Effect of π–π Stacking Interfacial Interaction on the Properties of Graphene/Poly(styrene-b-isoprene-b-styrene) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphene/SIS Composites
2.3. Characterization
3. Results
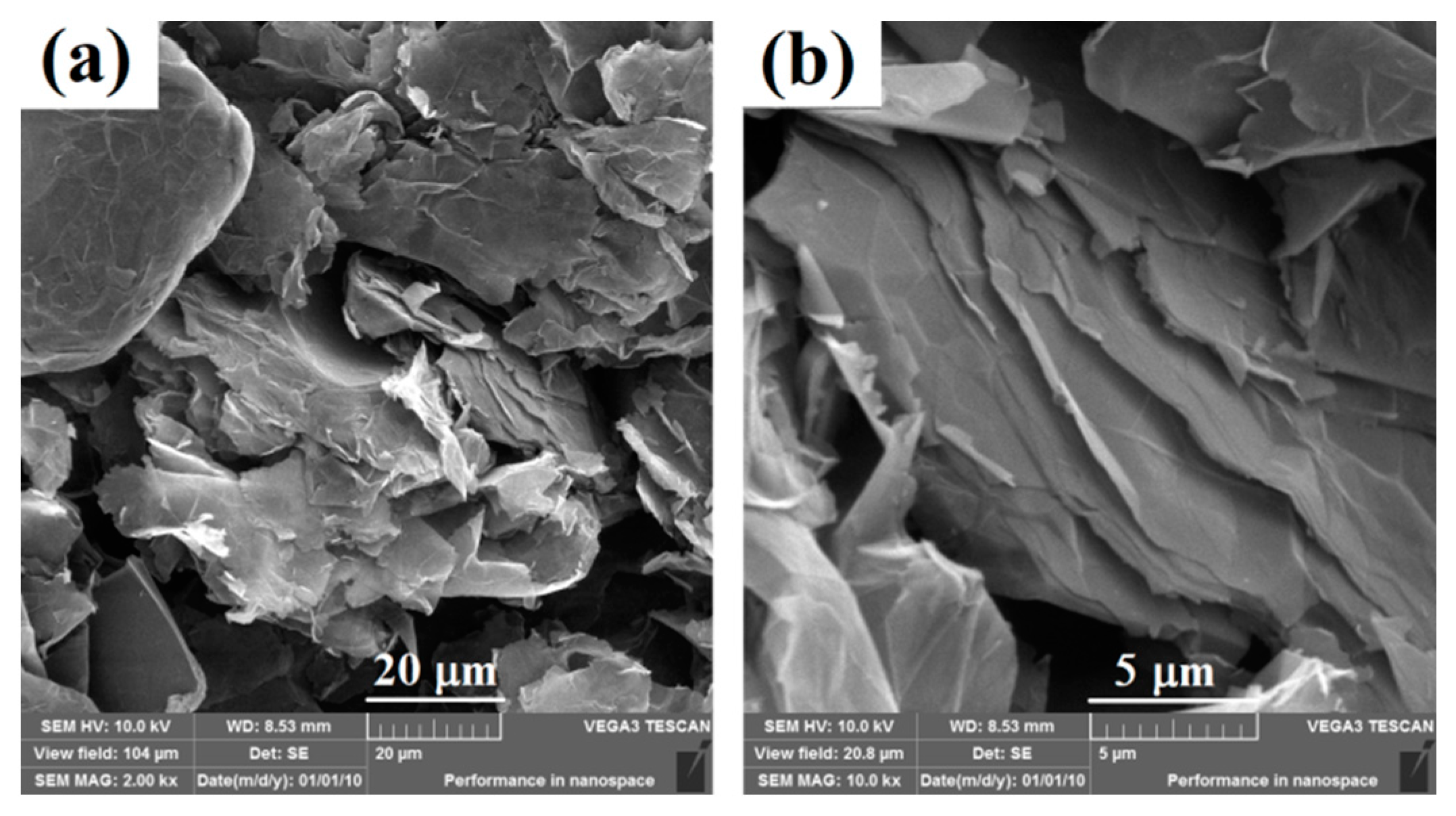
3.1. Morphology of Graphene
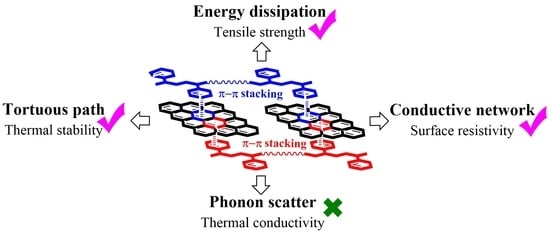
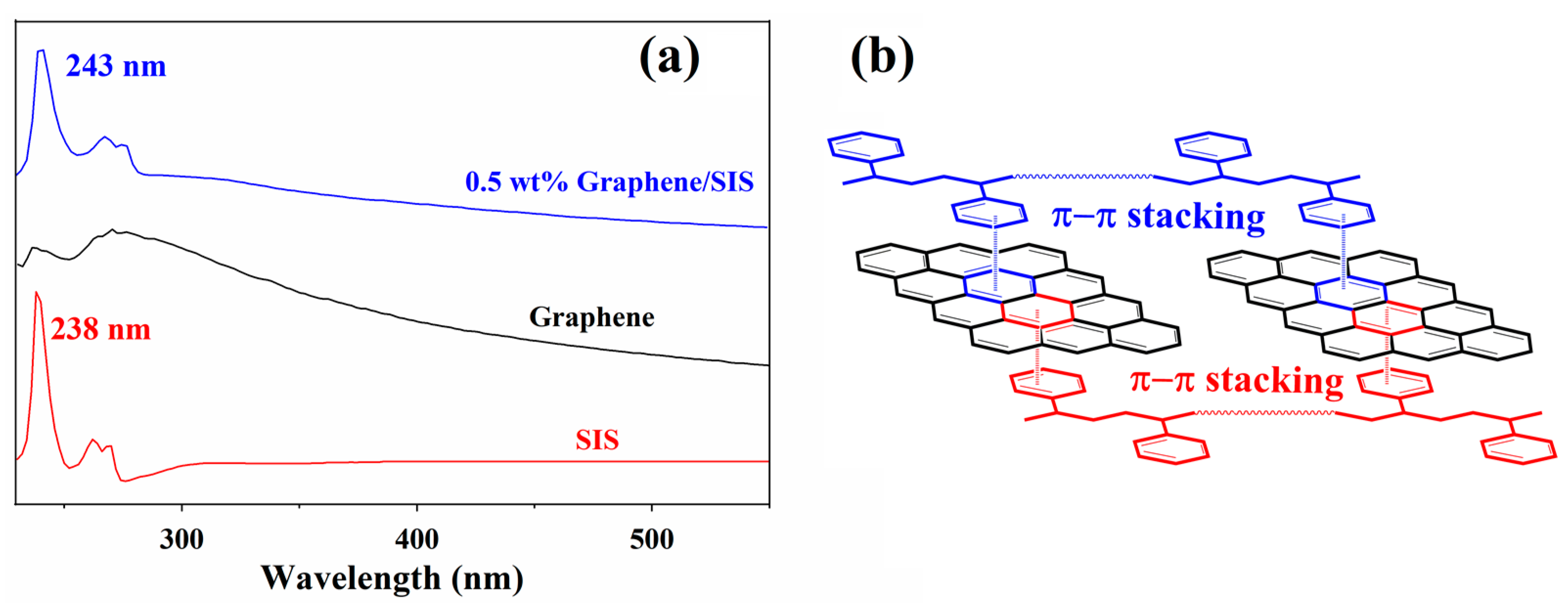
3.2. π–π Stacking Interaction between Graphene and SIS
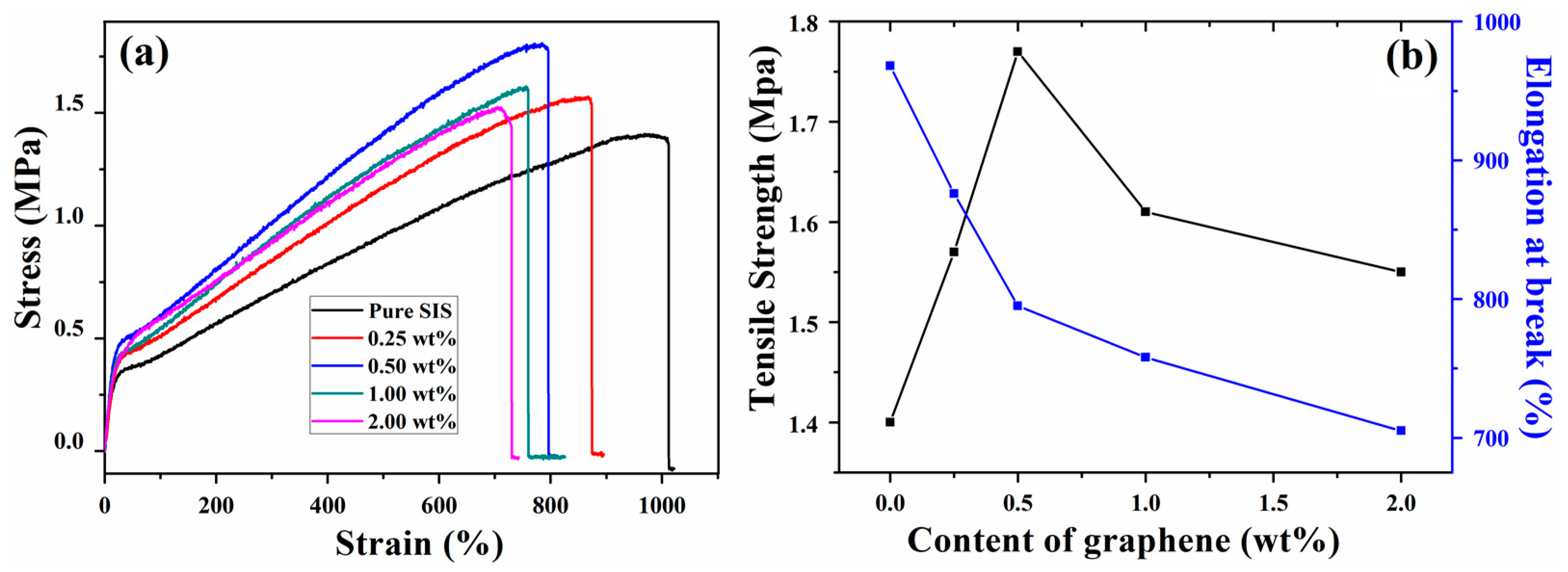
3.3. Mechanical Properties of Graphene/SIS Composites
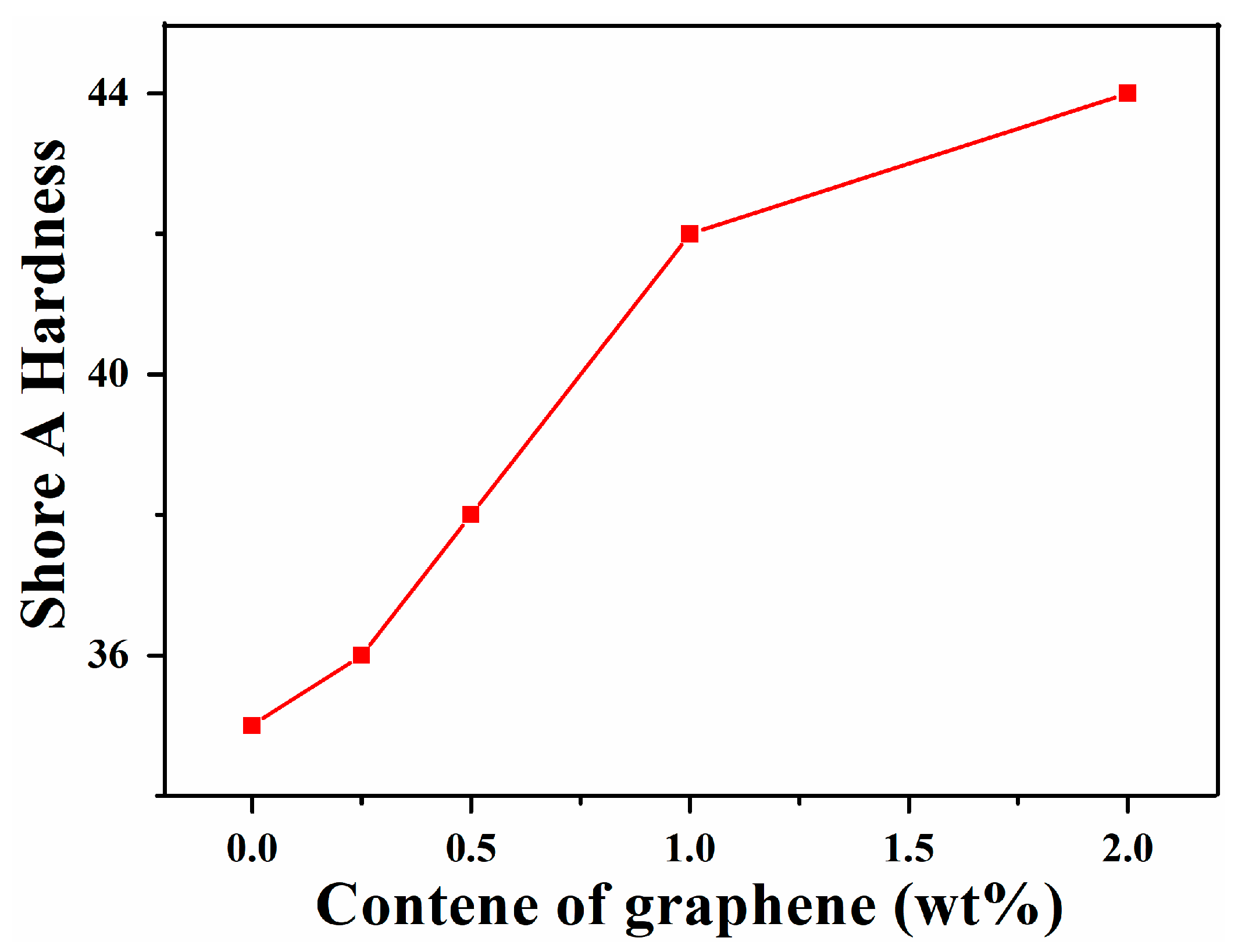
3.4. Shore A Hardness of Graphene/SIS Composites
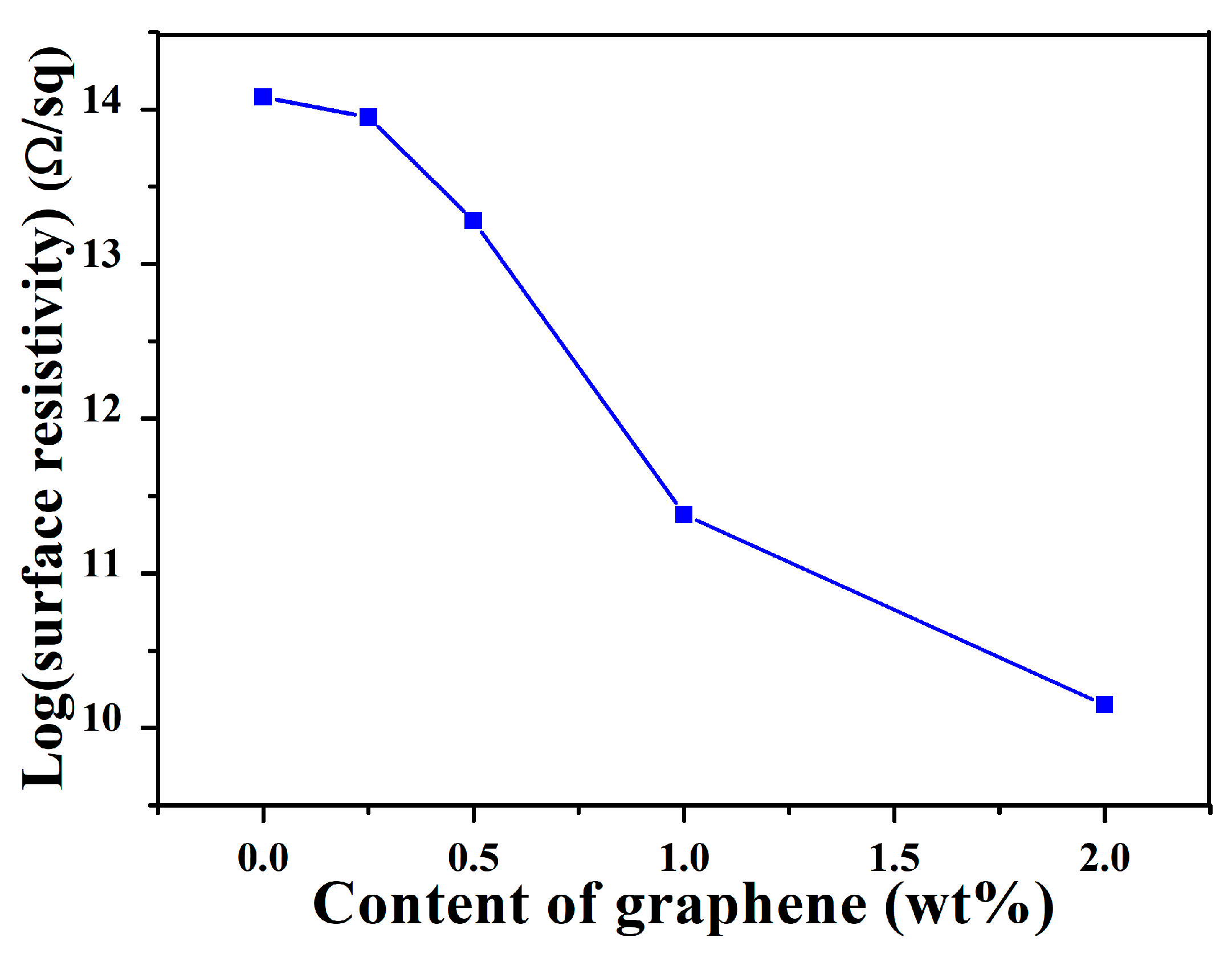
3.5. Surface Resistivity of Graphene/SIS Composites
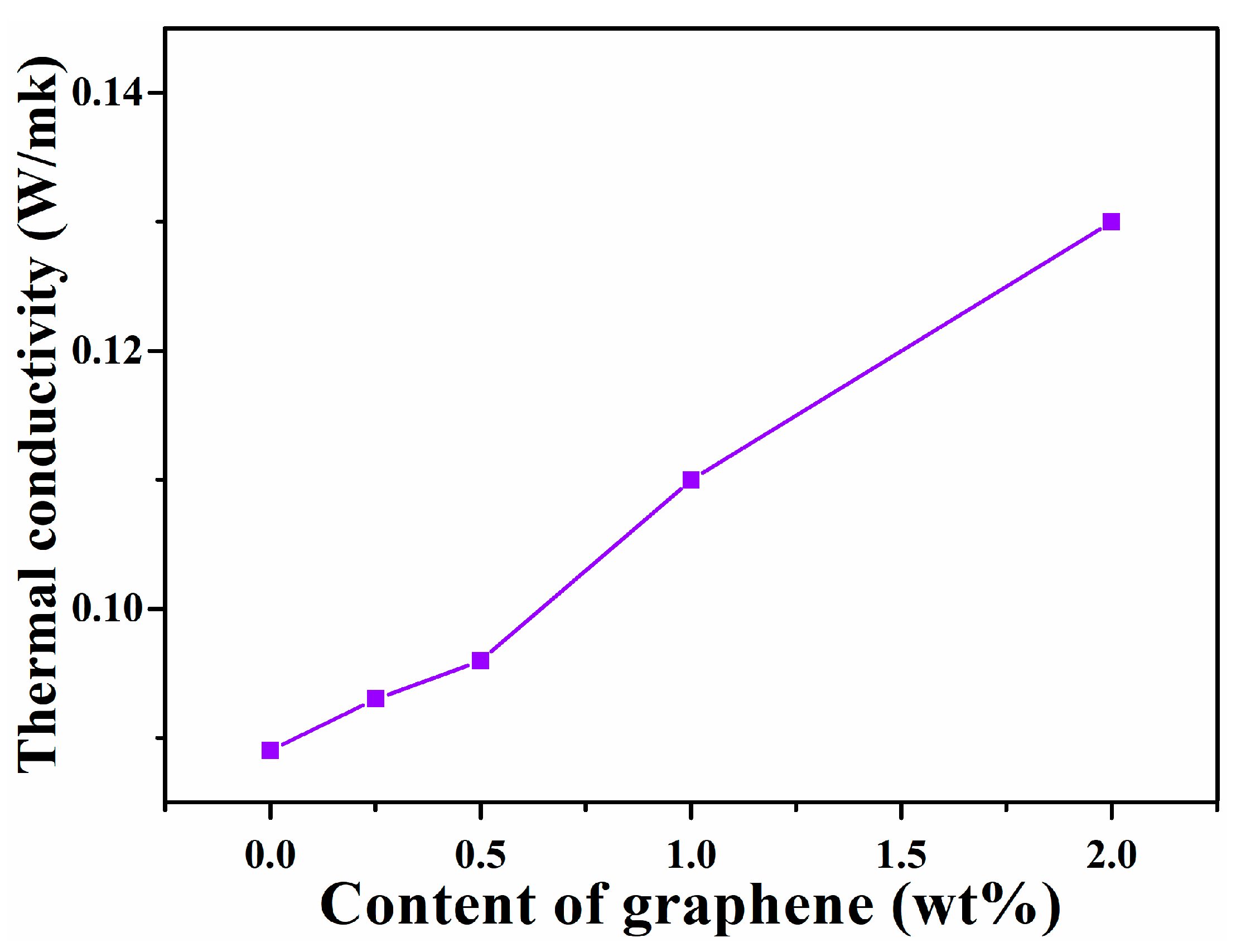
3.6. Thermal Conductivity of Graphene/SIS Composites
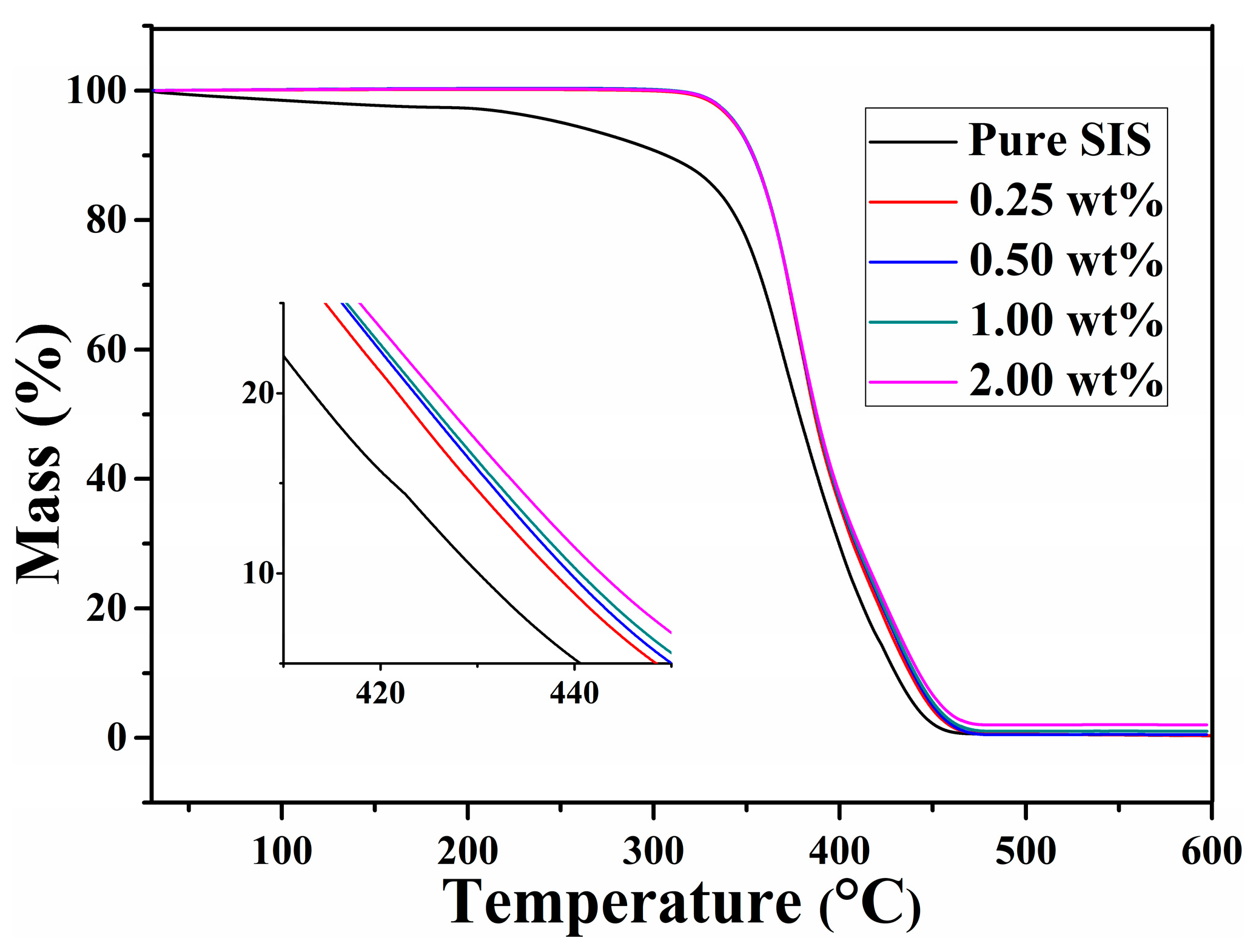
3.7. Thermal Stability of Graphene/SIS Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef]
- Christian, M.; Mazzaro, R.; Morandi, V. Bioinspired Design of Graphene-Based Materials. Adv. Funct. Mater. 2020, 30, 2007458. [Google Scholar] [CrossRef]
- Idumah, C.I.; Odera, S.R. Recent advancement in self-healing graphene polymer nanocomposites, shape memory, and coating materials. Polym. Technol. Mater. 2020, 59, 1167–1190. [Google Scholar] [CrossRef]
