Double-Antibody Sandwich Immunoassay and Plasmonic Coupling Synergistically Improved Long-Range SPR Biosensor with Low Detection Limit
Abstract
:1. Introduction
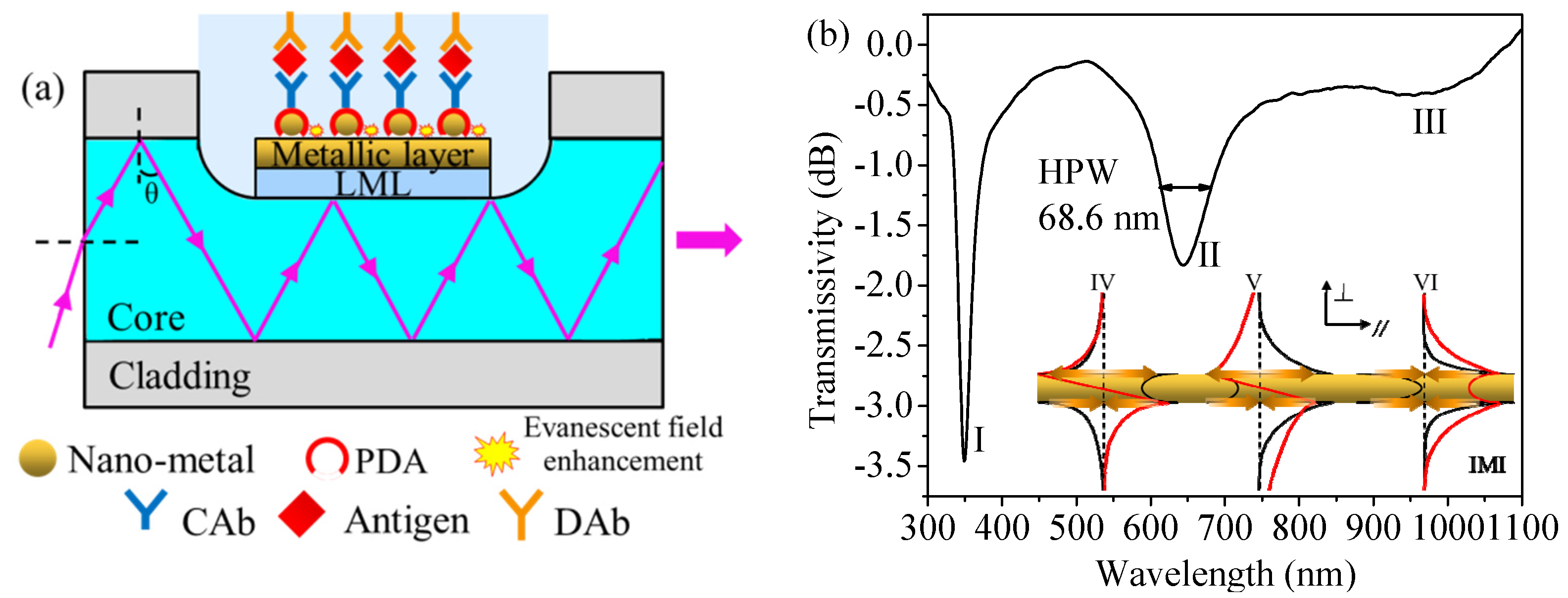
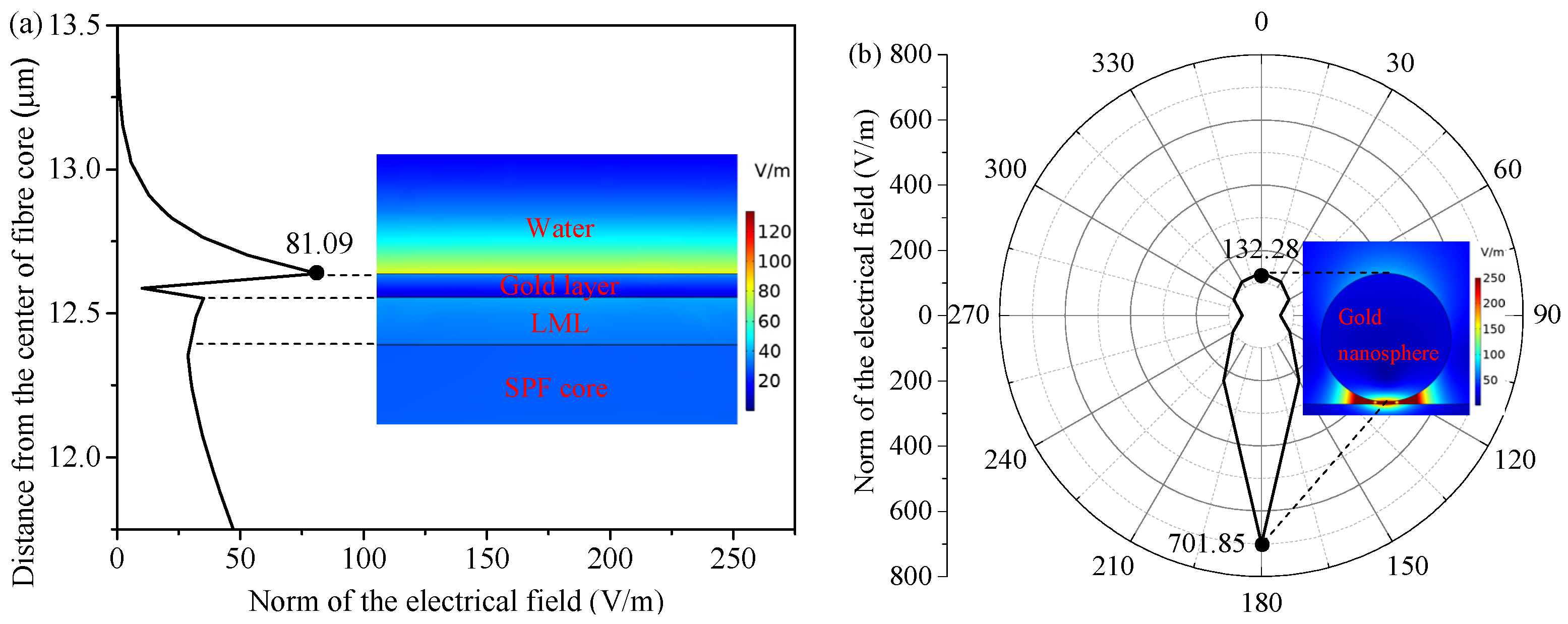
2. Sensing Principle and Simulation Analysis
3. Materials and Methods
3.1. Materials and Reagents
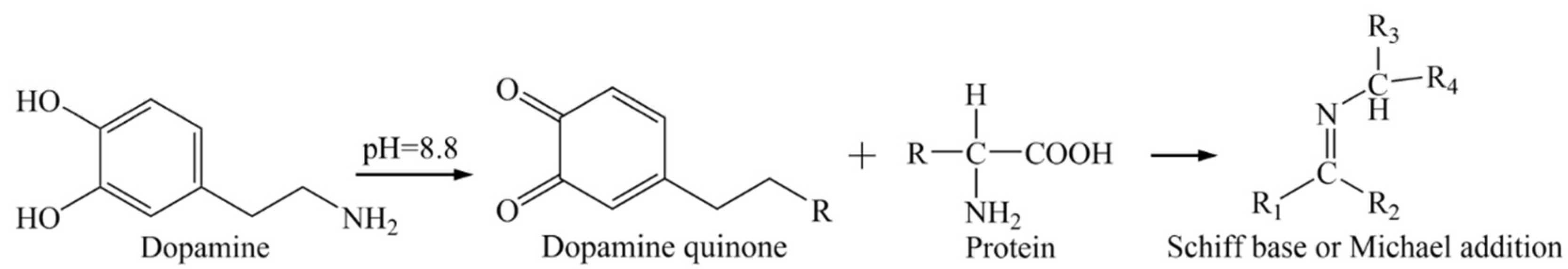
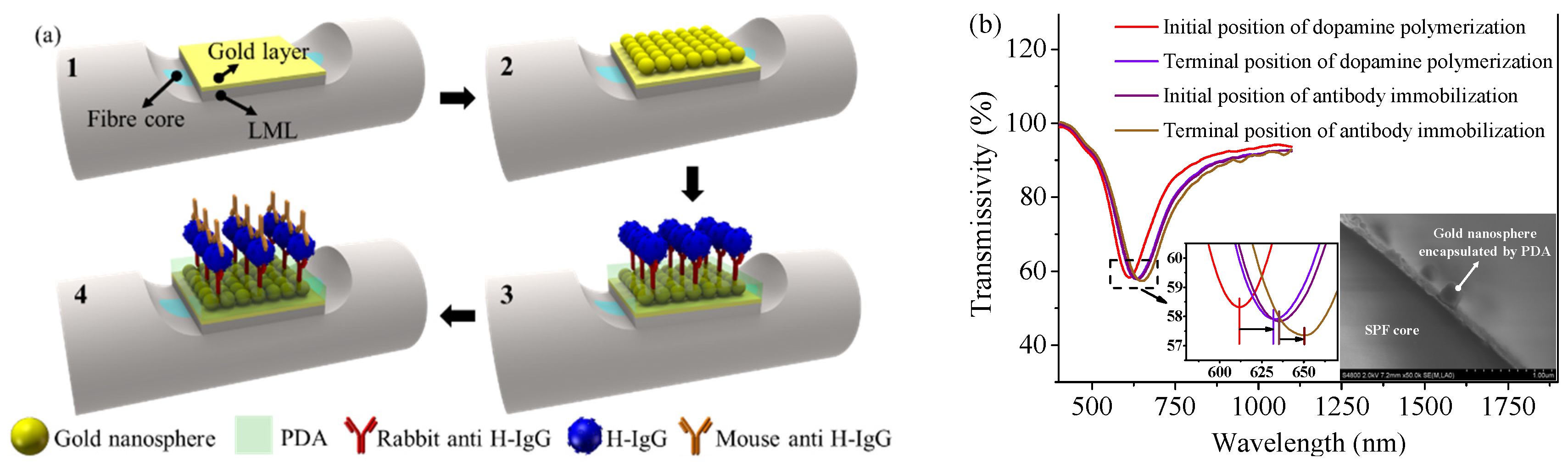
3.2. Manufacturing of Sensing Probe
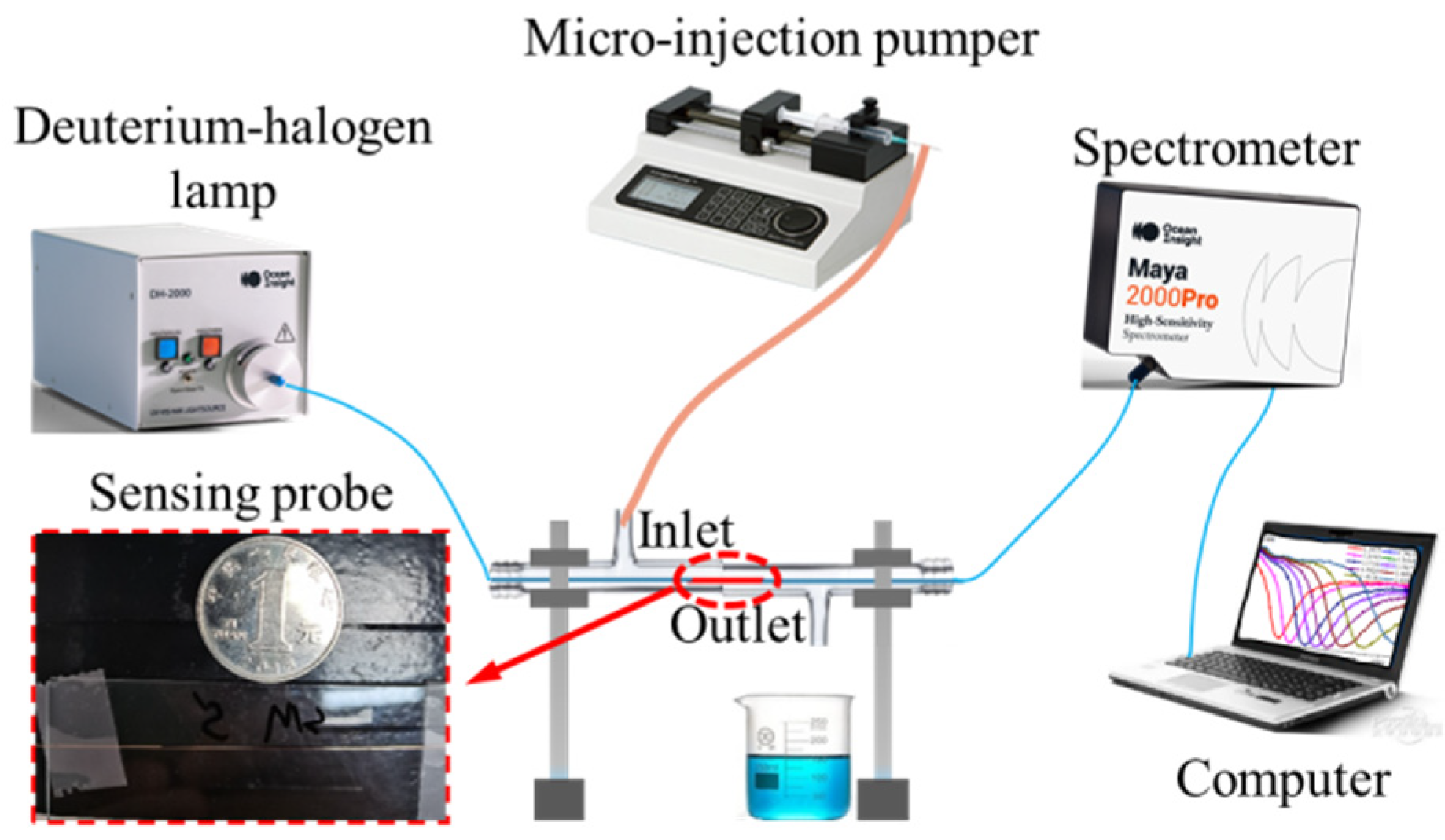
3.3. Experimental Setup
4. Results and Discussions
4.1. Refractive Index Sensing Experiment
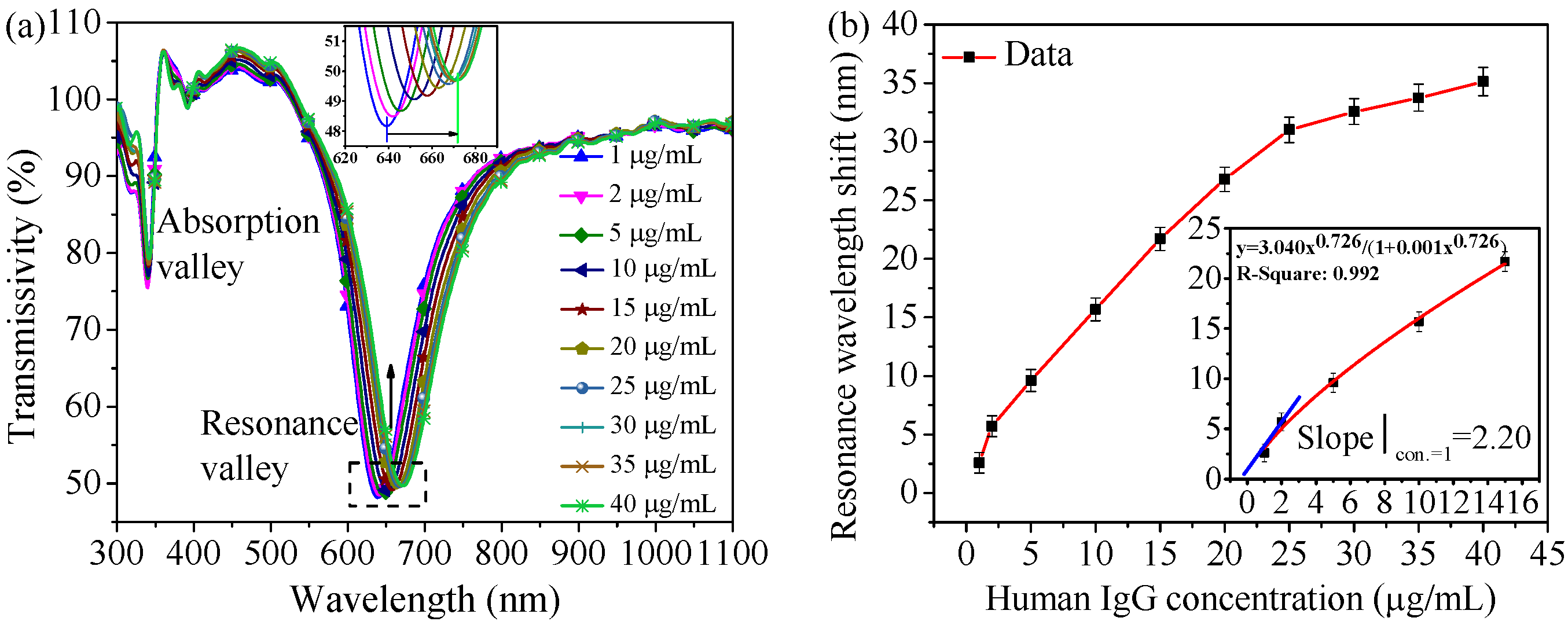
4.2. Human IgG Detection
4.2.1. PBS Matrix Sample Detection
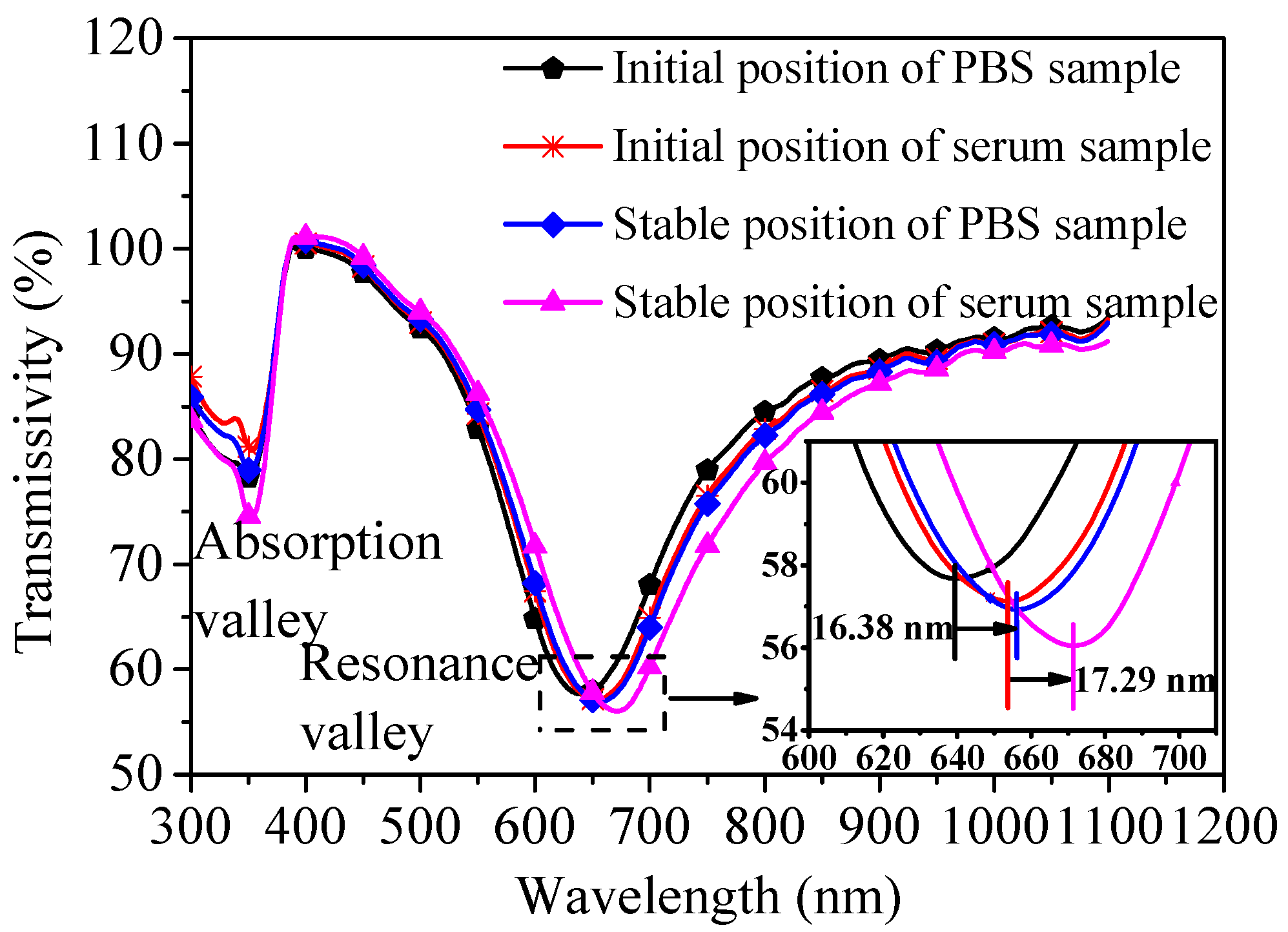
4.2.2. Serum Matrix Sample Detection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, B.T.; Niu, Y.X.; Zheng, S.W.; Yin, Y.H.; Ding, M. An optical fiber immunosensor with a low detection limit based on plasmon coupling enhancement. J. Lightwave Technol. 2020, 38, 3781–3788. [Google Scholar] [CrossRef]
- Yang, M.; Xiong, X.; He, R.; Luo, Y.H.; Tang, J.Y.; Dong, J.L.; Lu, H.H.; Yu, J.H.; Guan, H.Y.; Zhang, J.; et al. Halloysite nanotube-modified plasmonic interface for highly sensitive refractive index sensing. ACS Appl. Mater. Inter. 2018, 10, 5933–5940. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Gan, S.W.; Zhang, G.H.; Dai, X.Y. High sensitivity refractive index sensor based on surface plasmon resonance with topological insulator. Results Phys. 2019, 14, 102477. [Google Scholar] [CrossRef]
