Sulfur-Doped BiOCl with Enhanced Light Absorption and Photocatalytic Water Oxidation Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of S-BiOCl
2.3. Characterization
2.4. Pt Loading on S-BiOCl
2.5. Photocatalytic Water Oxidation Performance Measurements
2.6. Theoretical Calculation
3. Results and Discussion
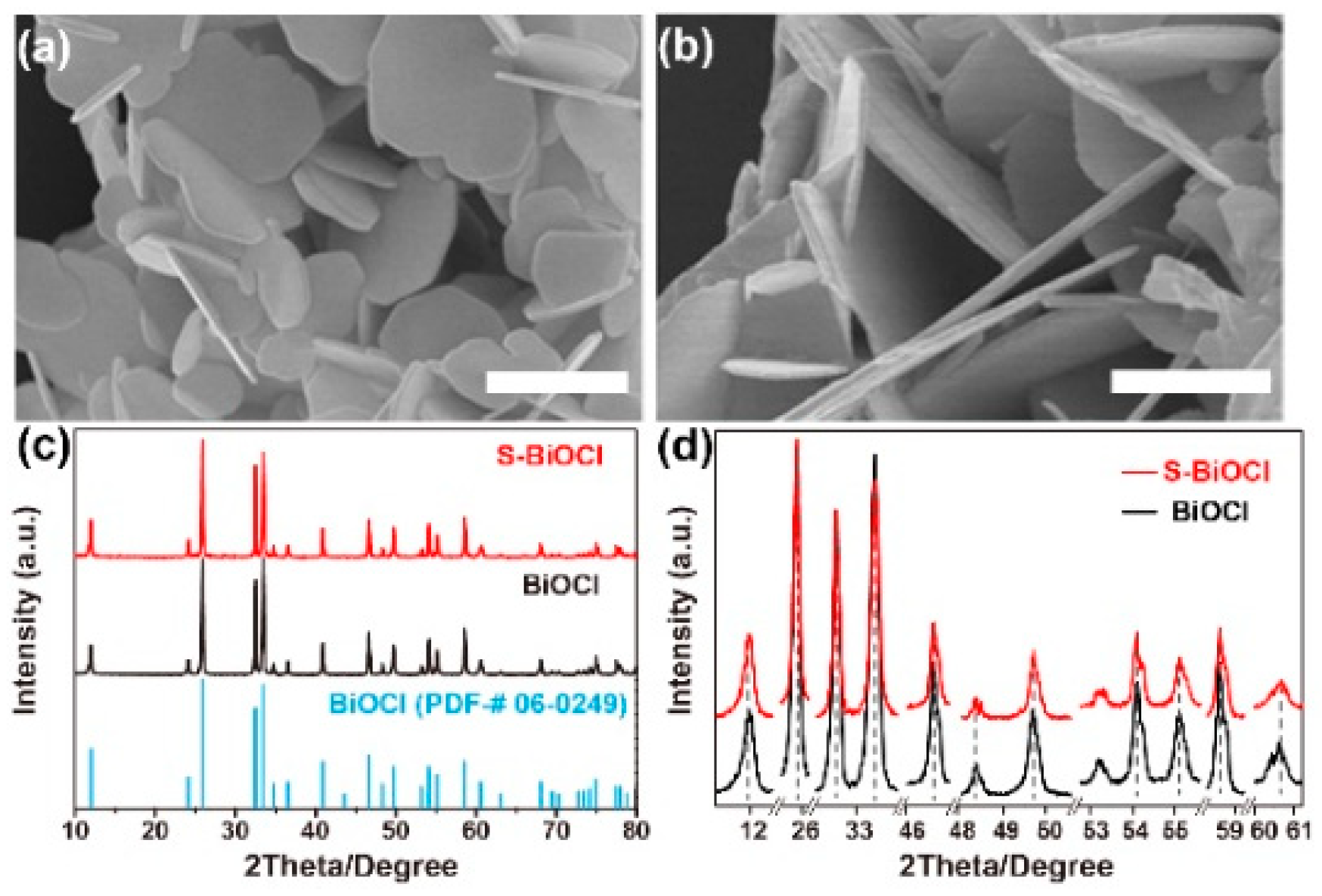
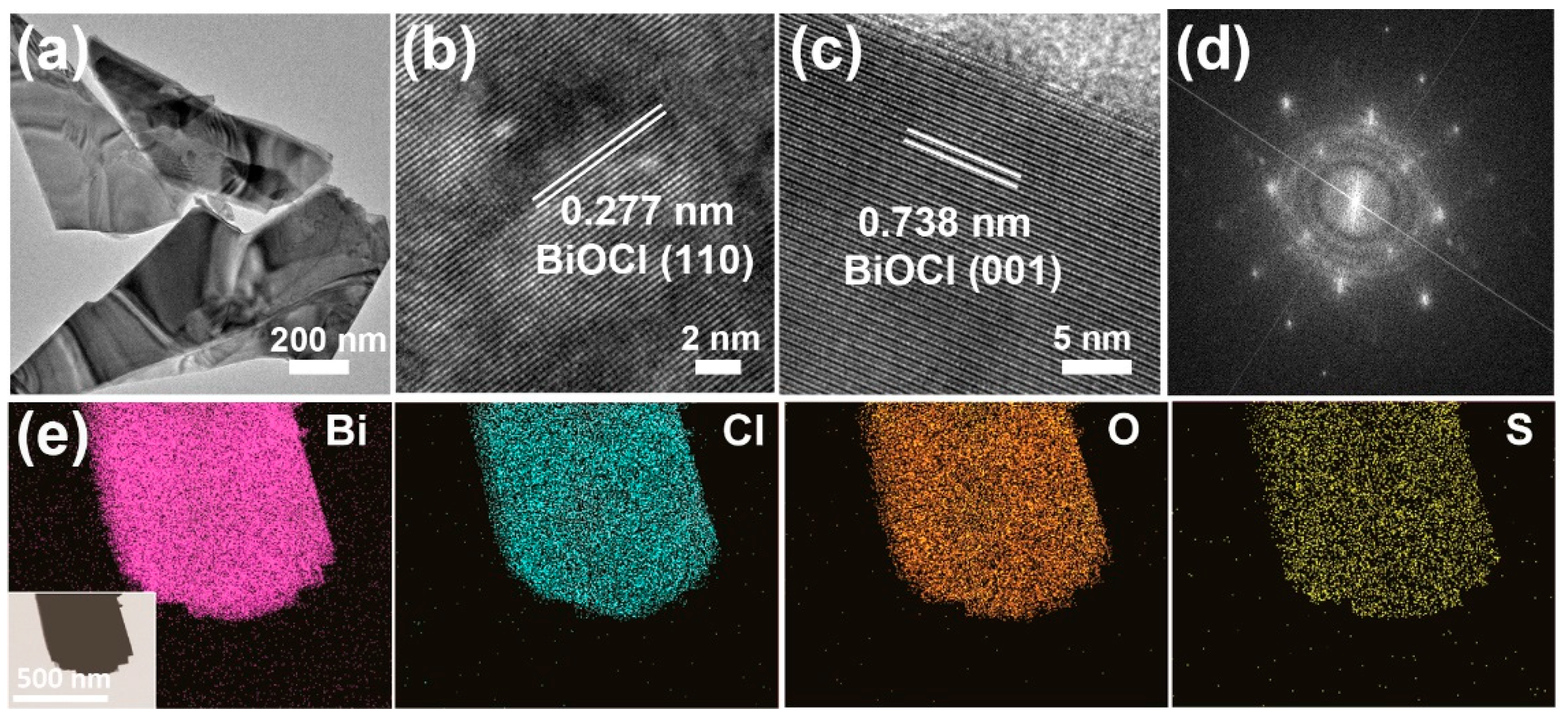
3.1. Preparation and Characterizations of S-BiOCl
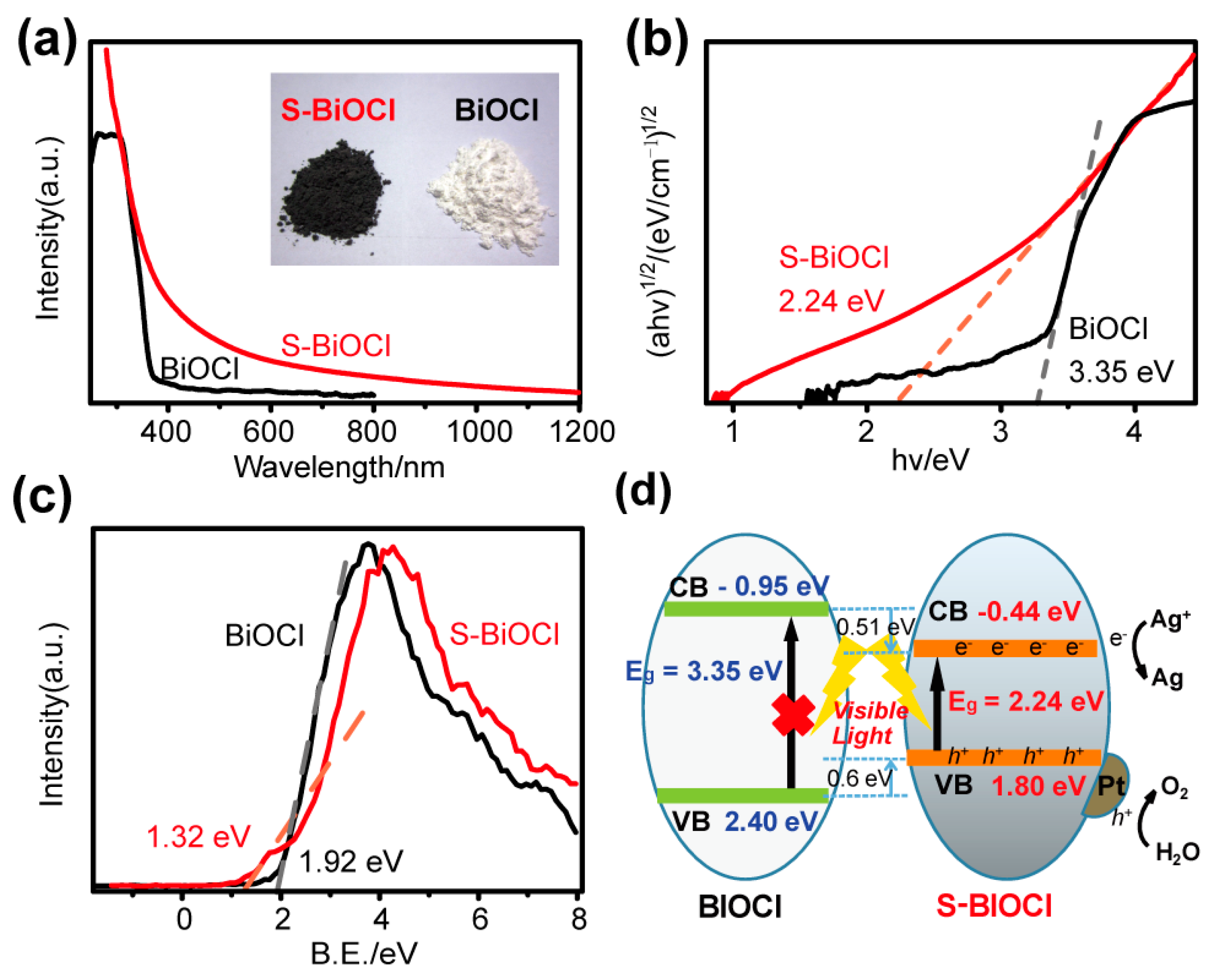
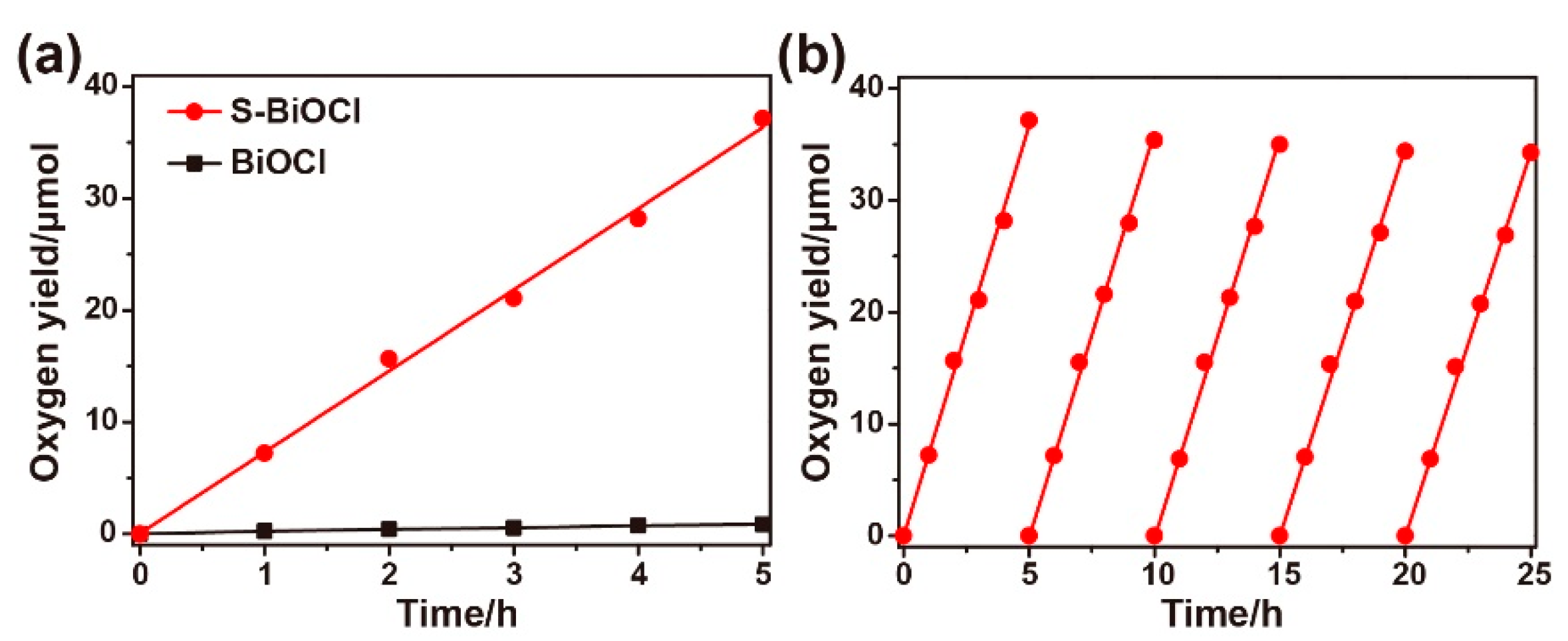
3.2. Band Structure and Photocatalytic Property of BS-CN
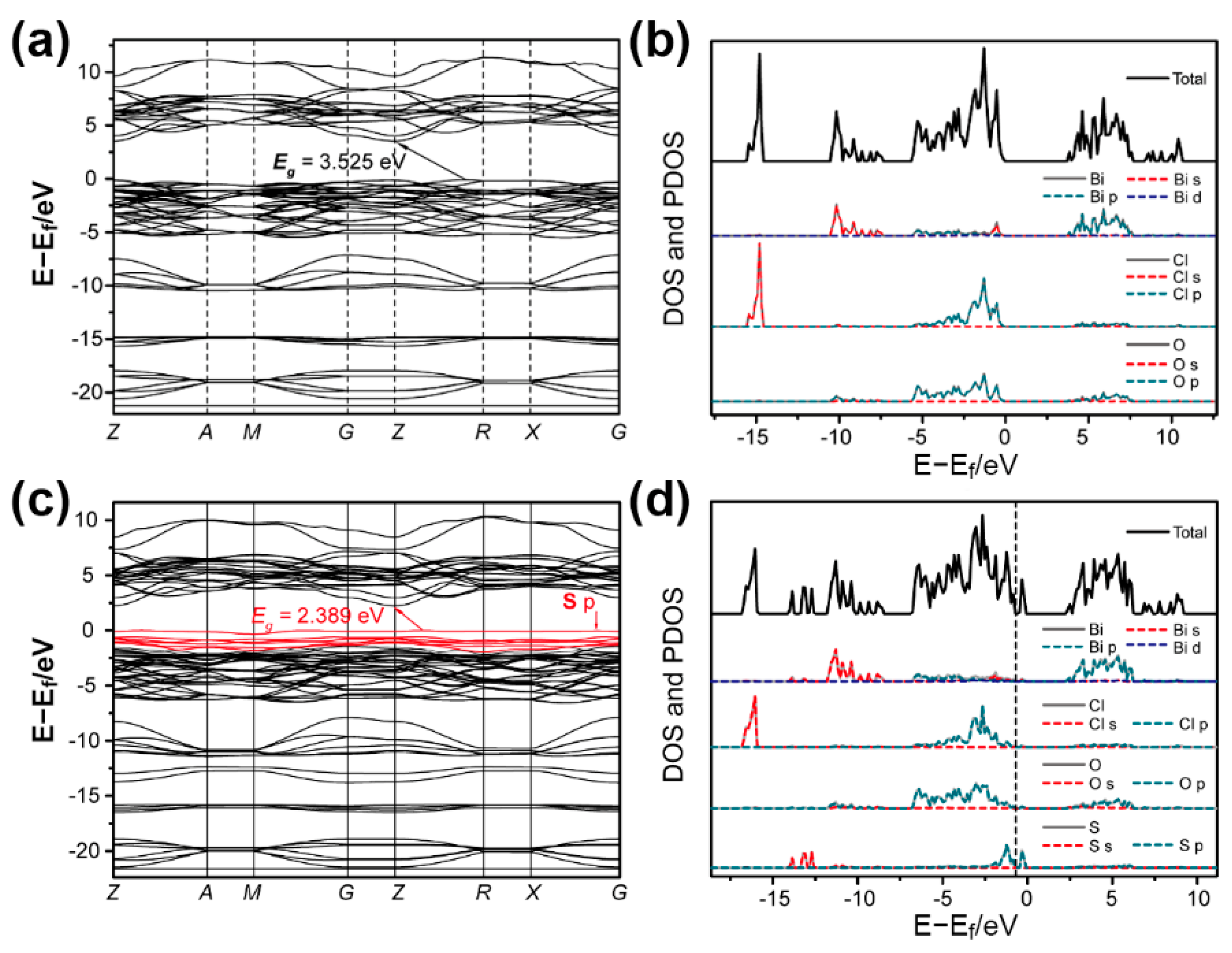
3.3. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Perovic, K.; dela Rosa, F.M.; Kovacic, M.; Kusic, H.; Stangar, U.L.; Fresno, F.; Dionysiou, D.D.; Bozic, A.L. Recent achievements in development of tio2-based composite photocatalytic materials for solar driven water purification and water splitting. Materials 2020, 13, 1338. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Chen, Z.; Shi, R.; Yang, X.; Zhang, T. Recent advances in conjugated polymers for visible-light-driven water splitting. Adv. Mater. 2020, 32, 1907296. [Google Scholar] [CrossRef]
- Wu, Y.; Bi, L. Research progress on catalytic water splitting based on polyoxometalate/semiconductor composites. Catalysts 2021, 11, 524. [Google Scholar] [CrossRef]
- Ng, B.-J.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S.-P. Z-scheme photocatalytic systems for solar water splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef] [PubMed]