- Gao, J.; Bao, F.; Wu, Q.; Ma, R.; Han, X.; Jin, D.; Chen, K.; He, J.; Guo, Z.; Yan, C. Multifunctionalgraphenefilled silicone encapsulant for high-performance light-emitting diodes. Mater. Today Commun. 2016, 7, 149–154. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, J.; Bao, F.; Jiang, S.; Zhang, X.; Ma, R. Covalent functionalization of graphene with polythiophene through a Suzuki coupling reaction. RSC Adv. 2015, 5, 42754–42761. [Google Scholar] [CrossRef]
- Gao, J.; Bao, F.; Zhu, Q.; Tan, Z.; Chen, T.; Cai, H.; Zhao, C.; Cheng, Q.; Yang, Y.; Ma, R. Attaching hexylbenzene and poly(9,9-dihexylfluorene) to brominated graphene via Suzuki coupling reaction. Polym. Chem. 2012, 4, 1672–1679. [Google Scholar] [CrossRef]
- Han, X.; Cheng, Q.; Bao, F.; Gao, J.; Yang, Y.; Chen, T.; Yan, C.; Ma, R. Synthesis of Low-Density Heat-Resisting Polystyrene/Graphite Composite Microspheres Used as Water Carrying Fracturing Proppants. Polym. Plast. Technol. Eng. 2014, 53, 1647–1653. [Google Scholar] [CrossRef]
- Gao, J.; Shen, K.; Bao, F.; Yin, J.; Wang, D.; Ma, R.; Yan, C.; Chen, T.; Wang, G.; Liu, X.; et al. Preparation and Characterization of a Graphene Oxide Film Modified by the Covalent Attachment of Polysiloxane. Polym. Plast. Technol. Eng. 2013, 52, 553–557. [Google Scholar] [CrossRef]
- Gao, J.; Bao, F.; Feng, L.; Shen, K.; Zhu, Q.; Wang, D.; Chen, T.; Ma, R.; Yan, C. Functionalized graphene oxide modified polysebacic anhydride as drug carrier for levofloxacin controlled release. RSC Adv. 2011, 1, 1737–1744. [Google Scholar] [CrossRef]