- Lu, M.D.; Zhu, H.; Bazuin, C.G.; Peng, W.; Masson, J.F. Polymer-templated gold nanoparticles on optical fibers for enhanced-sensitivity localized surface plasmon resonance biosensors. ACS Sens. 2019, 4, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Q.; Zhai, T.T.; Zhou, Y.; Yang, D.R.; Wang, F.B.; Xia, X.H. Localized surface plasmon resonance enhanced label-free photoelectrochemical immunoassay by Au-MoS2 nanohybrid. Electrochim. Acta 2018, 271, 361–369. [Google Scholar] [CrossRef]
- Yang, C.T.; Wu, L.; Bai, P.; Thierry, B. Investigation of plasmonic signal enhancement based on long range surface plasmon resonance with gold nanoparticle tags. J. Mater. Chem. C 2016, 4, 9897–9904. [Google Scholar] [CrossRef]
- Liu, K.; Xue, M.; Jiang, J.F.; Wang, T.; Chang, P.X.; Liu, T.G. Theoretical modeling of a coupled plasmon waveguide resonance sensor based on multimode optical fiber. Opt. Commun. 2018, 410, 552–558. [Google Scholar] [CrossRef]
- Zhang, H.T.; Geng, Y.J.; Xu, S.P.; Xu, W.Q.; Tian, Y.; Yu, J.; Deng, W.Y.; Yu, B.; Liu, Y. Surface plasmon field-enhanced Raman scattering based on evanescent field excitation of waveguide-coupled surface plasmon resonance configuration. J. Phys. Chem. C 2020, 124, 1640–1645. [Google Scholar] [CrossRef]
- Khosravi, R.; Rodriguez, C.; Mostowfi, F.; Sieben, V. Evaluation of crude oil asphaltene deposition inhibitors by surface plasmon resonance. Fuel 2020, 273, 117787. [Google Scholar] [CrossRef]
- Yu, H.X.; Han, R.X.; Su, J.; Chen, H.L.; Li, D.C. Multi-marker diagnosis method for early Hepatocellular Carcinoma based on surface plasmon resonance. Clin. Chim. Acta 2020, 502, 9–14. [Google Scholar] [CrossRef]
