Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
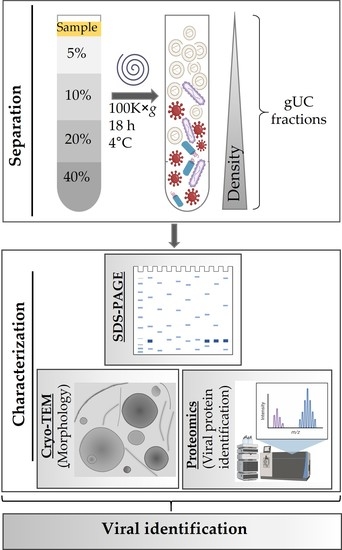
2.2. Isolation of Tomato Nanovesicles by Differential Centrifugation
2.3. Fractionation of Tomato Nanovesicles by Density Gradient Ultracentrifugation and Density Determination
2.4. Size-Exclusion Chromatography of Tomato-Derived Nanovesicles
2.5. Determination of Physical and Molecular Characteristics of Tomato-Derived NVs
2.6. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.7. Scanning Electron Microscopy (SEM)
2.8. LC–ESI–MS/MS Analysis
2.9. Bioinformatics
3. Results
3.1. Isolation and Characterization of Tomato-Derived Nanovesicles
3.2. Cryo-TEM Analysis Shows Viral Contamination in some Tomato Nanovesicles Samples
3.3. Proteomics Reveals the Identity of Viral Particles-Related Proteins in Tomato-Derived Nanovesicles
3.3.1. Sample 1 Contains Tomato Vesicles and Three Different Viral Particles
3.3.2. Sample 2 Contains Tomato Vesicles in the Low-Density and Viral Particles in the High-Density Sucrose Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. Peer J. 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef]
- Woith, E.; Melzig, M.F. Extracellular vesicles from fresh and dried plants—Simultaneous purification and visualization using gel electrophoresis. Int. J. Mol. Sci. 2019, 20, 357. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 1–18. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; Zhang, H. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2016, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Moubarak, M.; Fiume, I.; Turiák, L.; Pocsfalvi, G. Membrane transporters in citrus clementina fruit juice-derived nanovesicles. Int. J. Mol. Sci. 2019, 20, 6205. [Google Scholar] [CrossRef] [PubMed]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of Tomato-Derived Nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Şahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Cho, E.G.; Choi, S.Y.; Kim, H.; Choi, E.J.; Lee, E.J.; Park, P.J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-Derived Extracellular Vesicles Facilitate Anti-Senescence Effects in Human Skin Cells: An Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.; Lötvall, J.; Breakefield, X.O.; Andaloussi, S.E.L. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Cameron, G.T.; Geana, M.V. Symposium: Relative Bioactivity of Functional Foods and Related Dietary Supplements Functional Foods: Delivering Information to the Oncology Nurse 1, 2. J. Nutr. 2005, 1, 1253–1255. [Google Scholar] [CrossRef][Green Version]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.N.; Taheri, S.; Othman, R.Y.; Teo, C.H. Viral disease of tomato crops (Solanum lycopesicum L.): An overview. J. Plant Dis. Prot. 2020, 127, 725–739. [Google Scholar] [CrossRef]
- Caciagli, P. Vegetable Viruses. Encycl. Virol. 2008, 282–290. [Google Scholar] [CrossRef]
- Ambrós, S.; Martínez, F.; Ivars, P.; Hernández, C.; de la Iglesia, F.; Elena, S.F. Molecular and biological characterization of an isolate of Tomato mottle mosaic virus (ToMMV) infecting tomato and other experimental hosts in eastern Spain. Eur. J. Plant Pathol. 2017, 149, 261–268. [Google Scholar] [CrossRef]
