Using Gold-Nanorod-Filled Mesoporous Silica Nanobeads for Enhanced Radiotherapy of Oral Squamous Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of MSNbs, GSNbs, and GNSbs
2.1.1. Synthesis of Mesoporous Silica Nanobeads (MSNbs)
2.1.2. Synthesis of Gold-Seed-Filled Porous Silica Nanobeads (GSNbs)
2.1.3. Synthesis of Gold Nano-Sesame-Beads (GNSbs)
2.2. Characterization of MSNbs, GSNbs, and GNSbs
2.3. Cell Culture
2.4. Cell Viability
2.5. Cellular Uptake
2.6. Intracellular ROS Detection Using Flow Cytometry
2.7. DNA Double-Strand Break Monitoring
2.8. Cell Cycle Analysis
2.9. In Vivo Analysis
2.9.1. Animal Models
2.9.2. Evaluation of Therapeutic Efficacy of GNSbs
2.10. Statistical Analysis
3. Results
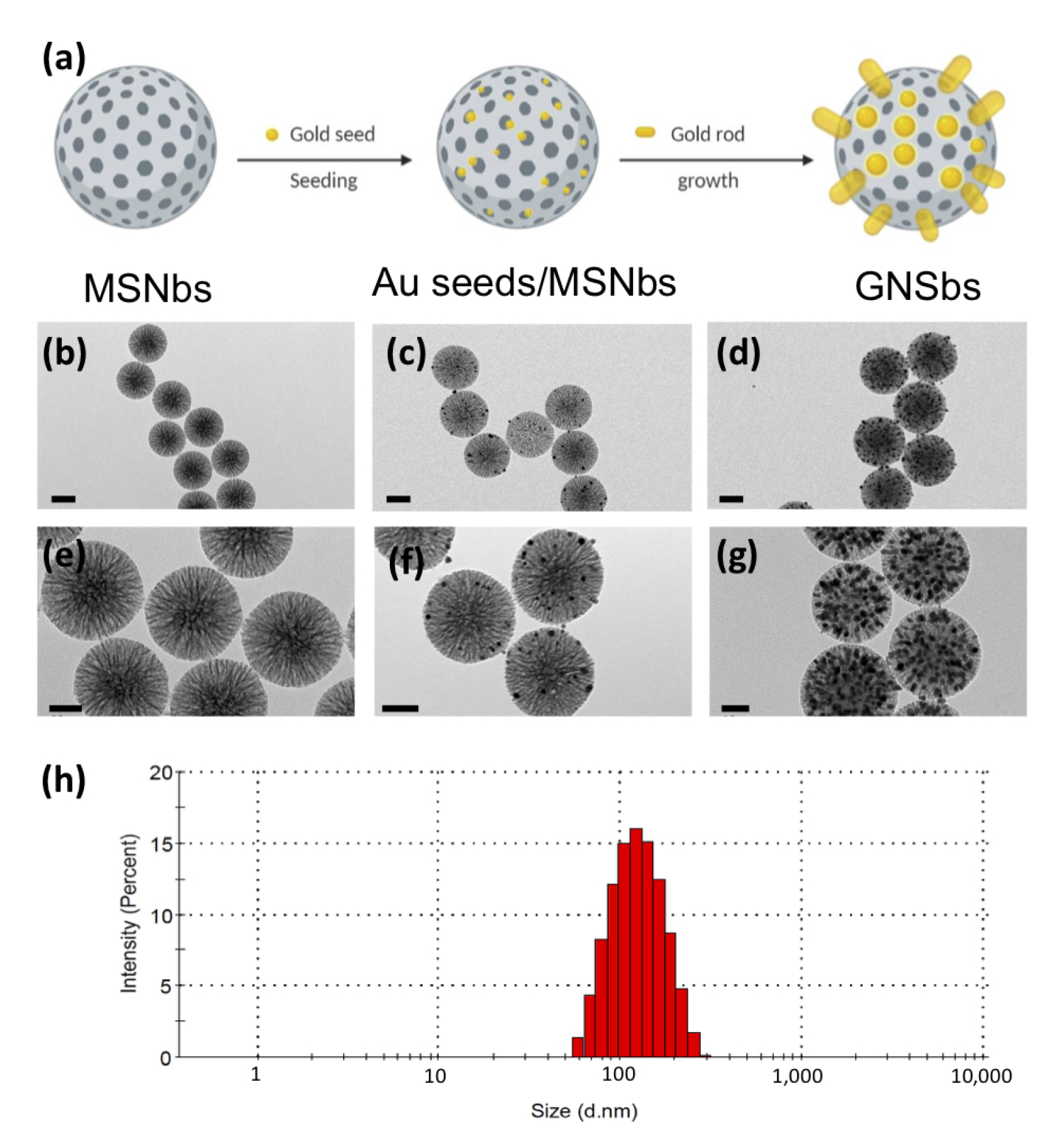
3.1. The Synthesis and Morphology of the Gold Nano-Sesame-Beads (GNSbs)
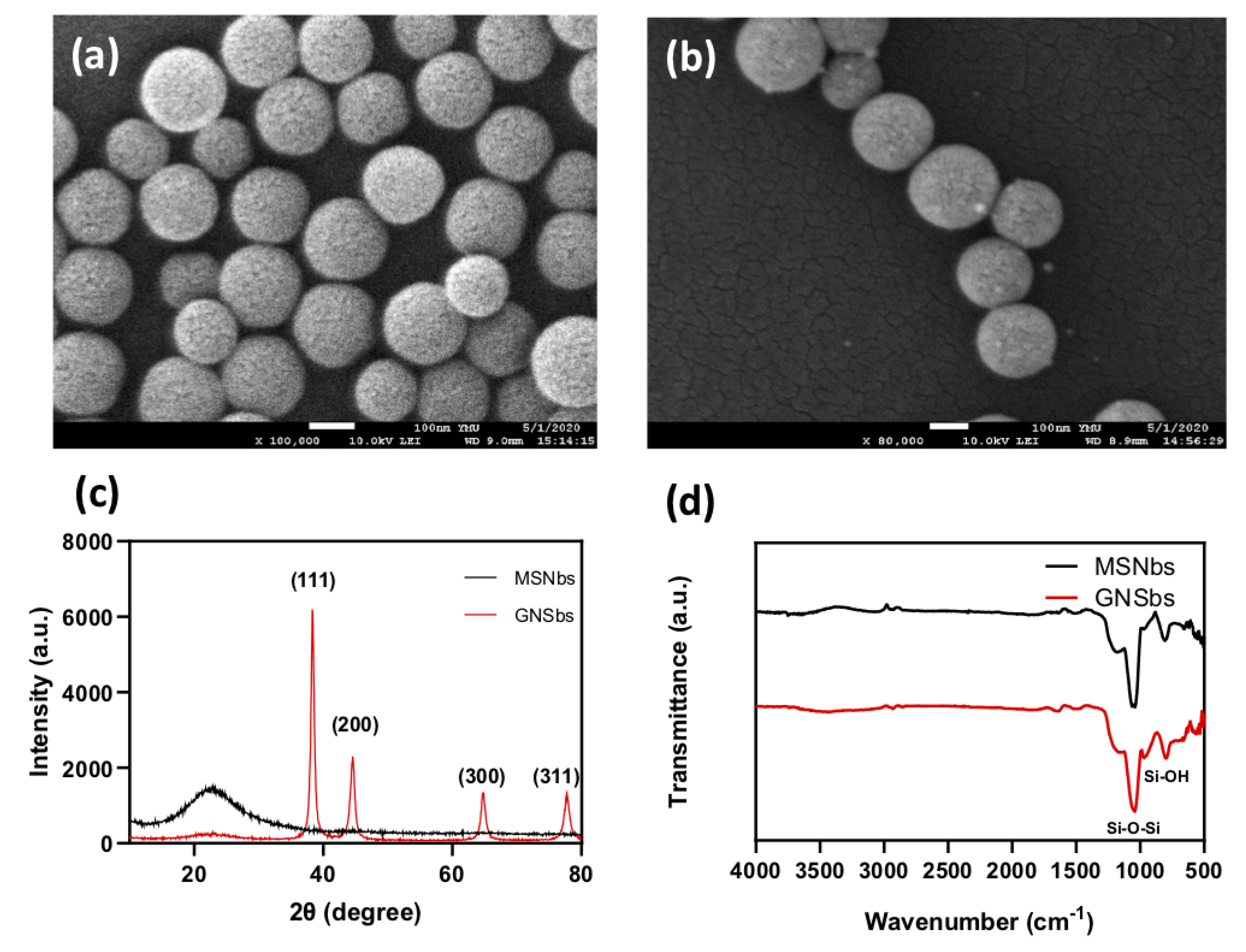
3.2. Physical Analysis of Gold Nano-Sesame-Beads (GNSbs)
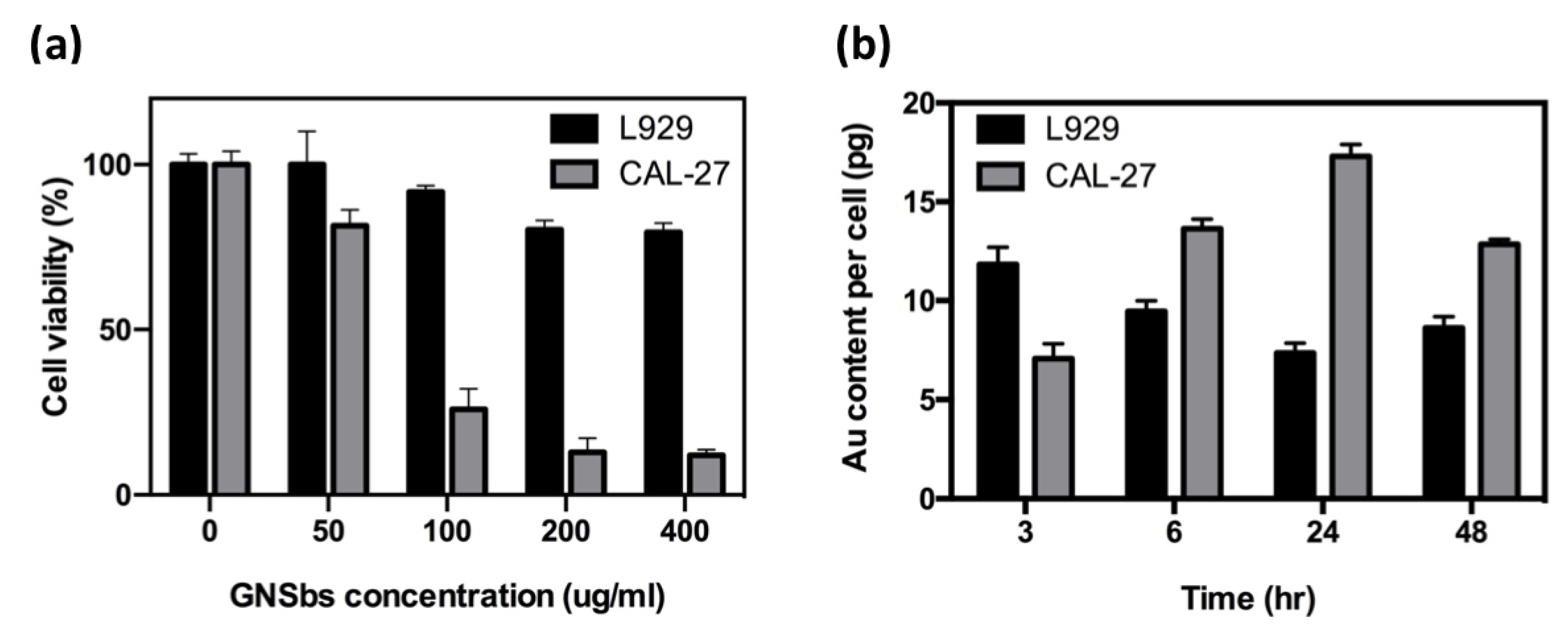
3.3. Effect of GNSbs on L929 and CAL-27 Cell Viability and Cellular Uptake
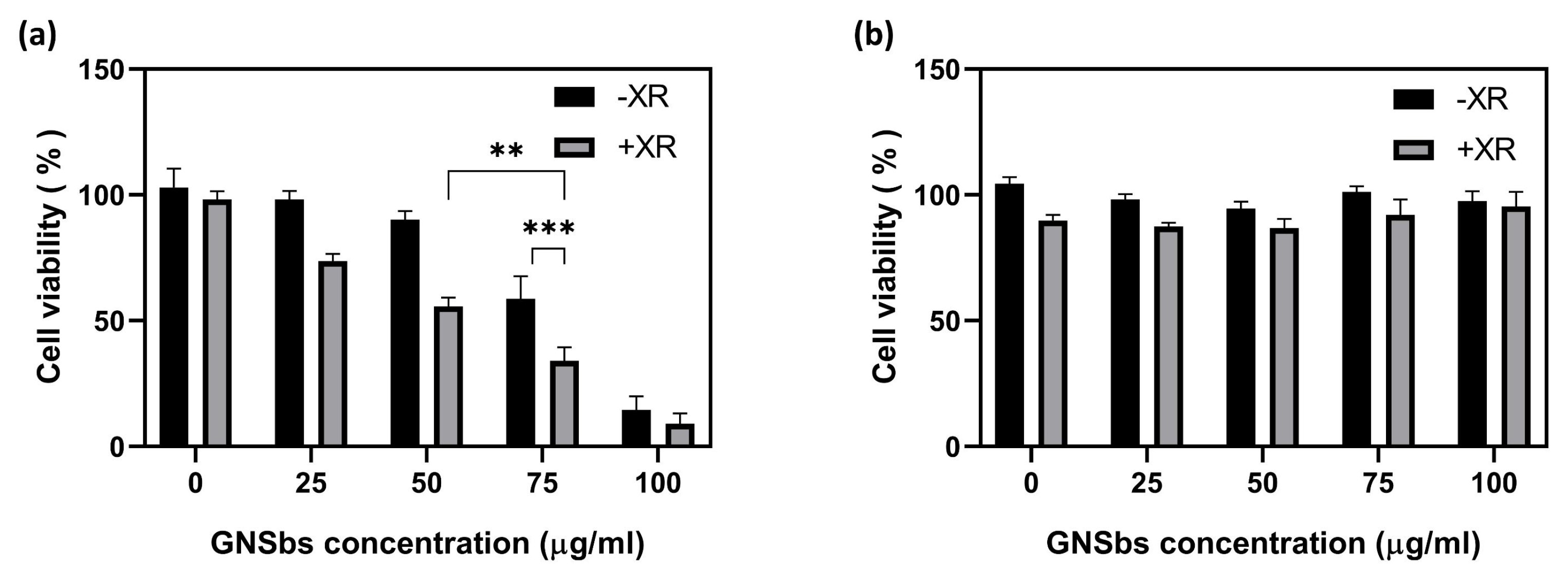
3.4. The Cytotoxicity on CAL-27 and L929 Cells Treated with or without the GNSbs under Radiation
3.5. The Intracellular ROS Levels in CAL27 and L929 Cells Treated with or without GNSbs under Radiation
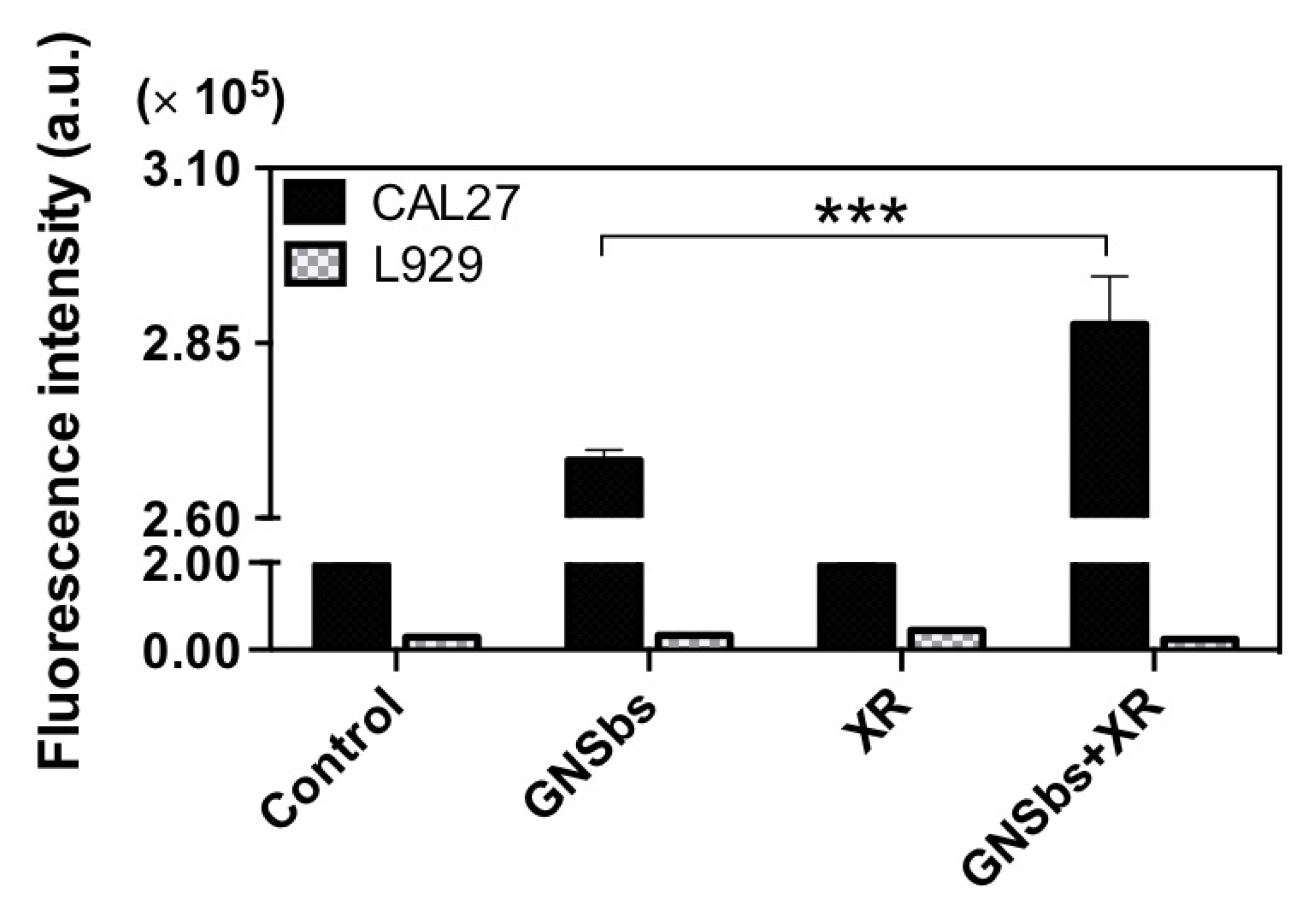