- Belen, S.M.; Sofia, N.T.; Romina, M.; Belen, A.M.; Santiago, C.; Julieta, F.L.M.; Pablo, R.; Cristina, V.; Martin, D.; Mauricio, D.; et al. Optimized surface plasmon resonance immunoassay for staphylococcal enterotoxin G detection using silica nanoparticles. Biochem. Biophys. Res. Commun. 2021, 558, 168–174. [Google Scholar] [CrossRef]
- Zubiate, P.; Urrutia, A.; Zamarreño, C.R.; Egea-Urra, J.; Fernández-Irigoyen, J.; Giannetti, A.; Baldini, F.; Díaz, S.; Matias, I.R.; Arregui, F.J.; et al. Fiber-based early diagnosis of venous thromboembolic disease by label-free D-dimer detection. Biosens. Bioelectron. X 2019, 2, 100026. [Google Scholar] [CrossRef]
- Singh, L.; Maccaferri, N.; Garoli, D.; Gorodetski, Y. Directional plasmonic excitation by helical nanotips. Nanomaterials 2021, 11, 1333. [Google Scholar] [CrossRef]
- Kravets, V.G.; Kabashin, A.V.; Barnes, W.L.; Grigorenko, A.N. Plasmonic surface lattice resonances: A review of properties and applications. Chem. Rev. 2018, 118, 5912–5951. [Google Scholar] [CrossRef]
- Esposito, F.; Sansone, L.; Srivastava, A.; Baldini, F.; Campopiano, S.; Chiavaioli, F.; Giordano, M.; Giannetti, A.; Iadicicco, A. Long period grating in double cladding fiber coated with graphene oxide as high-performance optical platform for biosensing. Biosens. Bioelectron. 2021, 172, 112747. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Zhang, D.; Li, S.; Zhang, Y.; Ma, P.Y.; Yu, Y.; Wang, X.H.; Song, D.Q. Ultrasensitive magnetic field-assisted surface plasmon resonance immunoassay for human cardiac troponin I. Biosens. Bioelectron. 2017, 96, 288–293. [Google Scholar] [CrossRef]
- Wang, F.M.; Liu, C.; Sun, Z.J.; Sun, T.; Liu, B.H.; Chu, P.K. A Highly sensitive SPR sensors based on two parallel PCFs for low refractive index detection. IEEE Photonics J. 2018, 10, 7104010. [Google Scholar] [CrossRef]
