Electrospun Semi-Alicyclic Polyimide Nanofibrous Membrane: High-Reflectance and High-Whiteness with Superior Thermal and Ultraviolet Radiation Stability for Potential Applications in High-Power UV-LEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
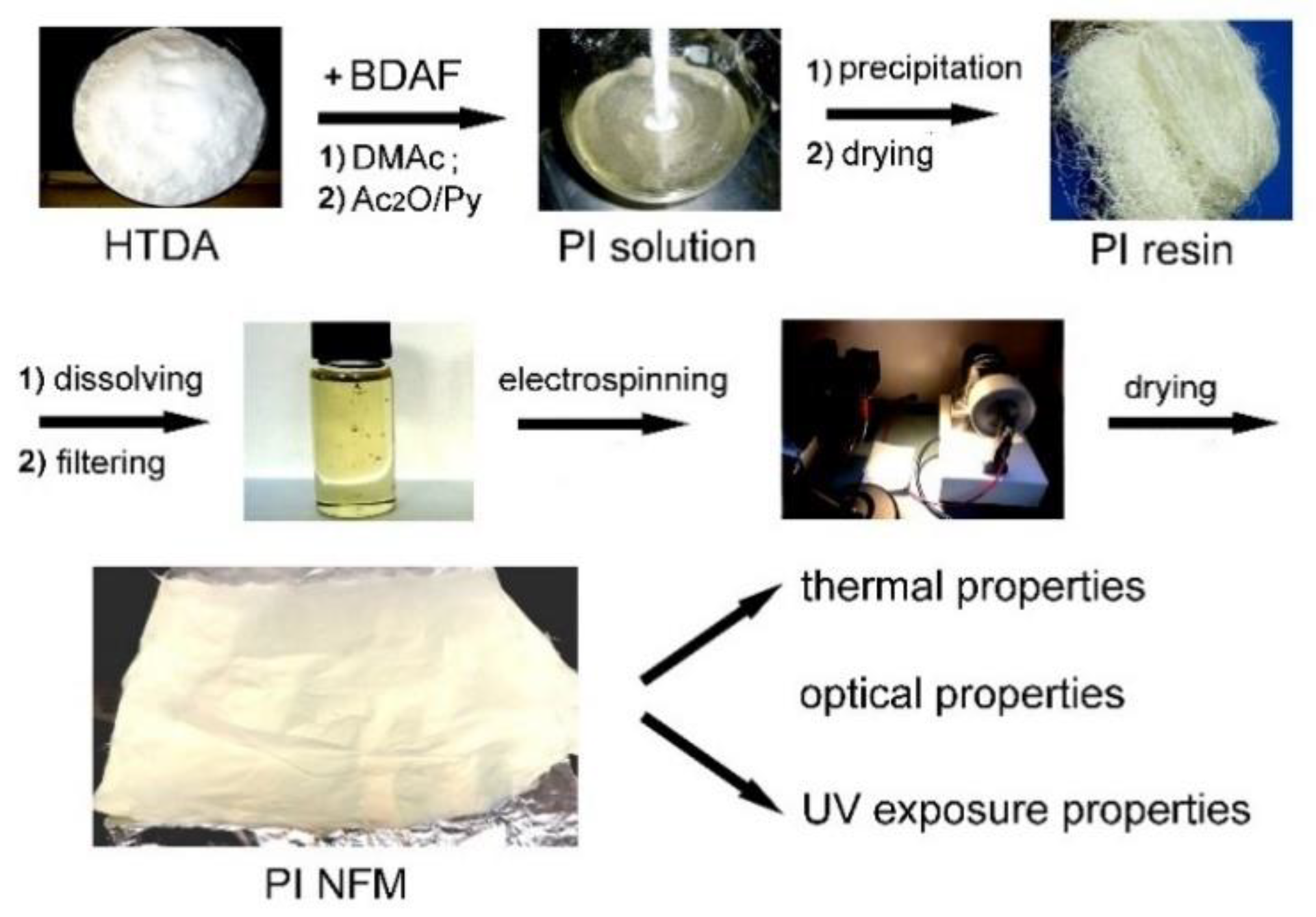
2.3. Synthesis of PI Resin
2.4. Fabrication of PI NFM via Electrospinning
3. Results and Discussion
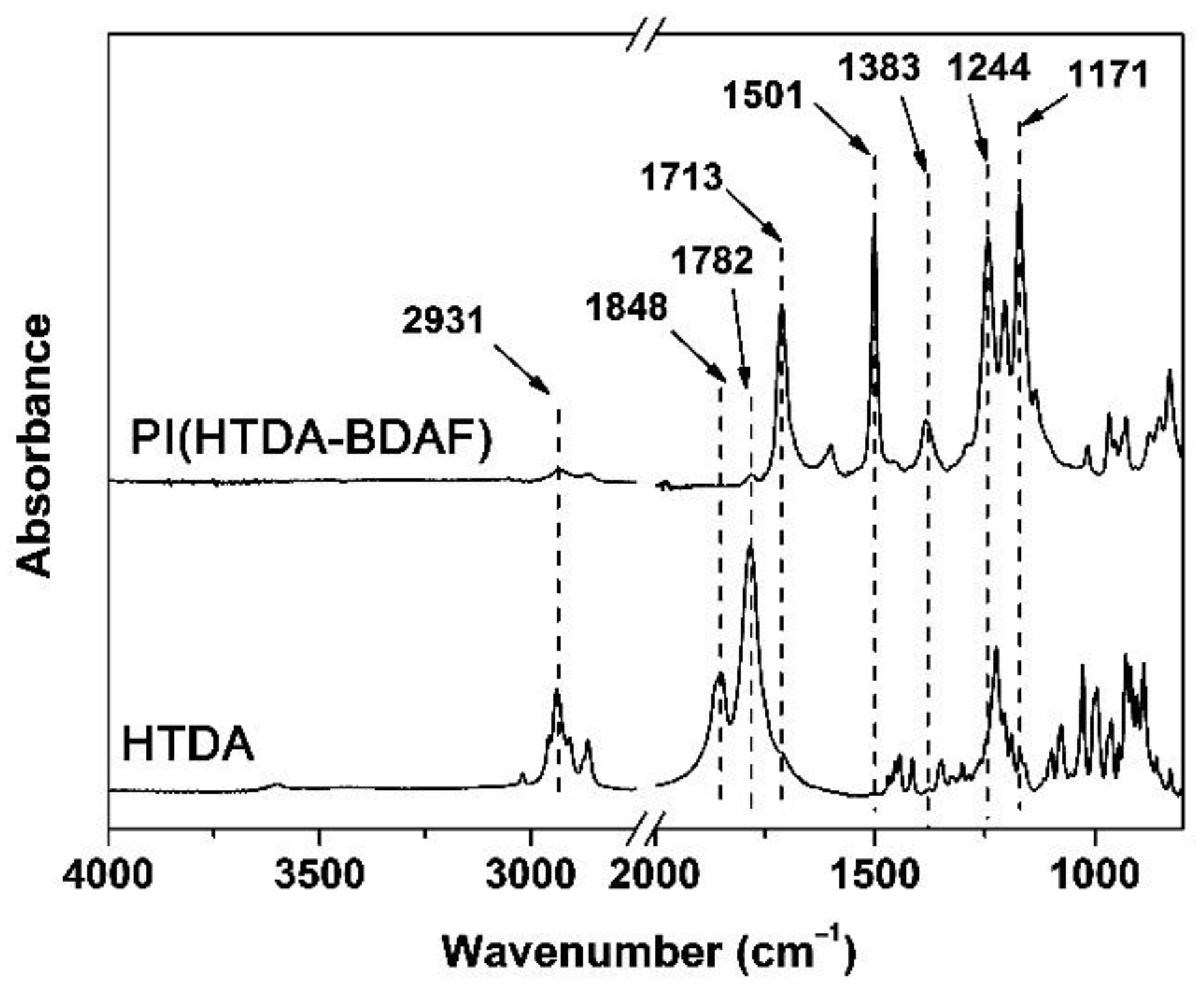
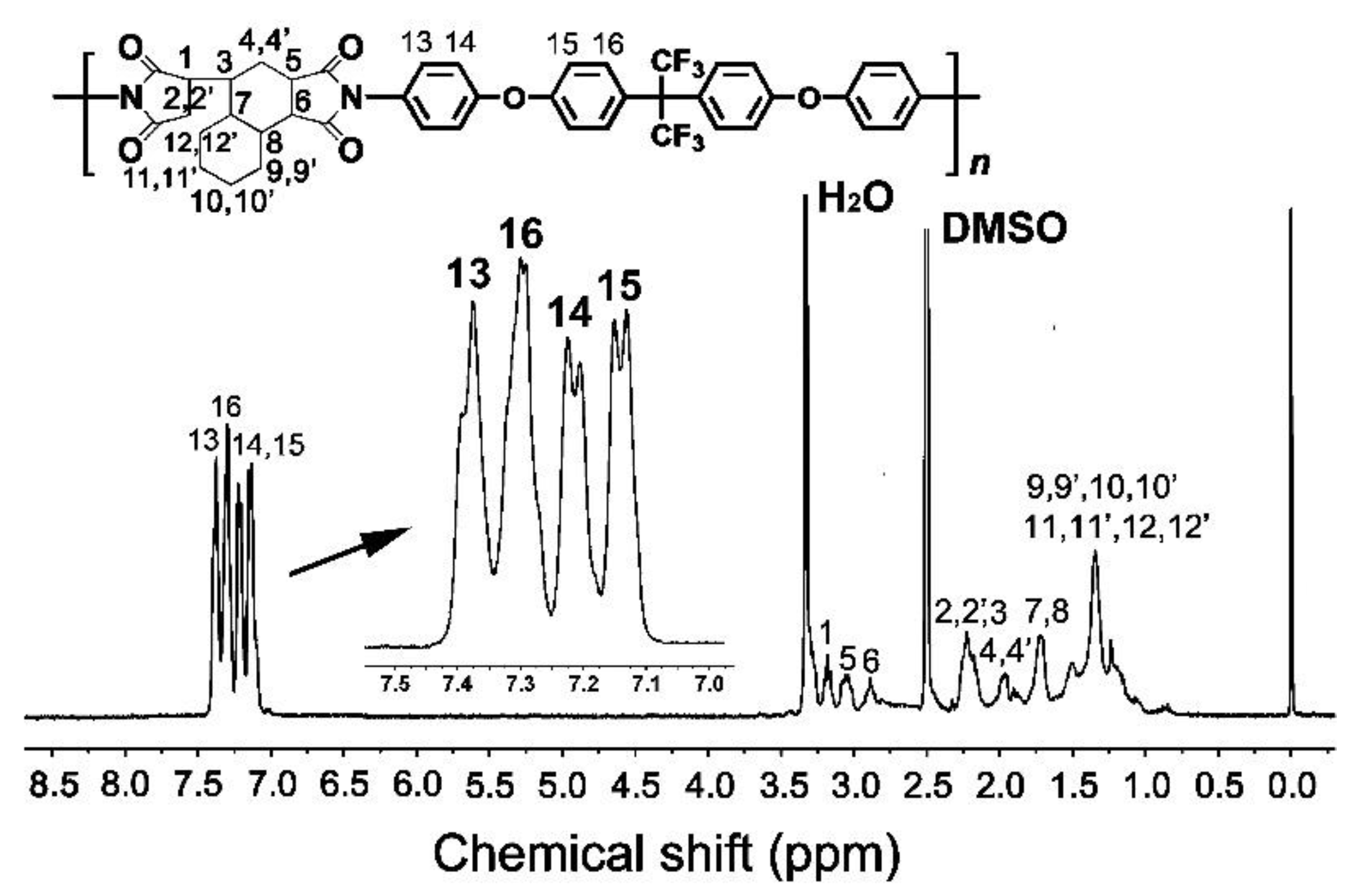
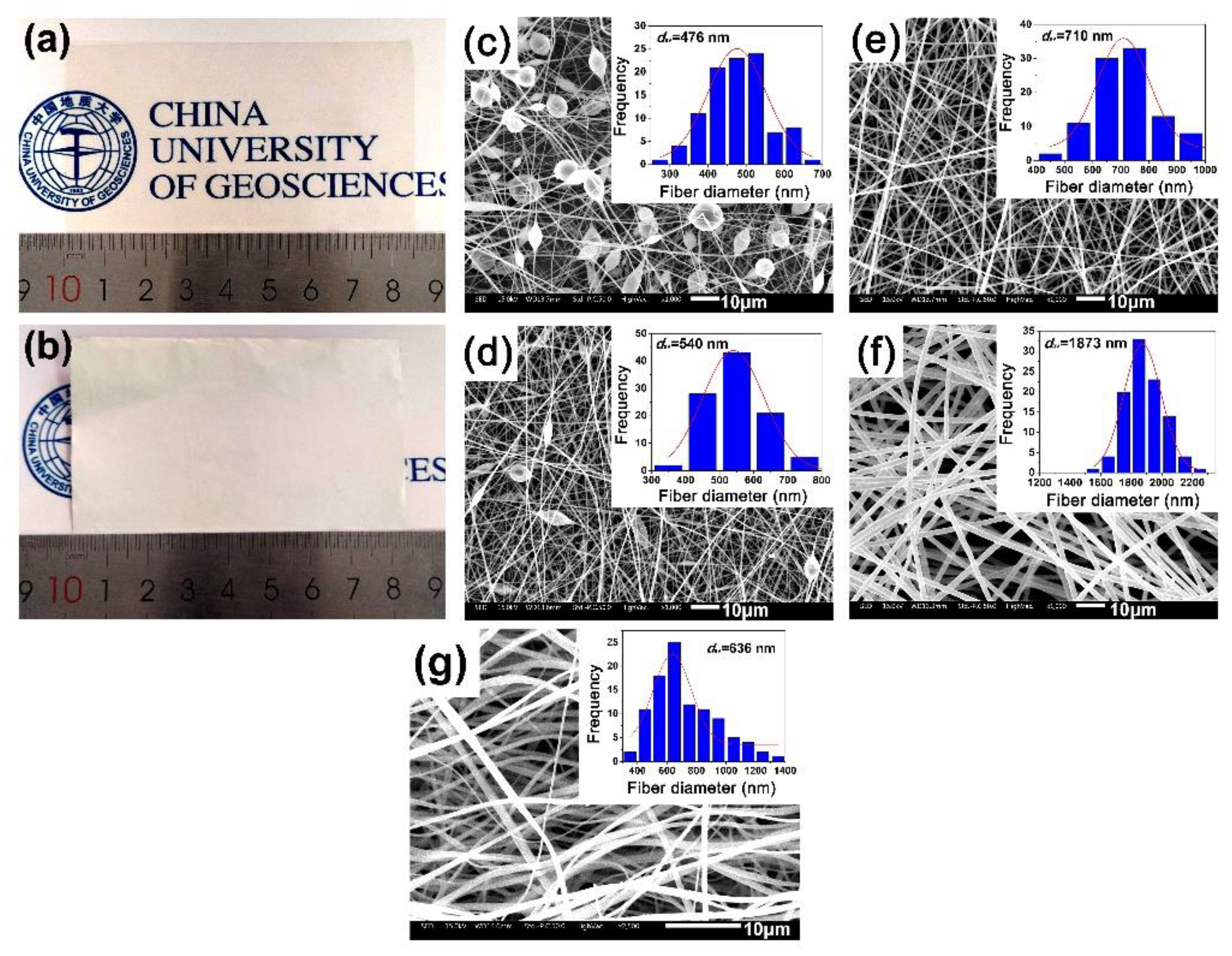
3.1. PI Resin Synthesis and Electrospun PI NFM Preparation
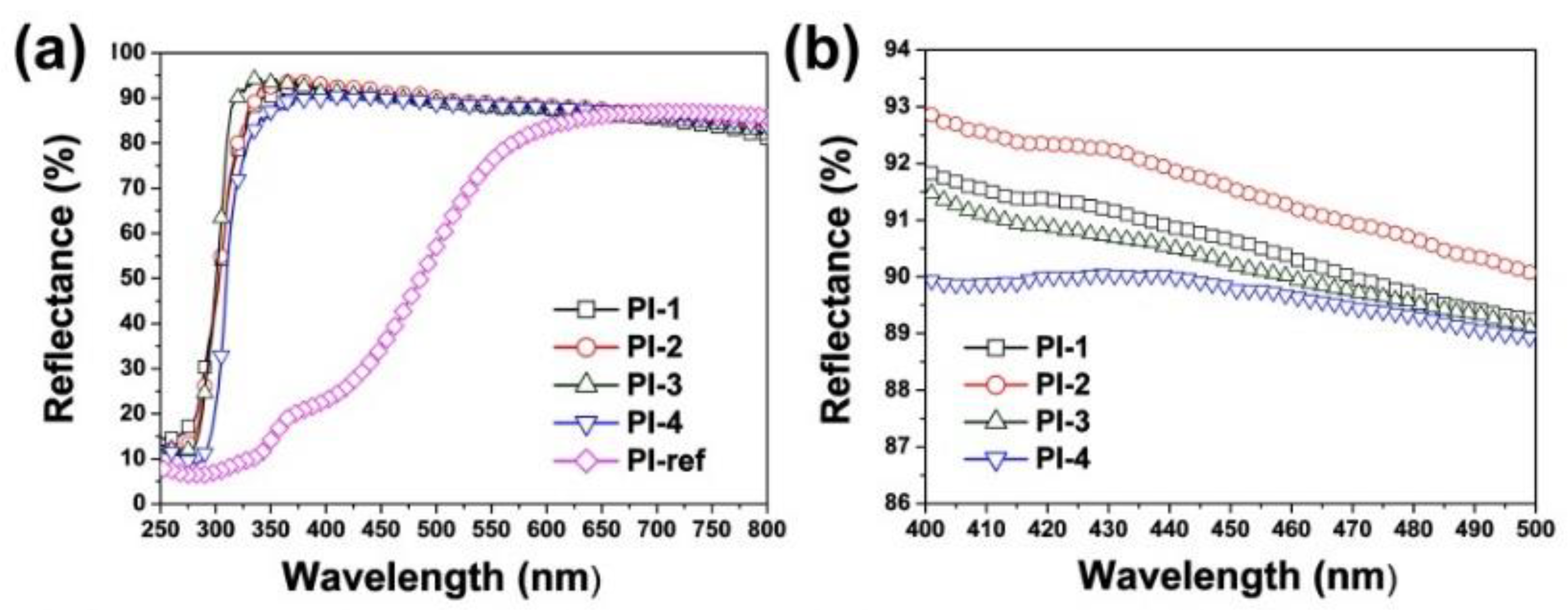
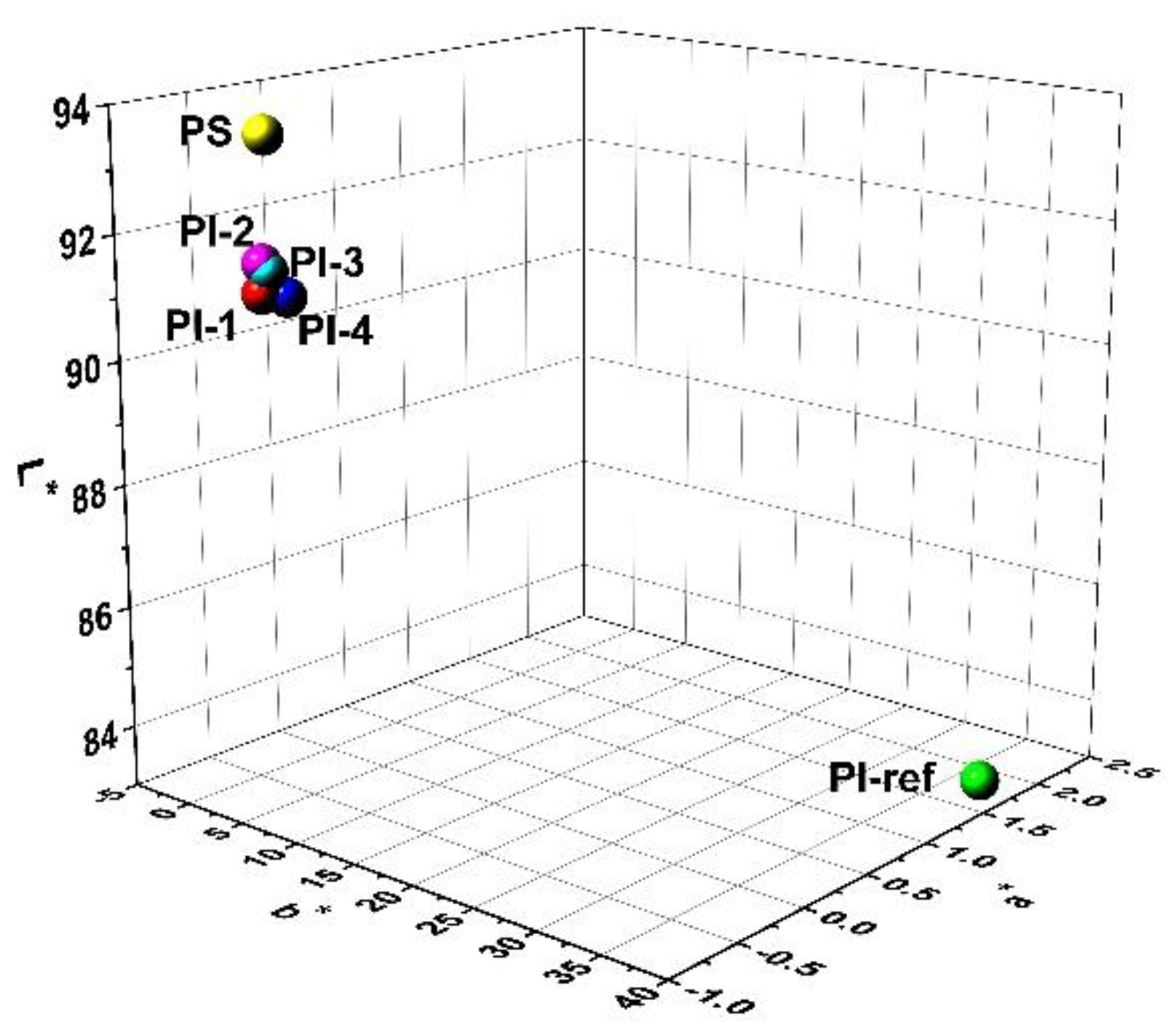
3.2. Optical Properties
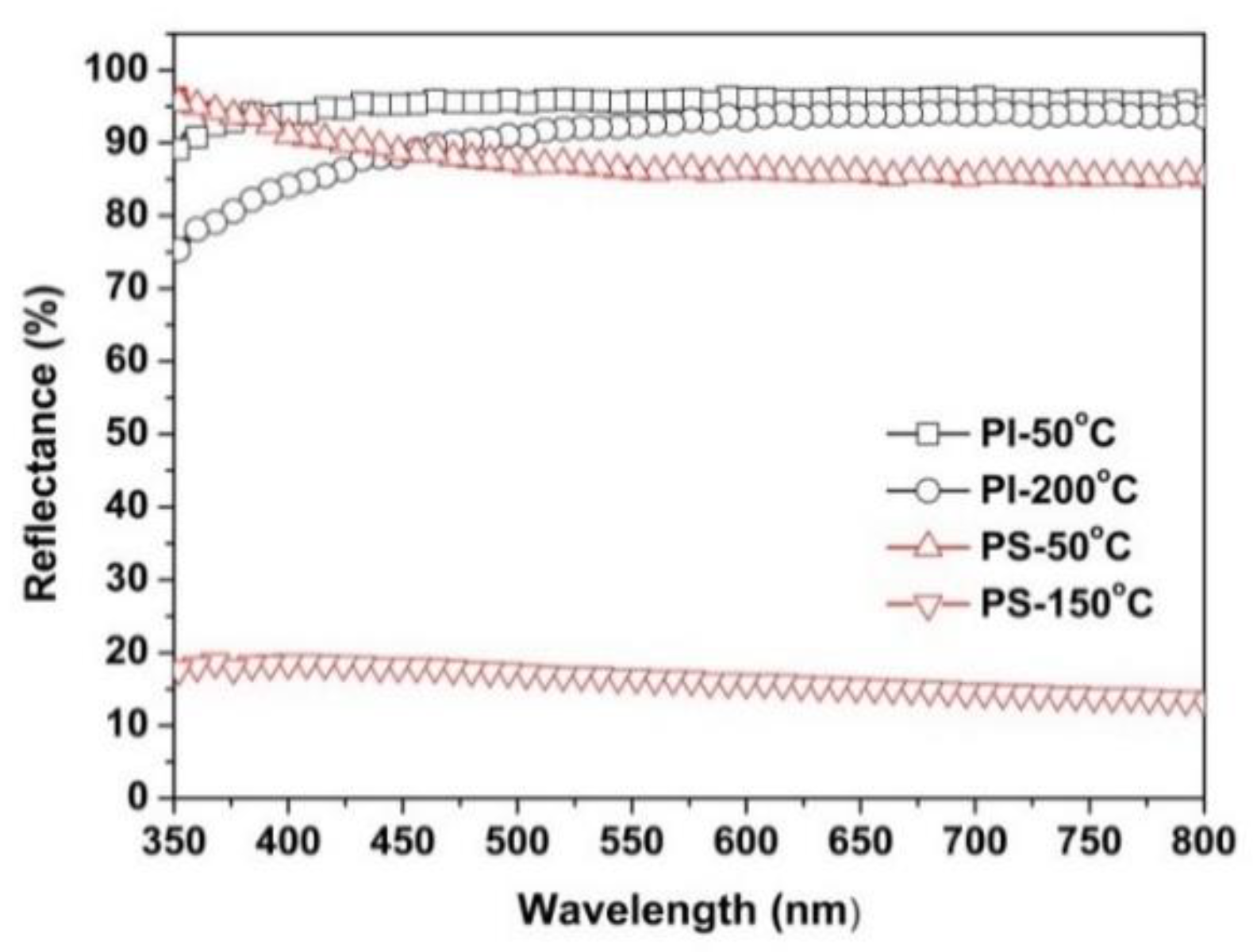
3.3. Thermal Properties
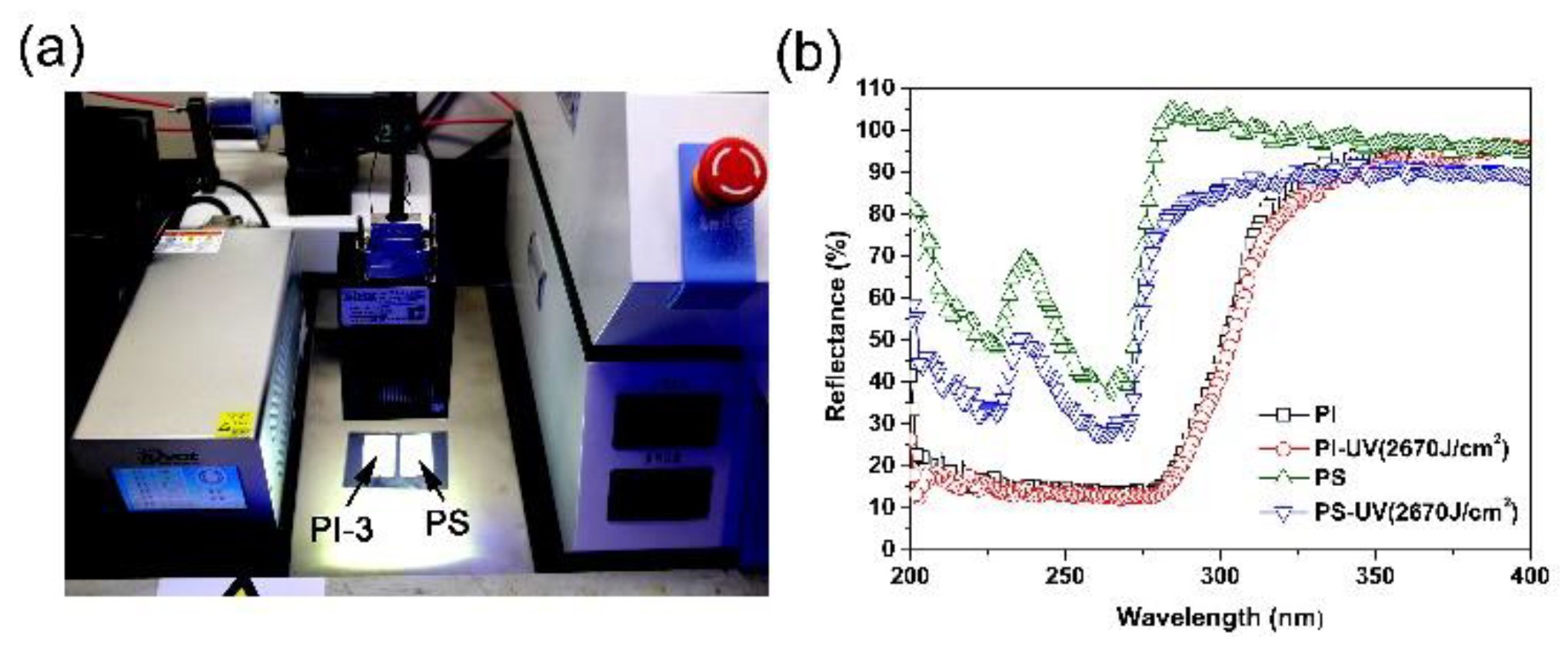
3.4. UV Exposure Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muramoto, Y.; Kimura, M.; Nouda, S. Development and future of ultraviolet light-emitting diodes: UV-LED will replace the UV lamp. Semicond. Sci. Technol. 2014, 29, 084004. [Google Scholar] [CrossRef] [Green Version]
- Espid, E.; Taghipour, F. UV-LED photo-activated chemical gas sensors: A review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 416–432. [Google Scholar] [CrossRef]