- Turina, M.; Geraats, B.P.J.; Ciuffo, M. First report of Tomato mottle mosaic virus in tomato crops in Israel. New Dis. Rep. 2016, 33, 1. [Google Scholar] [CrossRef]
- Oladokun, J.O.; Halabi, M.H.; Barua, P.; Nath, P.D. Tomato brown rugose fruit disease: Current distribution, knowledge and future prospects. Plant Pathol. 2019, 68, 1579–1586. [Google Scholar] [CrossRef]
- Wilstermann, A.; Ziebell, H. Tomato brown rugose fruit virus (ToBRFV). JKI Data Sheets Plant Dis. Diagn. 2019, 1, ISSN 2191-1398. [Google Scholar] [CrossRef]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Pappu, H.R.; Jones, R.A.C.; Jain, R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kwon, S.Y.; Kim, S.T. An insight into the tomato spotted wilt virus (TSWV), tomato and thrips interaction. Plant Biotechnol. Rep. 2018, 12, 157–163. [Google Scholar] [CrossRef]
- Turina, M.; Kormelink, R.; Resende, R.O. Resistance to Tospoviruses in Vegetable Crops: Epidemiological and Molecular Aspects. Annu. Rev. Phytopathol. 2016, 54, 347–371. [Google Scholar] [CrossRef]
- Abadkhah, M.; Koolivand, D.; Eini, O. A new distinct clade for Iranian Tomato spotted wilt virus isolates based on the polymerase, nucleocapsid, and non-structural genes. Plant Pathol. J. 2018, 34, 514–531. [Google Scholar] [CrossRef]
- Sether, D.M.; DeAngelis, J.D. Tomato Spotted Wilt Virus Host List and Bibliography; Special Report 888; Oregon State University: Corvallis, OR, USA, 1992. [Google Scholar]
- Mandal, B.; Jain, R.K. Can plant virus infect human being? Indian J. Virol. 2010, 21, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Balique, F.; Lecoq, H.; Raoult, D.; Colson, P. Can plant viruses cross the kingdom border and be pathogenic to humans? Viruses 2015, 7, 2074–2098. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, S.J.; Park, Y.J.; Kim, S.Y.; Ryu, C.M. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef]
- De Toledo Martins, S.; Alves, L.R. Extracellular Vesicles in Viral Infections: Two Sides of the Same Coin? Front. Cell. Infect. Microbiol. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Hoen, E.N.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Mammadova, R.; Ramos Juarez, A.P.; Bokka, R.; Trepiccione, F.; Capasso, G. COVID-19 and Extracellular Vesicles: An Intriguing Interplay. Kidney Blood Press. Res. 2020, 45, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Mcnamara, R.P.; Dittmer, D.P. Modern Techniques for the Isolation of Extracellular Vesicles and Viruses. J. Neuroimmune Pharmacol. 2020, 15, 459–472. [Google Scholar] [CrossRef]
- Hun, Y.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar]
- Balke, I.; Zeltins, A. Recent advances in the use of plant virus-like particles as vaccines. Viruses 2020, 12, 270. [Google Scholar] [CrossRef]
- Available online: https://biorender.com/ (accessed on 5 June 2021).
- Lešer, V.; Drobne, D.; Pipan, Ž.; Milani, M.; Tatti, F. Comparison of different preparation methods of biological samples for FIB milling and SEM investigation. J. Microsc. 2009, 233, 309–319. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havliš, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar] [CrossRef]
- Bioinformatics Made Easy (Version 1.4.12). Available online: https://www.biobam.com/omicsbox (accessed on 3 March 2019).