- Berini, P. Long-range surface plasmon polaritons. Adv. Opt. Photonics 2009, 1, 484–588. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2010, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Liu, B.H.; Zhu, X.S.; Tang, X.L.; Shi, Y.W. Long-range surface plasmon resonance sensor based on dielectric/silver coated hollow fiber with enhanced figure of merit. Opt. Lett. 2015, 40, 744–747. [Google Scholar] [CrossRef]
- Dastmalchi, B.; Tassin, P.; Koschny, T.; Soukoulis, C.M. A new perspective on plasmonics: Confinement and propagation length of surface plasmons for different materials and geometries. Adv. Opt. Mater. 2016, 4, 177–184. [Google Scholar] [CrossRef]
- Liu, F.; Wan, R.Y.; Li, Y.X.; Huang, Y.; Miura, Y.; Ohnishi, D.; Peng, J.D. Extremely high efficient coupling between long range surface plasmon polariton and dielectric waveguide mode. Appl. Phys. Lett. 2009, 95, 091104. [Google Scholar] [CrossRef] [Green Version]
- Shalabney, A.; Abdulhalim, I. Sensitivity-enhancement methods for surface plasmon sensors. Laser Photonics Rev. 2011, 5, 571–606. [Google Scholar] [CrossRef]
- Li, S.; Wu, Q.; Ma, P.Y.; Zhang, Y.; Song, D.Q.; Wang, X.H.; Sun, Y. A sensitive SPR biosensor based on hollow gold nanospheres and improved sandwich assay with PDA-Ag@Fe3O4/rGO. Talanta 2018, 180, 156–161. [Google Scholar] [CrossRef]
- Isaacs, S.; Abdulhalim, I. Long range surface plasmon resonance with ultra-high penetration depth for self-referenced sensing and ultra-low detection limit using diverging beam approach. Appl. Phys. Lett. 2015, 106, 193701. [Google Scholar] [CrossRef]
