Nanoarchitectonics for Hierarchical Fullerene Nanomaterials
Abstract
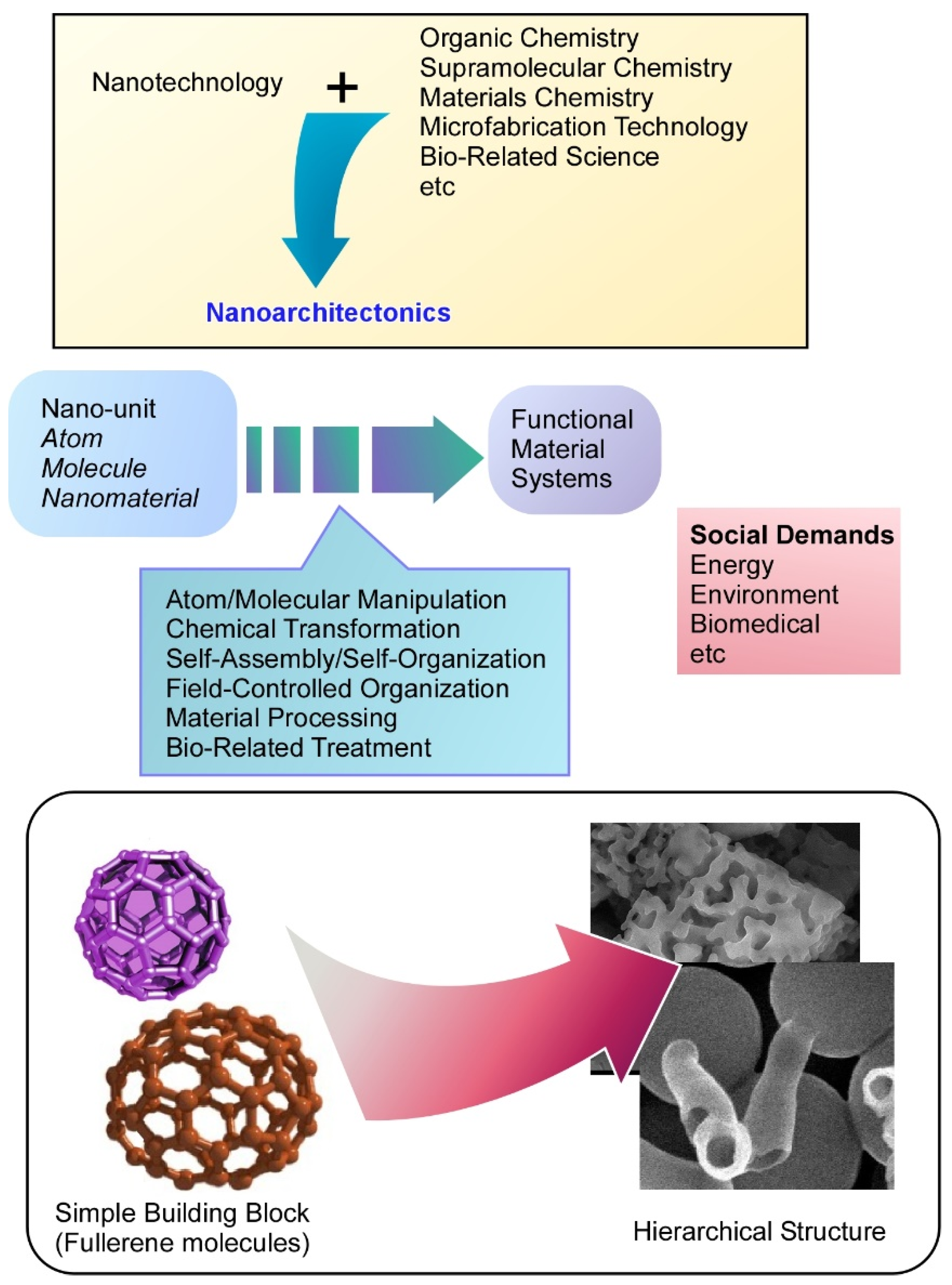
:1. Introduction
2. Hierarchically Structured Fullerene Assembly for Vapor Sensor Usage
2.1. Fullerene C70 Cube for Sensing Platform for Volatile Aromatic Solvent Vapor
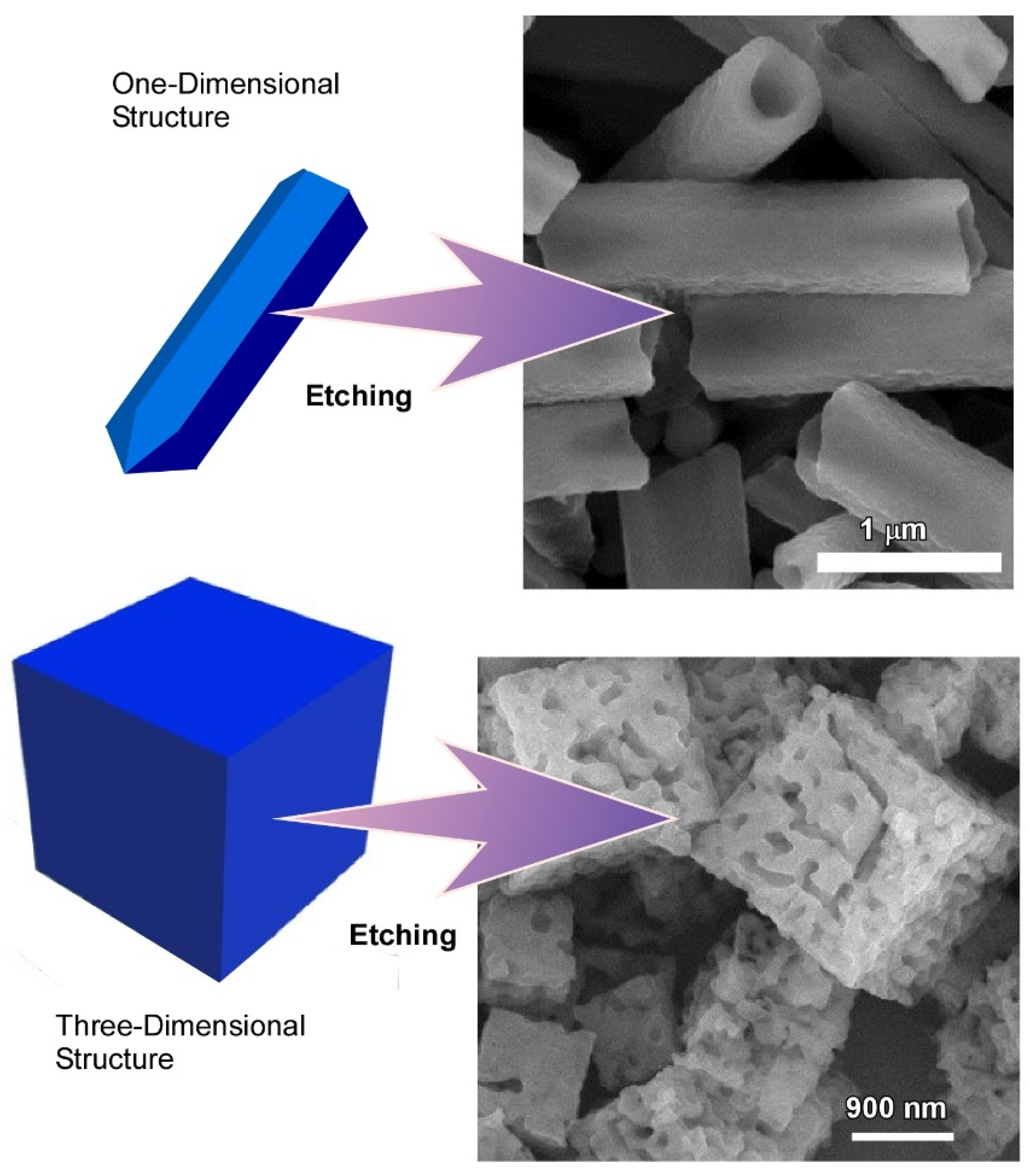
2.2. Dimension-Dependent Face-Selective Etching of Fullerene Assembly
2.3. Bitter Melon Shaped Nanoporous Fullerene C60 Assembly
3. Fullerene Assembly with Microscopic Recognition Capability
3.1. Hole-in-Cube Fullerene Assembly with Microscopic Recognition Capability
3.2. Fullerene Microhorns with Microscopic Recognition Properties
4. Fullerene Microstructure with Mesoporous Framework for Advanced Function
4.1. Mesoporous Fullerene C70 Cube with Enhanced Photoluminescence Property
4.2. Mesoporous Carbon Cubes for Supercapacitors
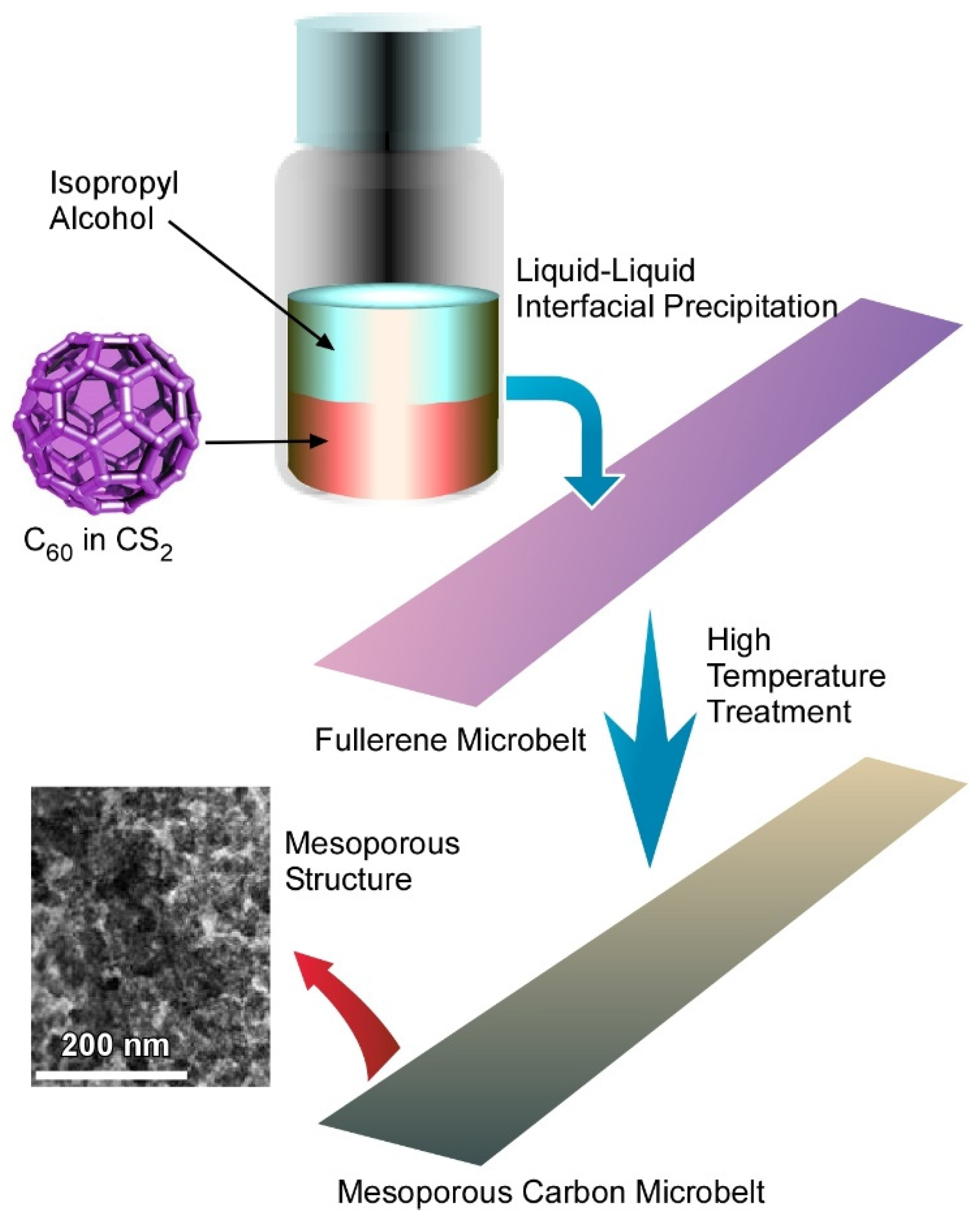
4.3. Quasi Two-Dimensional Mesoporous Carbon Microbelts for Supercapacitors
5. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Povie, G.; Segawa, Y.; Nishihara, T.; Miyauchi, Y.; Itami, K. Synthesis of a carbon nanobelt. Science 2017, 356, 172–175. [Google Scholar] [CrossRef]
- Muramatsu, W.; Hattori, T.; Yamamoto, H. Game Change from Reagent- to Substrate-Controlled Peptide Synthesis. Bull. Chem. Soc. Jpn. 2020, 93, 759–767. [Google Scholar] [CrossRef]
- Anderson, H.L.; Patrick, C.W.; Scriven, L.M.; Woltering, S.L. A Short History of Cyclocarbons. Bull. Chem. Soc. Jpn. 2021, 94, 798–811. [Google Scholar] [CrossRef]
- Nishihara, H.; Kyotani, T. Zeolite-templated carbons—Three-dimensional microporous graphene frameworks. Chem. Commun. 2018, 54, 5648–5673. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-B.; Jiang, X.-F.; Bando, Y. Blowing Route towards Advanced Inorganic Foams. Bull. Chem. Soc. Jpn. 2019, 92, 245–263. [Google Scholar] [CrossRef]
- Hosono, N.; Kitagawa, S. Modular Design of Porous Soft Materials via Self-Organization of Metal–Organic Cages. Acc. Chem. Res. 2018, 51, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.M.; Otake, K.-I.; Kitagawa, S. Transport properties in porous coordination polymers. Coord. Chem. Rev. 2020, 421, 213447. [Google Scholar] [CrossRef]
- Ariga, K.; Shionoya, M. Nanoarchitectonics for Coordination Asymmetry and Related Chemistry. Bull. Chem. Soc. Jpn. 2021, 94, 839–859. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-Catalyzed Living Radical Polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Luckham, S.I.J.; Nozaki, K. Toward the copolymerization of propylene with polar comonomers. Acc. Chem. Res. 2021, 54, 344–355. [Google Scholar] [CrossRef]
- Yamago, S. Photoactivation of Organotellurium Compounds in Precision Polymer Synthesis: Controlled Radical Polymerization and Radical Coupling Reactions. Bull. Chem. Soc. Jpn. 2020, 93, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, K.; Mori, T.; Ariga, K. Molecular imprinting: Materials nanoarchitectonics with molecular information. Bull. Chem. Soc. Jpn. 2018, 91, 1075–1111. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.; Zhou, J.; Jiao, T.; Feng, Y.; Zhang, L.; Chen, Y.; Bai, Z.; Peng, Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-Y.; Wang, C.-M.; Liao, W.-S. A Special Connection between Nanofabrication and Analytical Devices: Chemical Lift-Off Lithography. Bull. Chem. Soc. Jpn. 2019, 92, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Roy, N.; Suzuki, N.; Terashima, C.; Fujishima, A. Recent Improvements in the Production of Solar Fuels: From CO2 Reduction to Water Splitting and Artificial Photosynthesis. Bull. Chem. Soc. Jpn. 2019, 92, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y. Concentrated Battery Electrolytes: Developing New Functions by Manipulating the Coordination States. Bull. Chem. Soc. Jpn. 2020, 93, 109–118. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, H.; Hill, J.; Tsukube, H. Molecular recognition: From solution science to nano/materials technology. Chem. Soc. Rev. 2012, 41, 5800–5835. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Pang, P.; Lai, Y.; Zhang, Y.; Wang, H.; Conlan, X.A.; Barrow, C.J.; Yang, W. Recent Advancement of Biosensor Technology for the Detection of Microcystin-LR. Bull. Chem. Soc. Jpn. 2020, 93, 637–646. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Okano, T. Design of Temperature-Responsive Polymer-Grafted Surfaces for Cell Sheet Preparation and Manipulation. Bull. Chem. Soc. Jpn. 2019, 92, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Pou, P.; Abe, M.; Jelínek, P.; Perez, R.; Morita, S.; Custance, O. Chemical identification of individual surface atoms by atomic force microscopy. Nature 2007, 446, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, K.; Miwa, K.; Imada, H.; Imai-Imada, M.; Kawahara, S.; Takeya, J.; Kawai, M.; Galperin, M.; Kim, Y. Selective triplet exciton formation in a single molecule. Nature 2019, 570, 210–213. [Google Scholar] [CrossRef]
