Cu-Doped-ZnO Nanocrystals Induce Hepatocyte Autophagy by Oxidative Stress Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication and Characterization of CZON Nanocrystals
2.2. Animals Experiments
2.3. Single Dose Toxicity
2.4. Coefficients of Major Organs, Hematology and Blood Biochemical Assay
2.5. Histopathological Examinations
2.6. Biochemical Analysis of Oxidative Stress
2.7. TEM Imaging of Tissues
2.8. Western Blotting Analysis
2.9. Electron Paramagnetic Resonance Test of CZON
2.10. Cu content Analysis
2.11. StatisticsX
3. Results
3.1. Preparation and Characterization of CZON
3.2. Single Dose Toxicity of CZON
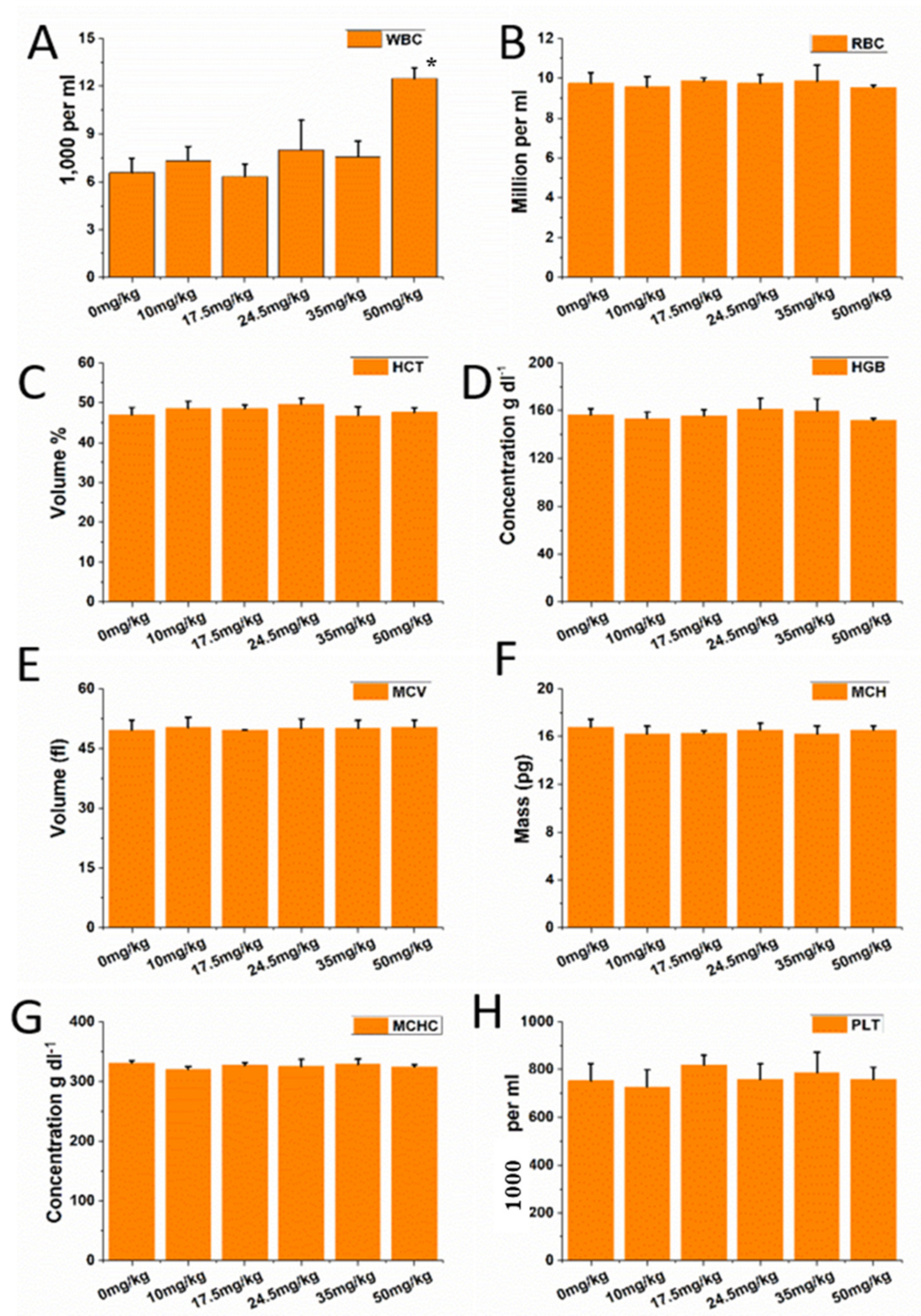
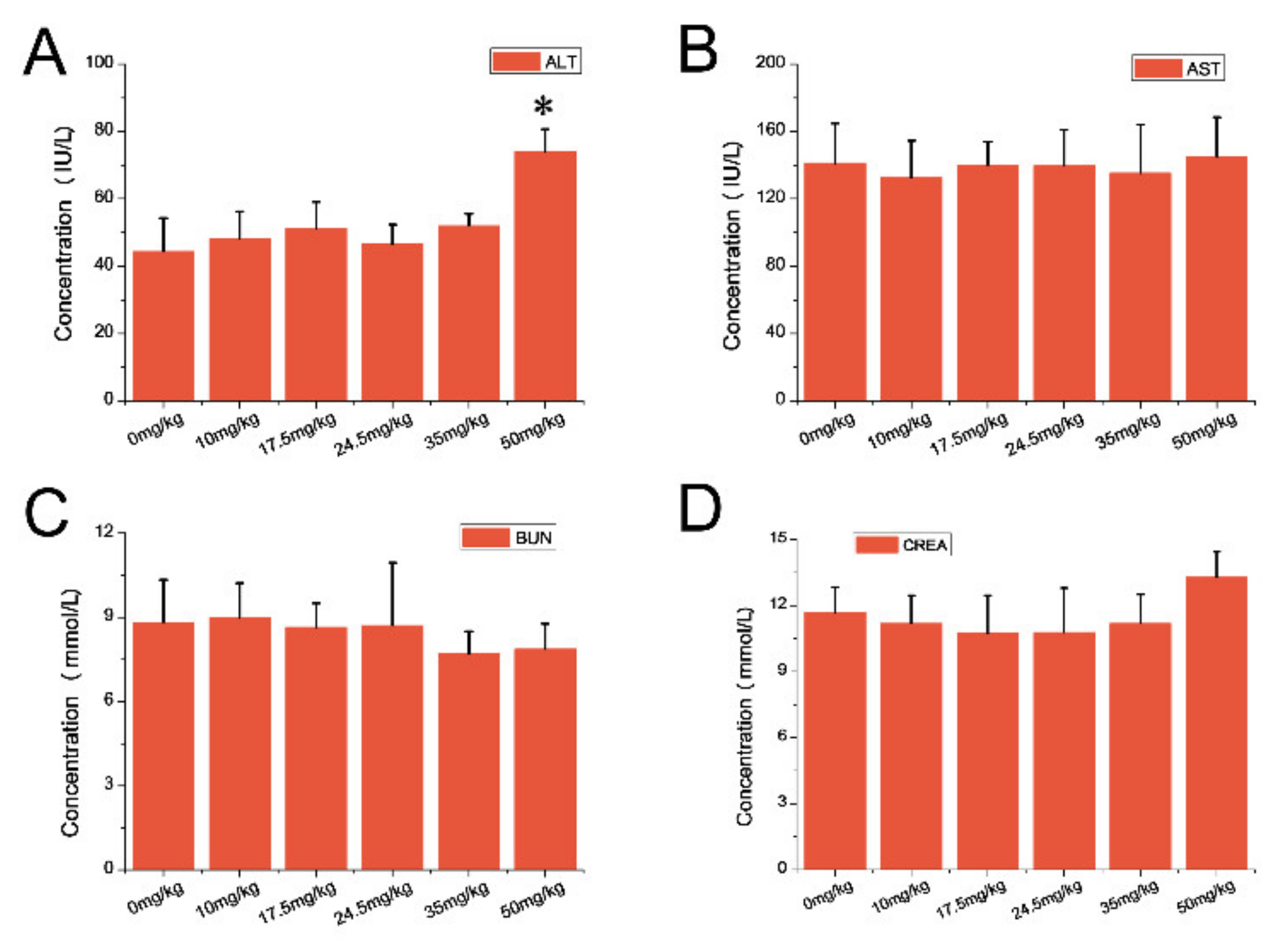
3.3. Hematology and Blood Biochemical Assay
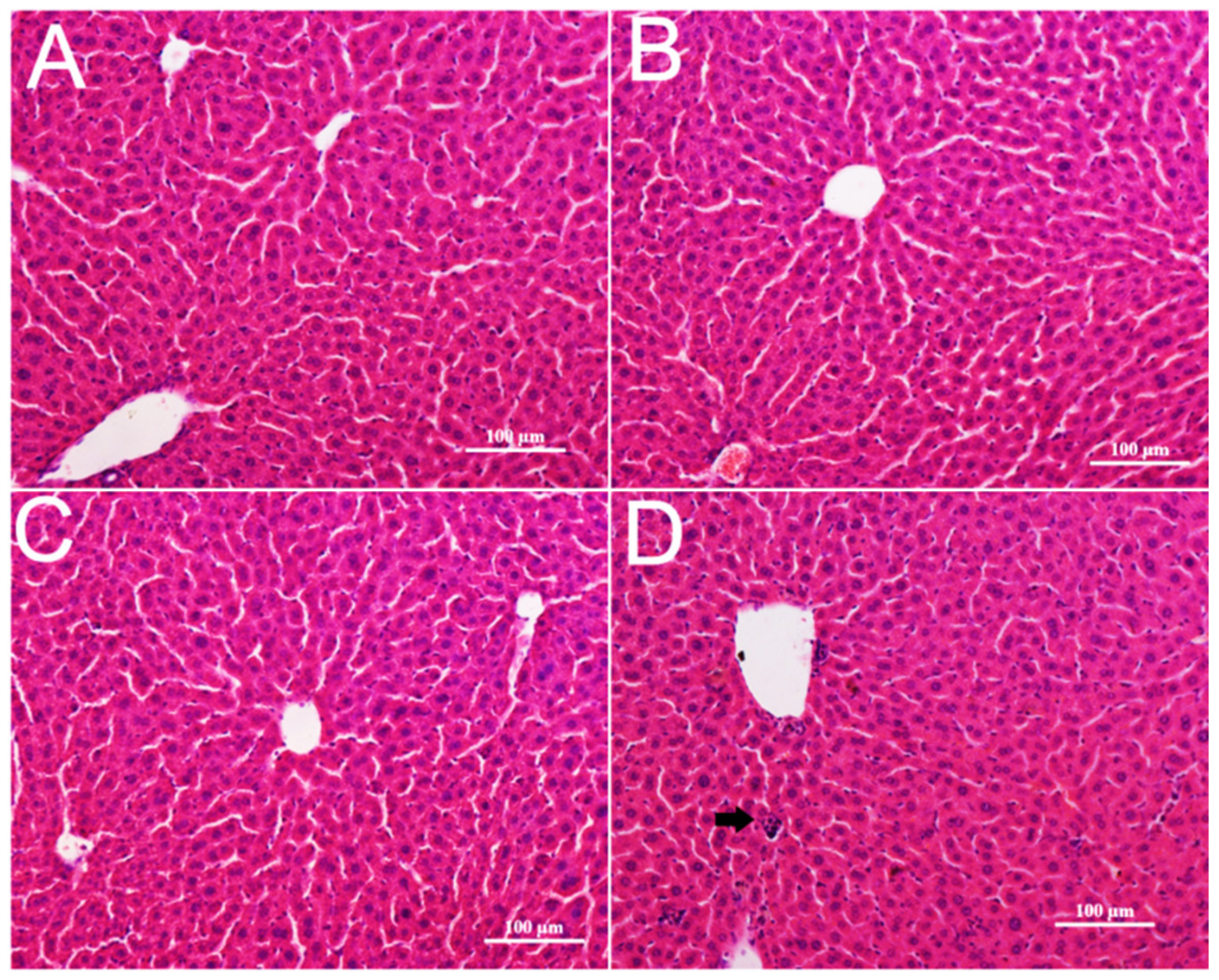
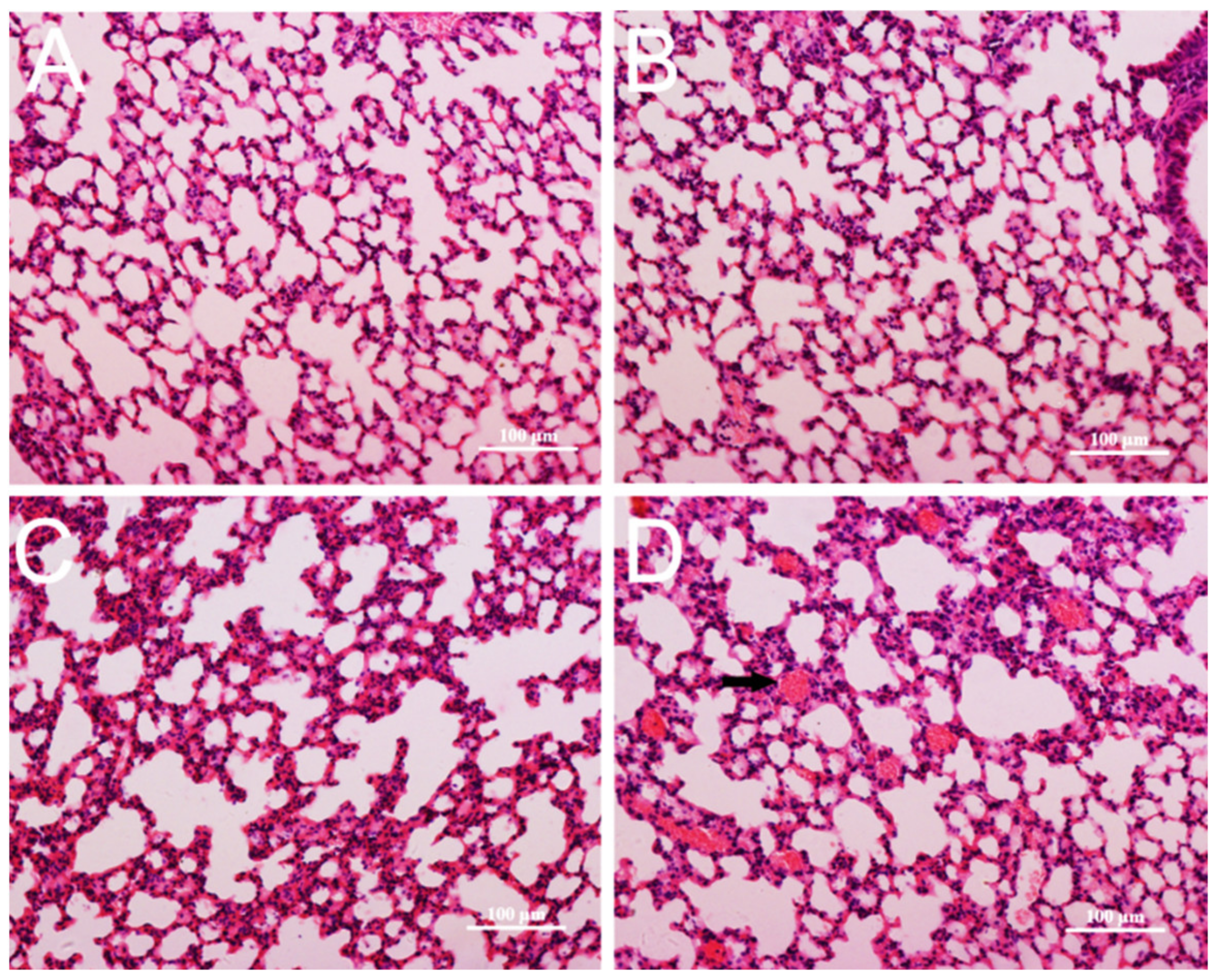
3.4. Pathological Changes in Mice
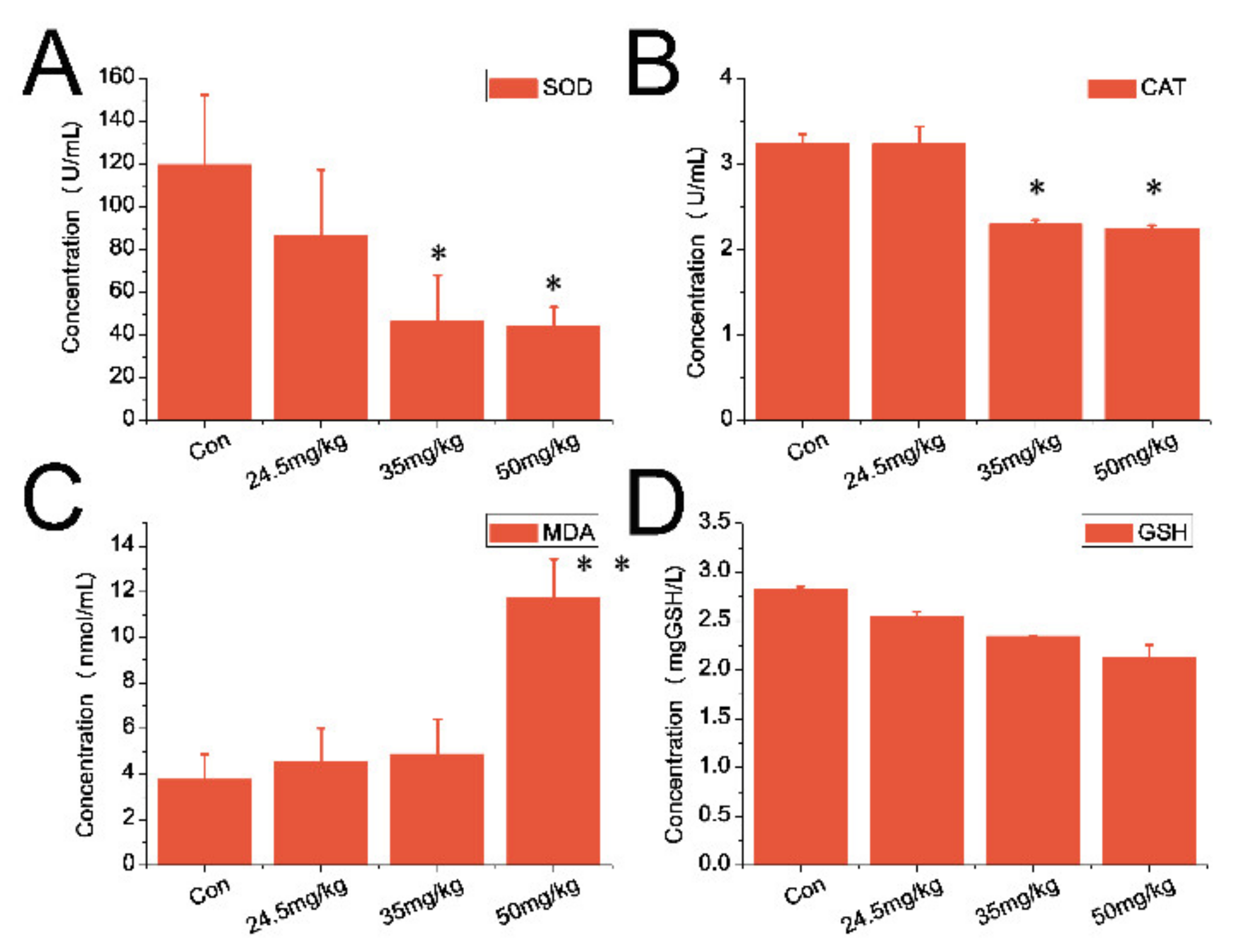
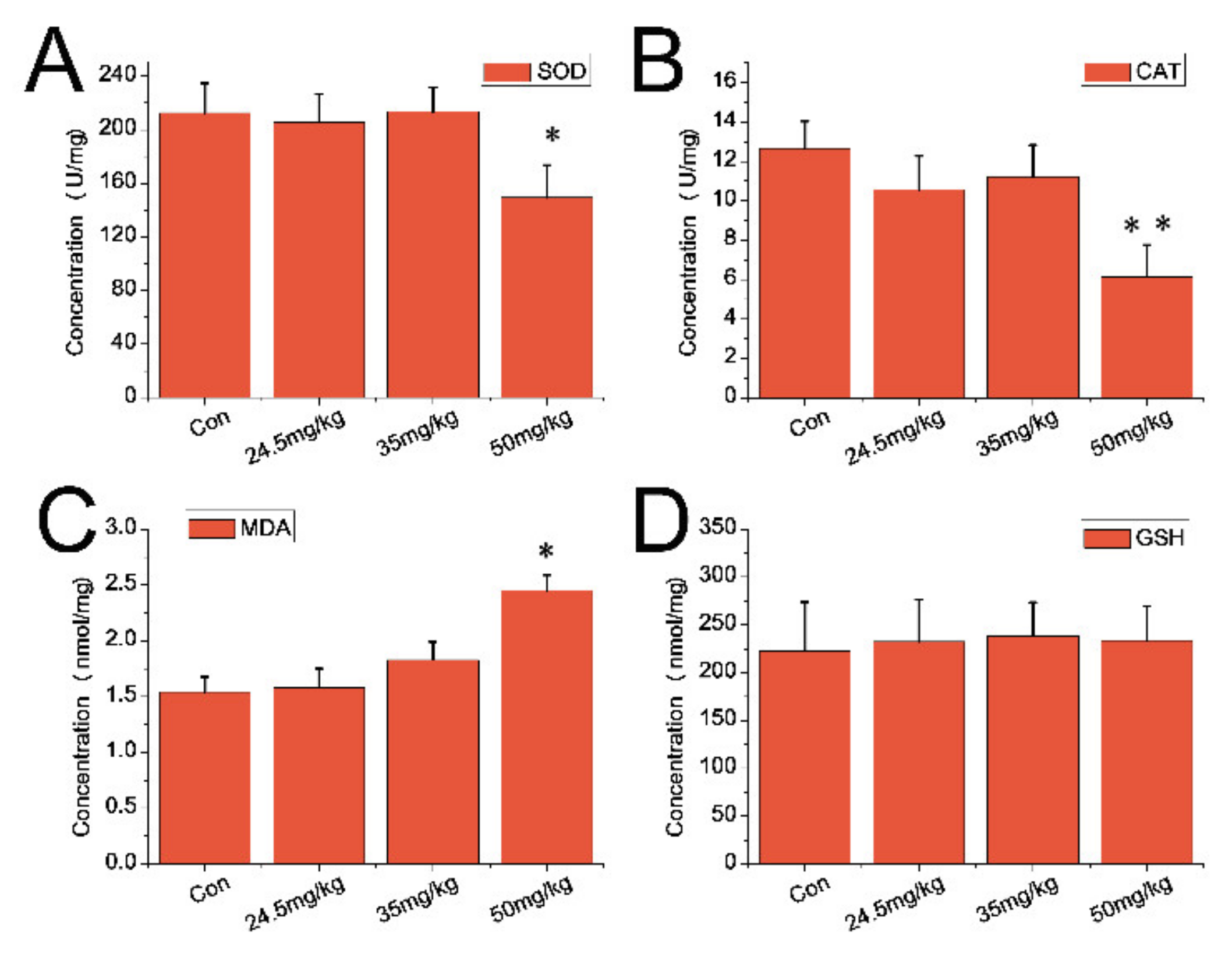
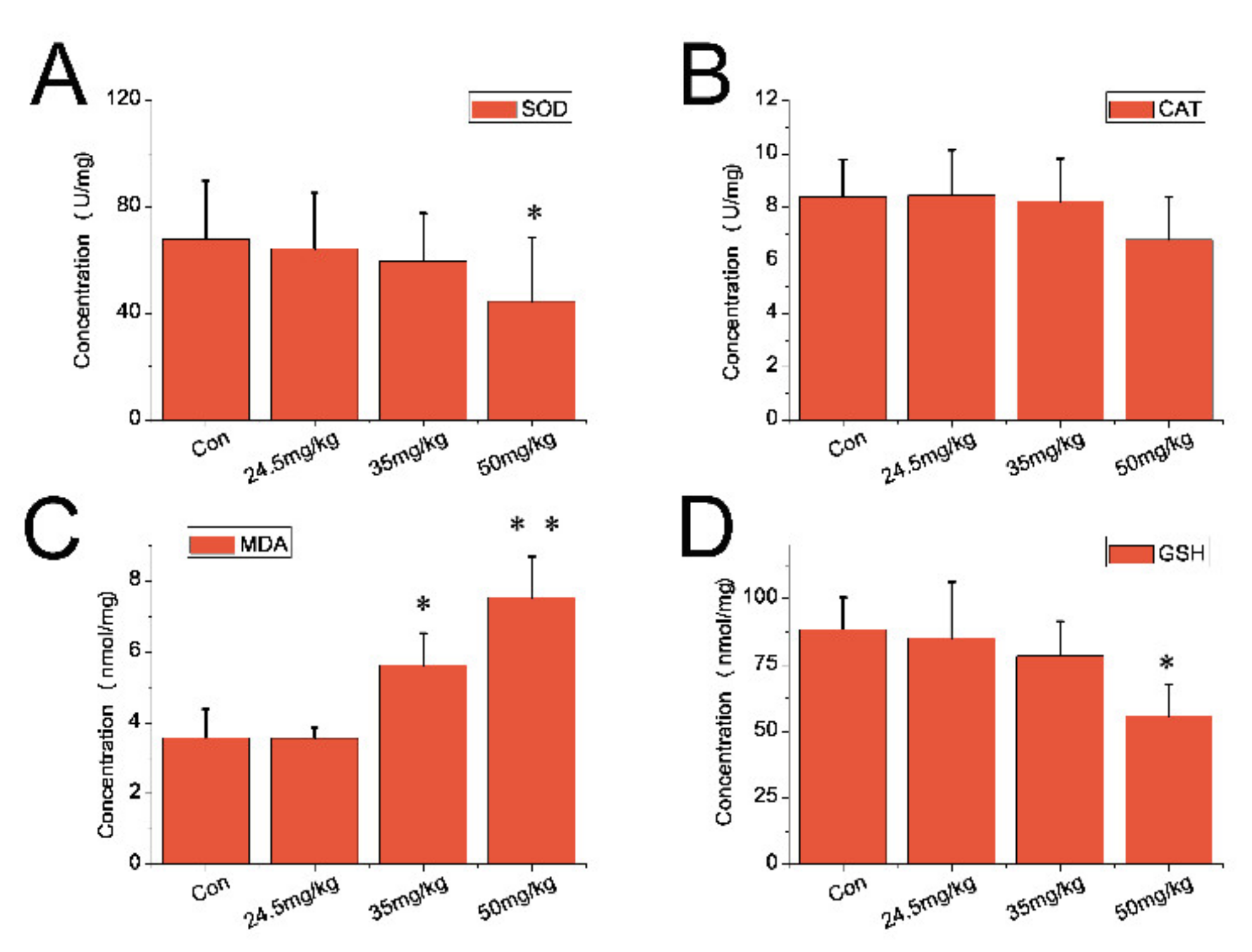
3.5. Indexes of Oxidative Stress
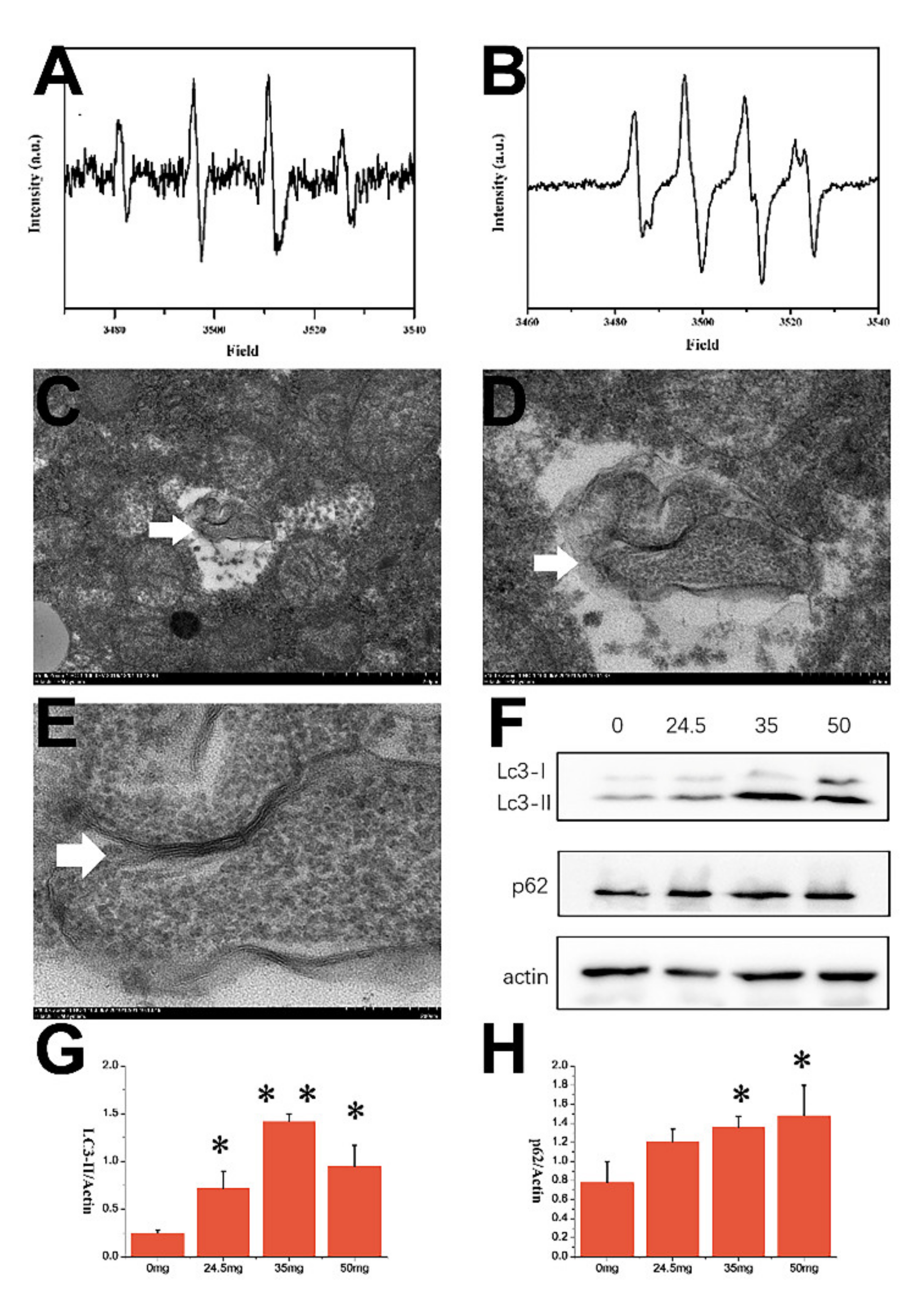
3.6. Electron Paramagnetic Resonance (EPR) Result of CZON
3.7. TEM and WB Results
3.8. Cu Content Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nano-materials in the environment: Behavior, fate, bioavailability, and effects-An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef] [PubMed]
- Eastlake, A.; Zumwalde, R.; Geraci, C. Can control banding be useful for the safe handling of nanomaterials? A systematic review. J. Nanoparticle Res. Interdiscip. Forum Nanoscale Sci. Technol. 2016, 18, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valsami-Jones, E.; Lynch, I. NANOSAFETY. How safe are nanomaterials? Science 2015, 350, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.-L.; Yang, S.-T.; Xing, G. Molecular toxicity of nanomaterials. J. Biomed. Nanotechnol. 2014, 10, 2828–2851. [Google Scholar] [CrossRef]