- Katagiri, S.; Tsukahara, Y.; Hasegawa, Y.; Wada, Y. Energy-transfer mechanism in photoluminescent terbium(III) complexes causing their temperature-dependence. Bull. Chem. Soc. Jpn. 2007, 80, 1492–1503. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.Y.; Wang, X.Z.; Niu, L.Y.; Zhao, W.M. A D-shaped fiber long-range surface plasmon resonance sensor with high Q-factor and temperature self-compensation. IEEE Trans. Instrum. Meas. 2019, 69, 2218–2224. [Google Scholar] [CrossRef]
- Ma, K.J.; Liu, L.; Zhang, P.F.; He, Y.H.; Peng, Q. Optimization of angle-pixel resolution for angular plasmonic biosensors. Sens. Actuators B Chem. 2019, 283, 188–197. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.H.; Jiang, J.F.; Xu, T.H.; Wang, S.; Chang, P.X.; Zhang, Z.; Ma, J.Y.; Liu, T.G. MoSe2-Au based sensitivity enhanced optical fiber surface plasmon resonance biosensor for detection of goat-anti-rabbit IgG. IEEE Access 2020, 8, 660–668. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Gouveia, C.A.J.; Jorge, P.A.S.; Baldini, F. Towards a uniform metrological assessment of grating-based optical fiber sensors: From refractometers to biosensors. Biosensors 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Quintela, A.; Alende, R.; Gude, F.; Campos, J.; Rey, J.; Meijide, L.M.; Fernandez-Merino, C.; Vidal, C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008, 151, 42–50. [Google Scholar] [CrossRef]
- Damborska, D.; Bertok, T.; Dosekova, E.; Holazova, A.; Lorencova, L.; Kasak, P.; Tkac, J. Nanomaterial-based biosensors for detection of prostate specific antigen. Microchim. Acta 2017, 184, 3049–3067. [Google Scholar] [CrossRef]
