Geopolymers vs. Cement Matrix Materials: How Nanofiller Can Help a Sustainability Approach for Smart Construction Applications—A Review
Abstract
:1. Introduction
- Raw materials preparation. After quarrying, principal raw materials (such as limestone and clay) are crushed to a maximum of about 7.5 cm in diameter by a two-step grinding treatment. In a “dry” production method, crushed aggregates are fed directly into the kiln. Conversely, the “wet” method consists of the mixing of ground material with water to form a slurry, a suspension of creamy consistency composed of water (35–50%) and fine particles. Next, the slurry is inserted into the kiln. “Wet” preparation ensures an easier control of the chemistry process and is suitable in the presence of moist raw aggregates. However, it has higher energy requirements due to the need for slurry water evaporation. On the other hand, the “dry” process is faster and less energy expensive [3].
- Clinker production. Ground ingredients are burned in the kiln at a temperature of about 1350 °C to 1500 °C. Various materials can be used as kiln fuels. Traditional sub-stances are fossil fuels, oil, coal, and gas. Secondary raw materials, including waste oils, plastics, waste tires, and sewage sludge, are considered alternative fuels for the cement industry [4]. Thermal treatment involves the partial fusion of raw materials, the breaking of their chemical bonds, and the recombination into new compounds. The result is a nodular-shaped clinker product.
- Final grinding process. After cooling, clinker nodules are crushed into a superfine powder by steel ball milling treatment. During this process, the clinker is mixed with a small amount of gypsum (3–5%) to produce PC. Gypsum prevents the flash setting of the cement and regulates its hardening.
- Use of alternative fuels or raw materials to mitigate CO2 emission for Portland cement manufacturing.
- Adoption of CO2-capturing technologies in the cement plants.
- Development of Portland-free alternative binders.
2. CO2 Emission Mitigation Strategies in the Cement Industry: An Overview
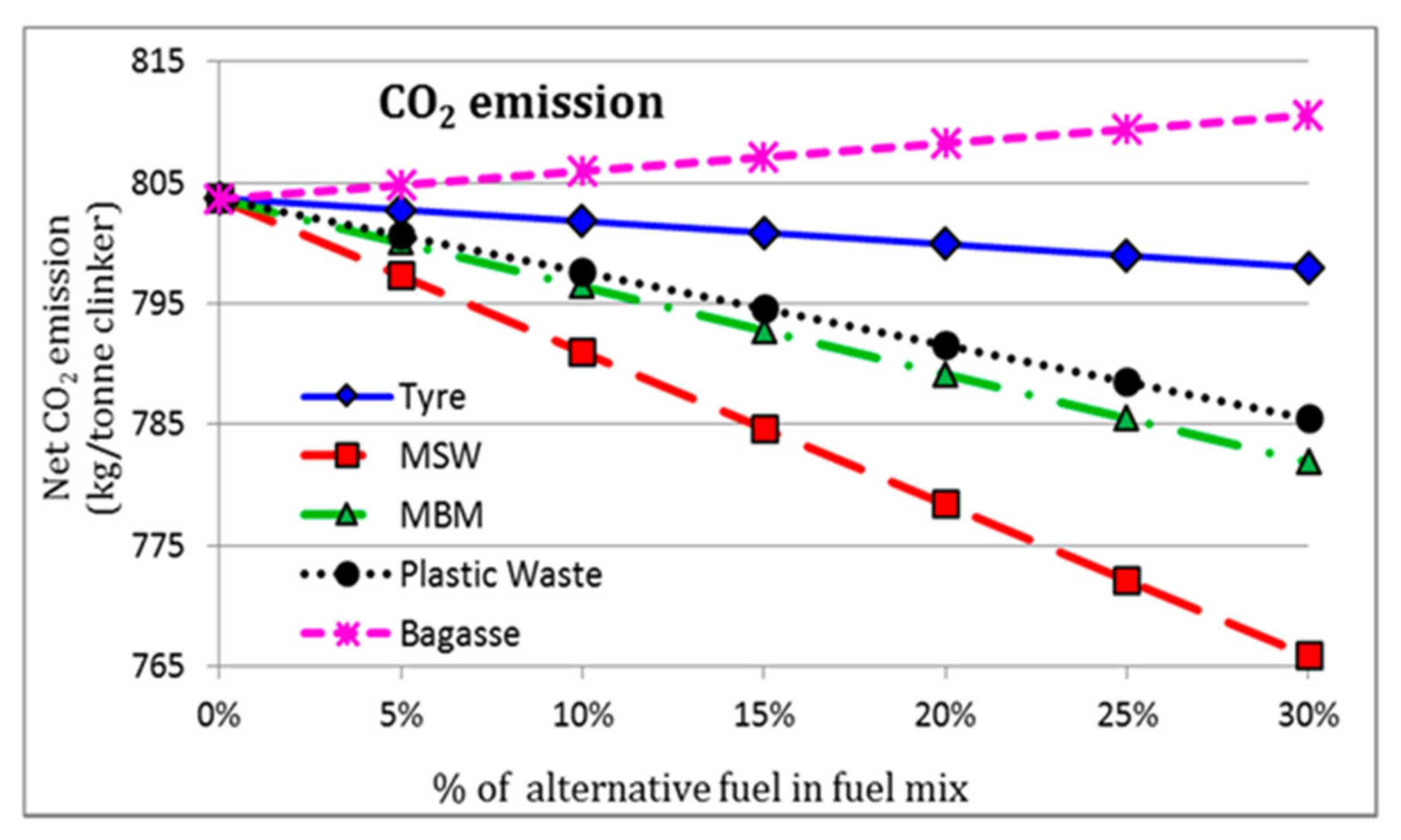
2.1. Alternative Fuels in PC Manufacturing
2.2. CO2-Capturing Technologies
2.2.1. Post-Combustion Amine Scrubbing
- Adverse effect of the gaseous and solid clinkering by-products including Nitrogen dioxide (NO2), Sulphur dioxide (SO2), Hydrochloric acid (HCl), and dust on the absorption efficiency of amine solvent.
- Additional amount of heat required to regenerate the solvent. This contribution remarkably penalizes the power plant efficiency.
- The large-scale application of amine-based systems is opposed by the toxicity of the amine chemical products.
2.2.2. Calcium (Ca) Looping Process
2.3. Alternative Cementitious Binders
2.3.1. CACs
- Refractory materials for industrial use in high-temperature processes.
- Repair material for infrastructural applications and protective coatings due to excellent resistance to chemical attack and abrasion.
- Printable materials in additive manufacturing (AM) technologies due to fast setting after deposition.
2.3.2. SSCs
2.3.3. MCs
- Urea hydrolysis. In a Ca-rich environment and an aqueous medium, a substrate solution is broken down into Ammonium (NH4+) and Carbonate (CO32−) by the bacterial enzyme (microbial urease), according to the following reaction:
- Calcite precipitation. Due to the breakdown of the substrate, the pH value around the surrounding environment increases. The presence of Ca ion results in CO32− precipitate as CaCO3 crystals.
- Ca-microorganism coordination. In addition to calcite precipitation, due to the complementary net charge, positive Ca ions deposit on the negative microorganism surface, generating nucleation sites on the cell wall.
- Generation of cementitious mass. CaCO3 crystals generate cementitious connections between the mineral aggregates. The presence of calcite sites protects the microorganism from the highly alkaline concrete environment and promotes the diffusion of the cementitious bridge until obtaining a single mass.
- High cost of bacterial cultures. The overall price of MC products is up to four times higher than conventional PC concrete. Besides, the manipulation of bacterial species requires skilled personnel, which increases the cost of R&D activities.
- Sensitivity of biological raw materials. MCs performance may significantly vary with geographic and environmental location. Besides, bacteria are considered dangerous to health and strict controls are required.
- Lack of standard. Nowadays, there are still no technical recommendations or protocols in place concerning the testing and acceptance criteria.
- Long-term durability. MCs are an emerging technology, and experimental research or analysis on the long-term (at least 50 years, as suggested by technical standards) durability and anti-corrosiveness of the bacterial-based cement products are not available.
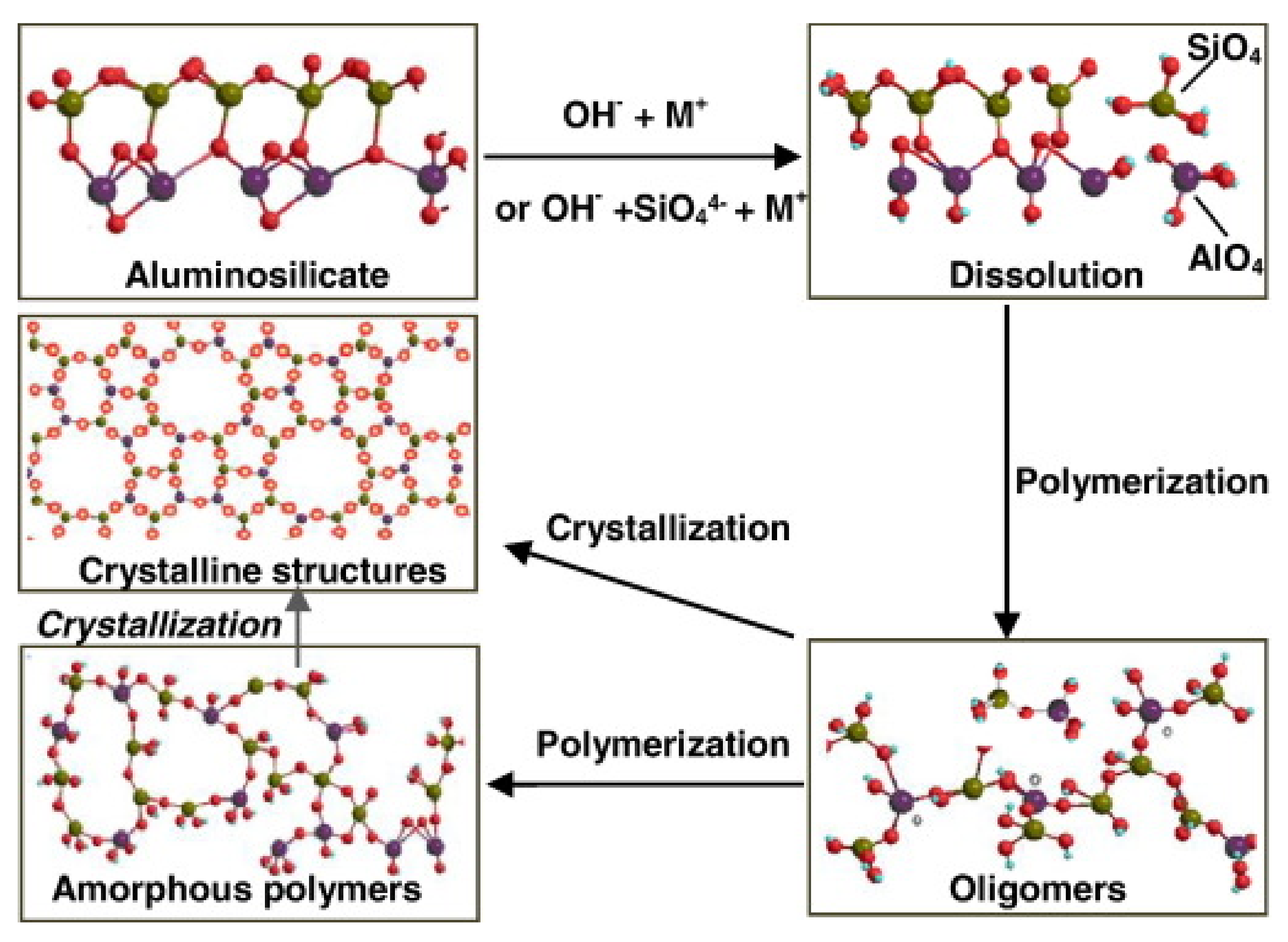
3. Geopolymer Cements: Basic Features and Novel Advances
- Preparation of the activator solution. The alkali solution is prepared at least one day prior to its use, employing distilled water to avoid contamination by unknown substances. Common molarity values investigated are in the range of 8 to 16 M.
