Systematic Analysis on the Effect of Sintering Temperature for Optimized Performance of Li0.15Ni0.45Zn0.4O2-Gd0.2Ce0.8O2-Li2CO3-Na2CO3-K2CO3 Based 3D Printed Single-Layer Ceramic Fuel Cell
Abstract
:1. Introduction
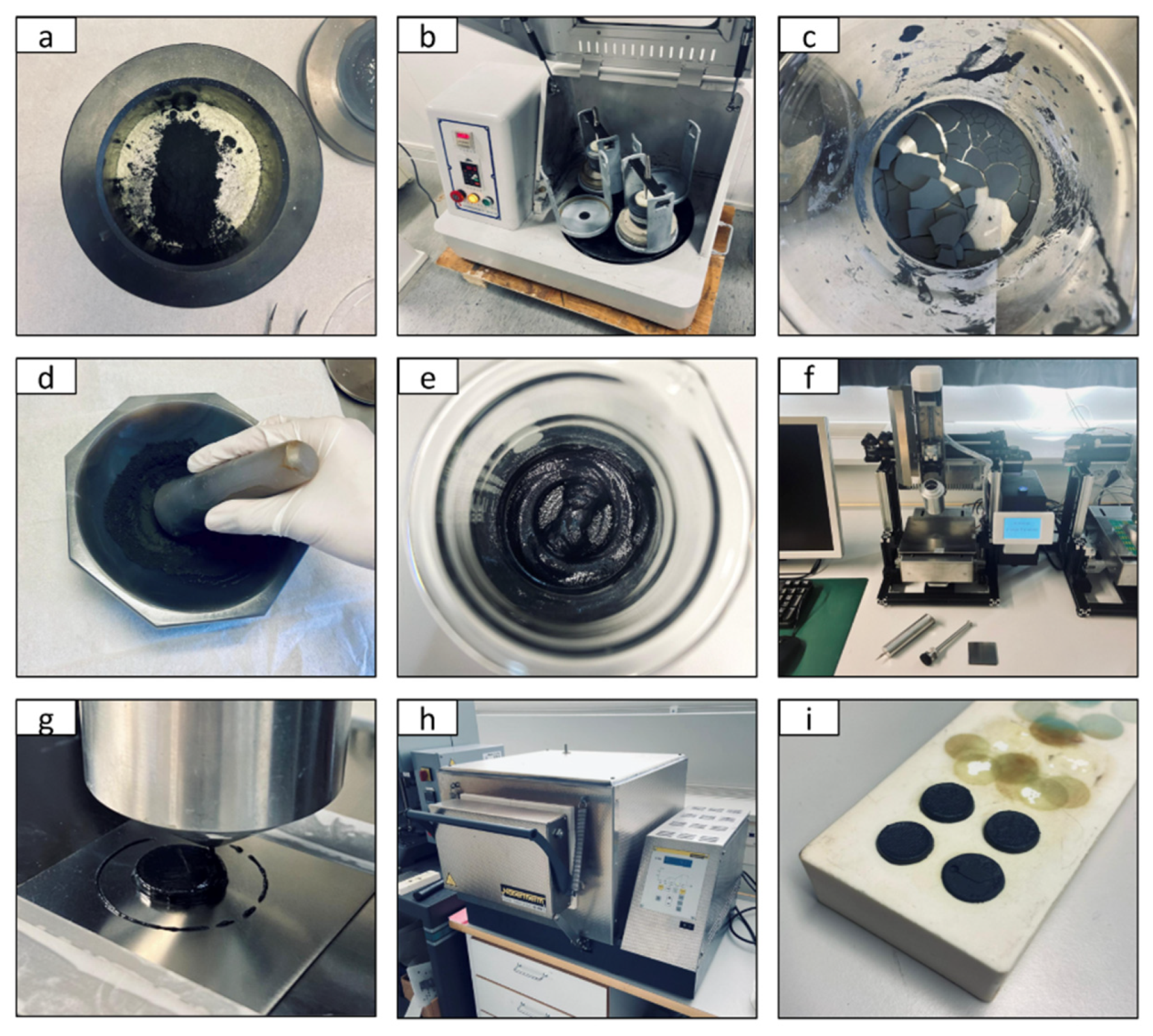
2. Materials and Methods
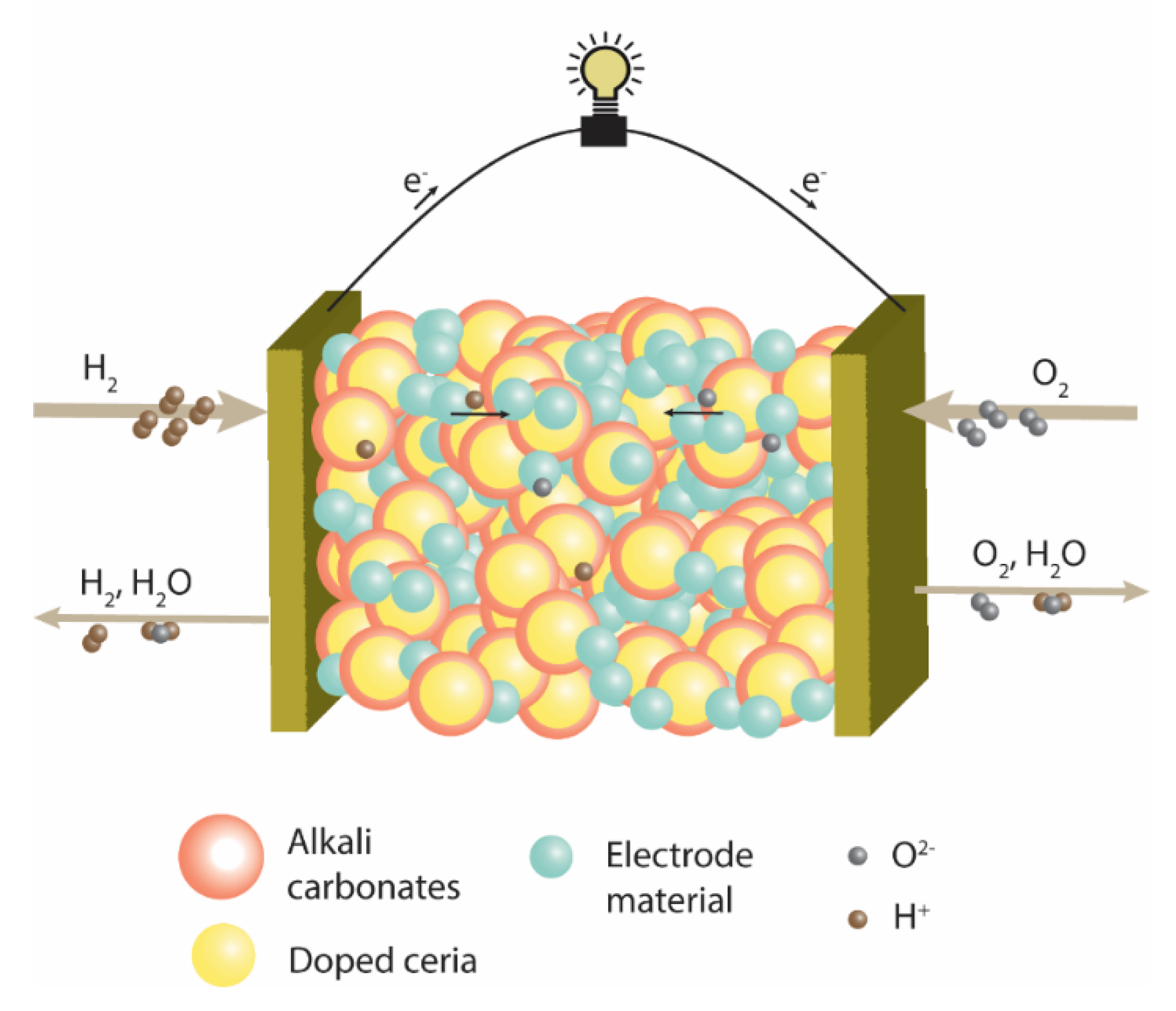
3. Results and Discussion
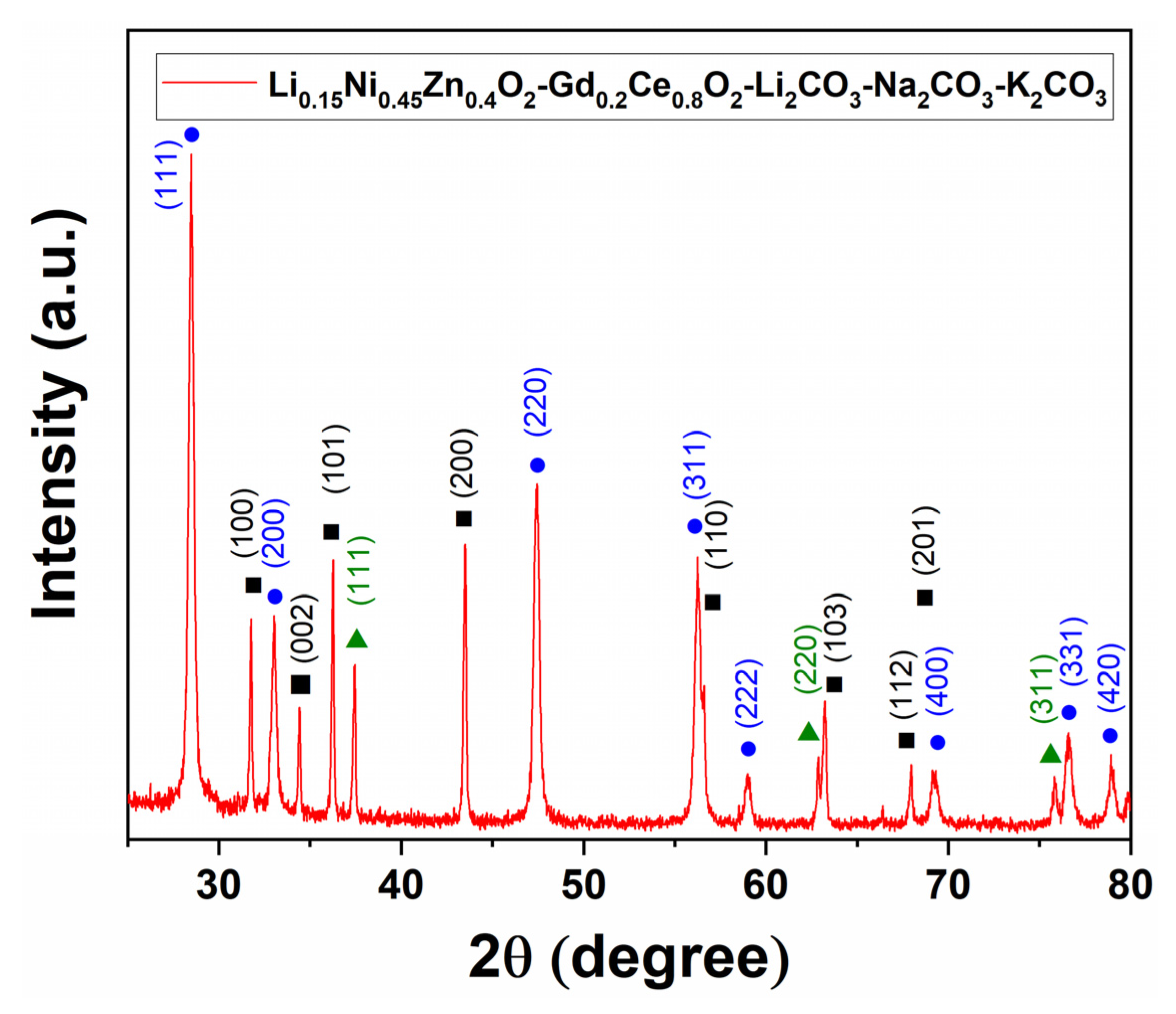
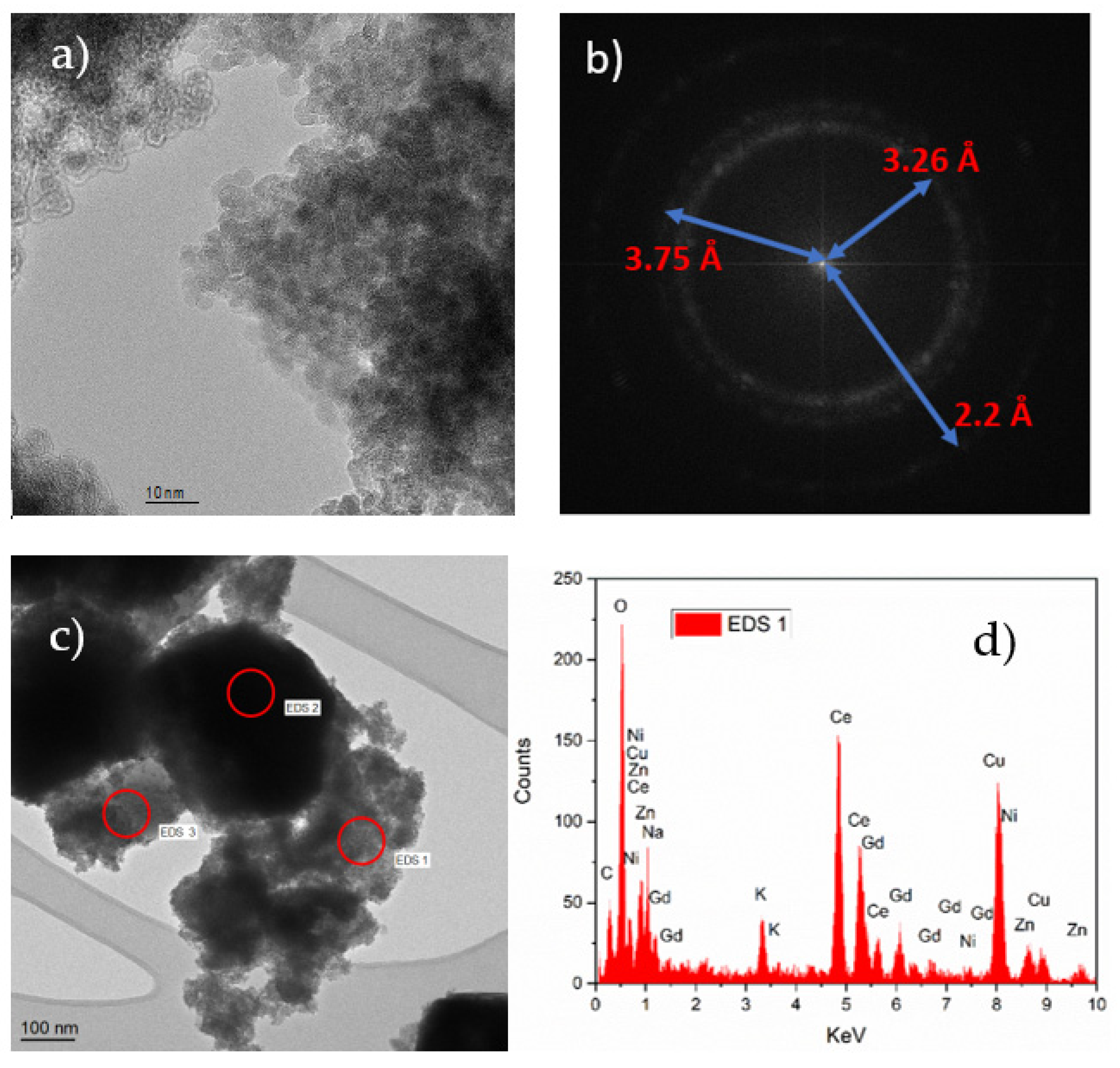
3.1. XRD and Microscopic Analysis of the Composite Material
3.2. Electrochemical Characterization
3.3. Structural and Elemental Analysis of the Printed Cell
3.4. Short-Term Stability Test of the Printed Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, B.; Ma, Y.; Wang, X.; Raza, R.; Qin, H.; Fan, L. A Fuel Cell with a Single Component Functioning Simultaneously as the Electrodes and Electrolyte. Electrochem. Commun. 2011, 13, 225–227. [Google Scholar] [CrossRef]
- Zhu, B.; Qin, H.; Raza, R.; Liu, Q.; Fan, L.; Patakangas, J.; Lund, P. A Single-Component Fuel Cell Reactor. Int. J. Hydrogen Energy 2011, 36, 8536–8541. [Google Scholar] [CrossRef]
- Zhu, B.; Lund, P.D.; Raza, R.; Ma, Y.; Fan, L.; Afzal, M.; Patakangas, J.; He, Y.; Zhao, Y.; Tan, W.; et al. Schottky Junction Effect on High Performance Fuel Cells Based on Nanocomposite Materials. Adv. Energy Mater. 2015, 5, 1401895. [Google Scholar] [CrossRef]
