Electrospun Nanofiber-Based Viroblock/ZnO/PAN Hybrid Antiviral Nanocomposite for Personal Protective Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
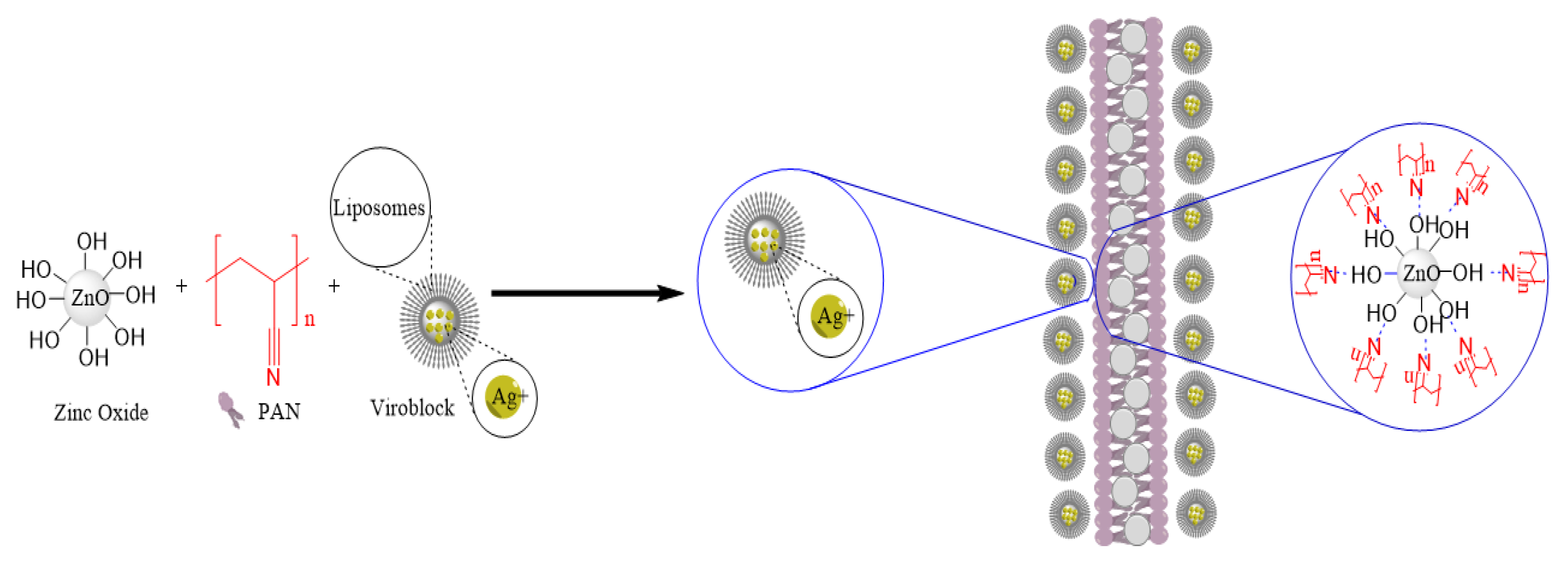
2.2. Preparation of Pristine PAN, PAN/ZnO, and VB-Loaded PAN/ZnO Electrospinning Solutions
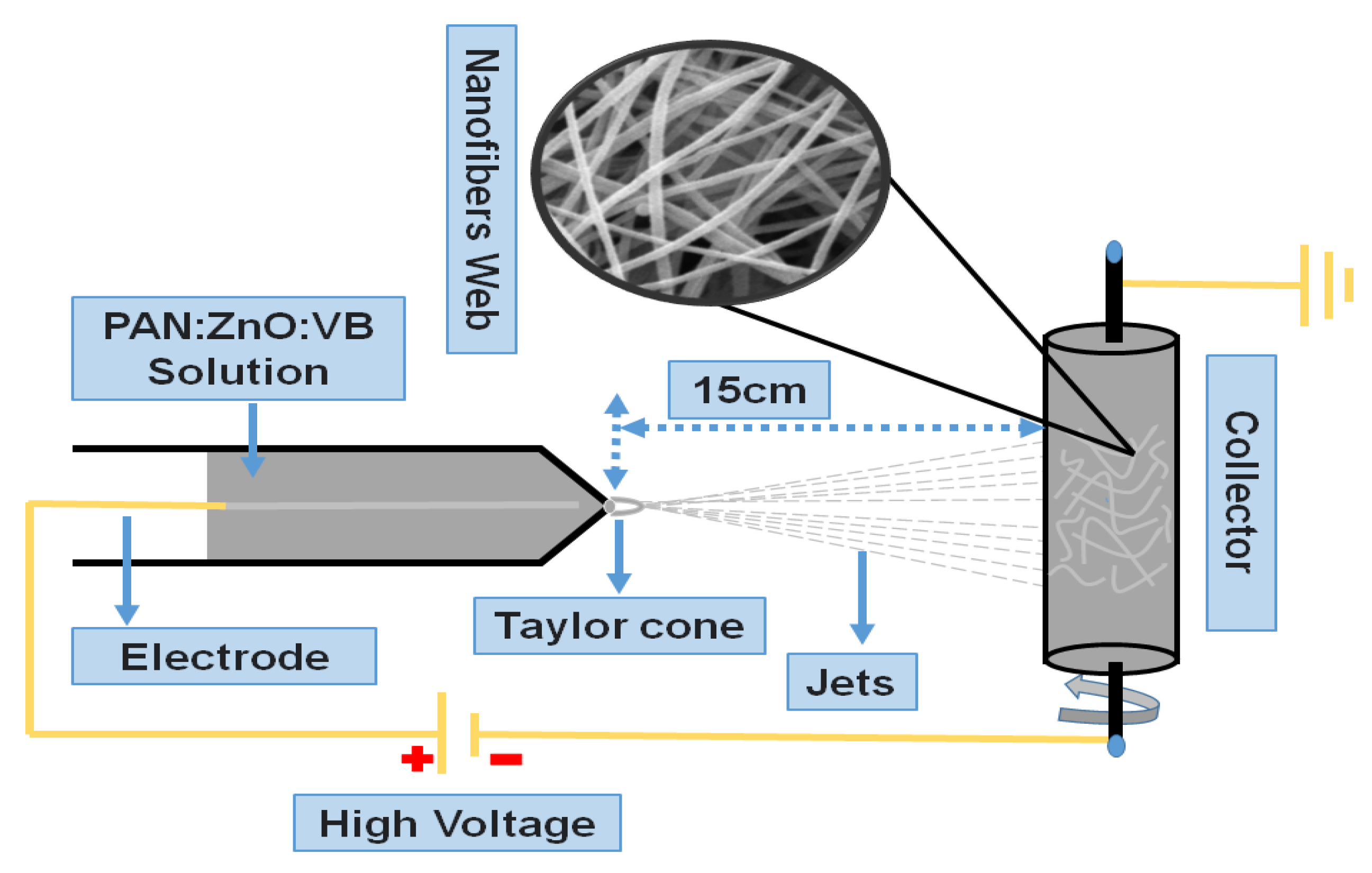
2.3. Fabrication of Electrospun Nanofibers
3. Characterization
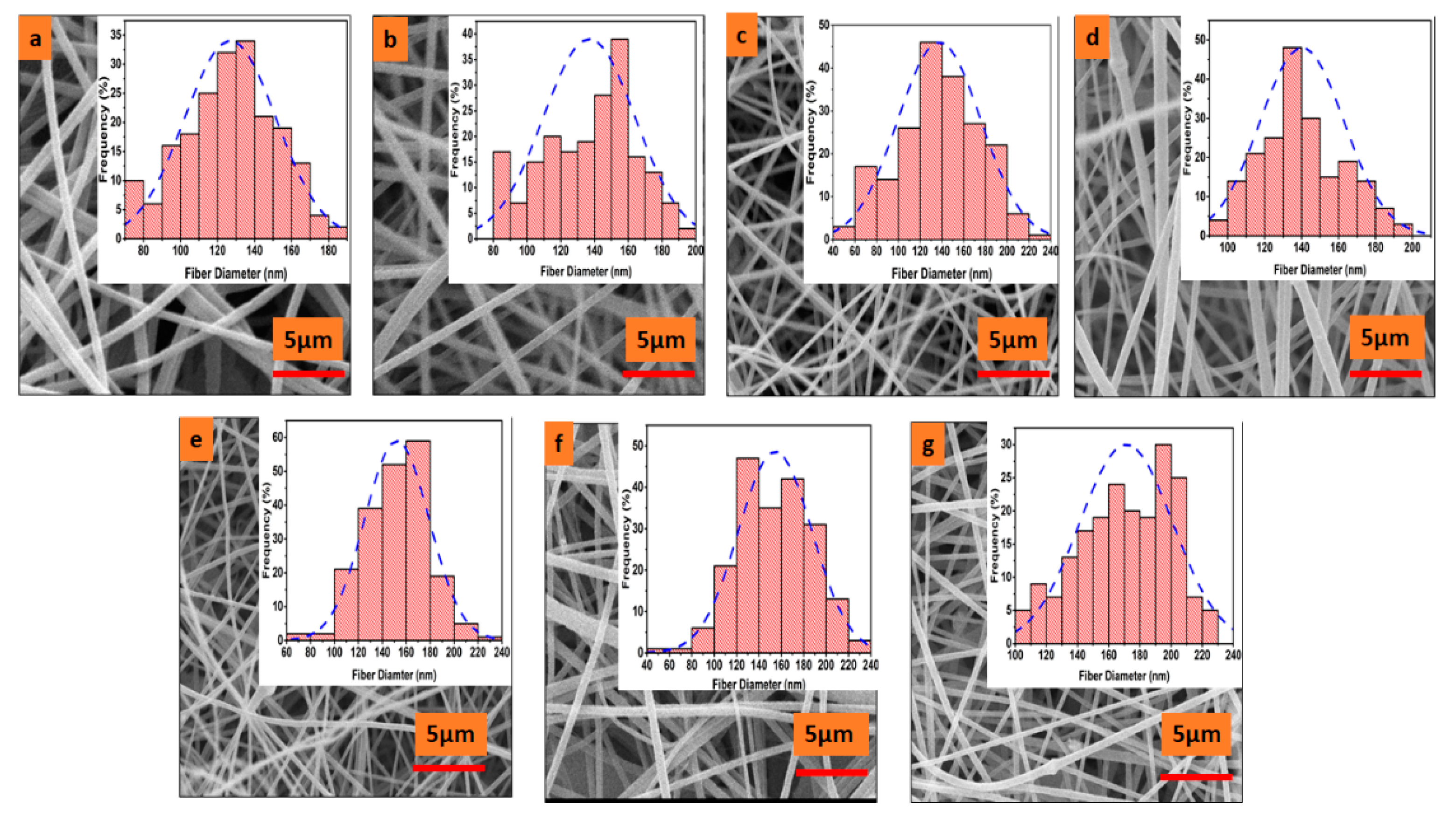
3.1. Surface Morphology
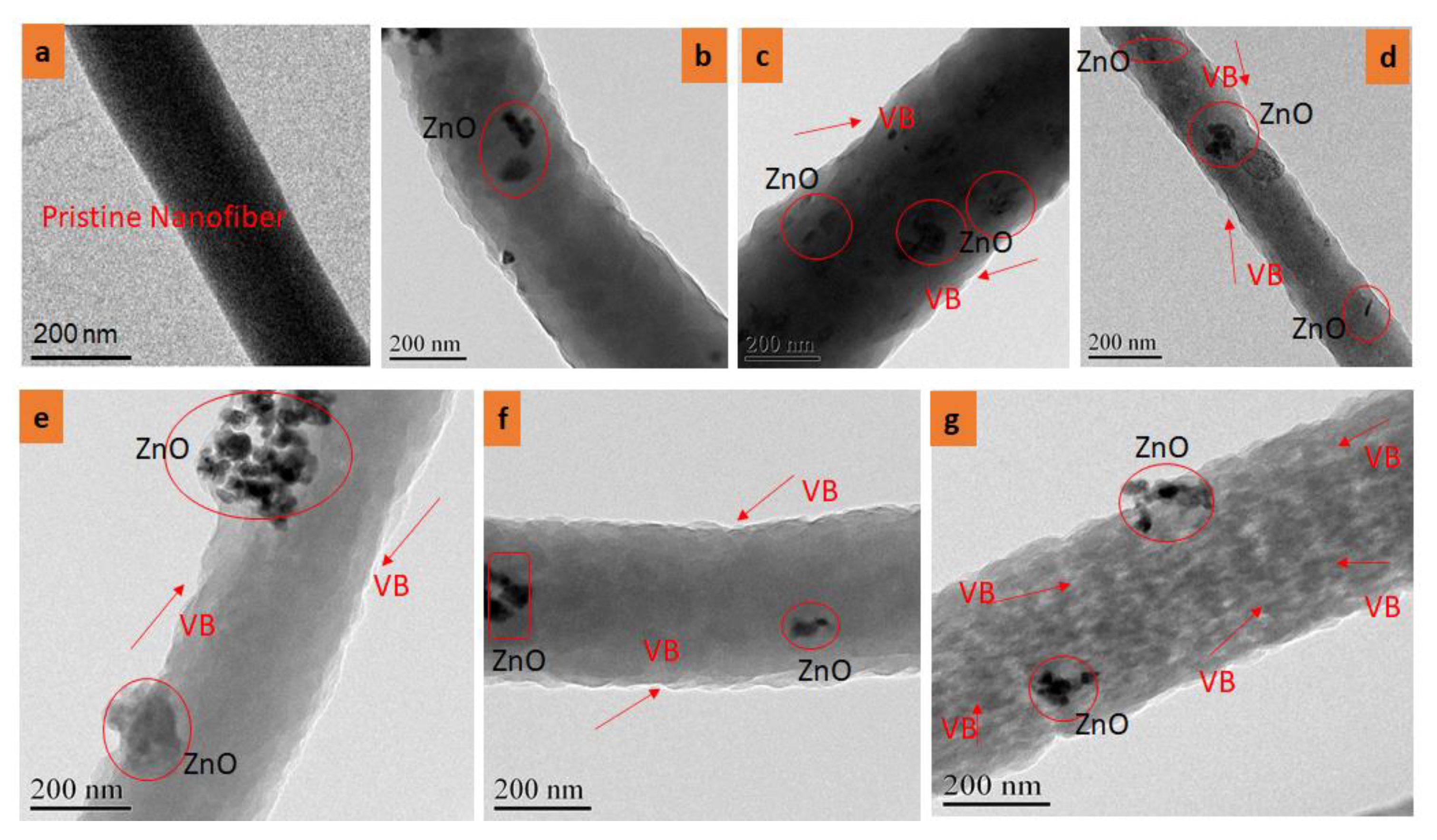
3.2. Transmission Electron Microscope (TEM) Analysis
3.3. Chemical Interaction (FTIR)
3.4. Antibacterial Activity Protocol
3.5. Assessment of Antiviral Activity
4. Results and Discussion
4.1. Surface Morphology of Electrospun Nanofibers
4.2. TEM Analysis of Electrospun Nanofibers
4.3. FTIR Analysis of Electrospun Nanofibers
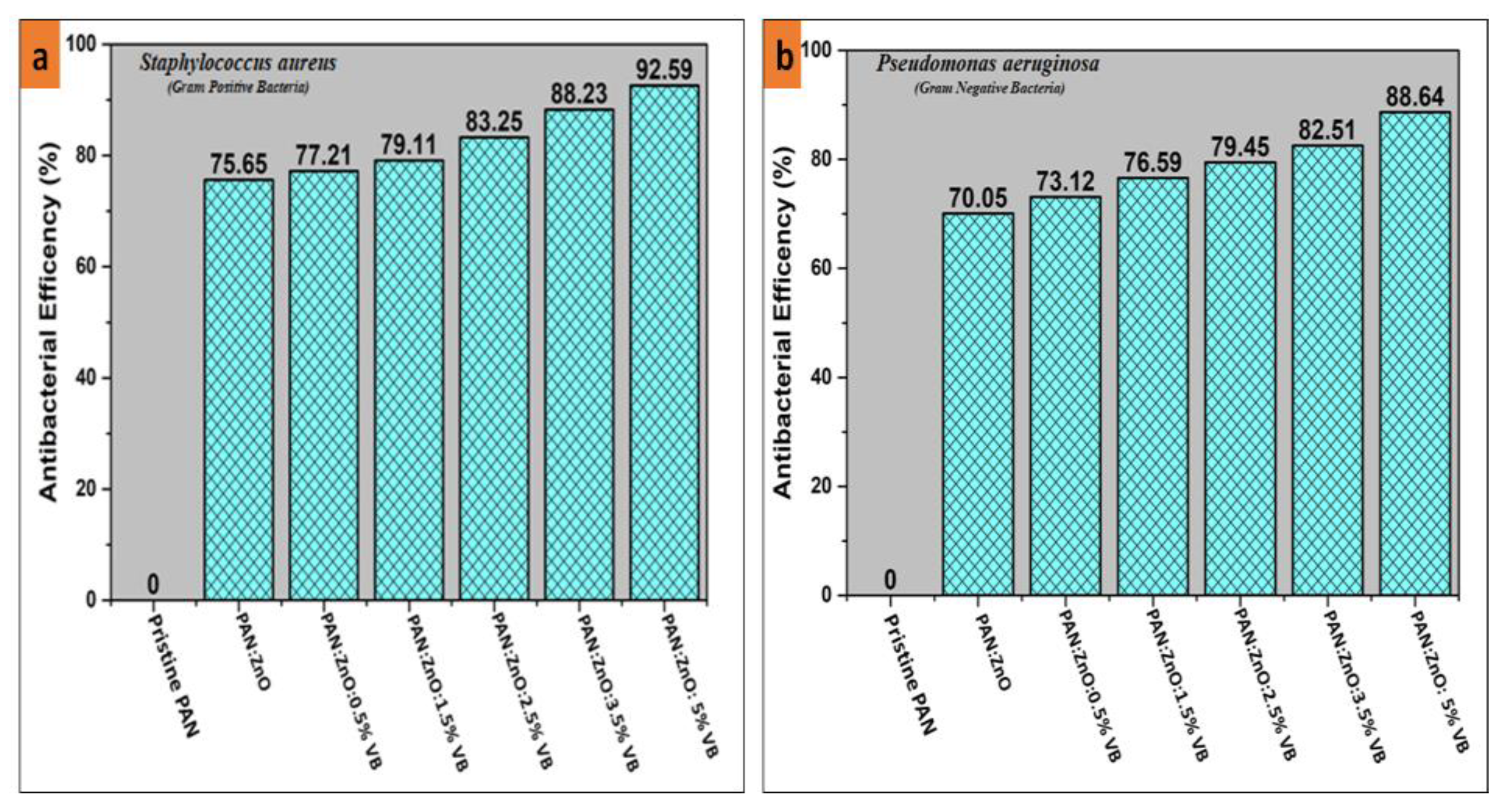
4.4. Antibacterial Activity of PAN/ZnO, PAN/ZnO-Loaded Viroblock Electrospun Nanofibers
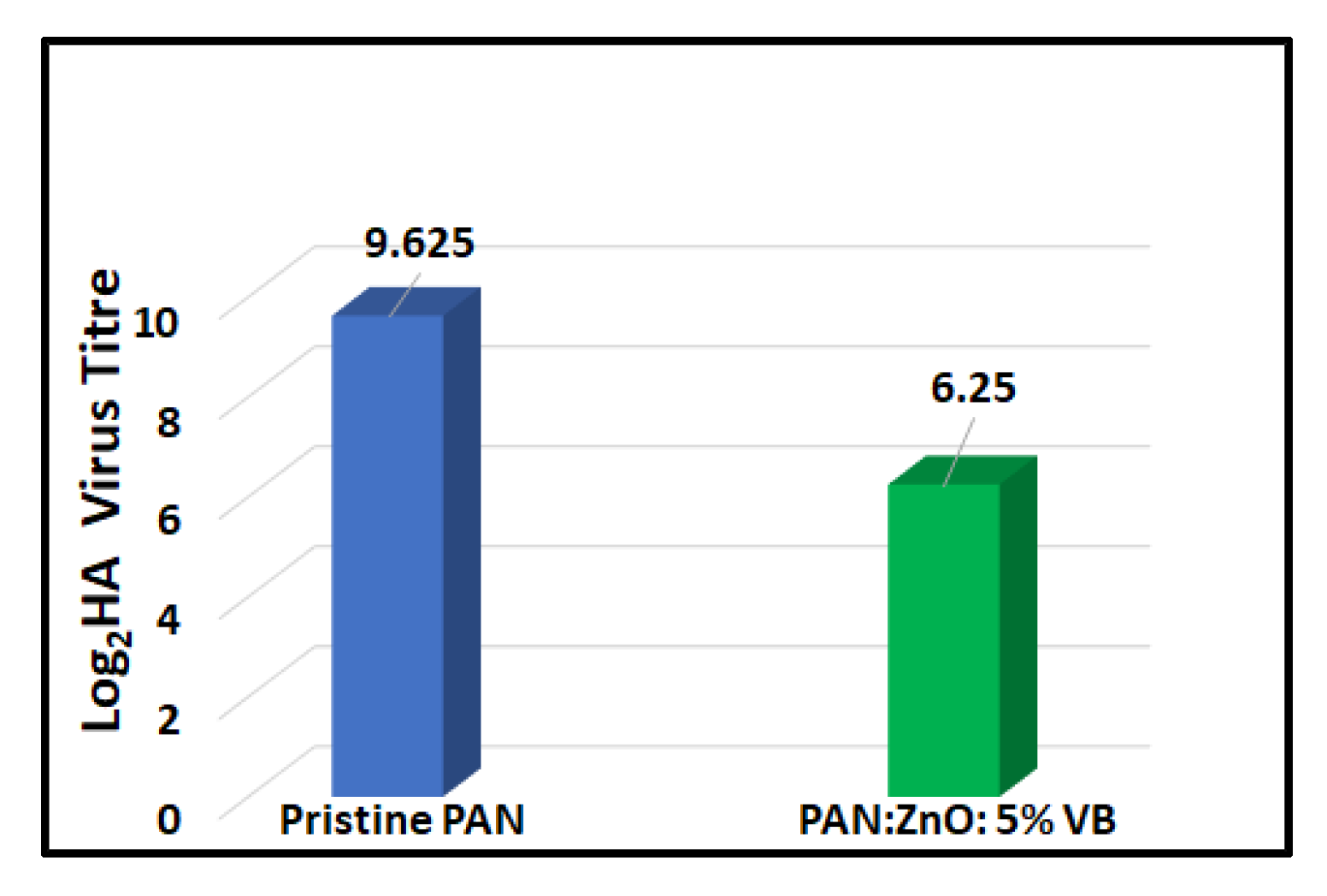
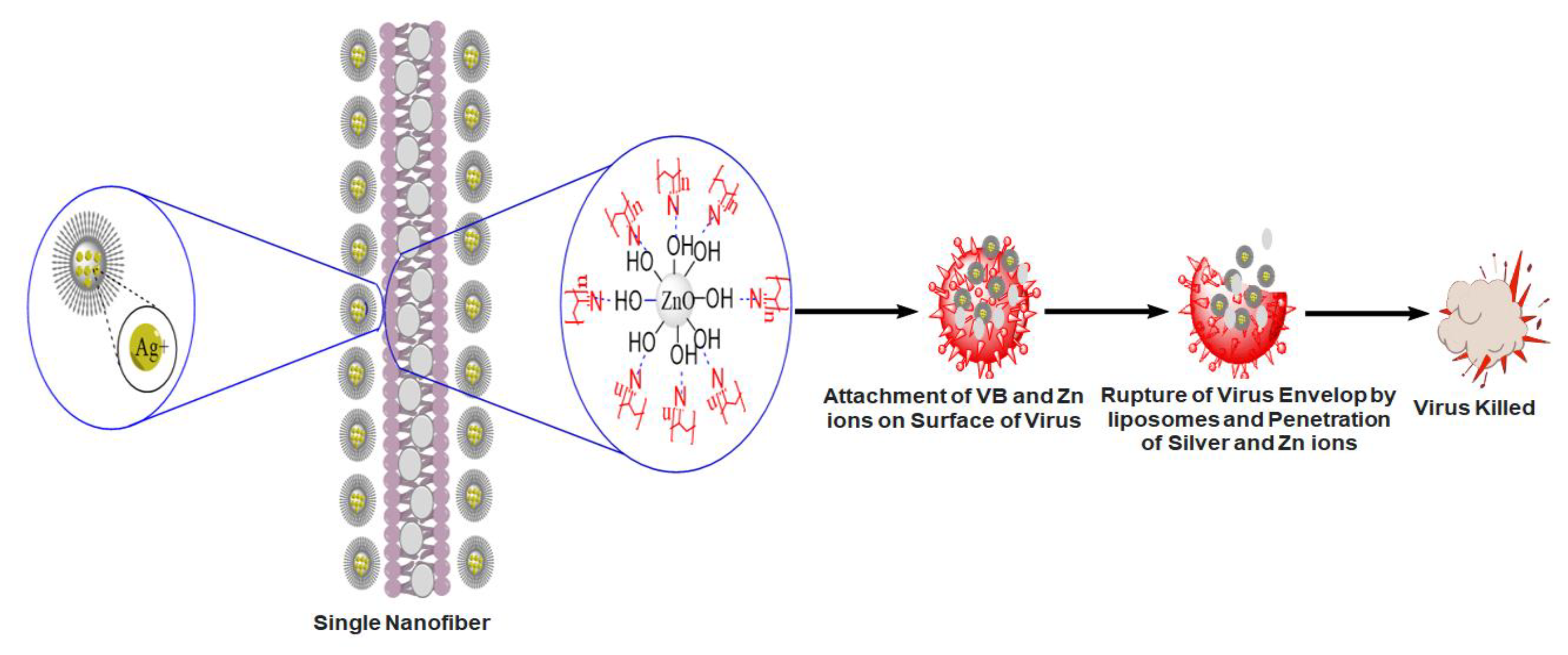
4.5. Antiviral Activity of Pristine PAN Nanofibers and 5% VB-Loaded PAN/ZnO Electrospun Nanofibers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asahi, K.; Undurraga, E.A.; Valdés, R.; Wagner, R. The effect of COVID-19 on the economy: Evidence from an early adopter of localized lockdowns. J. Glob. Health 2021, 11, 05002. [Google Scholar] [CrossRef]
- Agarwal, P.; Singh, R. Modelling of transmission dynamics of Nipah virus (Niv): A fractional order Approach. Phys. A Stat. Mech. Appl. 2020, 547, 124243. [Google Scholar] [CrossRef]
- Chauhan, S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed. J. 2020, 43, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Elduma, A.H.; LaBeaud, A.D.; Plante, J.A.; Plante, K.S.; Ahmed, A. High Seroprevalence of Dengue Virus Infection in Sudan: Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2020, 5, 120. [Google Scholar] [CrossRef] [PubMed]