- Shimizu, T.; Lungerich, D.; Stuckner, J.; Murayama, M.; Harano, K.; Nakamura, E. Real-Time Video Imaging of Mechanical Motions of a Single Molecular Shuttle with Sub-Millisecond Sub-Angstrom Precision. Bull. Chem. Soc. Jpn. 2020, 93, 1079–1085. [Google Scholar] [CrossRef]
- Onda, M.; Lvov, Y.; Ariga, K.; Kunitake, T. Sequential reaction and product separation on molecular films of glucoamylase and glucose oxidase assembled on an ultrafilter. J. Ferment. Bioeng. 1996, 82, 502–506. [Google Scholar] [CrossRef]
- Auxilia, F.M.; Ishihara, S.; Mandal, S.; Tanabe, T.; Saravanan, G.; Ramesh, G.V.; Umezawa, N.; Hara, T.; Xu, Y.; Hishita, S.; et al. Low-Temperature Remediation of NO Catalyzed by Interleaved CuO Nanoplates. Adv. Mater. 2014, 26, 4481–4485. [Google Scholar] [CrossRef] [Green Version]
- Harano, K. Self-Assembly Mechanism in Nucleation Processes of Molecular Crystalline Materials. Bull. Chem. Soc. Jpn. 2021, 94, 463–472. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for invitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef] [Green Version]
- Iida, K.; Uehigashi, Y.; Ichida, H.; Bu, H.-B.; Kim, D. Synthesis of Water-Soluble CuInS2 Quantum Dots by a Hydrothermal Method and Their Optical Properties. Bull. Chem. Soc. Jpn. 2019, 92, 930–936. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, Y.; Holmes, M.J. Progress in quantum-dot single photon sources for quantum information technologies: A broad spectrum overview. Appl. Phys. Rev. 2020, 7, 021309. [Google Scholar] [CrossRef]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2019, 120, 464–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitomo, H.; Ijiro, K. Controlled Nanostructures Fabricated by the Self-Assembly of Gold Nanoparticles via Simple Surface Modifications. Bull. Chem. Soc. Jpn. 2021, 94, 1300–1310. [Google Scholar] [CrossRef]
- Nakamura, S.; Mitomo, H.; Ijiro, K. Assembly and Active Control of Nanoparticles using Polymer Brushes as a Scaffold. Chem. Lett. 2021, 50, 361–370. [Google Scholar] [CrossRef]
- Kanemitsu, Y. Multiple Exciton Generation and Recombination in Carbon Nanotubes and Nanocrystals. Acc. Chem. Res. 2013, 46, 1358–1366. [Google Scholar] [CrossRef]
- Pileni, M.P. Au Supracrystal Growth Processes: Unexpected Morphologies. Bull. Chem. Soc. Jpn. 2019, 92, 312–329. [Google Scholar] [CrossRef] [Green Version]
- Saruyama, M.; Sato, R.; Teranishi, T. Transformations of Ionic Nanocrystals via Full and Partial Ion Exchange Reactions. Acc. Chem. Res. 2021, 54, 765–775. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Glotov, A.; Stavitskaya, A.; Chudakov, Y.; Ivanov, E.; Huang, W.; Vinokurov, V.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Mesoporous Metal Catalysts Templated on Clay Nanotubes. Bull. Chem. Soc. Jpn. 2019, 92, 61–69. [Google Scholar] [CrossRef]
- Shimizu, T.; Ding, W.; Kameta, N. Soft-Matter Nanotubes: A Platform for Diverse Functions and Applications. Chem. Rev. 2020, 120, 2347–2407. [Google Scholar] [CrossRef] [PubMed]
- Takahata, R.; Tsukuda, T. Ultrathin Gold Nanowires and Nanorods. Chem. Lett. 2019, 48, 906–915. [Google Scholar] [CrossRef]
- Miyajima, N.; Wang, Y.-C.; Nakagawa, M.; Kurata, H.; Imura, Y.; Wang, K.-H.; Kawai, T. Water-Phase Synthesis of Ultrathin Au Nanowires with a Two-Dimensional Parallel Array Structure. Bull. Chem. Soc. Jpn. 2020, 93, 1372–1377. [Google Scholar] [CrossRef]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The Use of Electrospun Organic and Carbon Nanofibers in Bone Regeneration. Nanomaterials 2020, 10, 562. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Imai, H.; Oaki, Y. Redox-Mediated High-Yield Exfoliation of Layered Composites into Nanosheets. Bull. Chem. Soc. Jpn. 2019, 92, 779–784. [Google Scholar] [CrossRef]