- Nalwa, H.S. A special issue on reviews in biomedical applications of nanomaterials, tissue engineering, stem cells, bioimaging, and toxicity. J. Biomed. Nanotechnol. 2014, 10, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, F.; Wang, J.; Zu, Y.; Gu, Z.; Zhao, Y. A Safe-by-design strategy towards safer nanomaterials in nano-medicines. Adv. Mater. 2019, 31, e1805391. [Google Scholar] [CrossRef]
- Farkas, J.; Booth, A.M. Are fluorescence-based chlorophyll quantification methods suitable for algae toxicity assessment of carbon nanomaterials? Nanotoxicology 2017, 11, 569–577. [Google Scholar] [CrossRef]
- Ng, C.T.; Yu, L.E.; Ong, C.N.; Bay, B.H.; Baeg, G.H. The use of Drosophila melanogaster as a model organism to study immune-nanotoxicity. Nanotoxicology 2018, 13, 429–446. [Google Scholar] [CrossRef]
- Dwivedi, S.; Saquib, Q.; Ahmad, B.; Ansari, S.M.; Azam, A.; Musarrat, J. Toxicogenomics: A new paradigm for nanotoxicity evaluation. Cell. Mol. Toxicol. Nanoparticles 2018, 1048, 143–161. [Google Scholar] [CrossRef]
- Lewis, R.W.; Bertsch, P.M.; McNear, D.H. Nanotoxicity of engineered nanomaterials (ENMs) to environmentally rel-evant beneficial soil bacteria—A critical review. Nanotoxicology 2019, 13, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Gajewicz, A. What if the number of nanotoxicity data is too small for developing predictive Nano-QSAR models? An alternative read-across based approach for filling data gaps. Nanoscale 2017, 9, 8435–8448. [Google Scholar] [CrossRef] [PubMed]
- Zangabad, P.S.; Karimi, M.; Mehdizadeh, F.; Malekzad, H.; Ghasemi, A.; Bahrami, S.; Zare, H.; Moghoofei, M.; Hekmatmanesh, A.; Hamblin, M.R. Nanocaged platforms: Modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale 2016, 9, 1356–1392. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Milosevic, A.; Hauser, D.; Lehner, R.; Blank, F.; Fink, A.; Rothen-Rutishauser, B. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv. Mater. 2018, 30, e1704307. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. Liver: Clearance of nanomaterials in the liver. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Lew, T.T.S.; Wong, M.H.; Kwak, S.-Y.; Sinclair, R.; Koman, V.B.; Strano, M.S. Rational design principles for the transport and subcellular distribution of nanomaterials into plant protoplasts. Small 2018, 14, e1802086. [Google Scholar] [CrossRef]