- Zhu, B.; Huang, Y.; Fan, L.; Ma, Y.; Wang, B.; Xia, C.; Afzal, M.; Zhang, B.; Dong, W.; Wang, H.; et al. Novel Fuel Cell with Nanocomposite Functional Layer Designed by Perovskite Solar Cell Principle. Nano Energy 2016, 19, 156–164. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, Y.; Wang, B.; Xia, C.; Dong, W.; Li, J.; Zhu, B. Mixed Ionic-Electronic Conductor Membrane based Fuel Cells by Incorporating Semiconductor Ni0.8Co0.15Al0.05LiO2−δ into the Ce0.8Sm0.2O2−δ-Na2CO3 Electrolyte. Int. J. Hydrogen Energy 2016, 41, 15346–15353. [Google Scholar] [CrossRef]
- Qin, H.; Zhu, B.; Raza, R.; Singh, M.; Fan, L.; Lund, P. Integration Design of Membrane Electrode Assemblies in Low Temperature Solid Oxide Fuel Cell. Int. J. Hydrogen Energy 2012, 37, 19365–19370. [Google Scholar] [CrossRef]
- Zhu, B.; Fan, L.; Lund, P. Breakthrough Fuel Cell Technology using Ceria-Based Multi-Functional Nanocomposites. Appl. Energy 2013, 106, 163–175. [Google Scholar] [CrossRef]
- Zhu, B.; Lund, P.; Raza, R.; Patankangas, J.; Huang, Q.; Fan, L.; Singh, M. A New Energy Conversion Technology based on Nano-Redox and Nano-Device Processes. Nano Energy 2013, 2, 1179–1185. [Google Scholar] [CrossRef]
- Asghar, M.I.; Jouttijärvi, S.; Jokiranta, R.; Valtavirta, A.-M.; Lund, P.D. Wide Bandgap Oxides for Low-Temperature Single-Layered Nanocomposite Fuel Cell. Nano Energy 2018, 53, 391–397. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, C.; Wang, B.; Tang, Y. Layered LiCoO2–LiFeO2 Heterostructure Composite for Semiconductor-Based Fuel Cells. Nanomaterials 2021, 11, 1224. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Y.; Zhang, W.; Wang, B.; Afzal, M.; Xia, C.; Zhu, B. Industrial Grade Rare-Earth Triple-Doped Ceria Applied for Advanced Low-Temperature Electrolyte Layer-Free Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 22273–22279. [Google Scholar] [CrossRef]