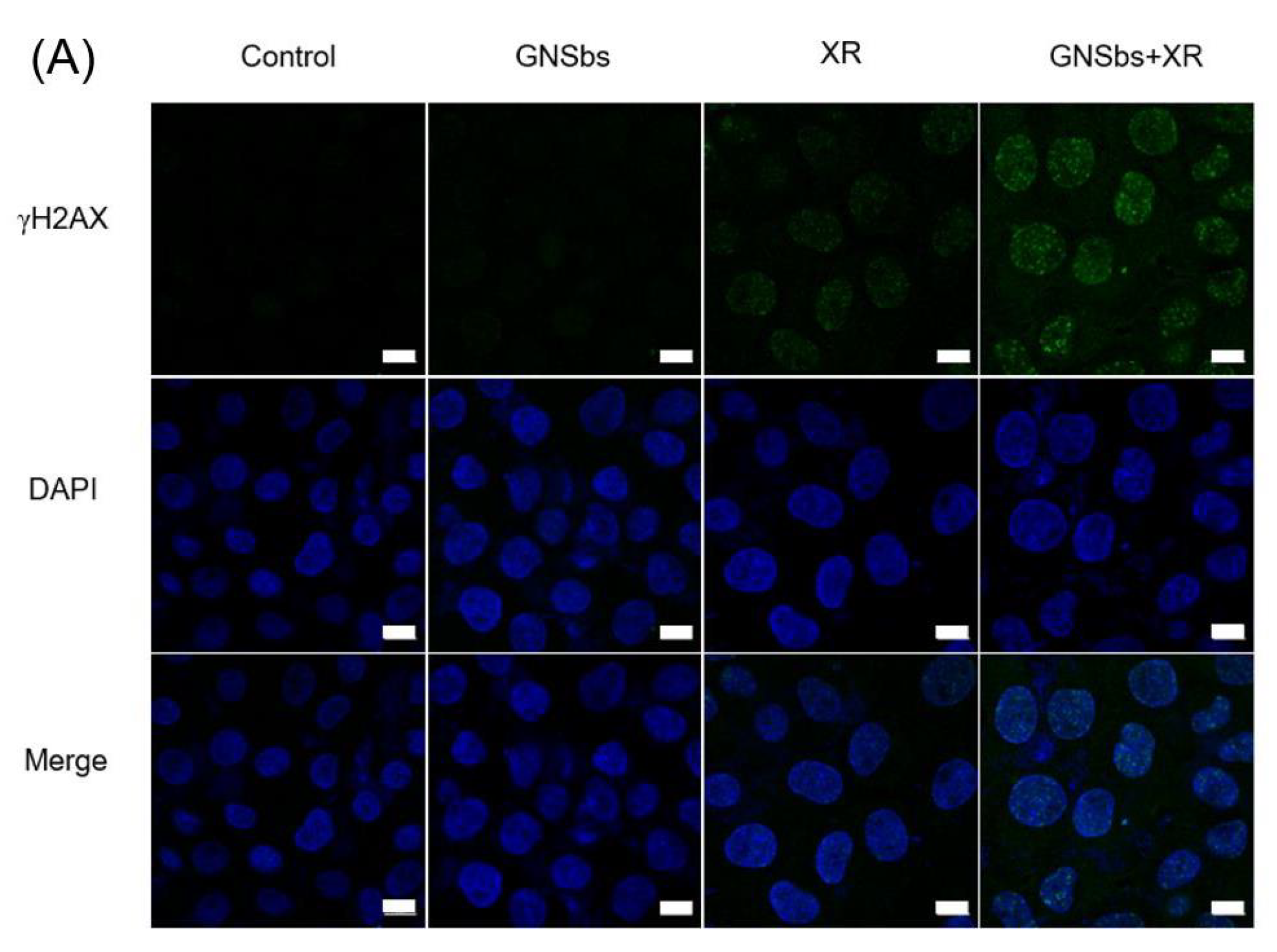
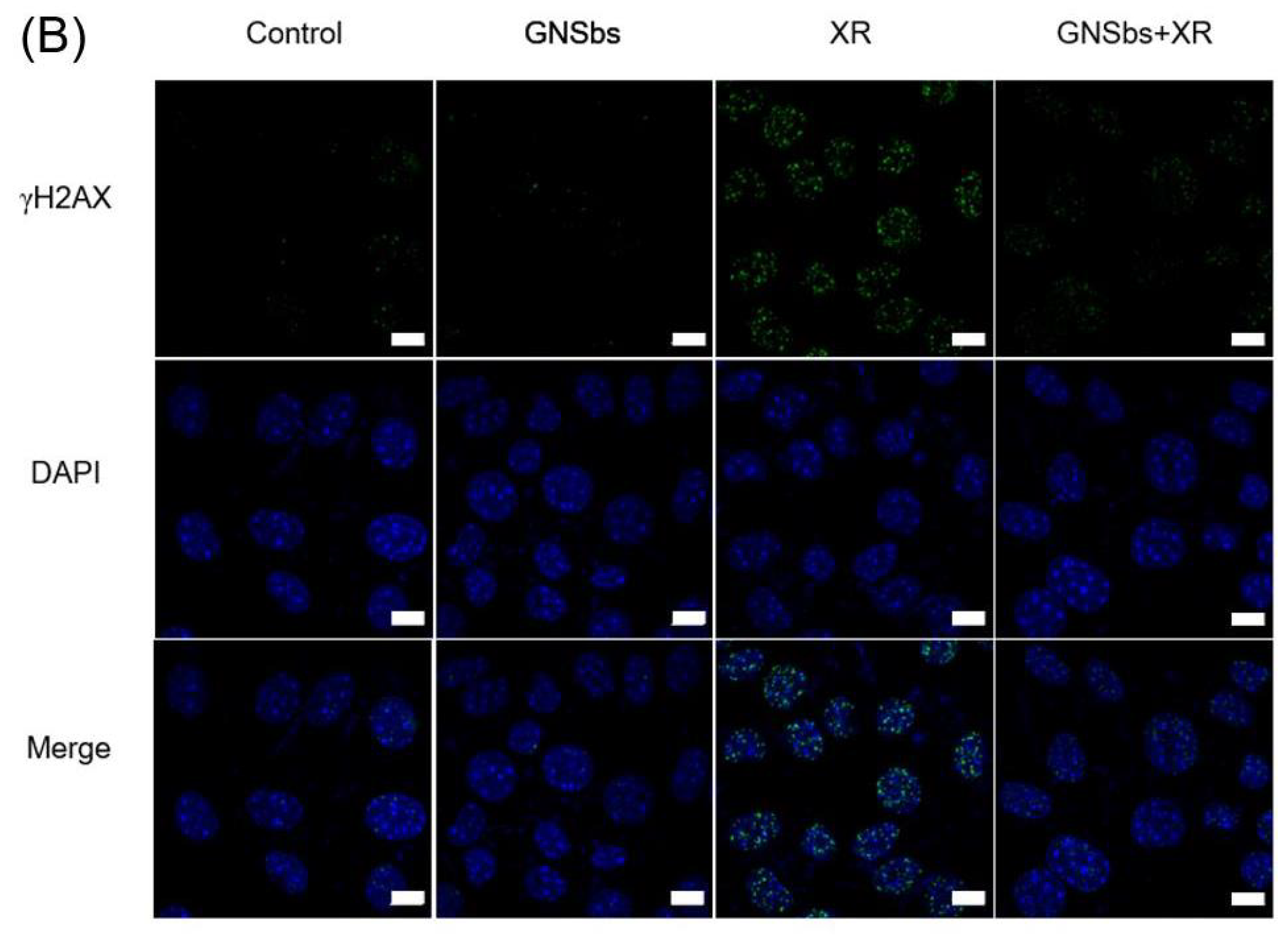
3.6. DNA Damage in CAL-27 and L929 Cells Treated with or without GNSbs under Radiation
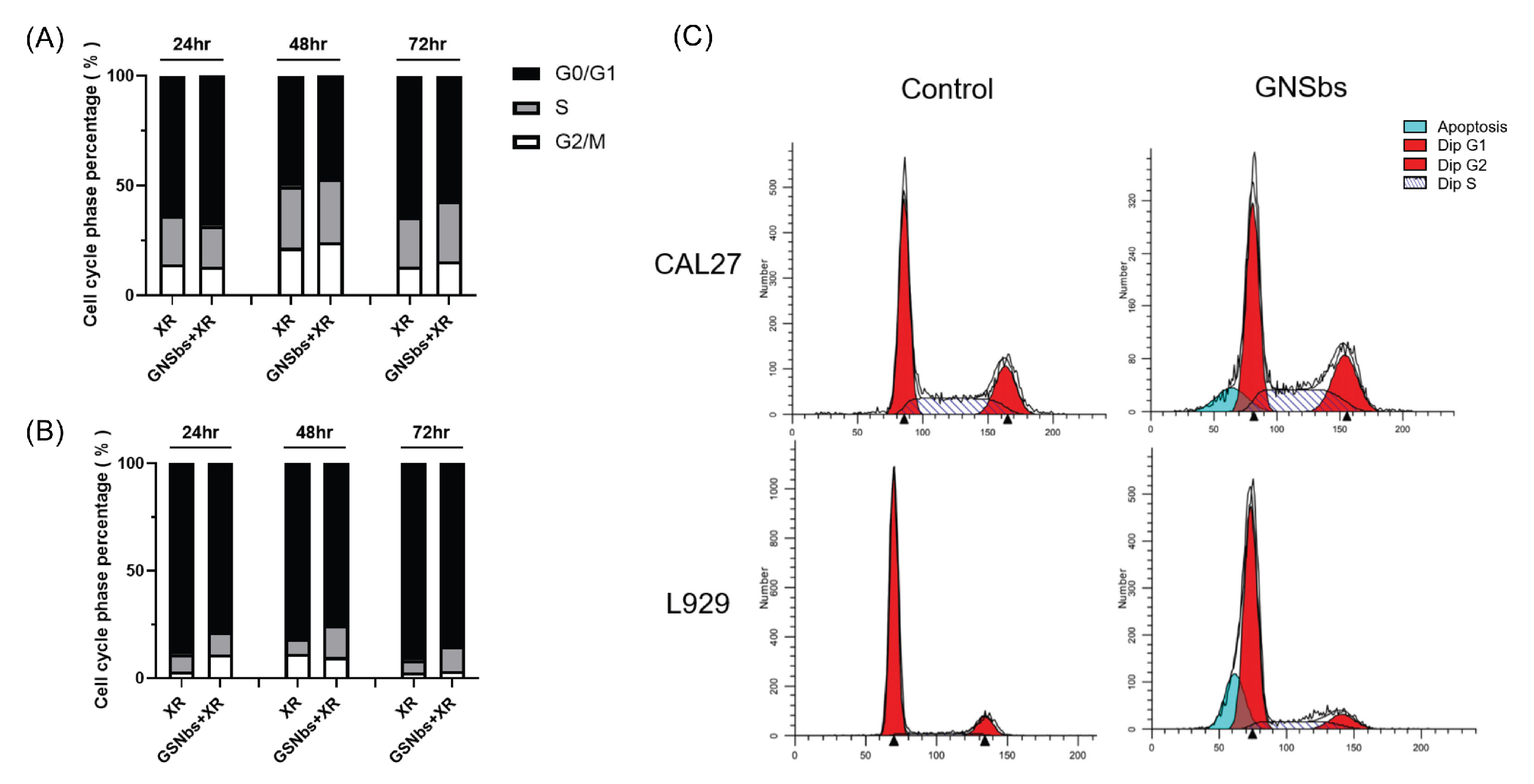
3.7. Cell Cycle Analysis of CAL-27 and L929 Treated with Irradiation and GNSbs
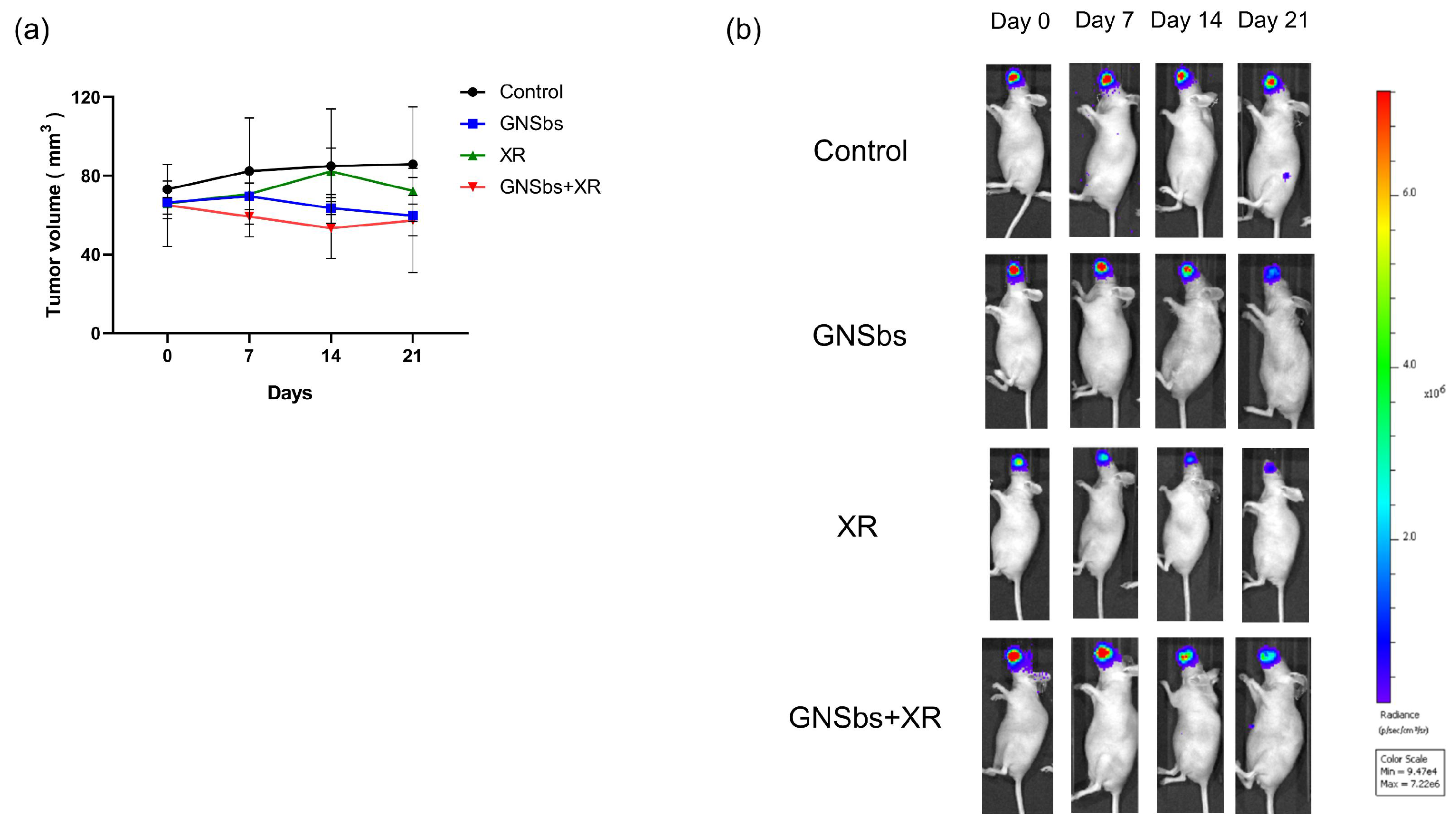
3.8. Therapeutic Efficacy of GNSbs and Irradiation in the CAL-27 Mouse Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.B.; Liu, I.Y.; Gornbein, J.A.; Nguyen, C.T. HPV-Positive Oropharyngeal Carcinoma: A Systematic Review of Treatment and Prognosis. Otolaryngol. Head Neck Surg. 2015, 153, 758–769. [Google Scholar] [CrossRef]
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Tolentino, E.d.S.; Centurion, B.S.; Ferreira, L.H.C.; Souza, A.P.d.; Damante, J.H.; Rubira-Bullen, I.R.F. Oral adverse effects of head and neck radiotherapy: Literature review and suggestion of a clinical oral care guideline for irradiated patients. J. Appl. Oral Sci. 2011, 19, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kashiwazaki, H.; Matsushita, T.; Sugita, J.; Shigematsu, A.; Kasashi, K.; Yamazaki, Y.; Kanehira, T.; Yamamoto, S.; Kondo, T.; Endo, T.; et al. Professional oral health care reduces oral mucositis and febrile neutropenia in patients treated with allogeneic bone marrow transplantation. Support. Care Cancer 2012, 20, 367–373. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.; Chen, W.; Li, Q. Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Kim, D.; El-Shall, H.; Dennis, D.; Morey, T. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf. B Biointerfaces 2005, 40, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, Y.; Wang, L.; Xu, L.; Bai, R.; Ji, Y.; Wu, X.; Zhao, Y.; Li, Y.; Chen, C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 2010, 31, 7606–7619. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.H.D.P.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Rausch, K.; Reuter, A.; Fischer, K.; Schmidt, M. Evaluation of nanoparticle aggregation in human blood serum. Biomacromolecules 2010, 11, 2836–2839. [Google Scholar] [CrossRef]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef]
- Albanese, A.; Chan, W.C.W. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef]
- Fernández-Lodeiro, A.; Djafari, J.; Fernández-Lodeiro, J.; Duarte, M.P.; Muchagato Mauricio, E.; Capelo-Martínez, J.L.; Lodeiro, C. Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications. Nanomaterials 2021, 11, 1338. [Google Scholar] [CrossRef]
- Peukert, D.; Kempson, I.; Douglass, M.; Bezak, E. Gold nanoparticle enhanced proton therapy: A Monte Carlo simulation of the effects of proton energy, nanoparticle size, coating material, and coating thickness on dose and radiolysis yield. Med. Phys. 2020, 47, 651–661. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Wang, S.; Xu, Z.; Wei, L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int. J. Nanomed. 2018, 13, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wu, S.H.; Hung, Y.; Mou, C.Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Chatterjee, A.; Magee, J. Theoretical Investigation of the Production of Strand Breaks in DNA by Water Radicals. Radiat. Prot. Dosim. 1985, 13, 137–140. [Google Scholar] [CrossRef]
- Banáth, J.P.; Olive, P.L. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 2003, 63, 4347–4350. [Google Scholar]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Zhang, J.; Zhou, M.; Shen, L.; Deng, W.; Liang, L.; Hu, R.; Yang, W.; Yao, Y.; et al. Radiosensitization by irinotecan is attributed to G2/M phase arrest, followed by enhanced apoptosis, probably through the ATM/Chk/Cdc25C/Cdc2 pathway in p53-mutant colorectal cancer cells. Int. J. Oncol. 2018, 53, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Sakamoto, Y.; Terasaki, O.; Kubota, Y.; Okubo, T.; Tatsumi, T. Periodic arrangement of silica nanospheres assisted by amino acids. J. Am. Chem. Soc. 2006, 128, 13664–13665. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Kim, S.G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chang, S.S.; Lee, C.L.; Wang, C.R.C. Gold Nanorods: Electrochemical Synthesis and Optical Properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- van der Zande, B.M.I.; Böhmer, M.R.; Fokkink, L.G.J.; Schönenberger, C. Colloidal Dispersions of Gold Rods: Synthesis and Optical Properties. Langmuir 2000, 16, 451–458. [Google Scholar] [CrossRef]
- Esumi, K.; Matsuhisa, K.; Torigoe, K. Preparation of Rodlike Gold Particles by UV Irradiation Using Cationic Micelles as a Template. Langmuir 1995, 11, 3285–3287. [Google Scholar] [CrossRef]
- Li, Z.; Kübel, C.; Pârvulescu, V.I.; Richards, R. Size tunable gold nanorods evenly distributed in the channels of mesoporous silica. ACS Nano 2008, 2, 1205–1212. [Google Scholar] [CrossRef]
- Chen, P.J.; Hu, S.H.; Fan, C.T.; Li, M.L.; Chen, Y.Y.; Chen, S.Y.; Liu, D.M. A novel multifunctional nano-platform with enhanced anti-cancer and photoacoustic imaging modalities using gold-nanorod-filled silica nanobeads. Chem. Commun. 2013, 49, 892–894. [Google Scholar] [CrossRef][Green Version]
- Paro, A.D.; Hossain, M.; Webster, T.J.; Su, M. Monte Carlo and analytic simulations in nanoparticle-enhanced radiation therapy. Int. J. Nanomed. 2016, 11, 4735–4741. [Google Scholar] [CrossRef]