- Borland, S.; Gracieux, P.; Jones, M.; Mallet, F.; Yugueros-Marcos, J. Influenza A Virus Infection in Cats and Dogs: A Literature Review in the Light of the “One Health” Concept. Front. Public Health 2020, 8, 83. [Google Scholar] [CrossRef]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Geller, C.; Varbanov, M.; Duval, R.E. Human Coronaviruses: Insights into Environmental Resistance and Its Influence on the Development of New Antiseptic Strategies. Viruses 2012, 4, 3044–3068. [Google Scholar] [CrossRef] [Green Version]
- Pathogenesis of Viral Infections and Diseases. In Fenner’s Veterinary Virology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–78.
- Howard, M.C. Understanding face mask use to prevent coronavirus and other illnesses: Development of a multidimensional face mask perceptions scale. Br. J. Health Psychol. 2020, 25, 912–924. [Google Scholar] [CrossRef]
- Patel, A.; D’Alessandro, M.M.; Ireland, K.J.; Burel, W.G.; Wencil, E.B.; Rasmussen, S.A. Personal Protective Equipment Supply Chain: Lessons Learned from Recent Public Health Emergency Responses. Health Secur. 2017, 15, 244–252. [Google Scholar] [CrossRef]
- Gralton, J.; McLaws, M.-L. Protecting healthcare workers from pandemic influenza: N95 or surgical masks? Crit. Care Med. 2010, 38, 657–667. [Google Scholar] [CrossRef]
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 Respirators Be Reused after Disinfection? How Many Times? ACS Nano 2020, 14, 6348–6356. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, C.D.; Radney, J.G.; Vicenzi, E.; Weaver, J.L. Filtration Efficiencies of Nanoscale Aerosol by Cloth Mask Materials Used to Slow the Spread of SARS-CoV-2. ACS Nano 2020, 14, 9188–9200. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Imoto, Y.; Kosaka, T.; Nishida, T.; Nakagawa, T.; Yamamoto, T.A. Antiviral Activity of Silver Nanoparticles Immobilized onto Textile Fabrics Synthesized by Radiochemical Process. MRS Adv. 2016, 1, 705–710. [Google Scholar] [CrossRef]
- Nozari, B.; Montazer, M.; Rad, M.M. In-Situ Synthesis of SiO2 Nanoparticles on Polyester Fabric as Benign Multi-Purpose Catalysts. Fibers Polym. 2018, 19, 2564–2573. [Google Scholar] [CrossRef]
- Shaheen, T.I.; El-Naggar, M.E.; Abdelgawad, A.; Hebeish, A. Durable antibacterial and UV protections of in situ synthesized zinc oxide nanoparticles onto cotton fabrics. Int. J. Biol. Macromol. 2016, 83, 426–432. [Google Scholar] [CrossRef]
- Shigita, S.; Tsurumi, H.; Naka, H. Anti-Viral Fiber, Process for Producing the Fiber, and Textile Product Comprising the Fiber. WO2005083171, 9 September 2007. [Google Scholar]
- Imai, K.; Ogawa, H.; Bui, V.N.; Inoue, H.; Fukuda, J.; Ohba, M.; Yamamoto, Y.; Nakamura, K. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antivir. Res. 2012, 93, 225–233. [Google Scholar] [CrossRef]
- Kumar, S.; Karmacharya, M.; Joshi, S.R.; Gulenko, O.; Park, J.; Kim, G.-H.; Cho, Y.-K. Photoactive Antiviral Face Mask with Self-Sterilization and Reusability. Nano Lett. 2021, 21, 337–343. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Fu, F.; Liu, X. Durable antimicrobial cotton textiles modified with inorganic nanoparticles. Cellulose 2016, 23, 2791–2808. [Google Scholar] [CrossRef]
- Yadav, A.; Prasad, V.; Kathe, A.A.; Raj, S.; Yadav, D.; Sundaramoorthy, C.; Vigneshwaran, N. Functional finishing in cotton fabrics using zinc oxide nanoparticles. Bull. Mater. Sci. 2006, 29, 641–645. [Google Scholar] [CrossRef]