- Jiang, G.; Li, X.; Che, Y.; Lv, Y.; Liu, F.; Wang, Y.; Zhao, C.; Wang, X. Antibacterial and anticorrosive properties of CuZnO@RGO waterborne polyurethane coating in circulating cooling water. Environ. Sci. Pollut. Res. Int. 2019, 26, 9027–9040. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the antibacteri-al mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef]
- Mantecca, P.; Moschini, E.; Bonfanti, P.; Fascio, U.; Perelshtein, I.; Lipovsky, A.; Chirico, G.; Bacchetta, R.; Del Giacco, L.; Colombo, A.; et al. Toxicity evaluation of a new Zn-doped CuO nanocomposite with highly effective antibacterial properties. Toxicol. Sci. 2015, 146, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Yu, G.; Shan, Z.; Li, Z. Phyto-mediated synthesized multifunctional Zn/CuO NPs hybrid nanoparticles for en-hanced activity for kidney cancer therapy: A complete physical and biological analysis. J. Photochem. Photobiol. B Biol. 2018, 186, 131–136. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnology 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Malka, E.; Perelshtein, I.; Lipovsky, A.; Shalom, Y.; Naparstek, L.; Perkas, N.; Patick, T.; Lubart, R.; Nitzan, Y.; Banin, E.; et al. Eradication of multi-drug resistant bacteria by a novel Zn-doped CuO nanocomposite. Small 2013, 9, 4069–4076. [Google Scholar] [CrossRef] [PubMed]
- Djurisic, A.B.; Leung, Y.H.; Ng, A.M.; Xu, X.Y.; Lee, P.K.; Degger, N.; Wu, R.S. Toxicity of metal oxide nanoparti-cles: Mechanisms, characterization, and avoiding experimental artefacts. Small 2015, 11, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, J.; Caviedes, P.; Palza, H. Sol–gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics. Biomed. Mater. 