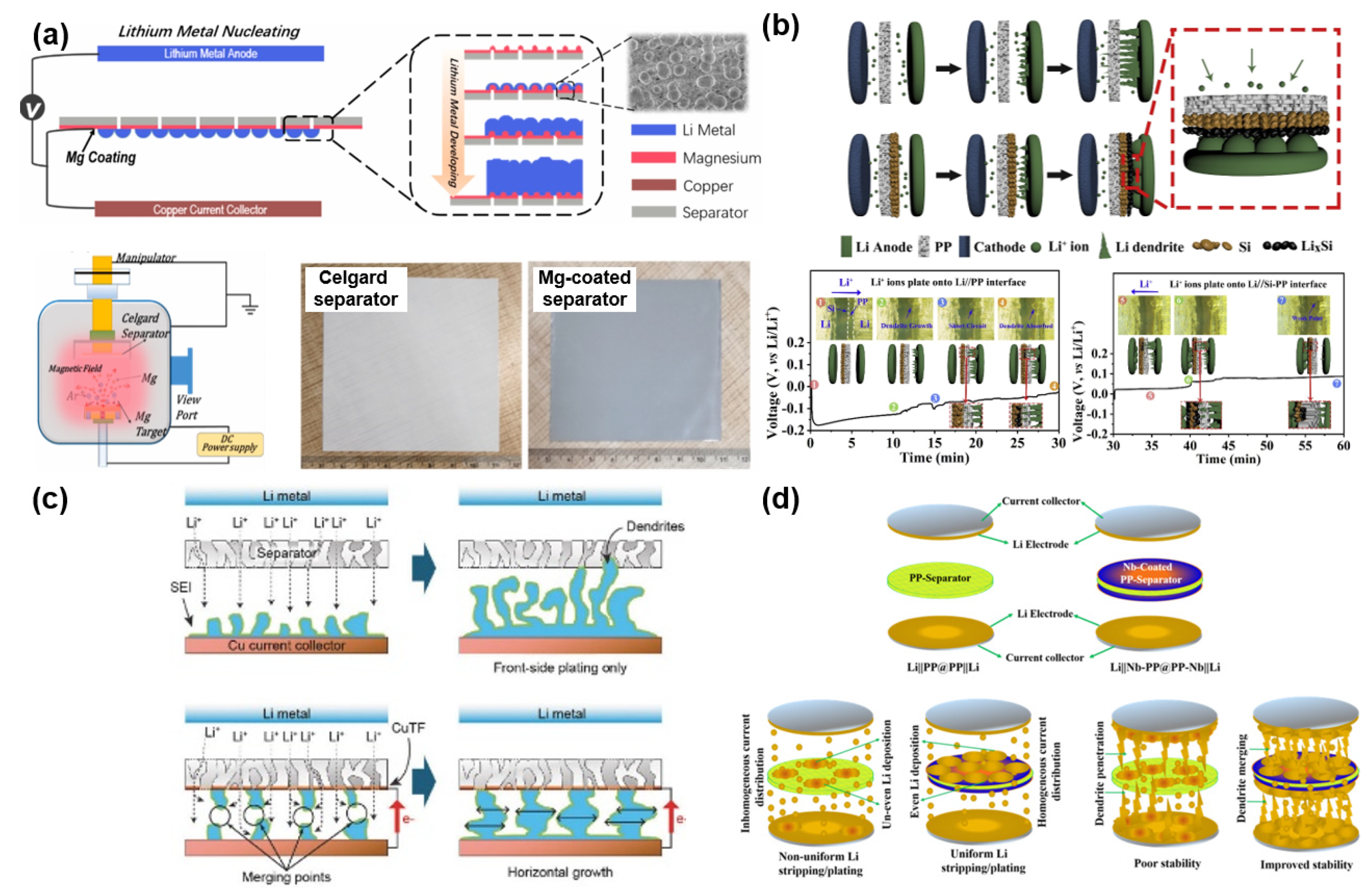
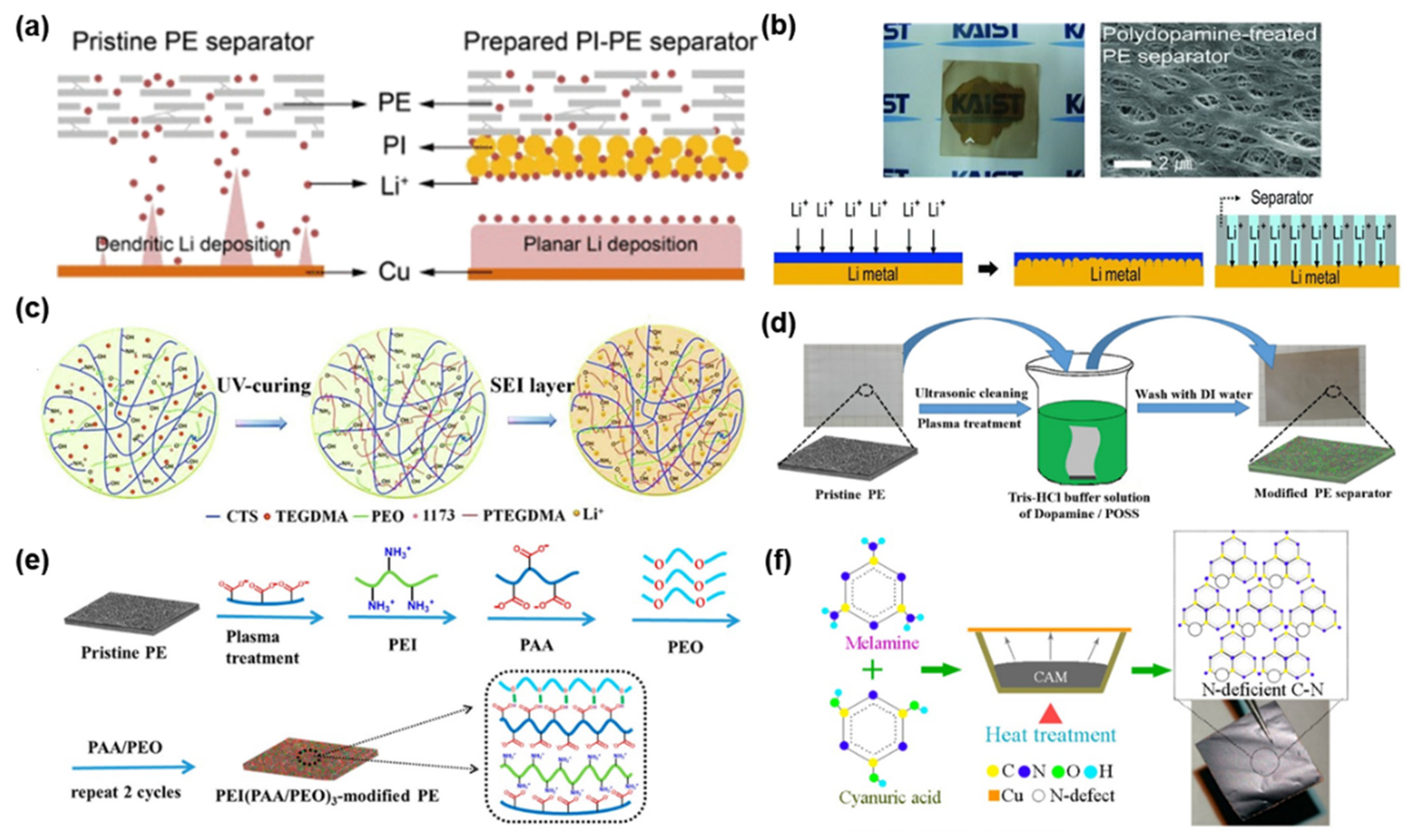

| Separator-Coating Materials | Separator & Fabrication Technique | Coulombic Efficiency, Current Density, Tested Cells | Ref. |
|---|---|---|---|
| 1. Thermally conductive materials | |||
| AlN nanopowder | PP, Tape-casting | 92% at 1st and 92% at 100th cycle, 0.5 mA cm−2, Li|Cu | [63] |
| BN nanopowder&carbon | PP, Tape-casting | 90% at 1st and 83.5% at 100th cycle, 0.5 mA cm−2, Li|Cu | [64] |
| BN nanosheets | PP/PE/PP, Spray coating | 88% at 1st and 92% at 100th cycle, 0.5 mA cm−2, Li|Cu | [69] |
| BN nanopowder/Graphene | PP, Tape-casting | 77% at 1st and 75% at 100th cycle, 1 mA cm−2, Li|Cu | [97] |
| 2. Metals | |||
| Cu | PE, Magnetron sputtering | 95.4% at 1st and 95.6% at 300th cycle, 0.4 mA cm−2, Li|Cu | [99] |
| Nb | PP, Magnetron sputtering | 97% at 120th cycle, 0.2 C, Li|LNMC | [100] |
| Mg | PP/PE, Magnetron sputtering | 97% at 1st and 94% at 400th cycle, 0.5 mA cm−2, Li|Cu | [101] |
| Si | PP, Tape-casting | 90% at 1st and 97.6% at 100th cycle, 0.5 mA cm−2, Li|Cu | [102] |
| 3. Polymers | |||
| Polyimide | PE, Electrospinning | 98.5% at 700th cycle, 1 mA cm−2, Li|Cu | [103] |