- Mai, Z.G.; Zhang, J.H.; Chen, Y.Z.; Wang, J.Q.; Hong, X.M.; Su, Q.N.; Li, X.J. A disposable fiber optic SPR probe for immunoassay. Biosens. Bioelectron. 2019, 144, 111621. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Lang, T.T.; Cao, B.B.; Jin, J.; Dong, R.Q.; Feng, H. Fiber optic SPR sensor for human immunoglobulin G measurement based on the MMF-NCF-MMF structure. Opt. Fiber Technol. 2018, 46, 179–185. [Google Scholar] [CrossRef]
- Vicente, A.; Santano, D.; Zubiate, P.; Urrutia, A.; Del Villar, I.; Zamarreno, C.R. Lossy mode resonance sensors based on nanocoated multimode-coreless-multimode fibre. Sens. Actuators B Chem. 2020, 304, 126955. [Google Scholar] [CrossRef]
- Socorro, A.B.; Del Villar, I.; Corres, J.M.; Arregui, F.J.; Matias, I.R. Fiber-optic immunosensor based on lossy mode resonances induced by indium tin oxide thin-films. In Proceedings of the 15th IEEE Sensors Conference, Orlando, FL, USA, 30 October–2 November 2016. [Google Scholar]
- Cheng, Z.; Wang, Q.; Zhu, A.S.; Qiu, F.M.; Niu, L.Y.; Jing, J.Y. Au-nanoshells modified surface field enhanced LRSPR biosensor with low LOD for highly sensitive hIgG sensing. Opt. Laser Technol. 2021, 134, 106656. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Zhang, D.; Li, S.; Wang, X.H.; Song, D.Q. Magnetic field-assisted SPR biosensor based on carboxyl-functionalized graphene oxide sensing film and Fe3O4-hollow gold nanohybrids probe. Biosens. Bioelectron. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, D.; Deng, X.Y.; Sun, Y.; Wang, X.H.; Ma, P.Y.; Song, D.Q. A novel surface plasmon resonance biosensor based on the PDA-AgNPs-PDA-Au film sensing platform for horse IgG detection. Spectrochim. Acta A 2018, 191, 290–295. [Google Scholar] [CrossRef] [PubMed]
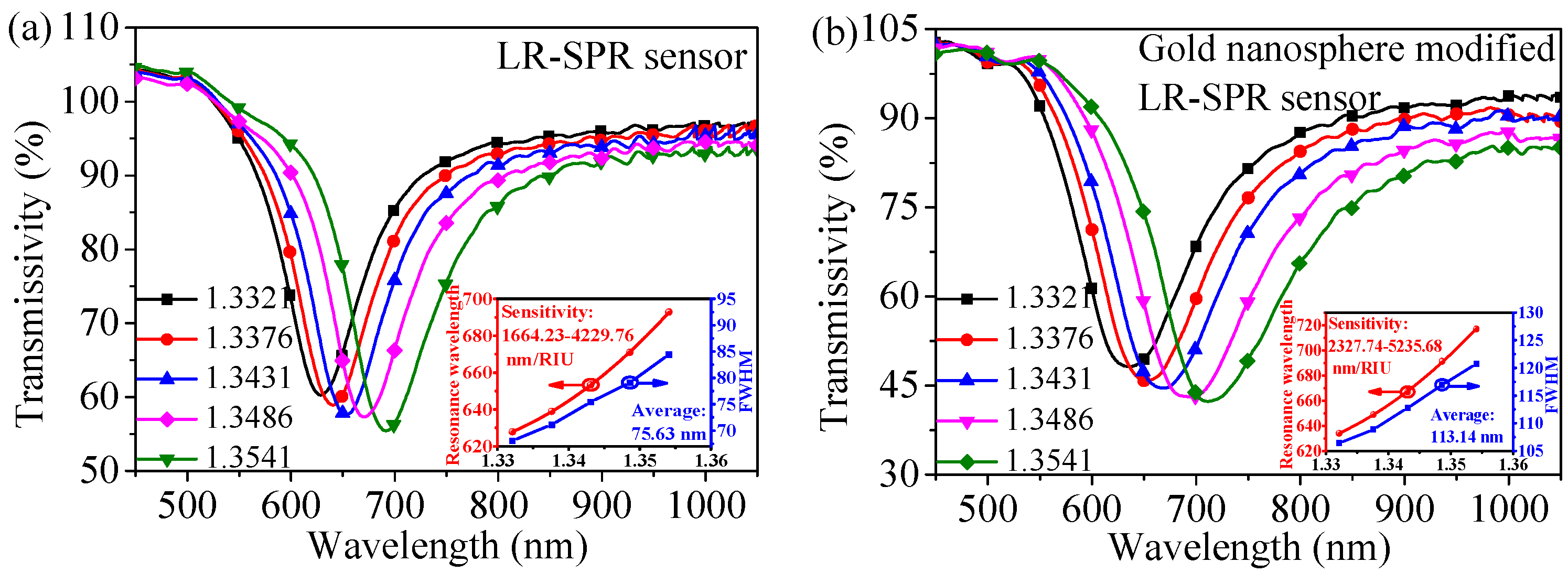