- Salam, A.; Khan, M.Q.; Hassan, T.; Hassan, N.; Nazir, A.; Hussain, T.; Azeem, M.; Kim, I.S. In-vitro assessment of appropriate hydrophilic scaffolds by co-electrospinning of poly(1,4 cyclohexane isosorbide terephthalate)/polyvinyl alcohol. Sci. Rep. 2020, 10, 19751. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Pires, R.; Faria, S.; Fonseca, N.A.C.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Immobilization of bioactive factor-loaded liposomes on the surface of electrospun nanofibers targeting tissue engineering. Biomater. Sci. 2014, 2, 1195–1209. [Google Scholar] [CrossRef]
- Cui, W.Y.; Yoo, H.J.; Li, Y.G.; Baek, C.; Min, J. Electrospun Nanofibers Embedded with Copper Oxide Nanoparticles to Improve Antiviral Function. J. Nanosci. Nanotechnol. 2021, 21, 4174–4178. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.O.; Islam, M.S.; Haque, M.A.; Rahman, M.A.; Hossain, M.T.; Hamid, M.A. Antibacterial activity of polyaniline coated silver nanoparticles synthesized from Piper betle leaves extract. Iran. J. Pharm. Res. 2016, 15, 591. [Google Scholar] [CrossRef]
- Ji, S.-M.; Tiwari, A.P.; Oh, H.J.; Kim, H.-Y. ZnO/Ag nanoparticles incorporated multifunctional parallel side by side nanofibers for air filtration with enhanced removing organic contaminants and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126564. [Google Scholar] [CrossRef]
- Kim, S.E.; Tiwari, A.P. Mussel-inspired polydopamine-enabled in situ-synthesized silver nanoparticle-anchored porous polyacrylonitrile nanofibers for wound-healing applications. Int. J. Polym. Mater. 2020, 1–10. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Shuvho, B.A.; Shahid, A.; Haque, A.M.; Kashem, M.A.; Lam, S.S.; Ong, H.C.; Uddin, A.; Mofijur, M. Prospect of biobased antiviral face mask to limit the coronavirus outbreak. Environ. Res. 2021, 192, 110294. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Ji, S.M.; Tiwari, A.P.; Kim, H.Y. Graphene Oxide Coated Zinc Oxide Core–Shell Nanofibers for Enhanced Photocatalytic Performance and Durability. Coatings 2020, 10, 1183. [Google Scholar] [CrossRef]
- HeiQ Viroblock-HeiQ Materials AG. Available online: https://heiq.com/products/functional-textile-technologies/heiq-viroblock/ (accessed on 16 August 2021).
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Dung, T.T.N.; Nam, V.N.; Nhan, T.T.; Ngoc, T.T.B.; Minh, L.Q.; Nga, B.T.T.; Le, V.P.; Quang, D.V. Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater. Res. Express 2019, 6, 1250g9. [Google Scholar] [CrossRef]
- Xiao, Y.; Cao, Y.; Xin, B.; Liu, Y.; Chen, Z.; Lin, L.; Sun, Y. Fabrication and characterization of electrospun cellulose/polyacrylonitrile nanofibers with Cu(II) ions. Cellulose 2018, 25, 2955–2963. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Recent developments in electrospun ZnO nanofibers: A short review. J. Eng. Fibers Fabr. 2020, 15, 155892501989968. [Google Scholar] [CrossRef] [Green Version]
- Iatsunskyi, I.; Vasylenko, A.; Viter, R.; Kempiński, M.; Nowaczyk, G.; Jurga, S.; Bechelany, M. Tailoring of the electronic properties of ZnO-polyacrylonitrile nanofibers: Experiment and theory. Appl. Surf. Sci. 2017, 411, 494–501. [Google Scholar] [CrossRef]
- Ali, S.W.; Rajendran, S.; Joshi, M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr. Polym. 2011, 83, 438–446. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.-L.; Ng, I.-S.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577. [Google Scholar] [CrossRef]