- Han, X.; Gao, J.; Chen, Z.; Tang, X.; Zhao, Y.; Chen, T. Correlation between microstructure and properties of graphene oxide/waterborne polyurethane composites investigated by positron annihilation spectroscopy. RSC Adv. 2020, 10, 32436–32442. [Google Scholar] [CrossRef]
- Han, X.B.; Gao, J.; Hu, G.W.; Tang, X.Q.; Chen, T. Effect of Hydrocarbon Polymer, Feed Ratio and Interfacial Interaction on the Liquid Exfoliation of Graphite. J. Nanopart. Res. 2020, 22, 341. [Google Scholar] [CrossRef]
- Han, X.; Gao, J.; Chen, T.; Zhao, Y. Interfacial interaction and steric repulsion in polymer-assisted liquid exfoliation to produce high-quality graphene. Chem. Pap. 2020, 74, 757–765. [Google Scholar] [CrossRef]
- Guo, B.; Tang, Z.; Zhang, L. Transport performance in novel elastomer nanocomposites: Mechanism, design and control. Prog. Polym. Sci. 2016, 61, 29–66. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, X.; Guo, B.; Zhang, L.; Jia, D. Preparation of butadiene–styrene–vinyl pyridine rubber–graphene oxide hybrids through co-coagulation process and in situ interface tailoring. J. Mater. Chem. 2012, 22, 7492–7501. [Google Scholar] [CrossRef]