- Maitani, M.M.; Yamada, T.; Mashiko, H.; Yoshimatsu, K.; Oshima, T.; Ohtomo, A.; Wada, Y. Microwave effects on Co–Pi cocatalysts deposited on α-Fe2O3 for application to photocatalytic oxygen evolution. ACS Appl. Mater. Interfaces 2017, 9, 10349–10354. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Chen, C.; Zhu, C.; Ji, M.; Xia, J.; Yan, C.; Hao, W.; Li, S.; Li, H.; Liu, Z. Bismuth vacancy mediated single unit cell Bi2WO6 nanosheets for boosting photocatalytic oxygen evolution. Appl. Catal. B Environ. 2018, 238, 119–125. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen vacancy-mediated photocatalysis of BiOCl: Reactivity, selectivity, and perspectives. Angew. Chem.-Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Chen, Z.; Qiu, M.; Hu, B.; Wang, X. Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater. Chemosphere 2021, 273, 128576. [Google Scholar] [CrossRef]
- Keramidas, Κ.G.; Voutsas, G.P.; Rentzeperis, P.I. The crystal structure of BiOCl. Z. Kristallogr. 1993, 205, 35–40. [Google Scholar] [CrossRef]
- Huang, W.L.; Zhu, Q. Electronic structures of relaxed BiOX (X = F, Cl, Br, I) photocatalysts. Comput. Mater. Sci. 2008, 43, 1101–1108. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Q.; Tang, G.; Zhu, W.; Luo, Y. Controllable synthesis of solar-light-driven BiOCl nanostructures for highly efficient photocatalytic degradation of carbamazepine. J. Solid State Chem. 2019, 277, 133–138. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Zhao, H. Fabricating visible-light photoactive 3D flower-like BiOCl nanostructures via a one-step solution chemistry method at room temperature. Appl. Surf. Sci. 2019, 479, 247–252. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, X.; Wu, C.; Chen, M.; Chen, Q.; Wang, Y.; Hu, S.; Xiang, J.; Liu, Q.; Zhang, X.; et al. Binary solvent controllable synthesis of BiOCl towards enhanced photocatalytic activity. J. Phys. Chem. Solids 2019, 135, 109119. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, C.; Liu, H.; Li, P.; Wei, F.; Jiang, Y.; Song, W. A Bi/BiOCl heterojunction photocatalyst with enhanced electron-hole separation and excellent visible light photodegrading activity. J. Mater. Chem. A 2014, 2, 1677–1681. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Wang, B.; Li, H.; Dong, W. Novel g-C3N4/BiOClxI1-x nanosheets with rich oxygen vacancies for enhanced photocatalytic degradation of organic contaminants under visible and simulated solar light. Appl. Catal. B Environ. 2019, 256, 117789. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Yang, X.; Chen, X.; Xu, X. Synthesis of SiC/BiOCl composites and its efficient photocatalytic activity. Catalysts 2020, 10, 946. [Google Scholar] [CrossRef]
- Kuila, A.; Saravanan, P.; Bahnemann, D.; Wang, C. Novel Ag decorated, BiOCl surface doped AgVO3 nanobelt ternary composite with Z-scheme homojunction-heterojunction interface for high prolific photo switching, quantum efficiency and hole mediated photocatalysis. Appl. Catal. B Environ. 2021, 293, 120224. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, L.; Zhang, M.; Li, Y.; Liu, S.; Lin, J.; Song, F.; Zhang, W.; Zhu, S.; Pan, J. Construction of a bioinspired hierarchical BiVO4/BiOCl heterojunction and its enhanced photocatalytic activity for phenol degradation. ACS Appl. Mater. Interfaces 2021, 13, 32906–32915. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, H.; Zhao, L.; Jian, L.; Liang, Q.; Xiao, X. Insight into facet-dependent photocatalytic H2O2 production on BiOCl nanosheets. New J. Chem. 2021, 45, 3335–3342. [Google Scholar] [CrossRef]
- Xu, T.; Yang, M.; Chen, C.; Duan, R.; Shen, Q.; Sun, C. Photocatalytic activation of C-Br bond on facet-dependent BiOCl with oxygen vacancies. Appl. Surf. Sci. 2021, 548, 149243. [Google Scholar] [CrossRef]
- Liu, W.; Zhong, D.; Dai, Z.; Liu, Y.; Wang, J.; Wang, Z.; Pan, J. Synergetic utilization of photoabsorption and surface facet in crystalline/amorphous contacted BiOCl-Bi2S3 composite for photocatalytic degradation. J. Alloys Compd. 2019, 780, 907–916. [Google Scholar] [CrossRef]
- Gnayem, H.; Sasson, Y. Hierarchical nanostructured 3D flowerlike BiOClxBr1-x semiconductors with exceptional visible light photocatalytic activity. ACS Catal. 2013, 3, 186–191. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Z.; Wang, Y.; Ding, Z.; Xu, X.; Peng, W.; Fan, J.; Zhou, X.; Liu, J. BiOCl0.875Br0.125/polydopamine functionalized PVDF membrane for highly efficient visible-light-driven photocatalytic degradation of roxarsone and simultaneous arsenic immobilization. Chem. Eng. J. 2020, 402, 126048. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Zhang, X.; Pang, H.; Hai, X.; Zhan, G.; Zhou, W.; Song, H.; Zhang, L.; Chen, H.; et al. In situ carbon homogeneous doping on ultrathin bismuth molybdate: A dual-purpose strategy for efficient molecular oxygen activation. Adv. Funct. Mater. 2017, 27, 1703923. [Google Scholar] [CrossRef]