- Lee, C.M.; Jin, C.H.; Ahn, C.H.; Cho, H.K.; Lim, J.H.; Hwang, S.M.; Joo, J. Enhanced Gas Sensing Performance of Hydrothermal MoS2 Nanosheets by Post-Annealing in Hydrogen Ambient. Bull. Chem. Soc. Jpn. 2019, 92, 1094–1099. [Google Scholar] [CrossRef]
- Eguchi, M.; Nugraha, A.S.; Rowan, A.E.; Shapter, J.; Yamauchi, Y. Adsorchromism: Molecular Nanoarchitectonics at 2D Nanosheets—Old Chemistry for Advanced Chromism. Adv. Sci. 2021, 8, 2100539. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-T.; Liu, M.; Yu, Y.; Li, A.-W.; Sun, H.-B. Laser-Structured Graphene/Reduced Graphene Oxide Films towards Bio-Inspired Superhydrophobic Surfaces. Bull. Chem. Soc. Jpn. 2019, 92, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Müllen, K.; Narita, A. Syntheses and Characterizations of Functional Polycyclic Aromatic Hydrocarbons and Graphene Nanoribbons. Bull. Chem. Soc. Jpn. 2020, 93, 490–506. [Google Scholar] [CrossRef]
- Ariga, K.; Watanabe, S.; Mori, T.; Takeya, J. Soft 2D nanoarchitectonics. NPG Asia Mater. 2018, 10, 90–106. [Google Scholar] [CrossRef]
- Ito, M.; Yamashita, Y.; Tsuneda, Y.; Mori, T.; Takeya, J.; Watanabe, S.; Ariga, K. 100 °C-Langmuir–Blodgett method for fabricating highly oriented, ultrathin films of polymeric semiconductors. ACS Appl. Mater. Interfaces 2020, 12, 56522–56529. [Google Scholar] [CrossRef]
- Kim, W.; Hwang, W.; Kim, N.H.; Kim, J.; Baek, K.; Kim, K. Permselective Two-Dimensional Polymer Film-Based Chemical Sensors. Bull. Chem. Soc. Jpn. 2021, 94, 869–871. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Tsujimura, S.; Morishita, T. Templated mesoporous carbons: Synthesis and applications. Carbon 2016, 107, 448–473. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Functionalized mesoporous SBA-15 silica: Recent trends and catalytic applications. Nanoscale 2020, 12, 11333–11363. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional Mesoporous Silica Nanomaterials for Catalysis and Environmental Applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified zeolites: Preparation, characterization, and applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef]
- Onishi, M.; Tsunoji, N.; Sadakane, M.; Sano, T. Synthesis of Phosphorus-Modified AFX Zeolite by the Hydrothermal Conversion of Tetraalkylphosphonium Hydroxide-Impregnated FAU Zeolite. Bull. Chem. Soc. Jpn. 2021, 94, 1–7. [Google Scholar] [CrossRef]
- Simancas, R.; Chokkalingam, A.; Elangovan, S.P.; Liu, Z.; Sano, T.; Iyoki, K.; Wakihara, T.; Okubo, T. Recent progress in the improvement of hydrothermal stability of zeolites. Chem. Sci. 2021, 12, 7677–7695. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2017, 30, e1703663. [Google Scholar] [CrossRef] [PubMed]
- Hosono, N. Design of Porous Coordination Materials with Dynamic Properties. Bull. Chem. Soc. Jpn. 2021, 94, 60–69. [Google Scholar] [CrossRef]
- Otake, K.; Kitagawa, H. Control of Proton-Conductive Behavior with Nanoenvironment within Metal–Organic Materials. Small 2021, 17, 2006189. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Azhar, A.; Li, Y.; Cai, Z.; Zakaria, M.B.; Masud, M.K.; Hossain, S.; Kim, J.; Zhang, W.; Na, J.; Yamauchi, Y.; et al. Nanoarchitectonics: A New Materials Horizon for Prussian Blue and Its Analogues. Bull. Chem. Soc. Jpn. 2019, 92, 875–904. [Google Scholar] [CrossRef]
- Horike, S.; Nagarkar, S.S.; Ogawa, T.; Kitagawa, S. A New Dimension for Coordination Polymers and Metal–Organic Frameworks: Towards Functional Glasses and Liquids. Angew. Chem. Int. Ed. 2019, 59, 6652–6664. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, K. Selective single-atom electrocatalysts: A review with a focus on metal-doped covalent triazine frameworks. Chem. Sci. 2020, 11, 8339–8349. [Google Scholar] [CrossRef]
- Jiang, D. Covalent Organic Frameworks: A Molecular Platform for Designer Polymeric Architectures and Functional Materials. Bull. Chem. Soc. Jpn. 2021, 94, 1215–1231. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minakawa, M.; Nakagawa, M.; Wang, K.-H.; Imura, Y.; Kawai, T. Controlling Helical Pitch of Chiral Supramolecular Nanofibers Composed of Two Amphiphiles. Bull. Chem. Soc. Jpn. 2020, 93, 1150–1154. [Google Scholar] [CrossRef]