- Hong, Y.; Bao, S.; Xiang, X.; Wang, X. Concentration-Dominated Orientation of Phenyl Groups at the Polystyrene/Graphene Interface. ACS Macro Lett. 2020, 9, 889–894. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Liao, R.; Yang, G.; Wu, X.; Tang, Z.; Guo, B.; Zhang, L.; Ma, Y.; Nie, Q.; et al. Rational design of covalent interfaces for graphene/elastomer nanocomposites. Compos. Sci. Technol. 2016, 132, 68–75. [Google Scholar] [CrossRef]
- Zhong, J.; Ding, Y.; Gao, F.; Wen, J.; Zhou, J.; Zheng, W.; Shen, L.; Fu, C.; Wang, B. Free volume correlation with electrical conductivity of polycarbonate/reduced graphene oxide nanocomposites studied by positron annihilation lifetime spectroscopy. J. Appl. Polym. Sci. 2019, 136, 48207. [Google Scholar] [CrossRef]
- Xue, G.; Zhong, J.; Gao, S.; Wang, B. Correlation between the free volume and thermal conductivity of porous poly(vinyl alcohol)/reduced graphene oxide composites studied by positron spectroscopy. Carbon 2016, 96, 871–878. [Google Scholar] [CrossRef]
- Hofmann, D.; Thomann, R.; Mülhaupt, R. Thermoplastic SEBS Elastomer Nanocomposites Reinforced with Functionalized Graphene Dispersions. Macromol. Mater. Eng. 2017, 303, 1700324. [Google Scholar] [CrossRef]
- Kashfipour, M.A.; Mehra, N.; Zhu, J. A review on the role of interface in mechanical, thermal, and electrical properties of polymer composites. Adv. Compos. Hybrid Mater. 2018, 1, 415–439. [Google Scholar] [CrossRef]
- Afzal, A.; Kausar, A.; Siddiq, M. Review Highlighting Physical Prospects of Styrenic Polymer and Styrenic Block Copolymer Reinforced with Carbon Nanotube. Polym. Plast. Technol. Eng. 2016, 56, 573–593. [Google Scholar] [CrossRef]