2015, 10, 025001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirzad-Siboni, M.; Jafari, A.J.; Farzadkia, M.; Esrafili, A.; Gholami, M. Enhancement of photocatalytic activity of Cu-doped ZnO nanorods for the degradation of an insecticide: Kinetics and reaction pathways. J. Environ. Manag. 2017, 186, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, H.; Guo, J.; Wang, Q.; Cao, J.; Wang, H.; Li, G.; Mao, J.; Zou, X.; Chen, D.; et al. Nano-hydroxyapatite modulates osteoblast differentiation through autophagy induction via mTOR signaling pathway. J. Biomed. Nanotechnol. 2019, 15, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, F.; Yang, T.; Zhou, W.; Liu, Y.; Man, N.; Zhang, L.; Jin, N.; Dou, Q.; Zhang, Y.; et al. Tuning the au-tophagy-inducing activity of lanthanide-based nanocrystals through specific surface-coating peptides. Nat. Mater. 2012, 11, 817–826. [Google Scholar] [CrossRef]
- Hawkins, S.J.; Crompton, L.A.; Sood, A.; Saunders, M.; Boyle, N.T.; Buckley, A.; Minogue, A.M.; McComish, S.F.; Jimenez-Moreno, N.; Cordero-Llana, O.; et al. Nanoparticle-induced neuronal toxicity across placental barriers is medi-ated by autophagy and dependent on astrocytes. Nat. Nanotechnol. 2018, 13, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, G.; Ye, M.; He, Y.; Li, Z.; Wang, X.; Ou, C.; Yang, Z.; Chen, M. Bifunctional supramolecular hydrogel alleviates myocardial ischemia/reperfusion injury by inhibiting autophagy and apoptosis. J. Biomed. Nanotechnol. 2018, 14, 1458–1470. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Wang, D.; Hao, M.; Kong, C.; Zhao, X.; Gao, Y.; Li, J.; Liu, B.; Yang, B.; et al. Inhibition of autoph-agy promoted apoptosis and suppressed growth of hepatocellular carcinoma upon photothermal exposure. J. Biomed. Nanotechnol. 2019, 15, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, R.; Sun, R.; Du, L.; Liu, L.; Zhang, K.; Ai, H.; Guo, Y. Superparamagnetic iron oxide nanoparticles as magnetic resonance imaging contrast agents and induced autophagy response in endothelial progenitor cells. J. Biomed. Nanotechnol. 2019, 15, 396–404. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Zhu, W.; Li, D.; Yang, L.; Duan, J.; Cai, Z.; Nie, Y.; Zhang, Y.; Gong, Q.; et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling. Biomaterials 2019, 203, 23–30. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, J.; Luo, Z.; Su, G.; Yue, T.; Yan, B. Remote induction of cell autophagy by 2D MoS2 nanosheets via per-turbing cell surface receptors and mTOR pathway from outside of cells. ACS Appl. Mater. Interfaces 2019, 11, 6829–6839. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Zhang, R.; Wang, J.; Su, T.; Tian, Z.; Han, D.; Zhao, C.; Fan, M.; Li, C.; et al. Quaternized chi-tosan/alginate-Fe3O4 magnetic nanoparticles enhance the chemosensitization of multidrug-resistant gastric carcinoma by regulating cell autophagy activity in mice. J. Biomed. Nanotechnol. 2016, 12, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhong, Z.; Kou, J.; Zheng, Y.; Liu, Z.; Jiang, Y.; Zhang, Z.; Gao, Z.; Cong, L.; Tian, Y.; et al. ROS generated by upconversion nanoparticle-mediated photodynamic therapy induces autophagy via PI3K/AKT/ mTOR signaling pathway in M1 peritoneal macrophage. Cell. Physiol. Biochem. 2018, 48, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Ma, Y.; Zhu, Y.; Yong, L.; Liu, X.; Wang, P.; Liang, C.; Yang, C.; Zhao, Z.; Hai, B.; et al. Cross talk between autophagy and apoptosis contributes to ZnO nanoparticle-induced human osteosarcoma cell death. Adv. Heal. Mater. 2018, 7, e1800332. [Google Scholar] [CrossRef]
- Chou, C.C.; Chen, W.; Hung, Y.; Mou, C.Y. Molecular elucidation of biological response to mesoporous silica nano-particles In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef]
- Ma, N.; Liu, P.; He, N.; Gu, N.; Wu, F.G.; Chen, Z. Action of gold nanospikes-based nanoradiosensitizers: Cellular internalization, radiotherapy, and autophagy. ACS Appl. Mater. Interfaces 2017, 9, 31526–31542. [Google Scholar] [CrossRef]