- Datta, S.; Kato, Y.; Higashiharaguchi, S.; Aratsu, K.; Isobe, A.; Saito, T.; Prabhu, D.D.; Kitamoto, Y.; Hollamby, M.J.; Smith, A.J.; et al. Self-assembled poly-catenanes from supramolecular toroidal building blocks. Nature 2020, 583, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Kubota, R.; Minami, T. Molecular self-assembled chemosensors and their arrays. Coord. Chem. Rev. 2020, 429, 213607. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Kise, R.; Fukumi, A.; Shioya, N.; Shimoaka, T.; Sonoyama, M.; Amii, H.; Takagi, T.; Kanamori, T.; Eda, K.; Hasegawa, T. Fluorous Property of a Short Perfluoroalkyl-Containing Compound Realized by Self-Assembled Monolayer Technique on a Silicon Substrate. Bull. Chem. Soc. Jpn. 2019, 92, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Hasobe, T. Organic-Inorganic Hybrid Molecular Architectures Utilizing Self-assembled Monolayers for Singlet Fission and Light Energy Conversion. Chem. Lett. 2021, 50, 615–622. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Hill, J. Mechanical Control of Nanomaterials and Nanosystems. Adv. Mater. 2011, 24, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Yamauchi, Y.; Mori, T.; Hill, J. 25th Anniversary Article: What Can Be Done with the Langmuir-Blodgett Method? Recent Developments and its Critical Role in Materials Science. Adv. Mater. 2013, 25, 6477–6512. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Li, J. Langmuir Nanoarchitectonics from Basic to Frontier. Langmuir 2018, 35, 3585–3599. [Google Scholar] [CrossRef]
- Ariga, K. Don’t forget Langmuir-Blodgett films 2020: Interfacial nanoarchitectonics with molecules, materials, and living objects. Langmuir 2020, 36, 7158–7180. [Google Scholar] [CrossRef]
- Rydzek, G.; Ji, Q.; Li, M.; Schaaf, P.; Hill, J.; Boulmedais, F.; Ariga, K. Electrochemical nanoarchitectonics and layer-by-layer assembly: From basics to future. Nano Today 2015, 10, 138–167. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K.; Ahn, E.; Park, M.; Kim, B. Layer-by-Layer Assembly: Recent Progress from Layered Assemblies to Layered Nanoarchitectonics. Chem. Asian J. 2019, 14, 2553–2566. [Google Scholar] [CrossRef]
- Alkekhia, D.; Hammond, P.T.; Shukla, A. Layer-by-Layer Biomaterials for Drug Delivery. Annu. Rev. Biomed. Eng. 2020, 22, 1–24. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Ariga, K.; Minami, K.; Ebara, M.; Nakanishi, J. What are the emerging concepts and challenges in NANO? Nanoarchitectonics, hand-operating nanotechnology and mechanobiology. Polym. J. 2016, 48, 371–389. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y. Nanoarchitectonics from Atom to Life. Chem. Asian J. 2020, 15, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s plenty of room at the bottom. Calif. Inst. Technol. J. Eng. Sci. 1960, 4, 23–36. [Google Scholar]
- Roukes, M. Plenty of room, indeed. Sci. Am. 2001, 285, 48–57. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J.; Bando, Y.; Aono, M. Forming nanomaterials as layered functional structures toward materials nanoarchitectonics. NPG Asia Mater. 2012, 4, e17. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K. Nanoarchitectonics: What’s coming next after nanotechnology? Nanoscale Horiz. 2021, 6, 364–378. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics Revolution and Evolution: From Small Science to Big Technology. Small Sci. 2020, 1, 2000032. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics at Interfaces for Regulations of Biorelated Phenomena: Small Structures with Big Effects. Small Struct. 2021, 2, 2100006. [Google Scholar] [CrossRef]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J. Nanoarchitectonics for Dynamic Functional Materials from Atomic-/Molecular-Level Manipulation to Macroscopic Action. Adv. Mater. 2015, 28, 1251–1286. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, M. Nanoarchitectonics through supramolecular gelation: Formation and switching of diverse nanostructures. Mol. Syst. Des. Eng. 2018, 4, 11–28. [Google Scholar] [CrossRef]
- Tirayaphanitchkul, C.; Imwiset, K.; Ogawa, M. Nanoarchitectonics through Organic Modification of Oxide Based Layered Materials; Concepts, Methods and Functions. Bull. Chem. Soc. Jpn. 2021, 94, 678–693. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Gong, Y.; Li, C.; Niu, L.; Xu, S.; Xu, X.; Pan, L.; Shapter, J.G.; Yamauchi, Y.; et al. Light-conversion phosphor nanoarchitectonics for improved light harvesting in sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100404. [Google Scholar] [CrossRef]