- Raw material mixing. One or more precursor types are mixed with the mineral aggregates (fine sand or coarse fraction), in dry conditions, for 2–3 min. The aggregate part occupies about 75–80% by mass of geopolymer concrete/mortar.
- Activator addition. The alkaline solution is added to the solid fractions and mixed for 3–5 min. To improve the rheology of the compound, in terms of workability, a part of water-reducing admixture could be added to the mixture. Firstly, the admixture is mixed with the alkali solution and then added to the solid dry material.
- Casting. The fresh material is cast in specific molds (cylinder, cubes, or beams) kept under vibration for 10 s.
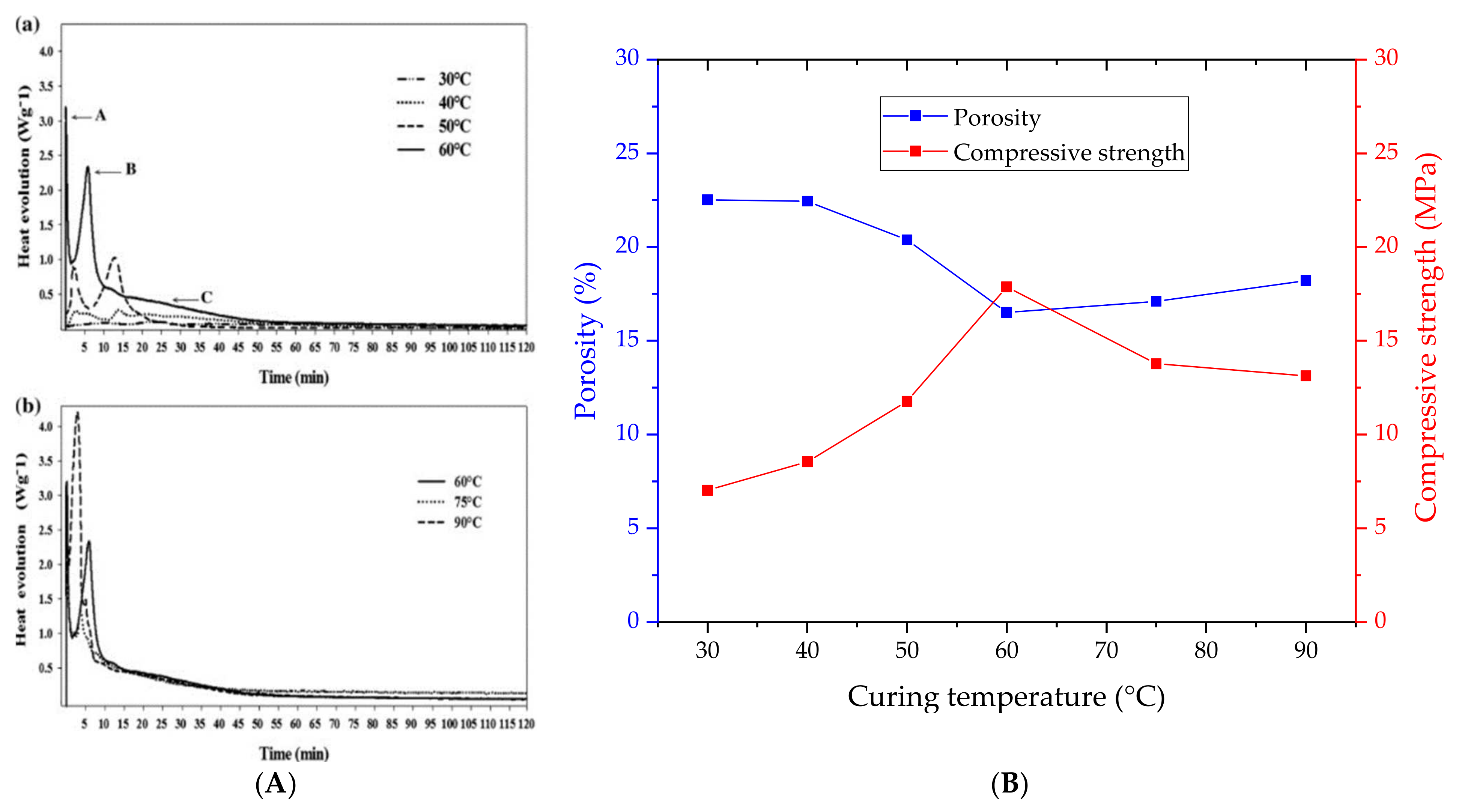
- Curing. The specimen curing can be performed under various temperature and time regimes. The material hardening can be completed