- Nie, X.; Chen, Y.; Mushtaq, N.; Rauf, S.; Wang, B.; Dong, W.; Wang, X.; Wang, H.; Zhu, B. The Sintering Temperature Effect on Electrochemical Properties of Ce0.8Sm0.05Ca0.15O2-δ (SCDC)-La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF) Heterostructure Pellet. Nanoscale Res. Lett. 2019, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Zhang, W.; Zhang, J.; Wang, B.; Xia, C.; Afzal, M.; Li, J.; Singh, M.; Zhu, B. Natural CuFe2O4 Mineral for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 17514–17521. [Google Scholar] [CrossRef]
- Asghar, M.I.; Yao, X.; Jouttijärvi, S.; Hochreiner, E.; Virta, R.; Lund, P.D. Intriguing Electrochemistry in Low-Temperature Single Layer Ceramic Fuel Cells Based on CuFe2O4. Int. J. Hydrogen Energy 2020, 45, 24083–24092. [Google Scholar] [CrossRef]
- Zhu, B.; Raza, R.; Qin, H.; Fan, L. Single-Component and Three-Component Fuel Cells. J. Power Sources 2011, 196, 6362–6365. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Q.; Zhu, Z.; Zhu, B.; Liu, X. Fabrication of Electrolyte-Free Fuel Cell with Mg0.4Zn0.6O/Ce0.8Sm0.2O2−δ–Li0.3Ni0.6Cu0.07Sr0.03O2−δ Layer. J. Power Sources 2014, 248, 577–581. [Google Scholar] [CrossRef]
- Dong, X.; Tian, L.; Li, J.; Zhao, Y.; Tian, Y.; Li, Y. Single Layer Fuel Cell Based on a Composite of Ce0.8Sm0.2O2−δ–Na2CO3 and a Mixed Ionic and Electronic Conductor Sr2Fe1.5Mo0.5O6−δ. J. Power Sources 2014, 249, 270–276. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, X.; Bai, Y.; Li, H.; Deng, X.; Niu, X.; Wu, X.; Zhou, D.; Lv, M.; Wang, Z.; et al. Electrical Conductivity Optimization in Electrolyte-Free Fuel Cells by Single-Component Ce0.8Sm0.2O2-δ–Li0.15Ni0.45Zn0.4 Layer. RSC Adv. 2012, 2, 3828–3834. [Google Scholar] [CrossRef]
- Hei, Y.; Huang, J.; Wang, C.; Mao, Z. Novel Doped Barium Cerate–Carbonate Composite Electrolyte Material for Low Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2014, 39, 14328–14333. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, H.; Raza, R.; Fan, L.; Li, Y.; Zhu, B. Advanced Electrolyte-Free Fuel Cells Based on Functional Nanocomposites of a Single Porous Component: Analysis, Modeling and Validation. RSC Adv. 2012, 2, 8036–8040. [Google Scholar] [CrossRef]
- Jouttijärvi, S.; Yao, X.; Asghar, M.I.; Etula, J.; Reinecke, A.-M.; Lippmann, W.; Lund, P.D. Carbonate Dual-Phase Improves the Performance of Single-Layer Fuel Cell Made from Mixed Ionic and Semiconductor Composite. BMC Energy 2020, 2, 4. [Google Scholar] [CrossRef]
- Jouttijärvi, S.V.; Asghar, M.I.; Lund, P.D. Investigation of Factors Affecting the Performance of a Single-Layer Nanocomposite Fuel Cell. Catal. Today 2021, 364, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Kim, J.-H.; Hwang, K.-T.; Hwang, H.-J.; Han, K.-S. Digital Inkjet Printing in Three Dimensions with Multiple Ceramic Compositions. J. Eur. Ceram. Soc. 2021, 41, 1490–1497. [Google Scholar] [CrossRef]