- Ramanathan, M.; Shrestha, L.; Mori, T.; Ji, Q.; Hill, J.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Kitao, T.; Uemura, T. Supramolecular chiral nanoarchitectonics. Adv. Mater. 2020, 32, 1905657. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, C.; Kaneti, Y.V.; Eguchi, M.; Lin, J.; Yamauchi, Y.; Na, J. Practical MOF Nanoarchitectonics: New Strategies for Enhancing the Processability of MOFs for Practical Applications. Langmuir 2020, 36, 4231–4249. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Liu, J.; Ariga, K. Catalytic nanoarchitectonics for environmentally compatible energy generation. Mater. Today 2015, 19, 12–18. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, A.; Krishnan, V. Ultrathin Au–Ag Heterojunctions on Nanoarchitectonics Based Biomimetic Substrates for Dip Catalysis. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1954–1966. [Google Scholar] [CrossRef]
- Chen, G.; Sciortino, F.; Ariga, K. Atomic Nanoarchitectonics for Catalysis. Adv. Mater. Interfaces 2020, 8, 2001395. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Pandeeswar, M.; Senanayak, S.P.; Govindaraju, T. Nanoarchitectonics of Small Molecule and DNA for Ultrasensitive Detection of Mercury. ACS Appl. Mater. Interfaces 2016, 8, 30362–30371. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Yang, W.; Ariga, K. Soft Nanoarchitectonics for Enantioselective Biosensing. Accounts Chem. Res. 2020, 53, 644–653. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 42, 6322–6345. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Atom/molecular nanoarchitectonics for devices and related applications. Nano Today 2019, 28, 100762. [Google Scholar] [CrossRef]
- Giussi, J.M.; Cortez, M.L.; Marmisollé, W.A.; Azzaroni, O. Practical use of polymer brushes in sustainable energy applications: Interfacial nanoarchitectonics for high-efficiency devices. Chem. Soc. Rev. 2019, 48, 814–849. [Google Scholar] [CrossRef]
- Ariga, K.; Ishihara, S.; Abe, H.; Li, M.; Hill, J. Materials nanoarchitectonics for environmental remediation and sensing. J. Mater. Chem. 2011, 22, 2369–2377. [Google Scholar] [CrossRef]
- Pham, T.; Qamar, A.; Dinh, T.; Masud, M.K.; Rais-Zadeh, M.; Senesky, D.G.; Yamauchi, Y.; Nguyen, N.; Phan, H. Nanoarchitectonics for Wide Bandgap Semiconductor Nanowires: Toward the Next Generation of Nanoelectromechanical Systems for Environmental Monitoring. Adv. Sci. 2020, 7, 2001294. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics Can Save Our Planet: Nanoarchitectonics for Energy and Environment. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2243–2244. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.-S. Interlayer Nanoarchitectonics of Two-Dimensional Transition-Metal Dichalcogenides Nanosheets for Energy Storage and Conversion Applications. Adv. Energy Mater. 2017, 7, 1700571. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Yan, M.; Yang, C.; He, H.; Jiang, Q.; Yang, L.; Lu, Z.; Sun, Z.; Xu, X.; Bando, Y.; et al. Graphene Nanoarchitectonics: Recent Advances in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. 2019, 31, e1903415. [Google Scholar] [CrossRef]
- Stulz, E. Nanoarchitectonics with Porphyrin Functionalized DNA. Accounts Chem. Res. 2017, 50, 823–831. [Google Scholar] [CrossRef]
- Liang, X.; Li, L.; Tang, J.; Komiyama, M.; Ariga, K. Dynamism of Supramolecular DNA/RNA Nanoarchitectonics: From Interlocked Structures to Molecular Machines. Bull. Chem. Soc. Jpn. 2020, 93, 581–603. [Google Scholar] [CrossRef]
- Ariga, K.; Fakhrullin, R. Nanoarchitectonics on living cells. RSC Adv. 2021, 11, 18898–18914. [Google Scholar] [CrossRef]
- Ariga, K.; Leong, D.T.; Mori, T. Nanoarchitectonics for Hybrid and Related Materials for Bio-Oriented Applications. Adv. Funct. Mater. 2017, 28, 1702905. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Q.; Yan, X. Self-Assembling Peptide-Based Nanoarchitectonics. Bull. Chem. Soc. Jpn. 2019, 92, 70–79. [Google Scholar] [CrossRef]
- Ariga, K.; Tsai, K.-C.; Shrestha, L.K.; Hsu, S.-H. Life science nanoarchitectonics at interfaces. Mater. Chem. Front. 2020, 5, 1018–1032. [Google Scholar] [CrossRef]