- Nazaktabar, A.; Lashkenari, M.S.; Araghi, A.; Ghorbani, M.; Golshahi, H. In vivo evaluation of toxicity and antiviral activity of polyrhodanine nanoparticles by using the chicken embryo model. Int. J. Biol. Macromol. 2017, 103, 379–384. [Google Scholar] [CrossRef]
- Fang, S.; Wang, W.; Yu, X.; Xu, H.; Zhong, Y.; Sui, X.; Zhang, L.; Mao, Z. Preparation of ZnO:(Al, La)/polyacrylonitrile (PAN) nonwovens with low infrared emissivity via electrospinning. Mater. Lett. 2015, 143, 120–123. [Google Scholar] [CrossRef]
- Yu, H.; Jiao, Z.; Hu, H.; Lu, G.; Ye, J.; Bi, Y. Fabrication of Ag3PO4–PAN composite nanofibers for photocatalytic applications. CrystEngComm 2013, 15, 4802–4805. [Google Scholar] [CrossRef]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Shah, A.P.; Jain, S.; Mokale, V.J.; Shimpi, N.G. High performance visible light photocatalysis of electrospun PAN/ZnO hybrid nanofibers. J. Ind. Eng. Chem. 2019, 77, 154–163. [Google Scholar] [CrossRef]
- Karim, S.A.; Mohamed, A.; Abdel-Mottaleb, M.; Osman, T.; Khattab, A. Visible light photocatalytic activity of PAN-CNTs/ZnO-NH2 electrospun nanofibers. J. Alloy. Compd. 2019, 772, 650–655. [Google Scholar] [CrossRef]
- Chauhan, D.; Afreen, S.; Mishra, S.; Sankararamakrishnan, N. Synthesis, characterization and application of zinc augmented aminated PAN nanofibers towards decontamination of chemical and biological contaminants. J. Ind. Eng. Chem. 2017, 55, 50–64. [Google Scholar] [CrossRef]
- Wasim, M.; Shi, F.; Liu, J.; Farooq, A.; Khan, S.U.; Salam, A.; Hassan, T.; Zhao, X. An overview of Zn/ZnO modified cellulosic nanocomposites and their potential applications. J. Polym. Res. 2021, 28, 338. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Kolawole, M.O.; Agunbiade, F.O.; Omorogie, M.O.; Koko, D.T.; Ugwuja, C.G.; Ugege, L.E.; Oyejide, N.E.; Günter, C.; Taubert, A. Novel metal-doped bacteriostatic hybrid clay composites for point-of-use disinfection of water. J. Environ. Chem. Eng. 2017, 5, 2128–2141. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Ray, S.S.; Maity, A. Synthesis and characterization of alginate beads encapsulated zinc oxide nanoparticles for bacteria disinfection in water. J. Colloid Interface Sci. 2018, 512, 686–692. [Google Scholar] [CrossRef]
- Rajavel, K.; Gomathi, R.; Manian, S.; Kumar, R.T.R. In Vitro Bacterial Cytotoxicity of CNTs: Reactive Oxygen Species Mediate Cell Damage Edges over Direct Physical Puncturing. Langmuir 2013, 30, 592–601. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Yang, H.; Liu, X.; Huang, Q.; Lu, Z. Antibacterial activity and mechanism of Ag/ZnO nanocomposite against anaerobic oral pathogen Streptococcus mutans. J. Mater. Sci. Mater. Med. 2017, 28, 23. [Google Scholar] [CrossRef]
- Patel, S.; Konar, M.; Sahoo, H.; Hota, G. Surface functionalization of electrospun PAN nanofibers with ZnO–Ag heterostructure nanoparticles: Synthesis and antibacterial study. Nanotechnology 2019, 30, 205704. [Google Scholar] [CrossRef] [PubMed]
- Sohrabnezhad, S.; Moghaddam, M.J.M.; Salavatiyan, T. Synthesis and characterization of CuO–montmorillonite nanocomposite by thermal decomposition method and antibacterial activity of nanocomposite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 125, 73–78. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Ray, S.S. Highly efficient inactivation of bacteria found in drinking water using chitosan-bentonite composites: Modelling and breakthrough curve analysis. Water Res. 2017, 111, 213–223. [Google Scholar] [CrossRef]