| Polydopamine | PE, Solution immersion | 97.1% at 1st cycle, 0.17 mA cm−2, Li|LiCoO2 | [104] |
| CTS-PEO-PTEGDMA | PP/PE/PP, Electrospraying | ~60% at 1st and 90% at 120th cycle, 0.5 mA cm−2, Li|Cu | [105] |
| PDA/POSS | PE, Dip coating | 98.6% at 200th cycle, 0.2 C, Li|LiCoO2 | [106] |
| PEI(PAA/PEO)3 | PE, Layer-by-layer (LBL) | 99.1% at 400th cycle, 0.2 C, Li|LiCoO2 | [107] |
| C-N polymer | PP, Pasting | ~72% at 1st and 95% at 450th cycle, 3 mA cm−2, Li|Cu | [108] |
| 4. Carbons | |||
| Graphene|Polydopamine | PP, Tape-casting | ~96.1% at 1st and 86.6% at 200th cycle, 0.5 mA cm−2, Li|Cu | [66] |
| VN-N-rGO | PP, Vacuum filtration | 92% at 1st and 97.1% at 55th cycle, 0.5 mA cm−2, Li|Cu | [110] |
| Functionalized nanocarbon | PP, Tape-casting | 96.07–97.41%, 1 mA cm−2, Li|Li | [111] |
| Cellulose-derived carbon | PP, Tape-casting | 36.7% at 1st and 77.1% at 120th cycle, 1 mA cm−2, Li|Cu | [125] |
| 5. Metal oxides | |||
| SiO2 nanosheet | PP, Tape-casting | 81.5% at 1st and 64% at 200th cycle, 1 mA cm−2, Li|Cu | [67] |
| SiO2 nanoparticles | PE, Tape-casting | - | [88] |
| ZrO2 | PE, Self-assembly | 97.8% at 400th cycle, 0.5 C, Li|LiCoO2 | [115] |
| Al2O3 particles | PE, Tape-casting | nearly 100% at 100th cycle, 0.2 C, Li|LiCoO2 | [116] |