- Ariga, K. There’s still plenty of room at the bottom. Chem. World 2021, 18, 5. [Google Scholar]
- Aono, M.; Ariga, K. The Way to Nanoarchitectonics and the Way of Nanoarchitectonics. Adv. Mater. 2015, 28, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K. Nanoarchitectonics: A navigator from materials to life. Mater. Chem. Front. 2016, 1, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond Self-Assembly: Challenges to Create Bio-Like Hierarchic Organization. Angew. Chem. Int. Ed. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Ji, Q.; Mori, T.; Miyazawa, K.; Yamauchi, Y.; Hill, J.; Ariga, K. Fullerene Nanoarchitectonics: From Zero to Higher Dimensions. Chem. Asian J. 2013, 8, 1662–1679. [Google Scholar] [CrossRef]
- Ariga, K.; Shrestha, L.K. Fullerene Nanoarchitectonics with Shape-Shifting. Materials 2020, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Maghfirah, A.; Ilmi, M.; Fajar, A.; Kadja, G. A review on the green synthesis of hierarchically porous zeolite. Mater. Today Chem. 2020, 17, 100348. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.M.; Kothandam, G.; Palanisami, T.; Al-Muhtaseb, A.H.; Karakoti, A.; Yi, J.; Bolan, N.; Vinu, A. A Review on the Synthesis and Applications of Nanoporous Carbons for the Removal of Complex Chemical Contaminants. Bull. Chem. Soc. Jpn. 2021, 94, 1232–1257. [Google Scholar] [CrossRef]
- Wu, G.; Jia, Z.; Zhou, X.; Nie, G.; Lv, H. Interlayer controllable of hierarchical MWCNTs@C@FexOy cross-linked composite with wideband electromagnetic absorption performance. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105687. [Google Scholar] [CrossRef]
- Li, L.; Zhang, B.; Wang, S.; Fan, F.; Chen, J.; Li, Y.; Fu, Y. Bimetallic NiCo Metal-Organic Framework-Derived Hierarchical Spinel NiCo2O4 Microflowers for Efficient Non-Enzymatic Glucose Sensing. Bull. Chem. Soc. Jpn. 2021, 94, 1118–1124. [Google Scholar] [CrossRef]
- Nakanishi, W.; Minami, K.; Shrestha, L.; Ji, Q.; Hill, J.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, T. Development of Fullerene Thin-Film Assemblies and Fullerene-Diamine Adducts towards Practical Nanocarbon-Based Electronic Materials. Bull. Chem. Soc. Jpn. 2019, 92, 1181–1199. [Google Scholar] [CrossRef]
- Minami, K.; Song, J.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics for fullerene biology. Appl. Mater. Today 2021, 23, 100989. [Google Scholar] [CrossRef]
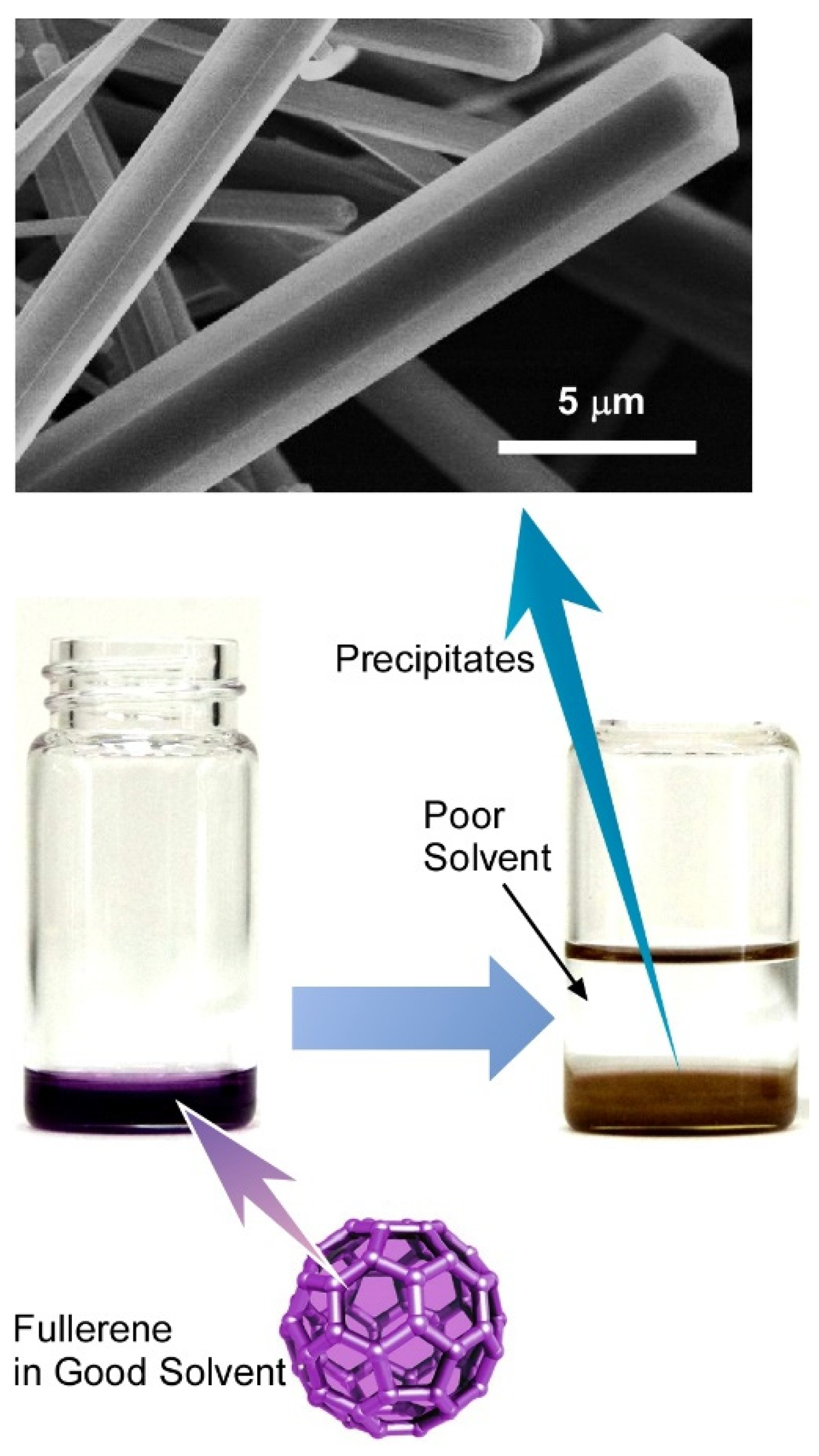
- Miyazawa, K.; Hamamoto, K.; Nagata, S.; Suga, T. Structural investigation of the C60/C70 whiskers fabricated by forming liquid–liquid interfaces of toluene with dissolved C60/C70 and isopropyl alcohol. J. Mater. Res. 2003, 18, 1096–1103. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis of fullerene nanowhiskers using the liquid–liquid interfacial precipitation method and their mechanical, electrical and superconducting properties. Sci. Technol. Adv. Mater. 2015, 16, 013502. [Google Scholar] [CrossRef] [PubMed]
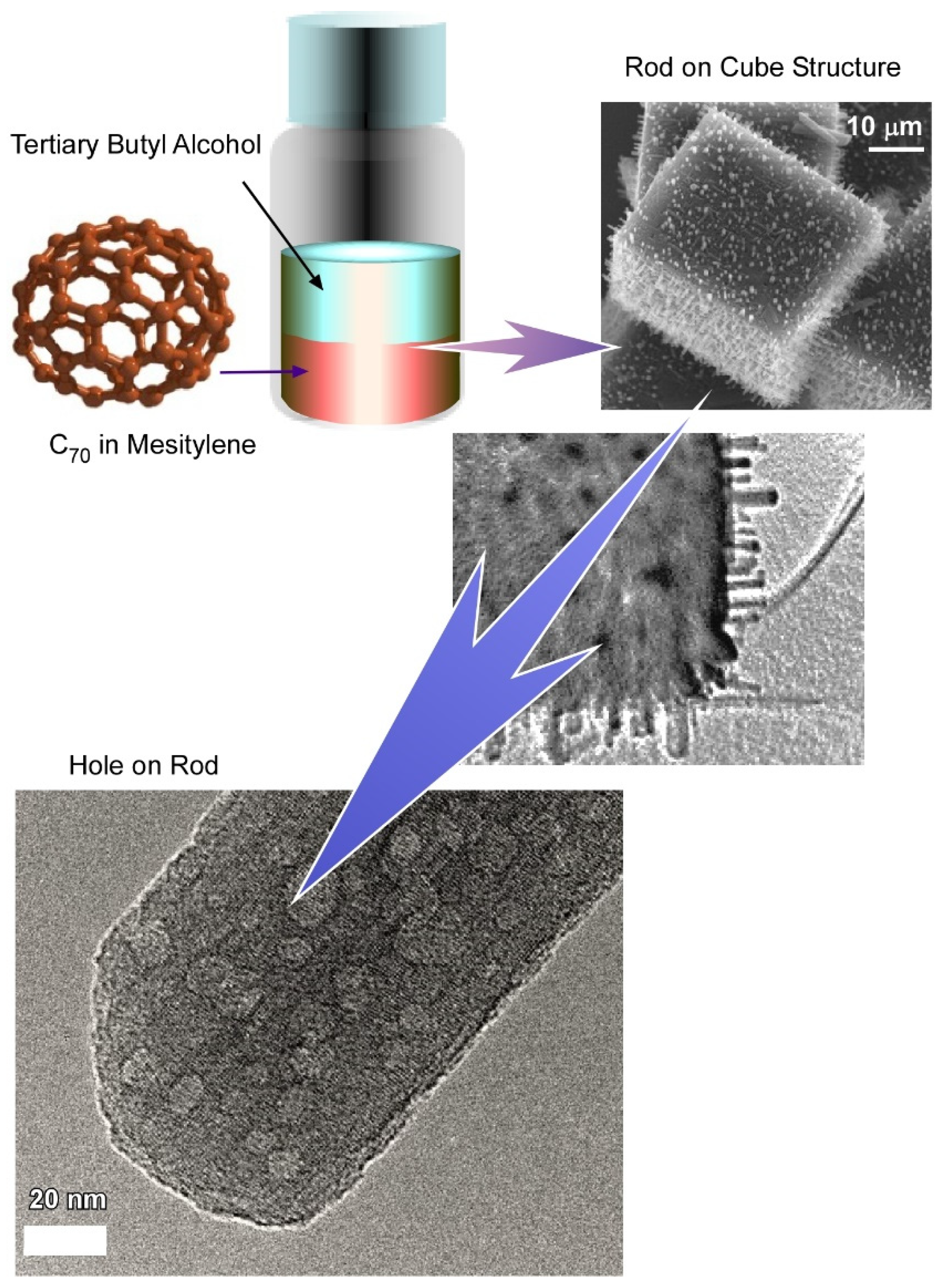
- Bairi, P.; Minami, K.; Nakanishi, W.; Hill, J.; Ariga, K.; Shrestha, L. Hierarchically Structured Fullerene C70 Cube for Sensing Volatile Aromatic Solvent Vapors. ACS Nano 2016, 10, 6631–6637. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-T.; Hsu, S.-H.; Maji, S.; Chahal, M.K.K.; Song, J.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Post-assembly dimension-dependent face-selective etching of fullerene crystals. Mater. Horiz. 2019, 7, 787–795. [Google Scholar] [CrossRef]
- Furuuchi, N.; Shrestha, R.G.; Yamashita, Y.; Hirao, T.; Ariga, K.; Shrestha, L.K. Self-Assembled Fullerene Crystals as Excellent Aromatic Vapor Sensors. Sensors 2019, 19, 267. [Google Scholar] [CrossRef] [Green Version]
- El-Mahdy, A.F.M.; Young, C.; Kim, J.; You, J.; Yamauchi, Y.; Kuo, S.-W. Hollow Microspherical and Microtubular [3 + 3] Carbazole-Based Covalent Organic Frameworks and Their Gas and Energy Storage Applications. ACS Appl. Mater. Interfaces 2019, 11, 9343–9354. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, B.; Hou, J.; Gu, M.; Chen, Y. In Situ Preparation and Unique Electrical Behaviors of Gold@Hollow Polyaniline Nanospheres through Recovery of Gold from Simulated e-Waste. Bull. Chem. Soc. Jpn. 2020, 93, 373–378. [Google Scholar] [CrossRef]