| Refractive Index | Sensitivity of LR-SPR Sensor (nm/RIU) | Sensitivity of Gold Nanosphere-Modified LR-SPR Sensor (nm/RIU) | Enhancement (nm/RIU) |
|---|---|---|---|
| 1.3321 | 1664.23 | 2327.74 | 663.51 |
| 1.3376 | 2303.42 | 3056.39 | 752.97 |
| 1.3431 | 2946.92 | 3786.73 | 839.81 |
| 1.3486 | 3587.85 | 4517.07 | 929.22 |
| 1.3541 | 4229.76 | 5235.68 | 1005.92 |
| Average | 2946.44 | 3784.72 | 838.23 |
| Principle | Method | Analyte | Concentration Range (μg/mL) | LOD (μg/mL) | Refs. |
|---|---|---|---|---|---|
| SPR | Gold layer/MoSe2 | Goat-anti-Rabbit IgG | 10–100 | 0.33 | [29] |
| Gold layer | Goat-anti-Mouse IgG | 0.50–10 | 0.10 | [33] | |
| Gold layer/PDDA-PSS | Human IgG | 25–1000 | 1.75 | [34] | |
| L-SPR | Gold nanoparticles arrays | Human IgG | 1–100 | 0.16 | [4] |
| MoS2/Gold nanoparticles | Human IgG | 6.26–626.32 | 0.62 | [5] | |
| LMR | SnO2−x | Goat-anti-Mouse IgG | 1–40 | 0.60 | [35] |
| ITO | Goat-anti IgG | 0.02–1.53 | - | [36] | |
| LR-SPR | MgF2/Gold layer | Human IgG | 0.3125–10 | 0.0032 | [28] |
| LML/Gold layer/Gold nanoshells | Human IgG | 1–40 | 0.20 | Previous work [37] | |
| Sandwich immunoassay and plasmonic coupling | 0.109 | This work |
| Method | Analyte | Spiked (μg/mL) | Repetition Rate (%) | Deviation from 100% (%) | Refs. |
|---|---|---|---|---|---|
| Gold layer/hollow gold nanospheres (HGNPs)/PDA wrapped magnetic carbon nanotube | Human cardiac troponin | 0.01 | 112.50 | +12.50 | [16] |
| 0.08 | 106.90 | +6.90 | |||
| 0.30 | 97.01 | −2.99 | |||
| Gold layer/HGNPs/PDA-Ag@Fe3O4-rGO | Rabbit IgG | 0.075 | 126.09 | +26.09 | [24] |
| 0.60 | 115.38 | +15.38 | |||
| 5.00 | 89.23 | −10.77 | |||