- Sun, J.; Wu, S.; Yang, S.-Z.; Li, Q.; Xiong, J.; Yang, Z.; Gu, L.; Zhang, X.; Sun, L. Enhanced photocatalytic activity induced by sp3 to sp2 transition of carbon dopants in BiOCl crystals. Appl. Catal. B Environ. 2018, 221, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.-Q.; Bai, Y.; Liu, J.-Y.; Fan, Z.; Hu, Y.-Q. N, C-codoped BiOCl flower-like hierarchical structures. Micro Nano Lett. 2012, 7, 876–879. [Google Scholar] [CrossRef]
- Pare, B.; Sarwan, B.; Jonnalagadda, S.B. Photocatalytic mineralization study of malachite green on the surface of Mn-doped BiOCl activated by visible light under ambient condition. Appl. Surf. Sci. 2011, 258, 247–253. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Chu, W.; Cen, W. Facile synthesis of Mn-doped BiOCl for metronidazole photodegradation: Optimization, degradation pathway, and mechanism. Chem. Eng. J. 2020, 400, 125813. [Google Scholar] [CrossRef]
- Xia, J.; Xu, L.; Zhang, J.; Yin, S.; Li, H.; Xu, H.; Di, J. Improved visible light photocatalytic properties of Fe/BiOCl microspheres synthesized via self-doped reactable ionic liquids. CrystEngComm 2013, 15, 10132–10141. [Google Scholar] [CrossRef]
- Wu, H.; Liu, X.; Xu, H.; Yang, X.; Ye, J. Efficient photodegradation of 2-chloro-4-nitrophenol over Fe-doped BiOCl nanosheets with oxygen vacancy. Catal. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Li, H.; He, W.; Yin, J.J. Self-doping and surface plasmon modification induced visible light photocatalysis of BiOCl. Nanoscale 2013, 5, 10573–10581. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, S.; Shang, H.; Wang, X.; Yang, Z.; Ai, Z.; Zhang, L. Surface hydrogen bond network spatially confined BiOCl oxygen vacancy for photocatalysis. Sci. Bull. 2020, 65, 1916–1923. [Google Scholar] [CrossRef]
- Wei, Z.; Li, W.; Hu, J.; Ma, X.; Zhu, Y. Interfacial internal electric field and oxygen vacancies synergistically enhance photocatalytic performance of bismuth oxychloride. J. Hazard. Mater. 2021, 402, 123470. [Google Scholar] [CrossRef]
- Li, Y.-L.; Liu, Y.; Mu, H.-Y.; Liu, R.-H.; Hao, Y.-J.; Wang, X.-J.; Hildebrandt, D.; Liu, X.; Li, F.-T. The simultaneous adsorption, activation and in situ reduction of carbon dioxide over Au-loading BiOCl with rich oxygen vacancies. Nanoscale 2021, 13, 2585–2592. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Jing, T.; Huang, B.; Wang, Z.; Zhang, X.; Qin, X.; Dai, Y. One-pot solvothermal synthesis of S doped BiOCl for solar water oxidation. RSC Adv. 2015, 5, 47261–47264. [Google Scholar] [CrossRef]
- Shang, J.; Chen, T.; Wang, X.; Sun, L.; Su, Q. Facile fabrication and enhanced photocatalytic performance: From BiOCl to element-doped BiOCl. Chem. Phys. Lett. 2018, 706, 483–487. [Google Scholar] [CrossRef]
- Wei, F.; Cao, C.; Sun, Y.; Yang, S.; Huang, P.; Song, W. Highly active and stable palladium nanoparticles encapsulated in a mesoporous silica yolk-shell nanoreactor for suzuki-miyaura reactions. ChemCatChem 2015, 7, 2475–2479. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Xiong, J.; Stadler, F.J. Facile template-free and fast refluxing synthesis of 3D desertrose-like BiOCl nanoarchitectures with superior photocatalytic activity. New J. Chem. 2013, 37, 3207–3213. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.; Lin, H.; Luo, B.; Chen, S. Novel Bi2S3-sensitized BiOCl with highly visible light photocatalytic activity for the removal of rhodamine B. Catal. Commun. 2012, 26, 204–208. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers. J. Hazard. Mater. 2012, 219, 26–34. [Google Scholar] [CrossRef]
- Bukhtiyarova, G.A.; Bukhtiyarov, V.I.; Sakaeva, N.S.; Kaichev, V.V.; Zolotovskii, B.P. XPS study of the silica-supported Fe-containing catalysts for deep or partial H2S oxidation. J. Mol. Catal. A Chem. 2000, 158, 251–255. [Google Scholar] [CrossRef]
- Malakooti, R.; Cademartiri, L.; Akcakir, Y.; Petrov, S.; Migliori, A.; Ozin, G.A. Shape-controlled Bi2S3 nanocrystals and their plasma polymerization into flexible film. Adv. Mater. 2006, 18, 2189–2194. [Google Scholar] [CrossRef]
- Lee, T.H.; Rabalais, J.W. X-ray photoelectron spectra and electronic structure of some diamine compounds. J. Electron. Spectrosc. Relat. Phenom. 1977, 11, 123–127. [Google Scholar] [CrossRef]
- Zhao, Y.; Chua, K.T.E.; Gan, C.K.; Zhang, J.; Peng, B.; Peng, Z.; Xiong, Q. Phonons in Bi2S3 nanostructures: Raman scattering and first-principles studies. Phys. Rev. B 2011, 84, 205330. [Google Scholar] [CrossRef] [Green Version]