- Jayaprakash, J.; Srinivasan, N.; Chandrasekaran, P.; Girija, E. Synthesis and characterization of cluster of grapes like pure and Zinc-doped CuO nanoparticles by sol–gel method. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2015, 136, 1803–1806. [Google Scholar] [CrossRef]
- Burgoyne, J.R. Oxidative stress impairs autophagy through oxidation of ATG3 and ATG7. Autophagy 2018, 14, 1092–1093. [Google Scholar] [CrossRef] [PubMed]
- Ruart, M.; Chavarria, L.; Camprecios, G.; Suarez-Herrera, N.; Montironi, C.; Guixe-Muntet, S.; Bosch, J.; Friedman, S.L.; Garcia-Pagan, J.C.; Hernandez-Gea, V. Impaired endothelial autophagy promotes liver fibrosis by aggravating the ox-idative stress response during acute liver injury. J. Hepatol. 2019, 70, 458–469. [Google Scholar] [CrossRef]
- O’Grady, S.M. Oxidative stress, autophagy and airway ion transport. Am. J. Physiol. Physiol. 2019, 316, C16–C32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.; Wan, J.; Guo, W.; Ou, C.; Liu, T.; Fu, C.; Zhang, Q.; Ren, X.; Liang, X.J.; Ren, J.; et al. Renal-clearable quater-nary chalcogenide nanocrystal for photoacoustic/magnetic resonance imaging guided tumor photothermal therapy. Biomaterials 2018, 159, 108–118. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Highly efficient photocatalytic degradation of organic dyes by Cu doped ZnO nanostructures. Phys. Chem. Chem. Phys. 2015, 17, 25172–25181. [Google Scholar] [CrossRef]
- Fkiri, A.; Wiem, S.; Sellami, B.; Saidani, M.A.; Khazri, A.; Smiri, L.-S. Facile synthesis of Cu-doped ZnO nanoparticle in triethyleneglycol: Photocatalytic activities and aquatic ecotoxicity. Environ. Technol. 2019, 41, 3745–3755. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Haidari, M.; Khan, W.; Fatima, A.; Jyoti, S.; Khanam, S.; Naz, F.; Rahul Ali, F.; Singh, B.R. Toxic potential of copper-doped ZnO nanoparticles inDrosophila melanogaster (Oregon R). Toxicol. Mech. Methods 2015, 25, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.S.R.; Pavitra, E.; Merchant, N.; Lee, H.; Prasad, G.L.V.; Nagaraju, G.P.; Huh, Y.S.; Han, Y.