- Munnawar, I.; Iqbal, S.S.; Anwar, M.N.; Batool, M.; Tariq, S.; Faitma, N.; Khan, A.L.; Khan, A.U.; Nazar, U.; Jamil, T.; et al. Synergistic effect of Chitosan-Zinc Oxide Hybrid Nanoparticles on antibiofouling and water disinfection of mixed matrix polyethersulfone nanocomposite membranes. Carbohydr. Polym. 2017, 175, 661–670. [Google Scholar] [CrossRef]
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar] [CrossRef]
- Firquet, S.; Beaujard, S.; Lobert, P.-E.; Sane, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salam, A.; Hassan, T.; Jabri, T.; Riaz, S.; Khan, A.; Iqbal, K.M.; Khan, S.u.; Wasim, M.; Shah, M.R.; Khan, M.Q.; et al. Electrospun Nanofiber-Based Viroblock/ZnO/PAN Hybrid Antiviral Nanocomposite for Personal Protective Applications. Nanomaterials 2021, 11, 2208. https://doi.org/10.3390/nano11092208
Salam A, Hassan T, Jabri T, Riaz S, Khan A, Iqbal KM, Khan Su, Wasim M, Shah MR, Khan MQ, et al. Electrospun Nanofiber-Based Viroblock/ZnO/PAN Hybrid Antiviral Nanocomposite for Personal Protective Applications. Nanomaterials. 2021; 11(9):2208. https://doi.org/10.3390/nano11092208
Chicago/Turabian StyleSalam, Abdul, Tufail Hassan, Tooba Jabri, Shagufta Riaz, Amina Khan, Kanwal Muhammad Iqbal, Saif ullah Khan, Muhammad Wasim, Muhammad Raza Shah, Muhammad Qamar Khan, and et al. 2021. "Electrospun Nanofiber-Based Viroblock/ZnO/PAN Hybrid Antiviral Nanocomposite for Personal Protective Applications" Nanomaterials 11, no. 9: 2208. https://doi.org/10.3390/nano11092208
APA StyleSalam, A., Hassan, T., Jabri, T., Riaz, S., Khan, A., Iqbal, K. M., Khan, S. u., Wasim, M., Shah, M. R., Khan, M. Q., & Kim, I.-S. (2021). Electrospun Nanofiber-Based Viroblock/ZnO/PAN Hybrid Antiviral Nanocomposite for Personal Protective Applications. Nanomaterials, 11(9), 2208. https://doi.org/10.3390/nano11092208