- Slater, J.C. Atomic radii in crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitsui, T.; Matsumura, M. Preparation of S-doped TiO2 photocatalysts and their photocatalytic activities under visible light. Appl. Catal. A Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Chai, S.Y.; Kim, Y.J.; Jung, M.H.; Chakraborty, A.K.; Jung, D.; Lee, W.I. Heterojunctioned BiOCl/Bi2O3, a new visible light photocatalyst. J. Catal. 2009, 262, 144–149. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Xiao, C.; Zhang, J.; Fan, S.; An, R.; Cheng, Q.; Xie, J.; Zhou, M.; Ye, B.; Xie, Y. Vacancy Associates promoting solar-driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets. J. Am. Chem. Soc. 2013, 135, 10411–10417. [Google Scholar] [CrossRef] [PubMed]

| Bi | O | Cl | S | Method |
|---|---|---|---|---|
| 30.7 | 38.8 | 25.3 | 5.2 | EDS-SEM |
| 32.1 | 33.2 | 26.2 | 8.5 | EDS-TEM |
| 29.0 | 38.8 | 22.7 | 9.5 | XPS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, R.; Liu, J.; Yuan, H.; Yu, Y. Sulfur-Doped BiOCl with Enhanced Light Absorption and Photocatalytic Water Oxidation Activity. Nanomaterials 2021, 11, 2221. https://doi.org/10.3390/nano11092221
Qi R, Liu J, Yuan H, Yu Y. Sulfur-Doped BiOCl with Enhanced Light Absorption and Photocatalytic Water Oxidation Activity. Nanomaterials. 2021; 11(9):2221. https://doi.org/10.3390/nano11092221
Chicago/Turabian StyleQi, Ruilian, Jian Liu, Huanxiang Yuan, and Yu Yu. 2021. "Sulfur-Doped BiOCl with Enhanced Light Absorption and Photocatalytic Water Oxidation Activity" Nanomaterials 11, no. 9: 2221. https://doi.org/10.3390/nano11092221
APA StyleQi, R., Liu, J., Yuan, H., & Yu, Y. (2021). Sulfur-Doped BiOCl with Enhanced Light Absorption and Photocatalytic Water Oxidation Activity. Nanomaterials, 11(9), 2221. https://doi.org/10.3390/nano11092221