-K. Targeting autophagy in gastrointestinal malignancy by using nanomaterials as drug delivery systems. Cancer Lett. 2018, 419, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Verma, S.K.; Das, T.; Panda, P.K.; Parashar, K.; Suar, M.; Parashar, S.K.S. Altered electrical properties with controlled copper doping in ZnO nanoparticles infers their cytotoxicity in macrophages by ROS induction and apoptosis. Chem. Biol. Interact. 2019, 297, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.H.; Chen, Z.Y.; Wang, Y.J.; Yan, S.J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.K.; Jha, E.; Panda, P.K.; Mukherjee, M.; Thirumurugan, A.; Makkar, H.; Das, B.; Parashar, S.K.S.; Suar, M. Mechanistic insight into ROS and neutral lipid alteration induced toxicity in the human model with fins (Danio rerio) by industrially synthesized titanium dioxide nanoparticles. Toxicol. Res. 2018, 7, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Hozayen, W.G.; Mahmoud, A.M.; Desouky, E.M.; El-Nahass, E.S.; Soliman, H.A.; Farghali, A.A. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed. Pharmacother. 2019, 109, 2527–2538. [Google Scholar] [CrossRef]
- Yue, L.; Zhao, J.; Yu, X.; Lv, K.; Wang, Z.; Xing, B. Interaction of CuO nanoparticles with duckweed (Lemna minor. L): Uptake, distribution and ROS production sites. Environ. Pollut. 2018, 243, 543–552. [Google Scholar] [CrossRef]
- Ghaemi, B.; Shaabani, E.; Najafi-Taher, R.; Jafari Nodooshan, S.; Sadeghpour, A.; Kharrazi, S.; Amani, A. Intracellular ROS induction by Ag@ZnO core-shell nanoparticles: Frontiers of permanent optically active holes in breast cancer theranostic. ACS Appl. Mater. Interfaces 2018, 10, 24370–24381. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.; Khan, M.M.; Alhadlaq, H.A.; Alshamsan, A. Cobalt iron oxide nanoparticles induce cytotoxicity and regulate the apoptotic genes through ROS in human liver cells (HepG2). Colloids Surf. B Biointerfaces 2016, 148, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.; Zine, K.; Fahima, K.; Abdelfattah, E.; Sharifudin, S.M.; Duduku, K. NiO nanoparticles induce cytotoxi-city mediated