entirely at room temperature or provide oven treatments between 30 °C to 90 °C, accelerating the curing. Typical treatment times are selected in the range from 6 h to 96 h.
3.1. Influence on Mechanical Strength Performance
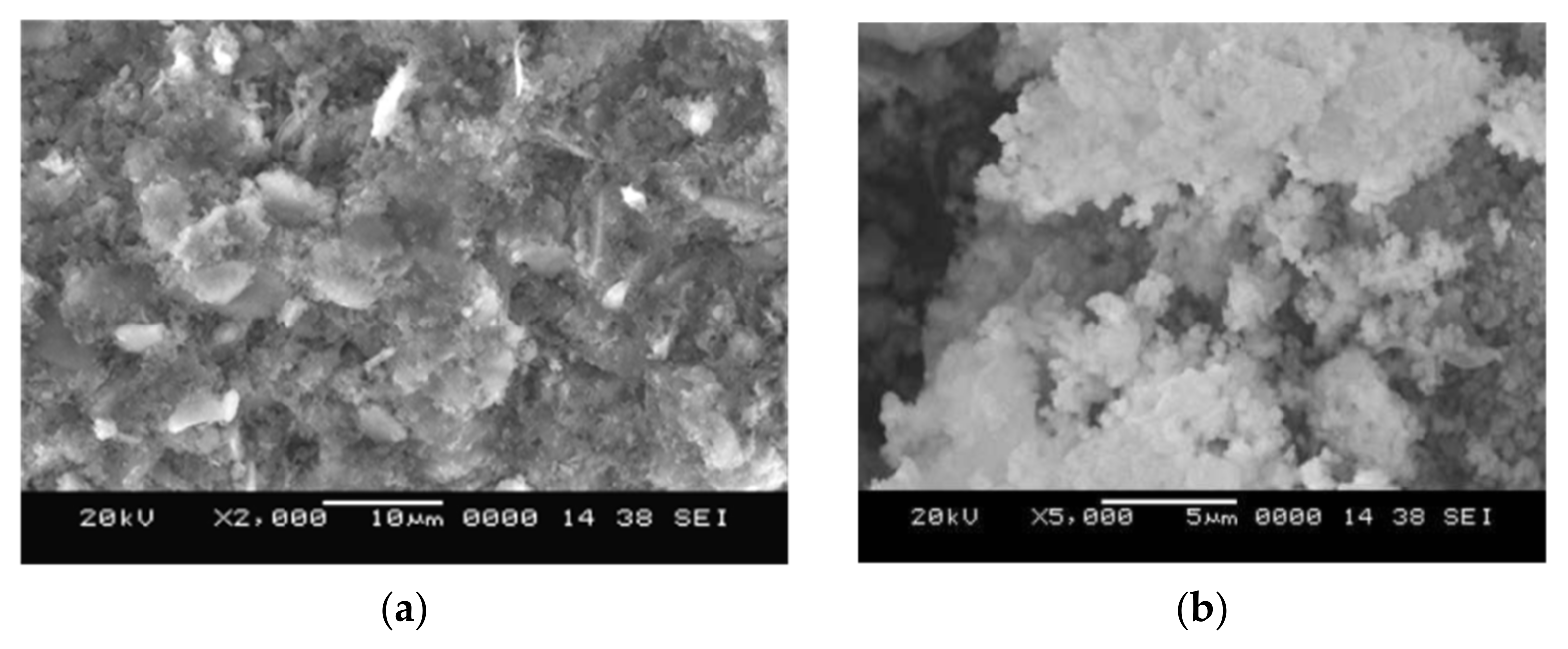
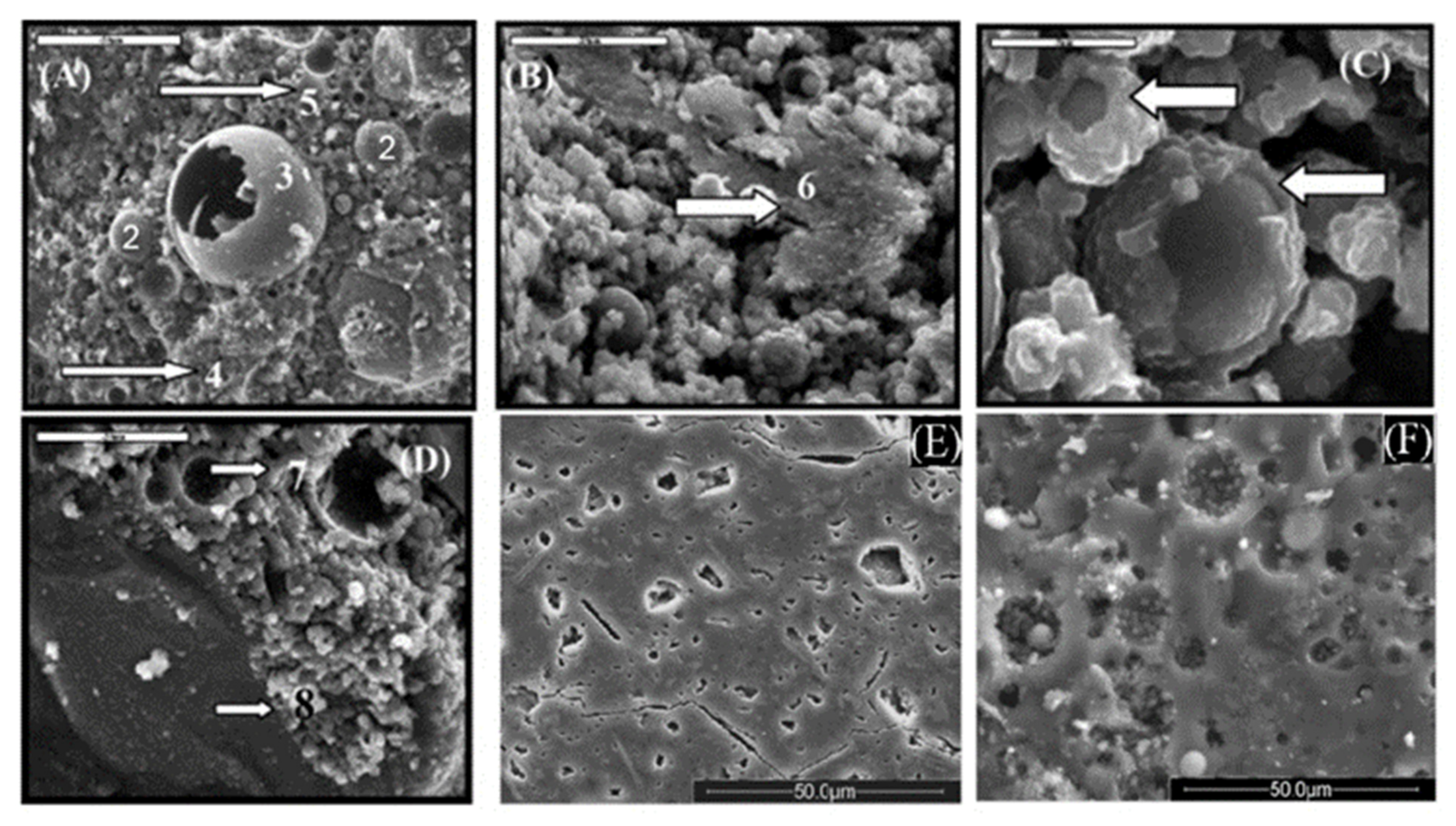
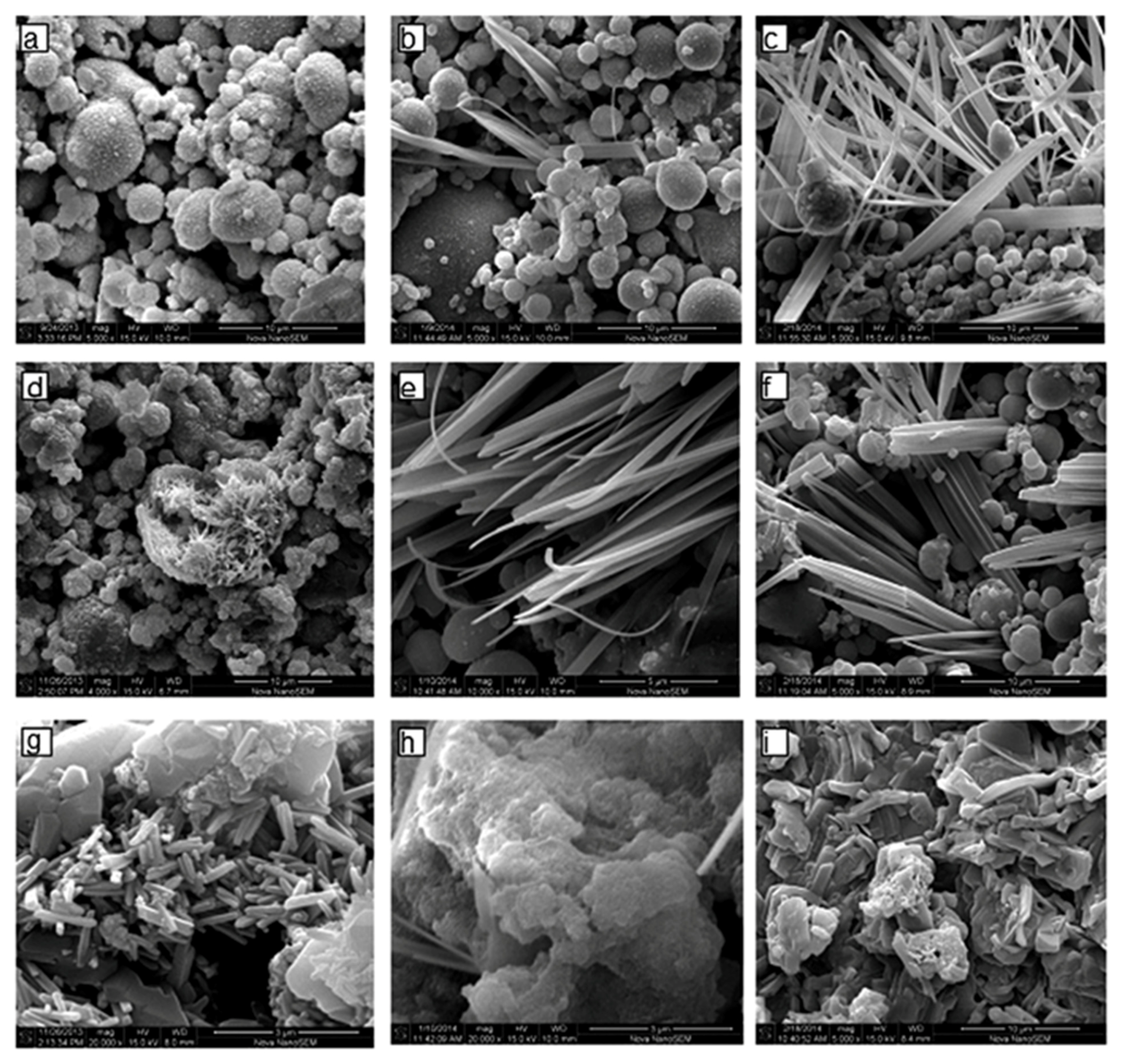
3.2. Influence on Microstructural Features
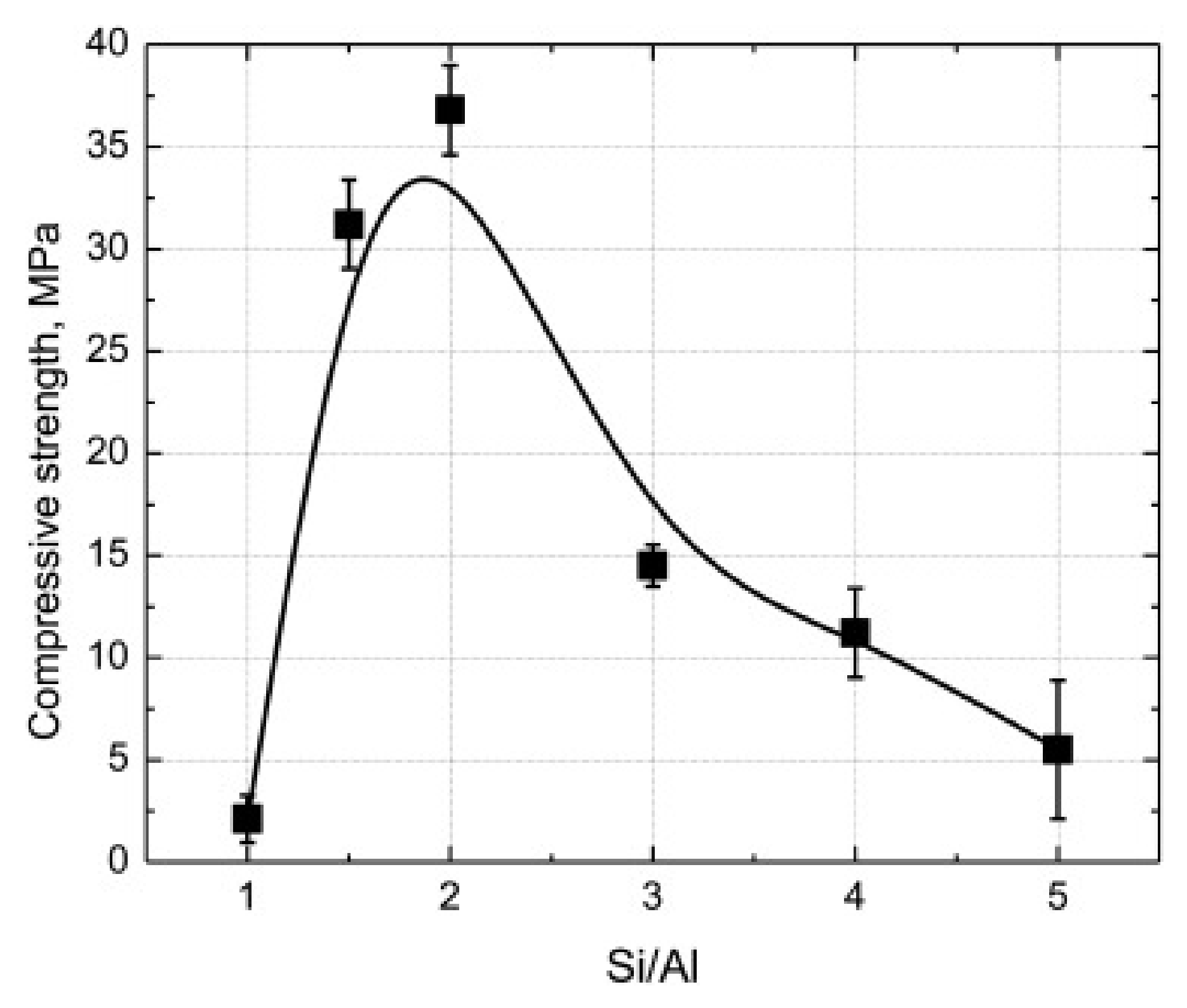
3.3. Influence on Mineralogical Characteristics
- 450 cm−1 bands associated with Si–O–Si bending vibration and common to both the precursor and geopolymer samples.
- A peak around 900–1200 cm−1 associated with Si–O–Si and Si–O–Al asymmetric stretching vibration. This peak shifts to shorter wavelengths in geopolymer samples than FA as a result of the activation process and different Si/Al ratio in the system.