- Leung, M.K.K.; Chow, J.C.L.; Chithrani, B.D.; Lee, M.J.G.; Oms, B.; Jaffray, D.A. Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med. Phys. 2011, 38, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.J.; Costea, D.E.; Gammon, L.; Fazil, B.; Biddle, A.; Mackenzie, I.C. Normal and malignant epithelial cells with stem-like properties have an extended G2 cell cycle phase that is associated with apoptotic resistance. BMC Cancer 2010, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wistuba, I.I.; Emmert-Buck, M.R.; Erickson, H.S. Squamous Cell Carcinoma—Similarities and Differences among Anatomical Sites. Am. J. Cancer Res. 2011, 1, 275–300. [Google Scholar] [CrossRef]
- Jahanbani, J.; Ghotbi, M.; Shahsavari, F.; Seydi, E.; Rahimi, S.; Pourahmad, J. Selective anticancer activity of superparamagnetic iron oxide nanoparticles (SPIONs) against oral tongue cancer using in vitro methods: The key role of oxidative stress on cancerous mitochondria. J. Biochem. Mol. Toxicol. 2020, 34, e22557. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, M.; Seydi, E.; Rahimi, S.; Tahmasebi, G.; Jahanbani, J.; Pourahmad, J. The selective toxicity of superparamagnetic iron oxide nanoparticles (SPIONs) on oral squamous cell carcinoma (OSCC) by targeting their mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Li, Y.; Sang, X.; Yuan, H.; Zheng, B. Stannic Oxide Nanoparticle Regulates Proliferation, Invasion, Apoptosis, and Oxidative Stress of Oral Cancer Cells. Front. Bioeng. Biotechnol. 