- Watanabe, Y.; Aiba, Y.; Ariyasu, S.; Abe, S. Molecular Design and Regulation of Metalloenzyme Activities through Two Novel Approaches: Ferritin and P450s. Bull. Chem. Soc. Jpn. 2020, 93, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Bairi, P.; Minami, K.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Intentional Closing/Opening of “Hole-in-Cube” Fullerene Crystals with Microscopic Recognition Properties. ACS Nano 2017, 11, 7790–7796. [Google Scholar] [CrossRef]
- Tang, Q.; Maji, S.; Jiang, B.; Sun, J.; Zhao, W.; Hill, J.P.; Ariga, K.; Fuchs, H.; Ji, Q.; Shrestha, L.K. Manipulating the Structural Transformation of Fullerene Microtubes to Fullerene Microhorns Having Microscopic Recognition Properties. ACS Nano 2019, 13, 14005–14012. [Google Scholar] [CrossRef]
- Bairi, P.; Tsuruoka, T.; Acharya, S.; Ji, Q.; Hill, J.P.; Ariga, K.; Yamauchi, Y.; Shrestha, L. Mesoporous fullerene C70 cubes with highly crystalline frameworks and unusually enhanced photoluminescence properties. Mater. Horiz. 2018, 5, 285–290. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Jiang, H.; Li, X.; Cheng, Y.; Meng, C. Designed mesoporous hollow sphere architecture metal (Mn, Co, Ni) silicate: A potential electrode material for flexible all solid-state asymmetric supercapacitor. Chem. Eng. J. 2019, 362, 818–829. [Google Scholar] [CrossRef]
- Saito, Y.; Ashizawa, M.; Matsumoto, H. Mesoporous Hydrated Graphene Nanoribbon Electrodes for Efficient Supercapacitors: Effect of Nanoribbon Dispersion on Pore Structure. Bull. Chem. Soc. Jpn. 2020, 93, 1268–1274. [Google Scholar] [CrossRef]
- Li, Y.; Henzie, J.; Park, T.; Wang, J.; Young, C.; Xie, H.; Yi, J.W.; Li, J.; Kim, M.; Kim, J.; et al. Fabrication of Flexible Microsupercapacitors with Binder-Free ZIF-8 Derived Carbon Films via Electrophoretic Deposition. Bull. Chem. Soc. Jpn. 2020, 93, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Nomura, K.; Nishihara, H.; Kobayashi, N.; Asada, T.; Kyotani, T. 4.4 V supercapacitors based on super-stable mesoporous carbon sheet made of edge-free graphene walls. Energy Environ. Sci. 2019, 12, 1542–1549. [Google Scholar] [CrossRef]
- Kim, G.; Shiraki, T.; Fujigaya, T. Thermal Conversion of Triazine-Based Covalent Organic Frameworks to Nitrogen-Doped Nanoporous Carbons and Their Capacitor Performance. Bull. Chem. Soc. Jpn. 2020, 93, 414–420. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Chaudhary, R.; Shrestha, R.G.; Shrestha, T.; Maji, S.; Ariga, K.; Shrestha, L.K. Washnut Seed-Derived Ultrahigh Surface Area Nanoporous Carbons as High Rate Performance Electrode Material for Supercapacitors. Bull. Chem. Soc. Jpn. 2021, 94, 565–572. [Google Scholar] [CrossRef]
- Shrestha, L.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous Carbon Tubes from Fullerene Crystals as the π-Electron Carbon Source. Angew. Chem. Int. Ed. 2014, 54, 951–955. [Google Scholar] [CrossRef]
- Sengottaiyan, C.; Jayavel, R.; Shrestha, R.G.; Subramani, T.; Maji, S.; Kim, J.H.; Hill, J.P.; Ariga, K.; Shrestha, L. Indium Oxide/Carbon Nanotube/Reduced Graphene Oxide Ternary Nanocomposite with Enhanced Electrochemical Supercapacitance. Bull. Chem. Soc. Jpn. 2019, 92, 521–528. [Google Scholar] [CrossRef]
- Baskar, A.V.; Ruban, A.M.; Davidraj, J.M.; Singh, G.; Al-Muhtaseb, A.H.; Lee, J.M.; Yi, J.; Vinu, A. Single-Step Synthesis of 2D Mesoporous C60/Carbon Hybrids for Supercapacitor and Li-Ion Battery Applications. Bull. Chem. Soc. Jpn. 2021, 94, 133–140. [Google Scholar] [CrossRef]
- Bairi, P.; Maji, S.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Mesoporous carbon cubes derived from fullerene crystals as a high rate performance electrode material for supercapacitors. J. Mater. Chem. A 2019, 7, 12654–12660. [Google Scholar] [CrossRef]
- Tang, Q.; Bairi, P.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Zeng, H.; Ji, Q.; Shrestha, L.K. Quasi 2D Mesoporous Carbon Microbelts Derived from Fullerene Crystals as an Electrode Material for Electrochemical Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 44458–44465. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Lee, M.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Technol. Adv. Mater. 2008, 9, 014109. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Kakuta, T.; Yamagishi, T.-A.; Yang, Y.-W.; Ogoshi, T. Molecular-Scale Porous Materials Based on Pillar[n]arenes. Chem 2018, 4, 2029–2053. [Google Scholar] [CrossRef] [Green Version]