- Broad bands around 1650 cm−1 and 3480 cm−1 assigned to water (–OH stretching vibration and O–H–O bending vibration, respectively. These peaks are not detectable in the FA sample, indicating the advent of geopolymerization process.
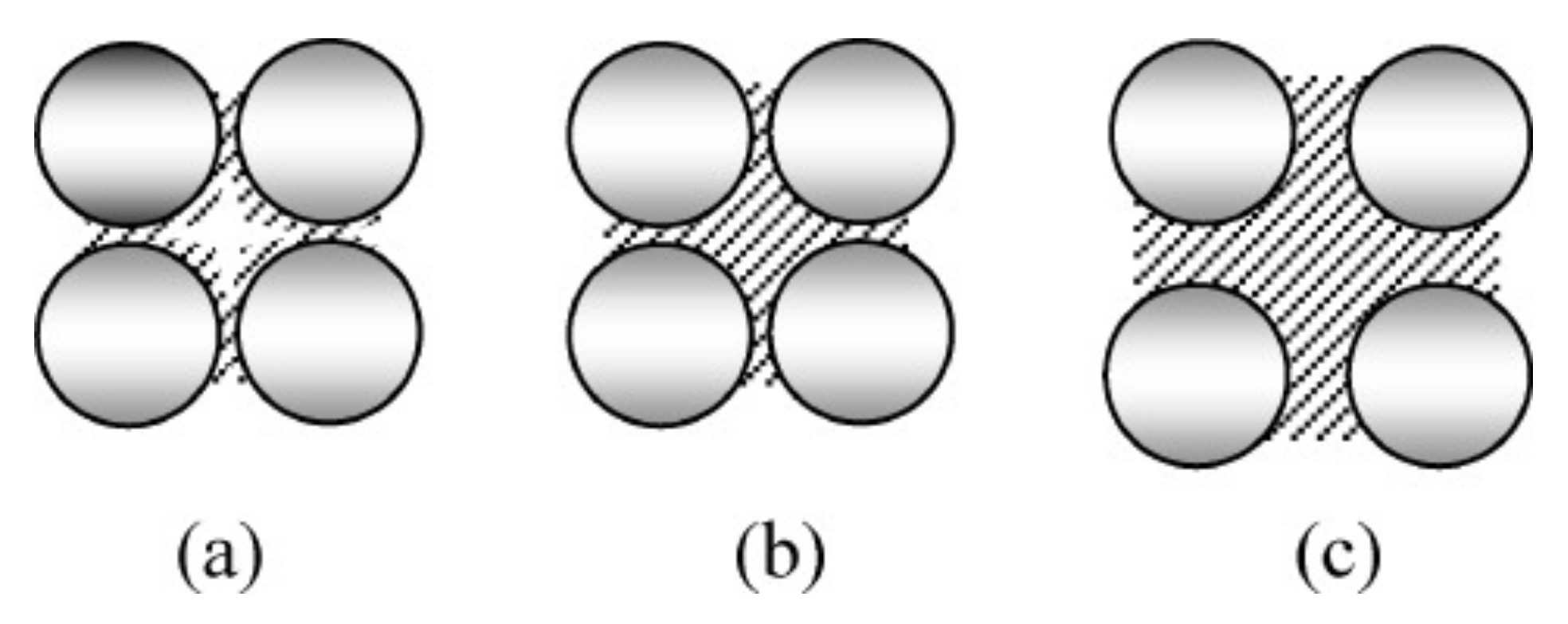
3.4. Influence on Porosity and Implications on Strength Properties
3.5. Influence of Nanostructured Materials on Geopolymer Performances
3.5.1. Nanoparticles
3.5.2. Nanofibers
3.5.3. Carbon-Based Nanostructured Sheet
- Fresh properties. The high specific surface area of CNTs decreases the workability and increases the viscosity of the fresh mixes. However, insignificant change in rheology can be reached for low CNT concentration (less than 0.2 wt%).
- Mechanical properties. An incorporation of a very small amount of CNT leads a strong mechanical enhancement: 0.5 wt% of nano-sized fillers corresponded to a mechanical strength 110–130% higher than plain geopolymer mix. CNTs act as reinforcing fillers due to the excellent mechanical behavior and crack bridging properties. Besides, it was demonstrated that a proper dosage of CNTs decreases the total porosity of the material. However, there will be a limit concentration (also defined as a percolation threshold), depending on the initial property of CNT and mix design of geopolymer samples, above which agglomeration and poor dispersion of CNTs will occur, leading to strong losses in mechanical strength.
- Durability. Addition of CNTs below the percolation threshold reduces the overall porosity and improves the microstructural compaction of the material, minimizing its hygrometric shrinkage and permeability to water and other aggressive agents.
- Smart properties. The presence of CNT in the geopolymer matrix generates a conductive network which improves the electrical conductivity properties. This peculiarity can potentially be exploited in self-sensing application for structural health monitoring.
3.6. Section Summary
4. Geopolymer vs. Ordinary PC Mixes: A Comparative Analysis
4.1. Geopolymer vs. Ordinary PC Mixes: Chemistry and Microstructural Analysis
4.2. Geopolymer vs. Ordinary PC Mixes: Mechanical Strength Properties
4.3. Geopolymer vs. Ordinary Portland Mixes: Durability Performance
4.3.1. Acid Attack Resistance: Discussion
4.3.2. Sulphate Attack Resistance: Discussion
4.3.3. Carbonation Resistance: Discussion
4.3.4. Water Sorptivity: Discussion
4.3.5. Freeze-Thaw Resistance: Discussion
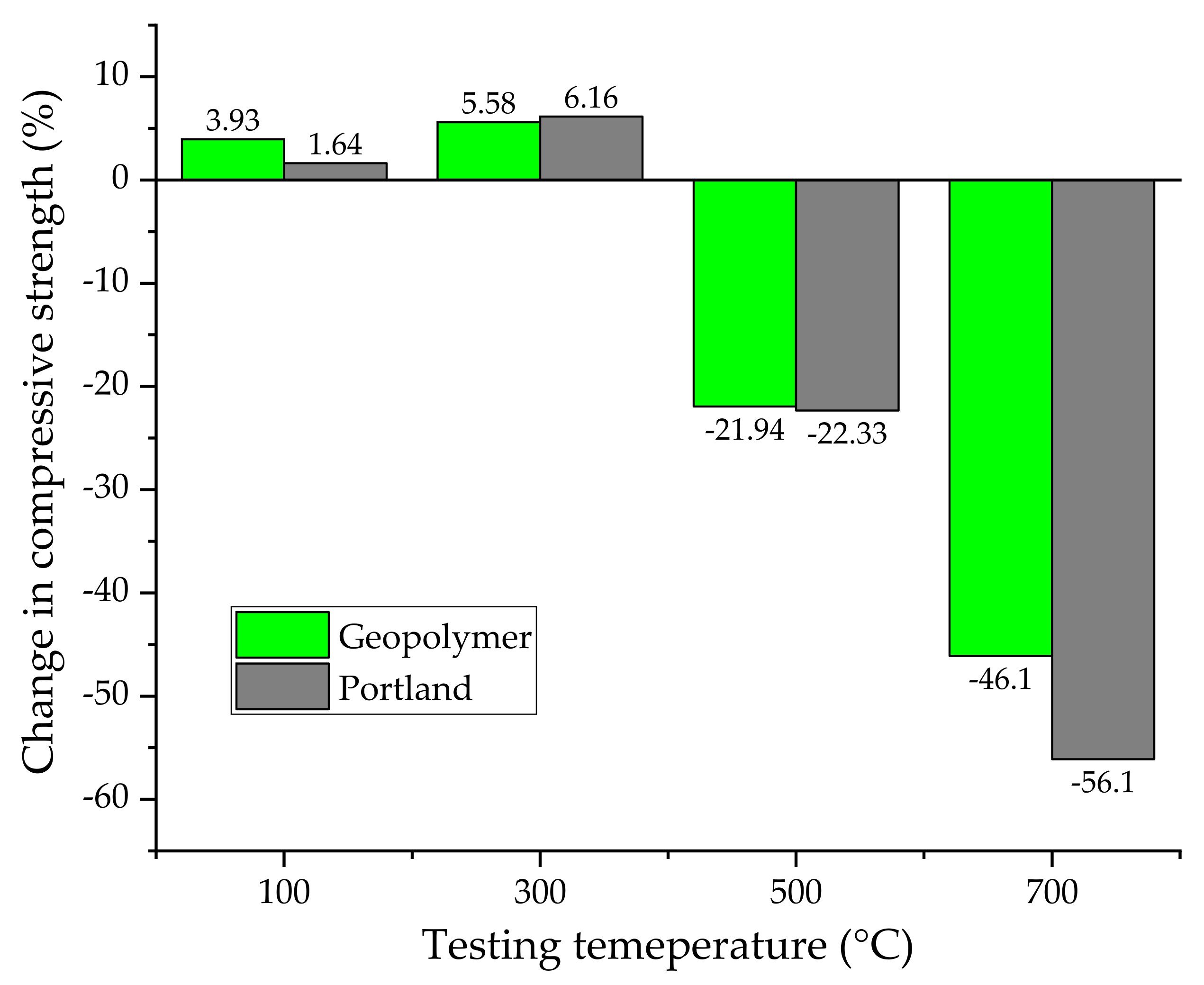
4.4. Geopolymer vs. Ordinary Portland Mixes: Fire Resistance and High-Temperature Behavior
4.4.1. Relationship between Thermal Stability and Pore Structure
4.4.2. Effect of the Alkaline Activator on Fire Performance
4.4.3. High-Temperature Performance of Ferrochrome Slag-Based Geopolymer Concrete
4.4.4. Influence of GBFS on Thermal Stability of FA-Based Geopolymer Concrete
4.5. Geopolymer vs. Ordinary Portland Mixes: Energy and Carbon-Emission Analysis
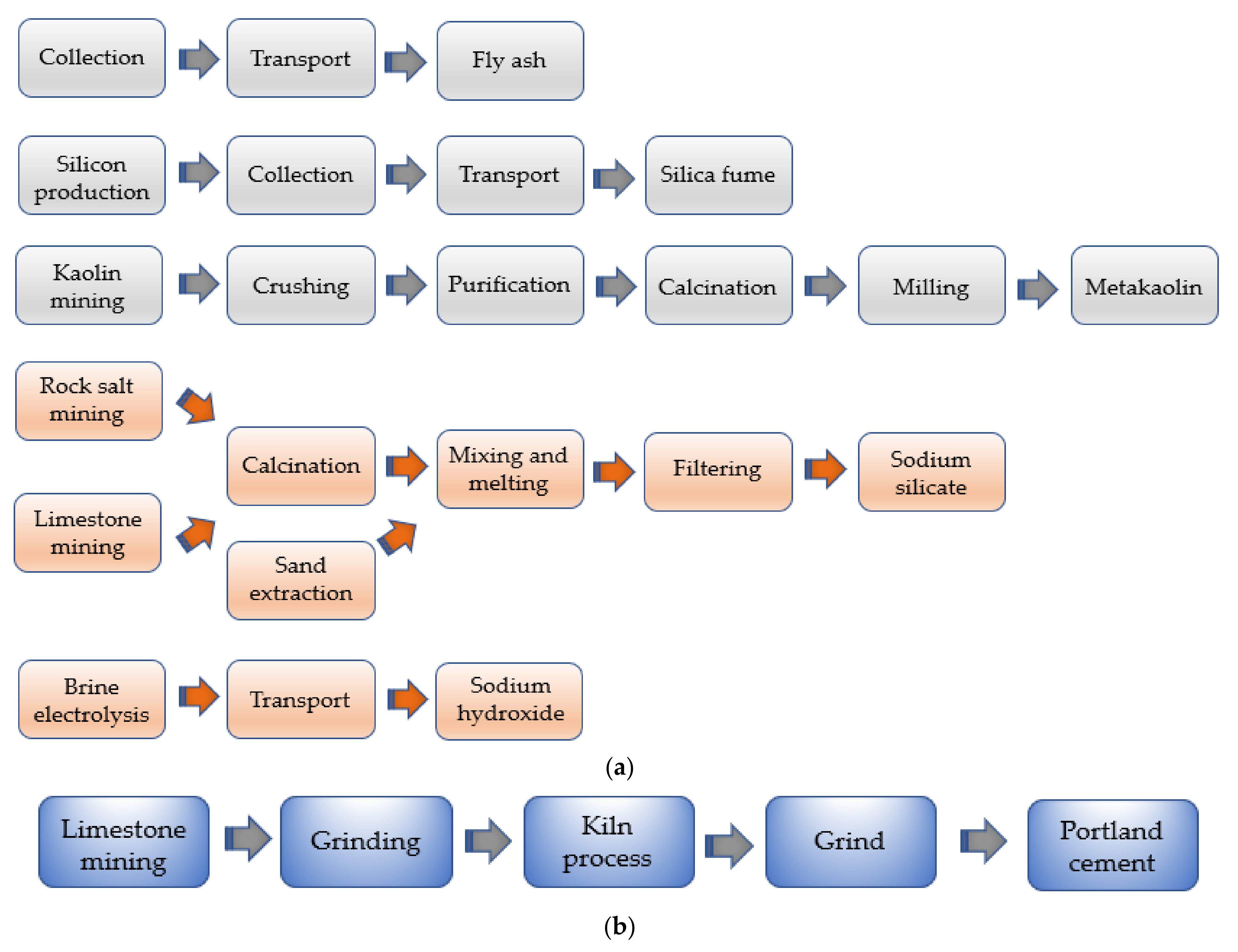
- Although the production cycle of geopolymer materials involves much more articulated stages than the “linear” flow of PC (see Figure 19), overall better performance in terms of CO2-emission and energy consumption is recorded, resulting in a valid sustainable alternative to the traditional concrete materials.