through ROS generation and impairing the antioxidant defense in the human lung epithelial cells (A549): Preventive effect of Pistacia lentiscus essential oil. Toxicol. Rep. 2018, 5, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Refsnes, M.; Skuland, T.; Lilleaas, E.; Ovrevik, J.; Lag, M. Concentration-dependent cytokine responses of silica nano-particles and role of ROS in human lung epithelial cells. Basic Clin. Pharmacol. Toxicol. 2019, 8, 6647. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Teng, X.; Huang, X.; Liu, H.; Chen, D.; Ren, J.; He, J.; Tang, F. Single and repeated dose toxicity of meso-porous hollow silica nanoparticles in intravenously exposed mice. Biomaterials 2011, 32, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Saowalak, K.; Titipun, T.; Somchai, T.; Chalermchai, P. Iron(III)-tannic molecular nanoparticles enhance autophagy effect and T1 MRI contrast in liver cell lines. Sci. Rep. 2018, 8, 304–314. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Q.; Wang, Y.; Duan, L.; Xu, X.; Hu, Y.; Yang, Y.; Zhang, L.; Liu, Z.; Bao, H.; Liu, T. Cu-Doped-ZnO Nanocrystals Induce Hepatocyte Autophagy by Oxidative Stress Pathway. Nanomaterials 2021, 11, 2081. https://doi.org/10.3390/nano11082081
Bai Q, Wang Y, Duan L, Xu X, Hu Y, Yang Y, Zhang L, Liu Z, Bao H, Liu T. Cu-Doped-ZnO Nanocrystals Induce Hepatocyte Autophagy by Oxidative Stress Pathway. Nanomaterials. 2021; 11(8):2081. https://doi.org/10.3390/nano11082081
Chicago/Turabian StyleBai, Qianyu, Yeru Wang, Luoyan Duan, Xiaomu Xu, Yusheng Hu, Yue Yang, Lei Zhang, Zhaoping Liu, Huihui Bao, and Tianlong Liu. 2021. "Cu-Doped-ZnO Nanocrystals Induce Hepatocyte Autophagy by Oxidative Stress Pathway" Nanomaterials 11, no. 8: 2081. https://doi.org/10.3390/nano11082081
APA StyleBai, Q., Wang, Y., Duan, L., Xu, X., Hu, Y., Yang, Y., Zhang, L., Liu, Z., Bao, H., & Liu, T. (2021). Cu-Doped-ZnO Nanocrystals Induce Hepatocyte Autophagy by Oxidative Stress Pathway. Nanomaterials, 11(8), 2081. https://doi.org/10.3390/nano11082081