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Kralj-Iglič, V.; Pocsfalvi, G.; Mesarec, L.; Šuštar, V.; Hägerstrand, H.; Iglič, A. Minimizing isotropic and deviatoric membrane energy—A unifying formation mechanism of different cellular membrane nanovesicle types. PLoS ONE 2020, 15, e0244796. [Google Scholar] [CrossRef]
- Quenouille, J.; Vassilakos, N.; Moury, B. Potato virus Y: A major crop pathogen that has provided major insights into the evolution of viral pathogenicity. Mol. Plant Pathol. 2013, 14, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Kežar, A.; Kavčič, L.; Polák, M.; Nováček, J.; Gutiérrez-Aguirre, I.; Žnidarič, M.T.; Coll, A.; Stare, K.; Gruden, K.; Ravnikar, M.; et al. Structural basis for the multitasking nature of the potato virus Y coat protein. Sci. Adv. 2019, 5, eaaw3808. [Google Scholar] [CrossRef]
- Fukuhara, T.; Tabara, M.; Koiwa, H.; Takahashi, H. Effect of asymptomatic infection with southern tomato virus on tomato plants. Arch. Virol. 2020, 165, 11–20. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant roots release small extracellular vesicles with antifungal activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10- to 17-nucleotide “Tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; Zhang, H.G. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Ito, Y.; Taniguchi, K.; Kuranaga, Y.; Eid, N.; Inomata, Y.; Lee, S.W.; Uchiyama, K. Uptake of micrornas from exosome-like nanovesicles of edible plant juice by rat enterocytes. Int. J. Mol. Sci. 2021, 22, 3749. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, H.M. CRC Handbook of Viruses: Mass-Molecular Weight Value and Related Properties; CRC Press LLC: Boca Raton, FL, USA, 1998. [Google Scholar]
- Hartmann, M.; Kim, D.; Bernsdorff, F.; Ajami-Rashidi, Z.; Scholten, N.; Schreiber, S.; Zeier, T.; Schuck, S.; Reichel-Deland, V.; Zeier, J. Biochemical Principles and Functional Aspects of Pipecolic Acid Biosynthesis in Plant Immunity. Plant Physiol. 2017, 174, 124–153. [Google Scholar] [CrossRef]








| No. | Accession | Description UniProt | PLGS Score | Peptides | Coverage (%) | Description OmicsBOX (Protein Blast) | Sim Mean |
|---|---|---|---|---|---|---|---|
| 1 | A0A3Q7IXE6 | Uncharacterized protein | 14,872 | 47 | 79 | patellin-3-like | 94.26 |
| 2 | Q84XW6 | V-ATPase 69 kDa subunit | 29,199 | 39 | 76 | V-type proton ATPase catalytic subunit A | 98.78 |
| 3 | P38416 | Linoleate 9S-lipoxygenase B | 9129 | 32 | 44 | putative linoleate 9S-lipoxygenase 5 | 90.26 |
| 4 | A0A3Q7ENA3 | Lipoxygenase | 9055 | 31 | 43 | putative linoleate 9S-lipoxygenase 5 | 90.1 |
| 5 | Q84XV7 | V-ATPase 69 kDa subunit | 19,723 | 29 | 51 | V-type proton ATPase catalytic subunit A | 98.78 |
| 6 | Q9XEX8 | Remorin 1 | 10,015 | 22 | 51 | remorin | 86.7 |
| 7 | A0A3Q7FE06 | V-type proton ATPase subunit a | 72,645 | 22 | 35 | V-type proton ATPase subunit a3 | 95.26 |
| 8 | Q9SPD5 | Plasma membrane ATPase | 6306 | 22 | 28 | plasma membrane atpase 1 | 99.29 |
| 9 | A0A3Q7IIS5 | Vacuolar proton pump subunit B | 24,212 | 21 | 62 | V-type proton ATPase subunit B2 | 98.52 |
| 10 | A0A3Q7IZ03 | Uncharacterized protein | 15,503 | 21 | 45 | heat shock cognate 70 kDa protein 2-like | 98.17 |
| 11 | A0A3Q7FX57 | Uncharacterized protein | 15,788 | 20 | 42 | heat shock cognate 70 kDa protein 2-like | 99.12 |
| 12 | A0A3Q7INZ6 | Uncharacterized protein | 44,097 | 18 | 48 | actin-7 | 99.19 |
| 13 | A0A3Q7GJM0 | Phosphoinositide phospholipase | 5957 | 18 | 38 | phosphoinositide phospholipase C 2-like | 95.35 |