- Due to the use of highly alkaline activating solutions, the water ecotoxicity and human toxicity are slightly higher than the PC mix. However, in recent years, more clean approaches were introduced, such as the “one-part” geopolymeric mixes, where the solid aluminosilicate precursors and activators are mixed just with water [107].
- Geopolymer cement production tends to be affected more by the transportation of raw materials than Portland. However, it is important to mention that the influence of transportation depends upon the demographic conditions and local availability of the resource. For instance, the effect in poorly populated countries will be greater than the densely populated countries, assuming that per capita demand and manufacturing of concrete is the same [108].
4.6. Section Summary
5. Conclusions
- By optimizing the mix design, comparable/superior strength properties to common Portland concrete mixes can be obtained. The best microstructural quality of the geopolymer matrix, in terms of reduced porosity and strengthening action of aluminosilicate particles, appears to be the main reason for this evidence.
- Overall greater chemical-physical durability (except for freezing and carbonation resistance). The lower permeability and the absence of Ca-rich hydration products (commonly found in Portland pastes) in geopolymer increase the inertia of the material against the permeation of corrosive agents and acid/sulphate attacks.
- Higher thermal stability. The ceramic nature of the geopolymers, instead of the hydrate feature, provides better resistance to high temperatures than PC mixes, allowing application in thermally hostile environments.
- In terms of sustainable production, some comparative LCA data show that geopolymer technology has a lower environmental impact than Portland manufacturing. According to the technical information reviewed in this work, carbon emission and energy consumption are reduced from 10% to 83% and from 50% to 67%, respectively. The variability is due to the availability of raw materials, type of cement formulation, and energy resources used in the production process.
Author Contributions
Funding
Conflicts of Interest
References
- Datis Export Group. Available online: https://datis-inc.com/ (accessed on 24 December 2020).
- Hanle, L.J.; Jayaraman, K.R.; Smith, J.S. CO2 Emissions Profile of the US Cement Industry; EPA: Washington, DC, USA, 2004. [Google Scholar]
- Barker, D.J.; Turner, S.A.; Napier-Moore, P.A.; Clark, M.; Davison, J.E. CO2 capture in the cement industry. Energy Procedia 2009, 1, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Kääntee, U.; Zevenhoven, R.; Backman, R.; Hupa, M. Cement manufacturing using alternative fuels and the advantages of process modelling. Fuel Process. Technol. 2004, 85, 293–301. [Google Scholar] [CrossRef]
- Geopolymer Institute. Available online: https://www.geopolymer.org/applications/global-warming (accessed on 24 December 2020).
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef] [Green Version]
- de Lena, E.; Spinelli, M.; Gatti, M.; Scaccabarozzi, R.; Campanari, S.; Consonni, S.; Cinti, G.; Romano, M.C. Techno-economic analysis of calcium looping processes for low CO2 emission cement plants. Int. J. Greenh. Gas Control. 2019, 82, 244–260. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S. Recent development on the uses of alternative fuels in cement manufacturing process. Fuel 2015, 145, 84–99. [Google Scholar] [CrossRef]
- Chatziaras, N.; Psomopoulos, C.S.; Themelis, N.J. Use of waste derived fuels in cement industry: A review. Manag. Environ. Qual. Int. J. 2016, 27, 178–193. [Google Scholar] [CrossRef]
- Rahman, A.; Rasul, M.G.; Khan, M.; Sharma, S.C. Assessment of energy performance and emission control using alternative fuels in cement industry through a process model. Energies 2017, 10, 1996. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.R.; Abanades, J.C. CO2 capture from the calcination of CaCO3 using iron oxide as heat carrier. J. Clean. Prod. 2016, 112, 1211–1217. [Google Scholar] [CrossRef]
- Ortiz Domínguez, C. The Calcium-Looping Process for Advancing in the Development of both CO2 Capture and Thermochemical Energy Storage Systems. Ph.D. Thesis, Escuela Técnica Superior de Ingeniería Universidad de Sevilla, Sevilla, Spain, July 2018. [Google Scholar]
- Romano, M.C.; Spinelli, M.; Campanari, S.; Consonni, S.; Marchi, M.; Pimpinelli, N.; Cinti, G. The calcium looping process for low CO2 emission cement plants. Energy Procedia 2014, 61, 500–503. [Google Scholar] [CrossRef] [Green Version]
- Vatopoulos, K.; Tzimas, E. Assessment of CO2 capture technologies in cement manufacturing process. J. Clean. Prod. 2012, 32, 251–261. [Google Scholar] [CrossRef]
- Gevaudan, J.P.; Osio-Norgaard, J.; Srubar, W.V., III. Alternative cements: Recent developments and future directions. In Proceedings of the AEI 2019: Integrated Building Solutions—The National Agenda, Tysons, VA, USA, 3–6 April 2019. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Maddalena, R.; Roberts, J.J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Cabiron, J.L.; Letourneux, R. High-performance concretes from calcium aluminate cements. Cem. Concr. Res. 1999, 29, 1215–1223. [Google Scholar] [CrossRef]
- Son, H.M.; Park, S.; Kim, H.Y.; Seo, J.H.; Lee, H.K. Effect of CaSO4 on hydration and phase conversion of calcium aluminate cement. Constr. Build. Mater. 2019, 224, 40–47. [Google Scholar] [CrossRef]
- Heikal, M.; Radwan, M.M.; Al-Duaij, O.K. Physico-mechanical characteristics and durability of calcium aluminate blended cement subject to different aggressive media. Constr. Build. Mater. 2015, 78, 379–385. [Google Scholar] [CrossRef]
- Nguyen, H.; Chang, T.; Shih, J.; Chen, C. Formulating for Innovative self-compacting concrete with low energy super-sulfated cement used for sustainability development. J. Mater. Sci. Chem. Eng. 2016, 4, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Brouwers, H.J.H.; Shui, Z.H. Three-dimensional computer modeling of slag cement hydration. J. Mater. Sci. 2007, 42, 9595–9610. [Google Scholar] [CrossRef]
- Lam, N.N. A study on super-sulfated cement using Dinh Vu phosphogypsum. In Proceedings of the 2nd International Conference on Sustainable Development in Civil, Urban and Transportation Engineering (CUTE 2018), Ho Chi Minh City, Vietnam, 17–19 April 2018. [Google Scholar] [CrossRef]
- Cabrera-Luna, K.; Maldonado-Bandala, E.E.; Nieves-Mendoza, D.; García, J.E. Supersulfated binders based on volcanic raw material: Optimization, microstructure and reaction products. Constr. Build. Mater. 2018, 176, 145–155. [Google Scholar] [CrossRef]
- Rong, H.; Qian, C.X.; Li, L.Z. Influence of molding process on mechanical properties of sandstone cemented by microbe cement. Constr. Build. Mater. 2012, 28, 238–243. [Google Scholar] [CrossRef]
- Rong, H.; Qian, C.X.; Li, L.Z. Study on microstructure and properties of sandstone cemented by microbe cement. Constr. Build. Mater. 2012, 36, 687–694. [Google Scholar] [CrossRef]
- Stanaszek-Tomal, E. Bacterial concrete as a sustainable building material? Sustainability 2020, 12, 696. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.; Phogat, A.; Singh, K. Bacterial concrete and effect of different bacteria on the strength and water absorption characteristics of concrete: A review. Int. J. Civ. Eng. Technol. 2016, 7, 43–56. Available online: http://www.iaeme.com/IJCIET/issues.asp?JType=IJCIET&VType=7&IType=5 (accessed on 30 December 2020).