| Al2O3 | PVDF-HFP, ALD | nearly 100% at 100th cycle, 0.2 C, Li|LiFePO4 | [117] |
| 6. Others | |||
| Li6.75La3Zr1.75Ta0.25O12 | PP, Tape-casting | 99.5% after 1000 cycles, 0.2 C, Li|LiFePO4 | [120] |
| Al-doped Li6.75La3Zr1.75Ta0.25O12 | PP, Vacuum filtration | 98% after 450 cycles, 0.5 mA cm−2, Li|Cu | [121] |
| Li6.4La3Zr1.4Ta0.6O12 | PP, Tape-casting | 97.5% after 300 cycles, 1 mA cm−2, Li|Cu | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, P.J. Surface-Functionalized Separator for Stable and Reliable Lithium Metal Batteries: A Review. Nanomaterials 2021, 11, 2275. https://doi.org/10.3390/nano11092275
Kim PJ. Surface-Functionalized Separator for Stable and Reliable Lithium Metal Batteries: A Review. Nanomaterials. 2021; 11(9):2275. https://doi.org/10.3390/nano11092275
Chicago/Turabian StyleKim, Patrick Joohyun. 2021. "Surface-Functionalized Separator for Stable and Reliable Lithium Metal Batteries: A Review" Nanomaterials 11, no. 9: 2275. https://doi.org/10.3390/nano11092275
APA StyleKim, P. J. (2021). Surface-Functionalized Separator for Stable and Reliable Lithium Metal Batteries: A Review. Nanomaterials, 11(9), 2275. https://doi.org/10.3390/nano11092275