- Gadea, C.; Hanniet, Q.; Lesch, A.; Marani, D.; Jensen, S.H.; Esposito, V. Aqueous Metal–Organic Solutions for YSZ Thin Film Inkjet Deposition. J. Mater. Chem. C 2017, 5, 6021–6029. [Google Scholar] [CrossRef] [Green Version]
- Lomberg, M.; Boldrin, P.; Tariq, F.; Offer, G.; Wu, B.; Brandon, N.P. Additive Manufacturing for Solid Oxide Cell Electrode Fabrication. ECS Trans. 2015, 68, 2119. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.; Shen, Z. Laser Sintering of Ceramics. J. Asian Ceram. Soc. 2013, 1, 315–321. [Google Scholar] [CrossRef]
- Masciandaro, S.; Torrell, M.; Leone, P.; Tarancón, A. Three-Dimensional Printed Yttria-Stabilized Zirconia Self-Supported Electrolytes for Solid Oxide Fuel Cell Applications. J. Eur. Ceram. Soc. 2019, 39, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Pesce, A.; Hornés, A.; Núñez, M.; Morata, A.; Torrell, M.; Tarancón, A. 3D Printing the next Generation of Enhanced Solid Oxide Fuel and Electrolysis Cells. J. Mater. Chem. A 2020, 8, 16926–16932. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Yu, F.; Zhang, W.; Meng, X.; Yang, N.; Liu, S. A Novel Fabrication of Yttria-Stabilized-Zirconia Dense Electrolyte for Solid Oxide Fuel Cells by 3D Printing Technique. Int. J. Hydrogen Energy 2019, 44, 6182–6191. [Google Scholar] [CrossRef]
- Xing, B.; Yao, Y.; Meng, X.; Zhao, W.; Shen, M.; Gao, S.; Zhao, Z. Self-Supported Yttria-Stabilized Zirconia Ripple-Shaped Electrolyte for Solid Oxide Fuel Cells Application by Digital Light Processing Three-Dimension Printing. Scr. Mater. 2020, 181, 62–65. [Google Scholar] [CrossRef]