- Sahoo, K.K.; Sathyan, A.K.; Kumari, C.; Sarkar, P.; Davis, R. Investigation of cement mortar incorporating Bacillus sphaericus. Int. J. Smart Nano Mater. 2016, 7, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Durga, C.S.S.; Ruben, N.; Chand, M.S.R.; Venkatesh, C. Evaluation of mechanical parameters of bacterial concrete. Ann. Chim. Sci. Matériaux 2019, 43, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Yoosathaporn, S.; Tiangburanatham, P.; Bovonsombut, S.; Chaipanich, A.; Pathom-Aree, W. A cost effective cultivation medium for biocalcification of Bacillus pasteurii KCTC 3558 and its effect on cement cubes properties. Microbiol. Res. 2016, 186, 132–138. [Google Scholar] [CrossRef]
- Alshalif, A.F.; Juki, M.I.; Othman, N.; Al-Gheethi, A.A.; Khalid, F.S. Improvement of mechanical properties of bio-concrete using enterococcus faecalis and Bacillus cereus strains. Environ. Eng. Res. 2019, 24, 630–637. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, A.D. Investigation into the optimal bacterial concentration for compressive strength enhancement of microbial concrete. Constr. Build. Mater. 2018, 183, 202–214. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer cement. A review. Geopolymer Inst. Tech. Pap. 2013, 21, 1–11. [Google Scholar]
- Longos, A., Jr.; Tigue, A.A.; Dollente, I.J.; Malenab, R.A.; Bernardo-Arugay, I.; Hinode, H.; Kurniawan, W.; Promentilla, M.A. Optimization of the mix formulation of geopolymer using nickel-laterite mine waste and coal fly ash. Minerals 2020, 10, 1144. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from kaolin in China: An overview. Appl. Clay Sci. 2016, 119, 31–41. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.; Rangan, B.V. On the development of fly ash-based geopolymer concrete. Mater. J. 2004, 101, 467–472. [Google Scholar]
- Sambucci, M.; Sibai, A.; Valente, M. Recent advances in geopolymer technology. A potential eco-friendly solution in the construction materials industry: A review. J. Compos. Sci. 2021, 5, 109. [Google Scholar] [CrossRef]
- Aredes, F.G.M.; Campos, T.M.B.; Machado, J.P.B.; Sakane, K.K.; Thim, G.P.; Brunelli, D.D. Effect of cure temperature on the formation of metakaolinite-based geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Wardhono, A. The effect of sodium hydroxide molarity on strength development of non-cement class C fly ash geopolymer mortar. J. Phys. Conf. Ser. 2018, 947, 012001. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patino, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Wallah, S.; Rangan, B.V. Low-Calcium Fly Ash-Based Geopolymer Concrete: Long-Term Properties; Res. Report-GC2; Curtin University: Bentley, Australia, 2006. [Google Scholar]
- Mustafa Al Bakri, A.M.; Faheem, M.; Tahir, M.; Sandhu, A.V.; Alida, A.; Salleh, M.A.A.M.; Ruzaidi, C.M. Microstructure studies on different types of geopolymer materials. Appl. Mech. Mater. 2013, 421, 384–389. [Google Scholar] [CrossRef]
- Nuruddin, M.F.; Malkawi, A.B.; Fauzi, A.; Mohammed, B.S.; Almattarneh, H.M. Evolution of Geopolymer Binders: A Review. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 133. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.K.; Maitra, S.; Mukherjee, S.; Kumar, S. Microstructural and morphological evolution of fly ash based geopolymers. Constr. Build. Mater. 2016, 111, 758–765. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Ishwarya, G.; Singh, B.; Deshwal, S.; Bhattacharyya, S.K. Effect of sodium carbonate/sodium silicate activator on the rheology, geopolymerization and strength of fly ash/slag geopolymer pastes. Cem. Concr. Compos. 2019, 97, 226–238. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Muniz-Villarreal, M.S.; Manzano-Ramirez, A.; Sampieri-Bulbarela, S.; Gasca-Tirado, R.J.; Reyes-Araiza, J.L.; Rubio-Avalos, J.C.; Perez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigo-Borras, V. The effect of temperature on the geopolymerization process of a metakaolin-based geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- Fansuri, H.; Prasetyoko, D.; Zhang, Z.; Zhang, D. The effect of sodium silicate and sodium hydroxide on the strength of aggregates made from coal fly ash using the geopolymerisation method. Asia Pac. J. Chem. Eng. 2012, 7, 73–79. [Google Scholar] [CrossRef]
- Ng, H.T.; Heah, C.Y.; Liew, Y.M.; Abdullah, M.M.A.B. The Effect of Various Molarities of NaOH Solution on Fly Ash Geopolymer Paste. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 2045, p. 020098. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Abdullah, M.M.A.B.; Cheng-Yong, H.; Hussin, K. Effect of sodium hydroxide molarity on physical, mechanical and thermal conductivity of metakaolin geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2017, 343, 012015. [Google Scholar] [CrossRef]
- Pouhet, R.; Cyr, M.; Bucher, R. Influence of the initial water content in flash calcined metakaolin-based geopolymer. Constr. Build. Mater. 2019, 201, 421–429. [Google Scholar] [CrossRef]
- Assaedi, H.; Alomayri, T.; Kaze, C.R.; Jindal, B.B.; Subaer, S.; Shaikh, F.; Alraddadi, S. Characterization and properties of geopolymer nanocomposites with different contents of nano-CaCO3. Constr. Build. Mater. 2020, 252, 119137. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Maiti, S.; Malik, M.A.; Xu, S. Modification of geopolymer with size controlled TiO2 nanoparticle for enhanced durability and catalytic dye degradation under UV light. J. Clean. Prod. 2020, 255, 120183. [Google Scholar] [CrossRef]
- Mahboubi, B.; Guo, Z.; Wu, H. Evaluation of durability behavior of geopolymer concrete containing Nano-silica and Nano-clay additives in acidic media. J. Civ. Eng. Mater. Appl. 2019, 3, 163–171. [Google Scholar] [CrossRef]
- Akono, A.T. Fracture behavior of metakaolin-based geopolymer reinforced with carbon nanofibers. Int. J. Ceram. Eng. Sci. 2020, 2, 234–242. [Google Scholar] [CrossRef]
- Rahman, A.S.; Shah, C.; Gupta, N. Simultaneous effects of rice husk silica and silicon carbide whiskers on the mechanical properties and morphology of sodium geopolymer. J. Compos. Mater. 2020, 54, 4611–4620. [Google Scholar] [CrossRef]
- Nurjaya, D.M.; Astutiningsih, S.; Zulfia, A. Thermal effect on flexural strength of geopolymer matrix composite with alumina and wollastonite as fillers. Int. J. Technol. 2015, 6, 462–470. [Google Scholar] [CrossRef]
- Amri, A.; Kurniawan, R.; Sutikno, S.; Yenti, S.R.; Rahman, M.M.; Hendri, Y.B. Compressive Strength of Coal Fly-ash Based Geopolymer with Integration of Graphene Nanosheets (GNs). J. Phys. Conf. Ser. 2020, 1655, 012005. [Google Scholar] [CrossRef]
- Su, Z.; Hou, W.; Sun, Z. Recent advances in carbon nanotube-geopolymer composite. Constr. Build. Mater. 2020, 252, 118940. [Google Scholar] [CrossRef]
- Long, W.J.; Ye, T.H.; Luo, Q.L.; Wang, Y.; Mei, L. Reinforcing mechanism of reduced graphene oxide on flexural strength of geopolymers: A synergetic analysis of hydration and chemical composition. Nanomaterials 2019, 9, 1723. [Google Scholar] [CrossRef] [Green Version]
- Guedes, M.; Evangelista, L.; de Brito, J.; Ferro, A.C. Microstructural characterization of concrete prepared with recycled aggregates. Microsc. Microanal. 2013, 19, 1222–1230. [Google Scholar] [CrossRef]
- Nuruddin, M.F.; Malkawi, A.B.; Fauzi, A.; Mohammed, B.S.; Al-Mattarneh, H.M. Effects of alkaline solution on the microstructure of hcfa geopolymers. Eng. Chall. Sustain. Future 2016, 501, 501–505. [Google Scholar]
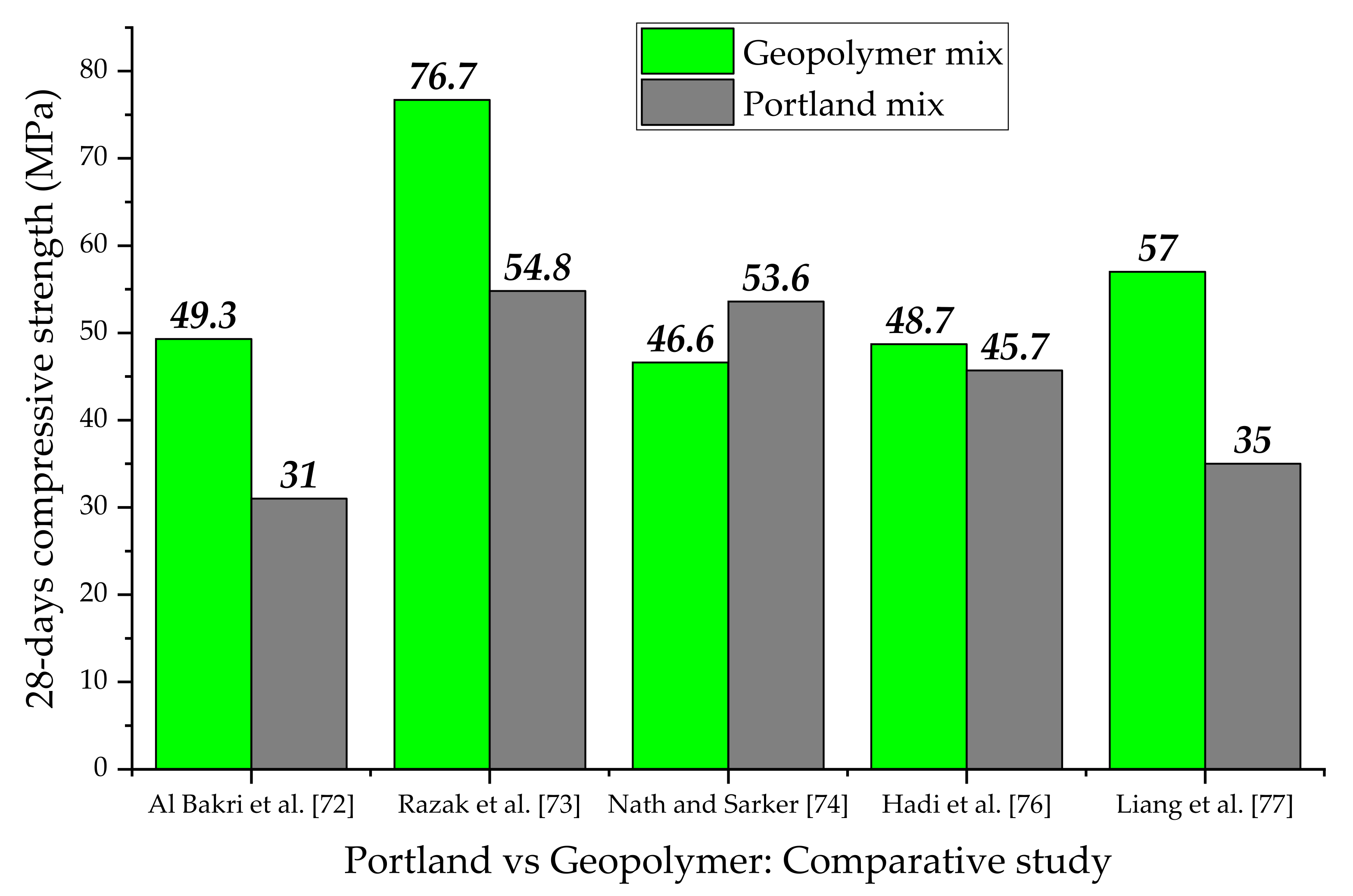