| Gold layer/Fe3O4-HGNPs | Human IgG | 0.03 | 116.67 | +16.67 | [38] |
| 0.125 | 110.34 | +10.34 | |||
| 0.50 | 106.84 | +6.84 | |||
| PDA/AgNPs/PDA/gold layer | Horse IgG | 5 | 107.14 | +7.14 | [39] |
| 10 | 111.11 | +11.11 | |||
| 20 | 108.33 | +8.33 | |||
| LML/gold layer/gold nanoshells | Human IgG | 15 | 107.62 | +7.62 | Previous work [37] |
| Sandwich immunoassay and plasmonic coupling | Human IgG | 10 | 105.56 | +5.56 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, J.; Liu, K.; Jiang, J.; Xu, T.; Wang, S.; Ma, J.; Zhang, Z.; Zhang, W.; Liu, T. Double-Antibody Sandwich Immunoassay and Plasmonic Coupling Synergistically Improved Long-Range SPR Biosensor with Low Detection Limit. Nanomaterials 2021, 11, 2137. https://doi.org/10.3390/nano11082137
Jing J, Liu K, Jiang J, Xu T, Wang S, Ma J, Zhang Z, Zhang W, Liu T. Double-Antibody Sandwich Immunoassay and Plasmonic Coupling Synergistically Improved Long-Range SPR Biosensor with Low Detection Limit. Nanomaterials. 2021; 11(8):2137. https://doi.org/10.3390/nano11082137
Chicago/Turabian StyleJing, Jianying, Kun Liu, Junfeng Jiang, Tianhua Xu, Shuang Wang, Jinying Ma, Zhao Zhang, Wenlin Zhang, and Tiegen Liu. 2021. "Double-Antibody Sandwich Immunoassay and Plasmonic Coupling Synergistically Improved Long-Range SPR Biosensor with Low Detection Limit" Nanomaterials 11, no. 8: 2137. https://doi.org/10.3390/nano11082137
APA StyleJing, J., Liu, K., Jiang, J., Xu, T., Wang, S., Ma, J., Zhang, Z., Zhang, W., & Liu, T. (2021). Double-Antibody Sandwich Immunoassay and Plasmonic Coupling Synergistically Improved Long-Range SPR Biosensor with Low Detection Limit. Nanomaterials, 11(8), 2137. https://doi.org/10.3390/nano11082137