- Kebbi, Y.; Muhammad, A.I.; Sant’Ana, A.S.; do Prado-silva, L.; Liu, D.; Ding, T. Recent advances on the application of UV-LED technology for microbial inactivation: Progress and mechanism. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3501–3527. [Google Scholar] [CrossRef]
- Liang, G.; Tang, Y.; Li, J.; Yuan, Y.; Zhang, J.; Li, Z.; Ding, X. Improving optical performance of NUV-LEDs by highly reflective BN/silicone structures. IEEE Photonics Technol. Lett. 2020, 32, 1345–1348. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, G.; Chen, J.; Yu, S.; Li, Z.; Rao, L.; Yu, B. Highly reflective nanofiber films based on electrospin-ning and their application on color uniformity and luminous efficacy improvement of white light-emitting diodes. Opt. Express 2017, 25, 20598–20611. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.; Ng, S.P.; Wong, K.H. Brilliant whiteness surfaces from electrospun nanofiber webs. Texture Res. J. 2009, 79, 771–779. [Google Scholar] [CrossRef]
- Syurik, J.; Jacucci, G.; Onelli, O.D.; Holscher, H.; Vignolini, S. Bio-inspired highly scattering networks via polymer phase separation. Adv. Funct. Polym. 2018, 28, 1706901. [Google Scholar] [CrossRef]