- Mat, A.; Canavar, M.; Timurkutluk, B.; Kaplan, Y. Investigation of Micro-Tube Solid Oxide Fuel Cell Fabrication using Extrusion Method. Int. J. Hydrogen Energy 2016, 41, 10037–10043. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, L.; Li, L.; Chen, J.; Liu, Y.; Li, Y.; Hao, L.; Wu, Y. 3D Printed Sm-Doped Ceria Composite Electrolyte Membrane for Low Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 13843–13851. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Ahn, S.-J.; Moon, J.; Kim, J.; Lee, H.-W. Direct-Write Fabrication of Solid Oxide Fuel Cells. ECS Proc. Vol. 2005, 2005, 489. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Ahn, S.-J.; Moon, J.; Kim, J.; Lee, H.-W. Direct-Write Fabrication of Integrated Planar Solid Oxide Fuel Cells. J. Electroceramics 2006, 17, 683–687. [Google Scholar] [CrossRef]
- Rosa, M.; Barou, C.; Esposito, V. Zirconia UV-Curable Colloids for Additive Manufacturing via Hybrid Inkjet Printing-Stereolithography. Mater. Lett. 2018, 215, 214–217. [Google Scholar] [CrossRef]
- Salari, F.; Najafabadi, A.B.; Ghatee, M.; Golmohammad, M. Hybrid Additive Manufacturing of the Modified Electrolyte-Electrode Surface of Planar Solid Oxide Fuel Cells. Int. J. Appl. Ceram. Technol. 2020, 17, 1554–1561. [Google Scholar] [CrossRef]
- Kostretsova, N.; Pesce, A.; Nuñez, M.; Morata, A.; Torrell, M.; Tarancón, A. Self-Supported Solid Oxide Fuel Cells by Multimaterial 3D Printing. ECS Trans. 2021, 103, 59. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Rahman, N.F.A.; Rahman, H.A.; Somalu, M.R.; Azmi, M.A.; Raharjo, J. Fabrication of High-Quality Electrode Films for Solid Oxide Fuel Cell by Screen Printing: A Review on Important Processing Parameters. Int. J. Energy Res. 2020, 44, 8296–8313. [Google Scholar] [CrossRef]