- Ahir, S.V.; Squires, A.M.; Tajbakhsh, A.R.; Terentjev, E. Infrared actuation in aligned polymer-nanotube composites. Phys. Rev. B 2006, 73, 085420. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.; Neelanchery, M.M.; Ushus, D. Graphene/poly(styrene-b-isoprene-b-styrene) nanocomposite optical actuators. J. Appl. Polym. Sci. 2013, 130, 3902–3908. [Google Scholar] [CrossRef]
- Song, P.; Xu, Z.; Wu, Y.; Cheng, Q.; Guo, Q.; Wang, H. Super-tough artificial nacre based on graphene oxide via synergistic interface interactions of π-π stacking and hydrogen bonding. Carbon 2017, 111, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Zhai, W.; Chen, C.; Lu, D.; Wang, J.; Zheng, W. Melt Blending In situ Enhances the Interaction between Polystyrene and Graphene through π–π Stacking. ACS Appl. Mater. Interfaces 2011, 3, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, S.; Lawson, B.P.; Stein, B.D.; Pink, M.; Carini, J.; Polezhaev, A.; Vlasov, E.; Zulfiqar, S.; Sarwar, M.I.; Bronstein, L.M. Elastomer based nanocomposites with reduced graphene oxide nanofillers allow for enhanced tensile and electrical properties. J. Polym. Res. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Palmeri, M.J.; Putz, K.W.; Brinson, L.C. Sacrificial Bonds in Stacked-Cup Carbon Nanofibers: Biomimetic Toughening Mechanisms for Composite Systems. ACS Nano 2010, 4, 4256–4264. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lei, Y.; Guo, B.; Zhang, L.; Jia, D. The use of rhodamine B-decorated graphene as a reinforcement in polyvinyl alcohol composites. Polymer 2012, 53, 673–680. [Google Scholar] [CrossRef]
- Liu, M.; Papageorgiou, D.; Li, S.; Lin, K.; Kinloch, I.A.; Young, R.J. Micromechanics of reinforcement of a graphene-based thermoplastic elastomer nanocomposite. Compos. Part A Appl. Sci. Manuf. 2018, 110, 84–92. [Google Scholar] [CrossRef]
- Mensah, B.; Kumar, D.; Lim, D.-K.; Kim, S.G.; Jeong, B.-H.; Nah, C. Preparation and properties of acrylonitrile-butadiene rubber-graphene nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42457. [Google Scholar] [CrossRef]
- Li, J.; Gunister, E.; Barsoum, I. Effect of graphene oxide as a filler material on the mechanical properties of LLDPE nanocomposites. J. Compos. Mater. 2019, 53, 2761–2773. [Google Scholar] [CrossRef]
- Yue, J.; Pan, J.; Deng, Y.; Li, J.; Bao, J. Enhanced properties of poly(styrene–b–ethylene–co–butylene–b–styrene) nanocomposites with in situ construction of interconnected graphene network. J. Appl. Polym. Sci. 2019, 136, 47118. [Google Scholar] [CrossRef]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. Effect of Graphene Oxide on the Properties of Its Composite with Polyaniline. ACS Appl. Mater. Interfaces 2010, 2, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shen, F.; Zhang, Y.; Huang, Z.; Muhammad, Y.; Hu, H.; Zhu, Y.; Yu, C.; Qin, Y. Graphene incorporated poly(vinyl chloride) composites prepared by mechanical activation with enhanced electrical and thermo–mechanical properties. J. Appl. Polym. Sci. 2019, 136, 48375. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Lin, Y.; Bao, H.; Wu, G.; Jiang, P.; Mai, Y.-W. Thermal conductivity of graphene-based polymer nanocomposites. Mater. Sci. Eng. R Rep. 2020, 142, 100577. [Google Scholar] [CrossRef]
- Park, J.; Sharma, J.; Monaghan, K.W.; Meyer, H.M.; Cullen, D.A.; Rossy, A.M.; Keum, J.K.; Wood, D.L.; Polizos, G. Styrene-Based Elastomer Composites with Functionalized Graphene Oxide and Silica Nanofiber Fillers: Mechanical and Thermal Conductivity Properties. Nanomaterials 2020, 10, 1682. [Google Scholar] [CrossRef]
- Jiang, F.; Cui, X.; Song, N.; Shi, L.; Ding, P. Synergistic effect of functionalized graphene/boron nitride on the thermal conductivity of polystyrene composites. Compos. Commun. 2020, 20, 100350. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–3045. [Google Scholar] [CrossRef]
- Gulotty, R.; Castellino, M.; Jagdale, P.; Tagliaferro, A.; Balandin, A.A. Effects of Functionalization on Thermal Properties of Single-Wall and Multi-Wall Carbon Nanotube-Polymer Nanocomposites. ACS Nano 2013, 7, 5114–5121. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Zhou, W.; Wang, Q.; Chu, Z.; Yang, L.; Yang, L.; Sun, J.; Zhao, L.; Xu, J.; Liang, Y.; et al. Structure dependence of water vapor permeation in polymer nanocomposite membranes investigated by positron annihilation lifetime spectroscopy. J. Membr. Sci. 2019, 549, 581–587. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Kong, H.; Chen, T.; Gao, J.; Zhao, Y.; Sang, Y.; Hu, G. Effect of π–π Stacking Interfacial Interaction on the Properties of Graphene/Poly(styrene-b-isoprene-b-styrene) Composites. Nanomaterials 2021, 11, 2158. https://doi.org/10.3390/nano11092158
Han X, Kong H, Chen T, Gao J, Zhao Y, Sang Y, Hu G. Effect of π–π Stacking Interfacial Interaction on the Properties of Graphene/Poly(styrene-b-isoprene-b-styrene) Composites. Nanomaterials. 2021; 11(9):2158. https://doi.org/10.3390/nano11092158
Chicago/Turabian StyleHan, Xiaobing, Hao Kong, Tao Chen, Jie Gao, Yuan Zhao, Yanan Sang, and Guowen Hu. 2021. "Effect of π–π Stacking Interfacial Interaction on the Properties of Graphene/Poly(styrene-b-isoprene-b-styrene) Composites" Nanomaterials 11, no. 9: 2158. https://doi.org/10.3390/nano11092158
APA StyleHan, X., Kong, H., Chen, T., Gao, J., Zhao, Y., Sang, Y., & Hu, G. (2021). Effect of π–π Stacking Interfacial Interaction on the Properties of Graphene/Poly(styrene-b-isoprene-b-styrene) Composites. Nanomaterials, 11(9), 2158. https://doi.org/10.3390/nano11092158