- Zeighami, F.; Tehran, M.A. Developing optically efficient nanofiber coatings inspired by Cyphochilus white beetles. J. Ind. Text. 2016, 46, 495–509. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Onelli, O.D.; Jacucci, G.; Lovikka, V.; Rojas, O.J.; Ikkala, O.; Vignolini, S. Anomalous-diffusion-assisted brightness in white cellulose nanofibril membranes. Adv. Mater. 2018, 30, 1704050. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kim, Y.; Kim, H.; Kim, Y.; Jin, J.; Bae, B. Ultraviolet light stable and transparent sol-gel methyl siloxane hybrid material for UV light-emitting diode (UV LED) encapsulant. ACS Appl. Mater. Interfaces 2015, 7, 1035–1039. [Google Scholar] [CrossRef]
- Islam, S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Liu, J.; Yin, L.; Huangfu, M.; Zhang, Y.; Wu, X.; Zhang, X. Preparation and characterization of electrospun polyimide microfibrous mats with high whiteness and high thermal stability from organo-soluble polyimides containing rigid-rod moieties. Fibers Polym. 2018, 19, 1706–1714. [Google Scholar] [CrossRef]
- Liaw, D.; Wang, K.; Huang, Y.; Lee, K.; Lai, J.; Ha, C. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films: Heterocyclic structures and nanocomposites. Compo. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional polyimide/polyhedral oligomeric silsesquioxane nanocomosites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; Pan, Y.; Wang, Y.; Zhang, H.; Xiao, R.; Zou, Z. Polyimide-based photocatalysts: Rational design for energy and environmental applications. J. Mater. Chem. A 2020, 8, 14441–14462. [Google Scholar] [CrossRef]
- Qi, L.; Jia, Y.; An, Y.; Zhi, X.; Zhang, Y.; Liu, J.; Li, J. Photo-patternable, high-speed electrospun ultrafine fibers fabricated by intrinsically negative photosensitive polyimide. ACS Omega 2021, 6, 18458–18464. [Google Scholar] [CrossRef]
- Qi, L.; Guo, C.; Huangfu, M.; Zhang, Y.; Yin, L.; Wu, L.; Liu, J.; Zhang, X. Enhancement of solvent resistance of polyimide electrospun mat via the UV-assisted electrospinning and photosensitive varnish. Polymers 2019, 11, 2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicyilmaz, A.S.; Bedeloglu, A.C. Applications of polyimide coatings: A review. SN Appl. Sci. 2021, 3, 363. [Google Scholar] [CrossRef]
- KauSar, A. Holistic insights on polyimide nanocomposite nanofiber. Polym. Plast. Technol. Mater. 2020, 59, 1621–1639. [Google Scholar] [CrossRef]