- Somalu, M.R.; Muchtar, A.; Daud, W.R.W.; Brandon, N.P. Screen-Printing Inks for the Fabrication of Solid Oxide Fuel Cell Films: A Review. Renew. Sustain. Energy Rev. 2017, 75, 426–439. [Google Scholar] [CrossRef]
- Myung, J.; Ko, H.J.; Im, C.H.; Moon, J.; Hyun, S.-H. Development of Solid Oxide Fuel Cells (SOFCs) by Tape-Casting and Single-Step Co-Firing of Monolithic Laminates. Int. J. Hydrogen Energy 2014, 39, 2313–2319. [Google Scholar] [CrossRef]
- Kwon, Y.; Han, Y. Fabrication of Electrolyte-Supported Solid Oxide Fuel Cells Using a Tape Casting Process. J. Ceram. Soc. Jpn. 2020, 128, 310–316. [Google Scholar] [CrossRef]
- Pattterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
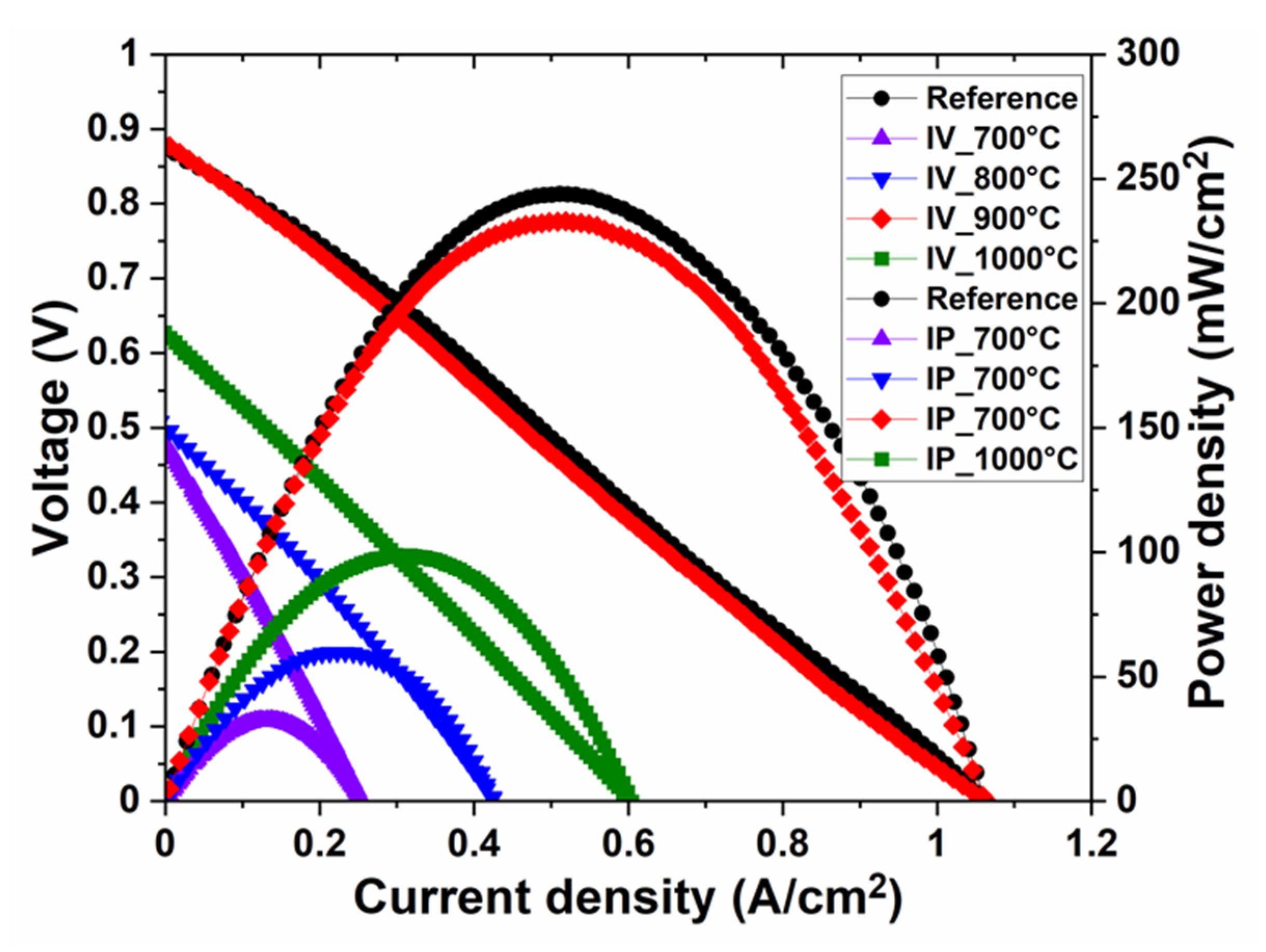
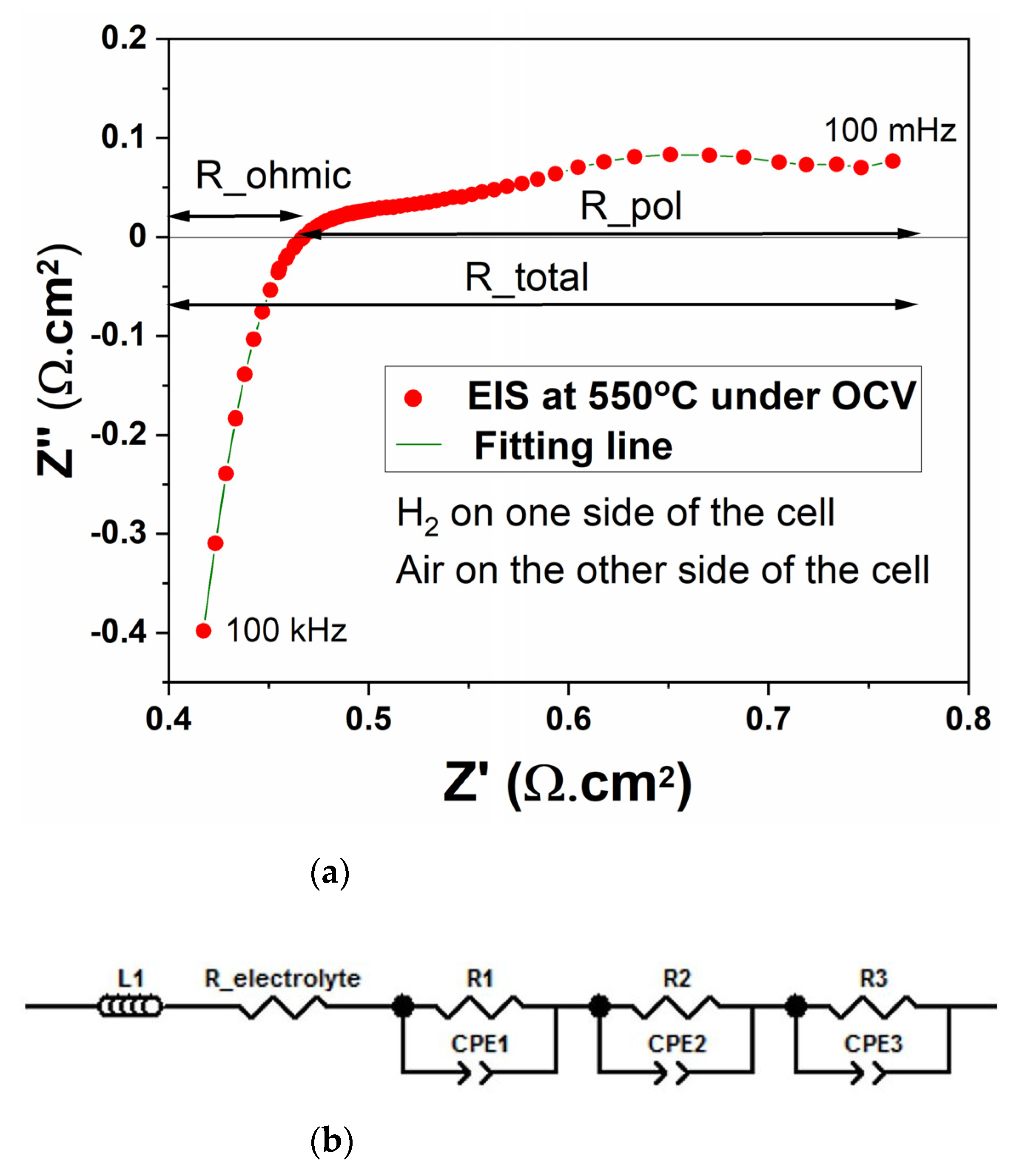
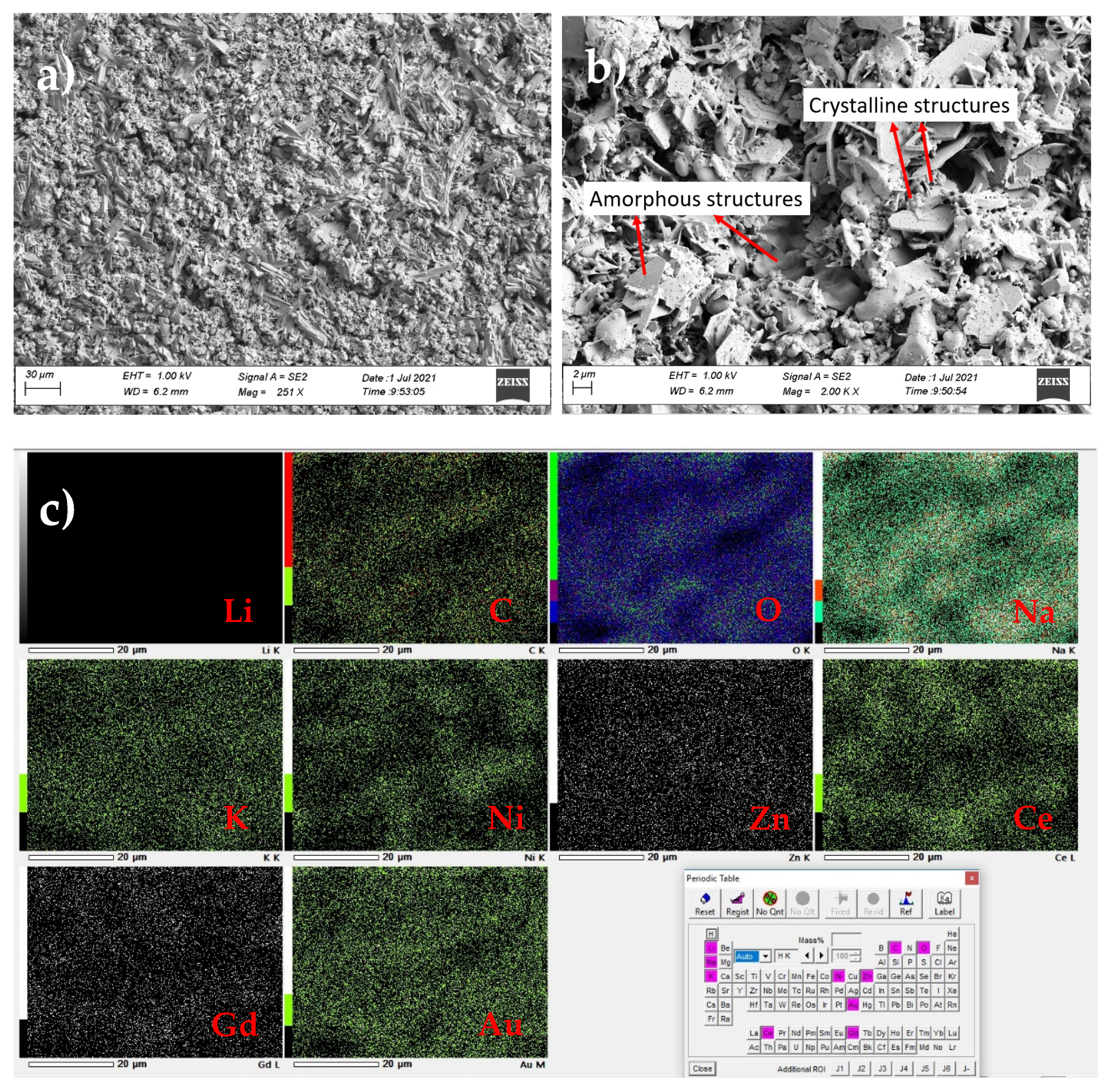

| Sintering Temperature (°C) | Max. Power Density (mW/cm2) | Total Cell Resistance (Ω·cm2) | BET Surface Area (m2/g) |
|---|---|---|---|
| 700 | 30 | 2.0 | 2.93 |
| 800 | 60 | 1.2 | 0.73 |
| 900 | 230 | 0.8 | 0.34 |
| 1000 | 150 | 0.7 | 0.18 |
| Reference | 240 | 0.8 | 0.39 |
| L1 (µH) | R_ohmic (Ω·cm2) | R1 (Ω·cm2) | R2 (Ω·cm2) | R3 (Ω·cm2) | R_pol (Ω·cm2) | R_total (Ω·cm2) |
|---|---|---|---|---|---|---|
| 0.59 ± 0.01 | 0.46 ± 0.03 | 0.07 ± 0.02 | 0.09 ± 0.01 | 0.21 ± 0.03 | 0.32 ± 0.04 | 0.78 ± 0.06 |