| 14 | A0A3Q7FJJ3 | Uncharacterized protein | 43,162 | 17 | 51 | actin-7 | 99.87 |
| 15 | A0A3Q7FRW6 | PHB domain-containing protein | 12,933 | 17 | 50 | hypersensitive-induced reaction 1 protein | 99.01 |
| 16 | A0A3Q7EZ16 | 14_3_3 domain-containing protein | 8764 | 17 | 58 | 14-3-3 protein 4 | 97.82 |
| 17 | A0A3Q7IYI9 | Uncharacterized protein | 14,977 | 16 | 35 | heat shock cognate 70 kDa protein | 98.88 |
| 18 | A0A3Q7FV11 | H(+)-exporting diphosphatase | 7652.922 | 16 | 13 | pyrophosphate-energized vacuolar membrane proton pump-like | 98.42 |
| 19 | A0A3Q7I767 | Fe2OG dioxygenase domain-containing protein | 7169.406 | 16 | 37 | 1-aminocyclopropane-1-carboxylate oxidase homolog | 83.31 |
| 20 | A0A3Q7HFP1 | Glycerophosphodiester phosphodiesterase | 6081.231 | 16 | 22 | glycerophosphodiester phosphodiesterase GDPDL4 | 88.91 |
| Name of the Virus | Genus of the Virus | Viral Characteristics | Sample | gUC Fraction(s) * | Name of Viral Protein(s) Identified | UniProt Accession No. | Coverage % of Viral Protein(s) Identified |
|---|---|---|---|---|---|---|---|
| Tomato brown rugose fruit virus (ToBRFV) | Tobamovirus | Single-stranded RNA rod-shaped particles of 300 nm in length and 17 nm in diameter [31] | S1 | 4 7 9 | Capsid protein | A0A0S2SZX3 | 55.3 54.7 55.4 |
| Tomato mosaic virus (ToMV) | Tobamovirus | Single-stranded RNA rod shaped structure, about 300 nm length and 18 nm radius [27] | S1 | 4 | Capsid protein | Q83482 | 6.4 |
| Tomato mottle mosaic virus (ToMMV) | Tobamovirus | Single-stranded RNA rod-shaped virus particles 300 nm in length [28,29] | S1 | 7 | Capsid protein | T1WEZ3 | 5.7 |
| Tomato spotted wilt virus (TSWV) | Orthotospovirus | Single-stranded RNA roughly spherical shaped with a diameter 80–120 nm and density of 1.207 g/mL [34] | S2 | B2 | Nucleoprotein | O55648 | 58.1 |
| NSs non-structural protein | E1Y5V2 | 19.9 | |||||
| Nucleocapsid protein | A0A0N9H8W3 | 56.7 | |||||
| Putative movement protein | A0A097PIF5 | 30.1 | |||||
| Potato virus Y (PVY) | Potyvirus | Single-stranded RNA, a filamentous, flexuous form, with a length of 730 nm and a diameter of 12 nm [53,54] | S2 | B1 | Putative coat protein | A0A0K2K0B0 | 18.3 |
| B2 | Genome polyprotein | P18247 | 9.3 | ||||
| Southern tomato virus (STV) | Amalgavirus | Double-stranded RNA, shape and size NA [55] | S2 | B2 | Putative coat protein | A0A0K2K0B0 | 18.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammadova, R.; Fiume, I.; Bokka, R.; Kralj-Iglič, V.; Božič, D.; Kisovec, M.; Podobnik, M.; Zavec, A.B.; Hočevar, M.; Gellén, G.; et al. Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach. Nanomaterials 2021, 11, 1922. https://doi.org/10.3390/nano11081922
Mammadova R, Fiume I, Bokka R, Kralj-Iglič V, Božič D, Kisovec M, Podobnik M, Zavec AB, Hočevar M, Gellén G, et al. Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach. Nanomaterials. 2021; 11(8):1922. https://doi.org/10.3390/nano11081922
Chicago/Turabian StyleMammadova, Ramila, Immacolata Fiume, Ramesh Bokka, Veronika Kralj-Iglič, Darja Božič, Matic Kisovec, Marjetka Podobnik, Apolonija Bedina Zavec, Matej Hočevar, Gabriella Gellén, and et al. 2021. "Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach" Nanomaterials 11, no. 8: 1922. https://doi.org/10.3390/nano11081922
APA StyleMammadova, R., Fiume, I., Bokka, R., Kralj-Iglič, V., Božič, D., Kisovec, M., Podobnik, M., Zavec, A. B., Hočevar, M., Gellén, G., Schlosser, G., & Pocsfalvi, G. (2021). Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach. Nanomaterials, 11(8), 1922. https://doi.org/10.3390/nano11081922