- Ni, H.; Liu, J.; Wang, Z.; Yang, S. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Kim, J.W.; Chang, J.H. Syntheses of colorless and transparent polyimide membranes for microfiltration. Polymers 2020, 12, 1610. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent trends on transparent colorless polyimides with balanced thermal and optical properties: Design and synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Choi, I.H.; Chang, J.H. Colorless polyimide nanocomposite films containing hexafluoroisopropylidene group. Polym. Adv. Technol. 2011, 22, 682–689. [Google Scholar] [CrossRef]
- Guo, C.; Wu, X.; Liu, J.; Zhang, Y.; Zhang, X. Preparation and properties of high-whiteness polyimide ultrafine fabrics by electrospinning from organo-soluble semi-alicyclic polyimide resins. J. Photopolym. Sci. Technol. 2018, 31, 27–36. [Google Scholar] [CrossRef]
- Guo, C.Y.; Wang, Q.W.; Liu, J.G.; Qi, L.; Huangfu, M.G.; Wu, X.; Zhang, Y.; Zhang, X.M. Electrospun polyimide ultrafine non-woven fabrics with high whiteness and good thermal stability from organo-soluble semi-alicyclic polyimides: Preparation and properties. Express Polym. Lett. 2019, 13, 724–738. [Google Scholar] [CrossRef]