| Element | Li | C | O | Na | K | Ni | Zn | Ce | Gd | Au | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass (%) | ND | 1.58 | 18.07 | 2.76 | 1.63 | 18.09 | 6.82 | 34.57 | 8.84 | 7.65 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, M.I.; Mäkinen, P.; Virtanen, S.; Maitre, A.; Borghei, M.; Lund, P.D. Systematic Analysis on the Effect of Sintering Temperature for Optimized Performance of Li0.15Ni0.45Zn0.4O2-Gd0.2Ce0.8O2-Li2CO3-Na2CO3-K2CO3 Based 3D Printed Single-Layer Ceramic Fuel Cell. Nanomaterials 2021, 11, 2180. https://doi.org/10.3390/nano11092180
Asghar MI, Mäkinen P, Virtanen S, Maitre A, Borghei M, Lund PD. Systematic Analysis on the Effect of Sintering Temperature for Optimized Performance of Li0.15Ni0.45Zn0.4O2-Gd0.2Ce0.8O2-Li2CO3-Na2CO3-K2CO3 Based 3D Printed Single-Layer Ceramic Fuel Cell. Nanomaterials. 2021; 11(9):2180. https://doi.org/10.3390/nano11092180
Chicago/Turabian StyleAsghar, Muhammad Imran, Pyry Mäkinen, Sini Virtanen, Anna Maitre, Maryam Borghei, and Peter D. Lund. 2021. "Systematic Analysis on the Effect of Sintering Temperature for Optimized Performance of Li0.15Ni0.45Zn0.4O2-Gd0.2Ce0.8O2-Li2CO3-Na2CO3-K2CO3 Based 3D Printed Single-Layer Ceramic Fuel Cell" Nanomaterials 11, no. 9: 2180. https://doi.org/10.3390/nano11092180
APA StyleAsghar, M. I., Mäkinen, P., Virtanen, S., Maitre, A., Borghei, M., & Lund, P. D. (2021). Systematic Analysis on the Effect of Sintering Temperature for Optimized Performance of Li0.15Ni0.45Zn0.4O2-Gd0.2Ce0.8O2-Li2CO3-Na2CO3-K2CO3 Based 3D Printed Single-Layer Ceramic Fuel Cell. Nanomaterials, 11(9), 2180. https://doi.org/10.3390/nano11092180