- Bakri, A.M.; Kamarudin, H.; Binhussain, M.; Nizar, I.K.; Rafiza, A.R.; Zarina, Y. Comparison of geopolymer fly ash and ordinary portland cement to the strength of concrete. Adv. Sci. Lett. 2013, 19, 3592–3595. [Google Scholar] [CrossRef]
- Razak, S.; Zainal, F.F.; Shamsudin, S.R. Effect of Porosity and Water Absorption on Compressive Strength of Fly Ash based Geopolymer and OPC Paste. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 957, p. 012035. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Flexural strength and elastic modulus of ambient-cured blended low-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2017, 130, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Hadi, M.N.; Zhang, H.; Parkinson, S. Optimum mix design of geopolymer pastes and concretes cured in ambient condition based on compressive strength, setting time and workability. J. Build. Eng. 2019, 23, 301–313. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, H.; Zhang, Z.; Wu, Q. Effect of rice husk ash addition on the compressive strength and thermal stability of metakaolin based geopolymer. Constr. Build. Mater. 2019, 222, 872–881. [Google Scholar] [CrossRef]
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W. Resistance of fly ash geopolymer binders to organic acids. Mater. Struct. 2020, 53, 1–18. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A.; Dong, P.; Bassim, N. Acid attack on geopolymer cement mortar based on waste-glass powder and calcium aluminate cement at mild concentration. Constr. Build. Mater. 2018, 193, 363–372. [Google Scholar] [CrossRef]
- Ganesan, N.; Abraham, R.; Raj, S.D. Durability characteristics of steel fibre reinforced geopolymer concrete. Constr. Build. Mater. 2015, 93, 471–476. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Abd Elmoaty, M.; Elshaboury, A.M. Magnesium sulfate resistance of geopolymer mortar. Constr. Build. Mater. 2018, 184, 111–127. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Jin, C. Use of slaked lime and Portland cement to improve the resistance of MSWI bottom ash-GBFS geopolymer concrete against carbonation. Constr. Build. Mater. 2018, 166, 290–300. [Google Scholar] [CrossRef]
- Pasupathy, K.; Sanjayan, J.; Rajeev, P. Evaluation of alkalinity changes and carbonation of geopolymer concrete exposed to wetting and drying. J. Build. Eng. 2021, 35, 102029. [Google Scholar] [CrossRef]
- Lavanya, G.; Jegan, J. Durability study on high calcium fly ash based geopolymer concrete. Adv. Mater. Sci. Eng. 2015, 2015, 731056. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Yuan, Y.; Cheng, Z.; Wen, T.; Li, J.; Li, F.; Ma, Z.J. Freeze-thaw resistance of Class F fly ash-based geopolymer concrete. Constr. Build. Mater. 2019, 222, 474–483. [Google Scholar] [CrossRef]
- Belforti, F.; Azarsa, P.; Gupta, R.; Dave, U. Effect of freeze-thaw on K-based geopolymer concrete and portland cement concrete. In Proceedings of the 6th Nirma University International Conference on Engineering, Ahmedabad, India, 23–25 November 2017. [Google Scholar]
- Goyal, S.; Kumar, M.; Sidhu, D.S.; Bhattacharjee, B. Resistance of mineral admixture concrete to acid attack. J. Adv. Concr. Technol. 2009, 7, 273–283. [Google Scholar] [CrossRef] [Green Version]
- El-Hachem, R.; Rozière, E.; Grondin, F.; Loukili, A. New procedure to investigate external sulphate attack on cementitious materials. Cem. Concr. Compos. 2012, 34, 357–364. [Google Scholar] [CrossRef]
- Silva, R.V.; Neves, R.; De Brito, J.; Dhir, R.K. Carbonation behaviour of recycled aggregate concrete. Cem. Concr. Compos. 2015, 62, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability performance of precast fly ash–based geopolymer concrete under atmospheric exposure conditions. J. Mater. Civ. Eng. 2018, 30, 04018007. [Google Scholar] [CrossRef]
- Hanžič, L.; Ilić, R. Relationship between liquid sorptivity and capillarity in concrete. Cem. Concr. Res. 2003, 33, 1385–1388. [Google Scholar] [CrossRef]
- Tuyan, M.; Mardani-Aghabaglou, A.; Ramyar, K. Freeze–thaw resistance, mechanical and transport properties of self-consolidating concrete incorporating coarse recycled concrete aggregate. Mater. Des. 2014, 53, 983–991. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Yong, H.C.; Ming, L.Y.; Hussin, K.; Kadir, A.A.; Azimi, E.A. Manufacturing of fire resistance geopolymer: A review. MATEC Web Conf. 2016, 78, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Ingenio. Available online: https://webapi.ingenio-web.it/immagini/file/byname?name=cU0BO01F4p.pdf (accessed on 15 June 2021).
- Lahoti, M.; Tan, K.H.; Yang, E.H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Elsayed, H.A.; AbdelMoied, S. Geopolymer synthesis by the alkali-activation of blastfurnace steel slag and its fire-resistance. HBRC J. 2018, 14, 159–164. [Google Scholar] [CrossRef] [Green Version]
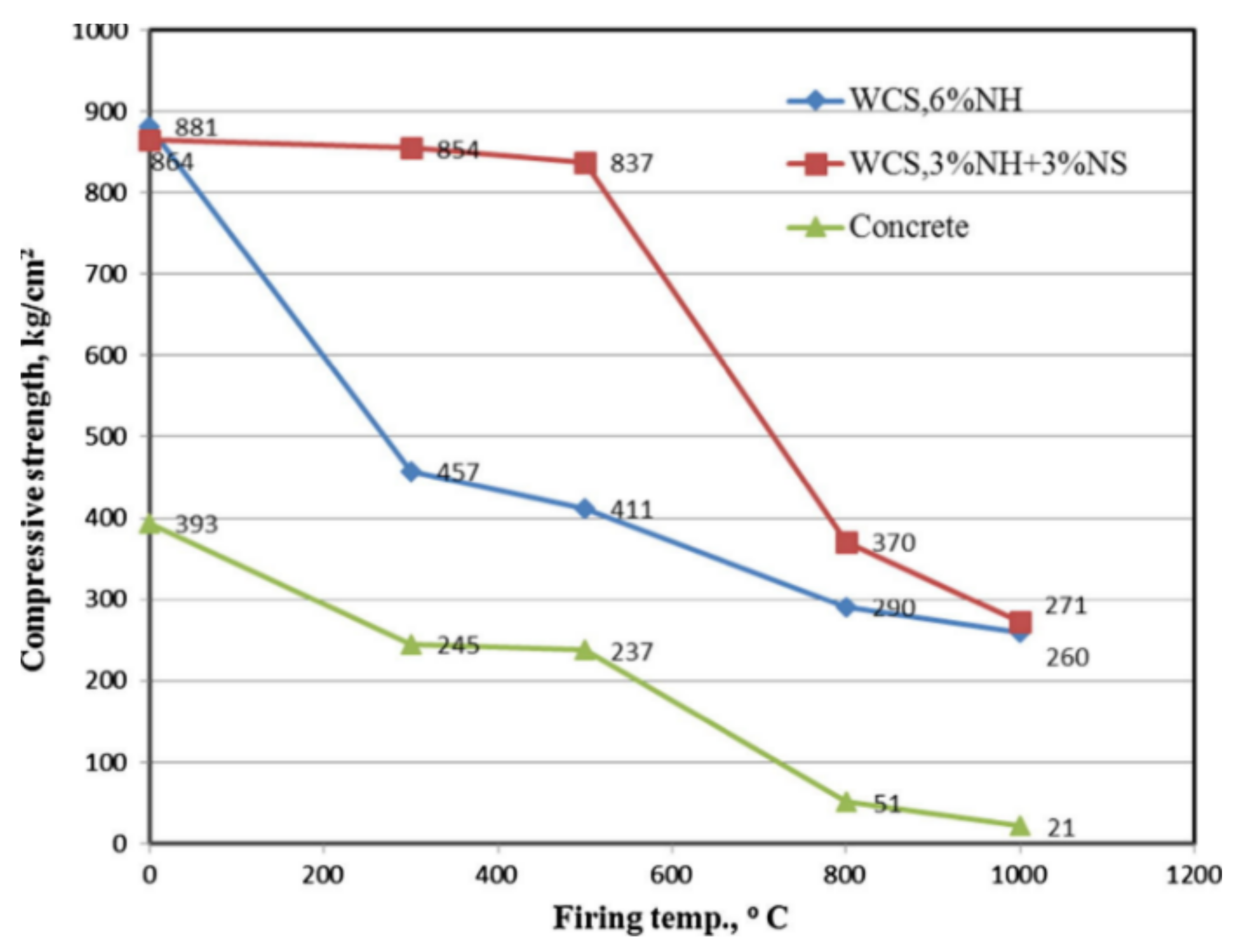
- Türkmen, İ.; Karakoç, M.B.; Kantarcı, F.; Maraş, M.M.; Demirboğa, R. Fire resistance of geopolymer concrete produced from Elazığ ferrochrome slag. Fire Mater. 2016, 40, 836–847. [Google Scholar] [CrossRef]
- Saavedra, W.G.V.; de Gutiérrez, R.M. Performance of geopolymer concrete composed of fly ash after exposure to elevated temperatures. Constr. Build. Mater. 2017, 154, 229–235. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; Van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Gomes, K.C.; Carvalho, M.; Diniz, D.D.P.; Abrantes, R.D.C.C.; Branco, M.A.; Carvalho Junior, P.R.O.D. Carbon emissions associated with two types of foundations: CP-II Portland cement-based composite vs. geopolymer concrete. Matéria 2019, 24. [Google Scholar] [CrossRef]
- Jamieson, E.; McLellan, B.; Van Riessen, A.; Nikraz, H. Comparison of embodied energies of ordinary portland cement with Bayer-derived geopolymer products. J. Clean. Prod. 2015, 99, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.L.; Kumar, R. Geopolymer: Cement for low carbon economy. Indian Concr. J. 2014, 88, 29–37. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Mejía-Arcila, J.; de Gutiérrez, R.M.; Martínez, E. Life cycle assessment (LCA) of an alkali-activated binary concrete based on natural volcanic pozzolan: A comparative analysis to OPC concrete. Constr. Build. Mater. 2018, 176, 103–111. [Google Scholar] [CrossRef]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean. Prod. 2020, 263, 121477. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, B.; Guo, S.; Long, G.; Xie, Y. Properties and characterization of green one-part geopolymer activated by composite activators. J. Clean. Prod. 2019, 220, 188–199. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental impact assessment of fly ash and silica fume based geopolymer concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Progress, current thinking and challenges in geopolymer foam concrete technology. Cem. Concr. Compos. 2021, 116, 103886. [Google Scholar] [CrossRef]
- Ranjbar, N.; Zhang, M. Fiber-reinforced geopolymer composites: A review. Cem. Concr. Compos. 2020, 107, 103498. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Ramakrishnan, S.; Sanjayan, J. Technologies for improving buildability in 3D concrete printing. Cem. Concr. Compos. 2021, 104144. [Google Scholar] [CrossRef]






| Bacterial Specie | 28 Days Compressive Strength (MPa) | Strength Rate Increase (%) | Ref. |
|---|---|---|---|
| Bacillus sphaericus | 6.1–7.3 | 3.4–23.4 | [31] |
| Bacillus subtilis | 62.1 | 20.3 | [32] |
| Bacillus pasteurii | 42.1 | 30.3 | [33] |
| Bacillus cereus | 33.8 | 11.0 | [34] |
| Si/Al Atomic Ratio | Applications |
|---|---|
| 1 | Bricks—Ceramics |
| 2 | Cement—Concrete |