- Yin, L.; Guo, C.; Qi, L.; Wang, X.; Zhang, N.; Wu, L.; Liu, J. Electrospun polyphenylquinoxaline fibrous membranes: A versatile filtering medium for separation of highly alkaline aqueous red mud pollutant. J. Polym. Res. 2020, 27, 321. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishigami, T.; Ishii, J.; Sugiura, K.; Fujii, M. Solution-processable transparent polyimides with low coefficients of thermal expansion and self-orientation behavior induced by solution casting. Eur. Polym. J. 2013, 49, 3657–3672. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, D.; Du, H.; Wu, X.; Zhang, Y.; Tan, Y.; Wu, L.; Liu, J.; Zhang, X. Reduced coefficients of linear thermal expansion of colorless and transparent semi-alicyclic polyimide films via incorporation of rigid-rod amide moiety: Preparation and properties. Polymers 2020, 12, 413. [Google Scholar] [CrossRef] [Green Version]
- Rieger, J. The glass transition temperature of polystyrene: Rresults of a round robin test. J. Therm. Anal. Calorim. 1996, 46, 965–972. [Google Scholar] [CrossRef]




| Solid Content (wt%) | Voltage Supply (kV) | Spinneret ID (mm) | |
|---|---|---|---|
| PI-1 | 25 | 12 | 0.50 |
| PI-2 | 30 | 15 | 0.50 |
| PI-3 | 30 | 15 | 0.21 |
| PI-4 | 35 | 18 | 0.50 |
| Sample | [η]inh 1 (dL/g) | Molecular Weight 2 (×104 g/mol) | Solubility 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | Mw | PDI | NMP | DMAc | DMF | CHCl3 | THF | ||
| PI (HTDA-BDAF) | 0.63 | 3.38 | 5.76 | 1.70 | ++ | ++ | ++ | +− | +− |
| Samples | R457 (%) 1 | L* 1 | a* 1 | b* 1 | WI1 |
|---|---|---|---|---|---|
| PI-1 | 90.4 | 91.02 | −0.49 | 1.24 | 90.92 |
| PI-2 | 91.3 | 91.49 | −0.48 | 1.19 | 91.39 |
| PI-3 | 90.2 | 91.38 | −0.47 | 1.62 | 91.22 |
| PI-4 | 89.5 | 90.94 | −0.38 | 2.07 | 90.70 |
| PI-ref 2 | 37.3 | 84.65 | 1.62 | 37.96 | 59.00 |
| PI-50 °C | 95.7 | 94.50 | −0.43 | 3.51 | 93.46 |
| PI-200 °C | 89.0 | 93.29 | −0.28 | 6.17 | 90.88 |
| PS | 88.4 | 93.52 | −0.49 | 1.78 | 93.26 |
| PS-50 °C | 88.1 | 93.37 | −0.46 | 1.79 | 93.23 |
| PS-150 °C | 18.1 | ND 3 | ND 3 | ND 3 | ND 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, X.; Wang, H.; Wei, X.; Zhang, Y.; An, Y.; Qi, H.; Liu, J. Electrospun Semi-Alicyclic Polyimide Nanofibrous Membrane: High-Reflectance and High-Whiteness with Superior Thermal and Ultraviolet Radiation Stability for Potential Applications in High-Power UV-LEDs. Nanomaterials 2021, 11, 1977. https://doi.org/10.3390/nano11081977