2020, 8, 768. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey Inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Tse, K.; Zahedi, P.; Harding, S.M.; Zafarana, G.; Jaffray, D.A.; Bristow, R.G.; Allen, C. Hypoxia and cellular localization influence the radiosensitizing effect of gold nanoparticles (AuNPs) in breast cancer cells. Radiat. Res. 2014, 182, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Mackey, M.A.; El-Sayed, M.A. Chemosensitization of cancer cells via gold nanoparticle-induced cell cycle regulation. Photochem. Photobiol. 2014, 90, 306–312. [Google Scholar] [CrossRef]
- Roa, W.; Zhang, X.; Guo, L.; Shaw, A.; Hu, X.; Xiong, Y.; Gulavita, S.; Patel, S.; Sun, X.; Chen, J.; et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology 2009, 20, 375101. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wang, Y.; Liu, Z.; Fu, L.; Hu, L. Enhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound gold nanoparticles at megavoltage radiation energies. J. Nanopart. Res. 2013, 15, 1642. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Y.; Li, X.; Hu, L. Thioglucose-bound gold nanoparticles increase the radiosensitivity of a triple-negative breast cancer cell line (MDA-MB-231). Breast Cancer 2015, 22, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, T.; Li, P.; Zhou, C.; Cui, D.; Pang, B.; Ren, Q.; Fu, S. RGD-conjugated gold nanorods induce radiosensitization in melanoma cancer cells by downregulating α(v)β3 expression. Int. J. Nanomed. 2012, 7, 915–924. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-H.; Chen, M.-H.; Li, C.-Y.; Tung, F.-I.; Chen, S.-Y.; Liu, T.-Y. Using Gold-Nanorod-Filled Mesoporous Silica Nanobeads for Enhanced Radiotherapy of Oral Squamous Carcinoma. Nanomaterials 2021, 11, 2235. https://doi.org/10.3390/nano11092235
Chen M-H, Chen M-H, Li C-Y, Tung F-I, Chen S-Y, Liu T-Y. Using Gold-Nanorod-Filled Mesoporous Silica Nanobeads for Enhanced Radiotherapy of Oral Squamous Carcinoma. Nanomaterials. 2021; 11(9):2235. https://doi.org/10.3390/nano11092235
Chicago/Turabian StyleChen, Mei-Hsiu, Ming-Hong Chen, Chia-Ying Li, Fu-I Tung, San-Yuan Chen, and Tse-Ying Liu. 2021. "Using Gold-Nanorod-Filled Mesoporous Silica Nanobeads for Enhanced Radiotherapy of Oral Squamous Carcinoma" Nanomaterials 11, no. 9: 2235. https://doi.org/10.3390/nano11092235
APA StyleChen, M.-H., Chen, M.-H., Li, C.-Y., Tung, F.-I., Chen, S.-Y., & Liu, T.-Y. (2021). Using Gold-Nanorod-Filled Mesoporous Silica Nanobeads for Enhanced Radiotherapy of Oral Squamous Carcinoma. Nanomaterials, 11(9), 2235. https://doi.org/10.3390/nano11092235