| 3 | Heat resistant composites (600–100 °C)—Foundry equipment |
| >3 | Sealant materials (200–600 °C) |
| Process Parameters | SEM Analysis | EDS Analysis | ||
|---|---|---|---|---|
| Alkali molarity | Curing temperature | Morphological features | Si/Al ratio | Na/Al ratio |
| 6 M | 27 °C | Spherical particles with partially reacted surface. Few Na-rich elongated grains (<5 μm size). Open pores throughout the matrix. | 1.3–1.7 | 0.2–3.0 |
| 6 M | 45 °C | More reacted surface with reaction product. Numerous Na-rich elongated grains (5–10 μm size). Open pores and few fibrous grains. | 1.3–1.7 | 0.2–4.0 |
| 6 M | 60 °C | Surface fully altered into reaction product. Numerous Na-rich fibrous grains (up to 20 μm size). Lower pores amount. | 1.3–1.6 | 0.4–5.0 |
| 8 M | 27 °C | Well reacted surface with reaction products. Al-rich short prismatic grains (2 μm size) occurring in clusters on broken cenosphere. Na-rich dense gel. | 1.2–2.1 | 0.5–3.5 |
| 8 M | 45 °C | Fully reacted particles enveloped with reaction product. Numerous Na rich fibrous grains (up to 20 μm size). Na-rich dense gel. | 1.3–2.1 | 0.3–3.5 |
| 8 M | 60 °C | Fully reacted particles, reaction product on the crust. Numerous Na-rich fibrous grains larger than the fibers obtained at 45 °C. Low porosity and compact structure. | 1.3–2.1 | 0.15–3.5 |
| 10 M | 27 °C | Fully reacted particles covered with reaction product. Al-rich short prismatic grains (2 μm size) occurring in clusters. Na-rich dense gel. | 0.6–2.3 | 0.15–3.5 |
| 10 M | 45 °C | Fully reacted particles covered with reaction product. Fibrous grains (up to 50 μm size). Na-rich dense gel. | 1.1–3.0 | 0.12–3.8 |
| 10 M | 60 °C | Fully reacted FA particles. Na-rich gel particles are fusing together to form a dense structure. Si-rich very fine particles (0.5–2 μm size). | 1.2–4.0 | 0.2–3.5 |
| Ref. | Molarity Level (M) | Porosity (%) | Compressive Strength (MPa) |
|---|---|---|---|
| [57] | 6—8—10—12—14 | 21.5—20.0—22.5—22.2—22.5 | 32.5—36.0—27.5—27.5—27.5 |
| [58] | 12—16—18 | 11.6—11.1—11.4 | 18.0—27.0—21.0 |
| [59] | 6—8—10—12—14 | 19.0—24.0—17.5—18.0—17.8 | 1.0—9.0—14.5—8.0—6.5 |
| Durability Indicator | Test Description | Aluminosilicate Precursor | Geopolymer Performance | Ref. |
|---|---|---|---|---|
| Acid attack resistance | Exposure to 0.1–0.5 M organic acid solution (acetic and lactic acids) for 56 days and evaluation of compressive strength changes and percentage of mass loss | FA | Better | [79] |
| Acid attack resistance | Exposure to sulfuric acid and hydrochloric acid (pH = 3) for 2 years and evaluation of compressive strength changes and percentage of mass loss | Waste-glass powder (WGP) | Better | [80] |
| Sulphate attack resistance | Exposure to 3% sodium sulphate solution for 6 months and evaluation of compressive strength changes and percentage of mass loss | FA | Better | [81] |
| Sulphate attack resistance | Exposure to 10% solution of magnesium sulfate for 1 year and evaluation of compressive strength changes and percentage of mass loss | FA-GBFS | Better | [82] |
| Carbonation resistance | Accelerated carbonation test: treatment in a carbonation chamber (CO2 mass fraction of 20%, temperature of 20 ± 2 °C, and relative humidity of 70 ± 5%) for 2 months and evaluation of carbonation depth | GBFS | Worst | [83] |
| Carbonation resistance | Accelerated carbonation test: treatment in a carbonation chamber (CO2 concentration of 1%, temperature of 23° C, and relative humidity of 65%) for 6 months and evaluation of carbonation depth | FA-GBFS | Worst | [84] |
| Water sorptivity | Immersion in water for 2 h and gain mass measurement at regular interval of 30 min, resulting the penetration of the water into the material | FA | Better | [81] |
| Water sorptivity | Immersion in water for 45 days and gain mass measurement at regular interval of 30 min, resulting the penetration of the water into the material | FA | Better | [85] |
| Freeze-thaw resistance | Exposure to 125 freeze-thaw cycles, according to ASTM C666 method. Hereinafter, evaluation of compressive strength changes and percentage of mass loss was performed | FA-GBFS | Worst | [86] |
| Freeze-thaw resistance | Exposure to 3 h temperature cycling from −18 °C to 4 °C for 27 cycles. Hereinafter, evaluation of compressive strength changes percentage of mass loss was performed | FA | Worst | [87] |
| Indicator | Portland Concrete | Geopolymer Concrete |
|---|---|---|
| Combustibility | Incombustible | Incombustible |
| Thermal conductivity range | 1.0–4.0 W/mK | 0.2–0.4 W/mK |
| Thermal stability | Portland is a hydrate compound. Degradation of hydration products (CH, CSH, CaCO3) occurs between 200 °C and 800 °C. | Maintenance of microstructural stability under high temperatures. Geopolymer cement has a glass (ceramic-like) structure and the resistance to heat/fire is better. |
| Porosity development | Porosity degree gradually increase as a function of increasing thermal stressing, inducing drop in mechanical strength. | Between 500 °C and 900 °C generally occurs the densification of the matrix: geopolymer gel sinters, causing a reduction in void fractions, stronger bonding between particles, and increase in strength. |
| Pore structure | The chief cause for spalling is the rise in pore pressure due to common close porosity in Portland concrete matrix. | Geopolymer concrete has more interconnected pores: it suggests that water vapor could escape from the geopolymer matrix more quickly than in PC concrete, resulting in lower internal pore pressure and low damage after vaporization. |
| Degradation condition | Risk of spalling from 200 °C. Structurally not useful above 600 °C. | Lower amount of chemically-bound water implies less susceptibility to spalling. The decomposition mode is to melt over 1000 °C, rather than explosively release water or dehydrate to a powder in Portland concrete. |
| Geopolymer Cement | PC | Ref. | ||
|---|---|---|---|---|
| CO2 emission | Embodied energy | CO2 emission | Embodied energy | |
| 271–404 kg/ton of cement | / | 760 kg/ton of cement | / | [100] |
| 239.8 kg/m3 of concrete | / | 418.8 kg/m3 of concrete | / | [101] |
| / | 0.33 GJ/ton of concrete | / | 1.01 GJ/ ton of concrete | [102] |
| 150–250 kg/ton of cement | 2.2–2.4 GJ/ton of cement | 800–900 kg/ ton of cement | 4.0–4.4 GJ/ton of cement | [103] |
| 320 kg/m3 of concrete | / | 354 kg/m3 of concrete | / | [104] |
| 197.2–210.9 kg/m3 of concrete | / | 371.7–381.2 kg/m3 of concrete | / | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, M.; Sambucci, M.; Sibai, A. Geopolymers vs. Cement Matrix Materials: How Nanofiller Can Help a Sustainability Approach for Smart Construction Applications—A Review. Nanomaterials 2021, 11, 2007. https://doi.org/10.3390/nano11082007
Valente M, Sambucci M, Sibai A. Geopolymers vs. Cement Matrix Materials: How Nanofiller Can Help a Sustainability Approach for Smart Construction Applications—A Review. Nanomaterials. 2021; 11(8):2007. https://doi.org/10.3390/nano11082007
Chicago/Turabian StyleValente, Marco, Matteo Sambucci, and Abbas Sibai. 2021. "Geopolymers vs. Cement Matrix Materials: How Nanofiller Can Help a Sustainability Approach for Smart Construction Applications—A Review" Nanomaterials 11, no. 8: 2007. https://doi.org/10.3390/nano11082007
APA StyleValente, M., Sambucci, M., & Sibai, A. (2021). Geopolymers vs. Cement Matrix Materials: How Nanofiller Can Help a Sustainability Approach for Smart Construction Applications—A Review. Nanomaterials, 11(8), 2007. https://doi.org/10.3390/nano11082007