Zhi X, Wang H, Wei X, Zhang Y, An Y, Qi H, Liu J. Electrospun Semi-Alicyclic Polyimide Nanofibrous Membrane: High-Reflectance and High-Whiteness with Superior Thermal and Ultraviolet Radiation Stability for Potential Applications in High-Power UV-LEDs. Nanomaterials. 2021; 11(8):1977. https://doi.org/10.3390/nano11081977
Chicago/Turabian StyleZhi, Xinxin, Huasen Wang, Xinying Wei, Yan Zhang, Yuancheng An, Haoran Qi, and Jingang Liu. 2021. "Electrospun Semi-Alicyclic Polyimide Nanofibrous Membrane: High-Reflectance and High-Whiteness with Superior Thermal and Ultraviolet Radiation Stability for Potential Applications in High-Power UV-LEDs" Nanomaterials 11, no. 8: 1977. https://doi.org/10.3390/nano11081977
APA StyleZhi, X., Wang, H., Wei, X., Zhang, Y., An, Y., Qi, H., & Liu, J. (2021). Electrospun Semi-Alicyclic Polyimide Nanofibrous Membrane: High-Reflectance and High-Whiteness with Superior Thermal and Ultraviolet Radiation Stability for Potential Applications in High-Power UV-LEDs. Nanomaterials, 11(8), 1977. https://doi.org/10.3390/nano11081977