- Akutagawa, T. Chemical Design and Physical Properties of Dynamic Molecular Assemblies. Bull. Chem. Soc. Jpn. 2021, 94, 1400–1420. [Google Scholar] [CrossRef]
- Zhou, H.; Yamada, T.; Kimizuka, N. Supramolecular Thermocells Based on Thermo-Responsiveness of Host–Guest Chemistry. Bull. Chem. Soc. Jpn. 2021, 94, 1525–1546. [Google Scholar] [CrossRef]
- Endo, M.; Sugiyama, H. DNA Origami Nanomachines. Molecules 2018, 23, 1766. [Google Scholar] [CrossRef] [Green Version]
- Fukunaga, K.; Tsutsumi, H.; Mihara, H. Self-Assembling Peptides as Building Blocks of Functional Materials for Biomedical Applications. Bull. Chem. Soc. Jpn. 2019, 92, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Govindaraju, T. Amino Acids and Peptides as Functional Components in Arylenediimide-Based Molecular Architectonics. Bull. Chem. Soc. Jpn. 2019, 92, 1883–1901. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Wang, H.-F.; Huang, J.-Q.; Qian, W.; Wei, F.; Qiao, S.-Z.; Zhang, Q. 3D Hierarchical Porous Graphene-Based Energy Materials: Synthesis, Functionalization, and Application in Energy Storage and Conversion. Electrochem. Energy Rev. 2019, 2, 332–371. [Google Scholar] [CrossRef]
- Maeda, K.; Mallouk, T.E. Two-Dimensional Metal Oxide Nanosheets as Building Blocks for Artificial Photosynthetic Assemblies. Bull. Chem. Soc. Jpn. 2019, 92, 38–54. [Google Scholar] [CrossRef]
- Percec, V.; Xiao, Q. Helical Self-Organizations and Emerging Functions in Architectures, Biological and Synthetic Macromolecules. Bull. Chem. Soc. Jpn. 2021, 94, 900–928. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Darder, M.; Boutahala, M.; Aranda, P.; Ruiz-Hitzky, E. Composite Nanoarchitectonics: Alginate Beads Encapsulating Sepiolite/Magnetite/Prussian Blue for Removal of Cesium Ions from Water. Bull. Chem. Soc. Jpn. 2021, 94, 122–132. [Google Scholar] [CrossRef]
- Yamashita, M. Next Generation Multifunctional Nano-Science of Advanced Metal Complexes with Quantum Effect and Nonlinearity. Bull. Chem. Soc. Jpn. 2021, 94, 209–264. [Google Scholar] [CrossRef]
- Sumita, M.; Tamura, R.; Homma, K.; Kaneta, C.; Tsuda, K. Li-Ion Conductive Li3PO4-Li3BO3-Li2SO4 Mixture: Prevision through Density Functional Molecular Dynamics and Machine Learning. Bull. Chem. Soc. Jpn. 2019, 92, 1100–1106. [Google Scholar] [CrossRef]
- Toyao, T.; Maeno, Z.; Takakusagi, S.; Kamachi, T.; Takigawa, I.; Shimizu, K.-I. Machine Learning for Catalysis Informatics: Recent Applications and Prospects. ACS Catal. 2019, 10, 2260–2297. [Google Scholar] [CrossRef]
- Chen, C.; Zuo, Y.; Ye, W.; Li, X.; Deng, Z.; Ong, S.P. A Critical Review of Machine Learning of Energy Materials. Adv. Energy Mater. 2020, 10, 1903242. [Google Scholar] [CrossRef]
- Maji, S.; Shrestha, L.; Ariga, K. Nanoarchitectonics for Nanocarbon Assembly and Composite. J. Inorg. Organomet. Polym. Mater. 2019, 30, 42–55. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, S.; Lai, Z.; Yao, X.; Zhao, Y.; Zhang, H.; Chen, H. Two-dimensional C60 nano-meshes via crystal transformation. Nanoscale 2019, 11, 8692–8698. [Google Scholar] [CrossRef]
- Maji, S.; Shrestha, R.G.; Lee, J.; Han, S.A.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Macaroni fullerene crystals-derived mesoporous carbon tubes as a high rate performance supercapacitor electrode material. Bull. Chem. Soc. Jpn. 2021, 94, 1502–1509. [Google Scholar] [CrossRef]
- Han, F.; Wang, R.; Feng, Y.; Wang, S.; Liu, L.; Li, X.; Han, Y.; Chen, H. On demand synthesis of hollow fullerene nanostructures. Nat. Commun. 2019, 10, 1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics for Hierarchical Fullerene Nanomaterials. Nanomaterials 2021, 11, 2146. https://doi.org/10.3390/nano11082146
Maji S, Shrestha LK, Ariga K. Nanoarchitectonics for Hierarchical Fullerene Nanomaterials. Nanomaterials. 2021; 11(8):2146. https://doi.org/10.3390/nano11082146
Chicago/Turabian StyleMaji, Subrata, Lok Kumar Shrestha, and Katsuhiko Ariga. 2021. "Nanoarchitectonics for Hierarchical Fullerene Nanomaterials" Nanomaterials 11, no. 8: 2146. https://doi.org/10.3390/nano11082146
APA StyleMaji, S., Shrestha, L. K., & Ariga, K. (2021). Nanoarchitectonics for Hierarchical Fullerene Nanomaterials. Nanomaterials, 11(8), 2146. https://doi.org/10.3390/nano11082146