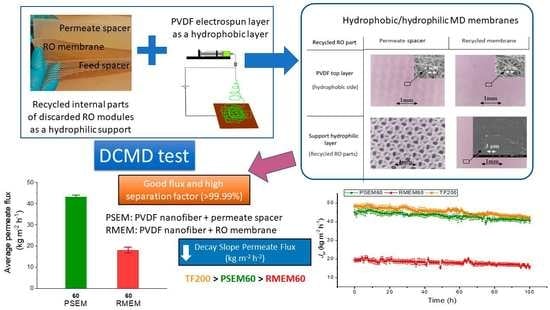
Electrospun Nanostructured Membrane Engineering Using Reverse Osmosis Recycled Modules: Membrane Distillation Application
Abstract
1. Introduction
2. Materials and Methods
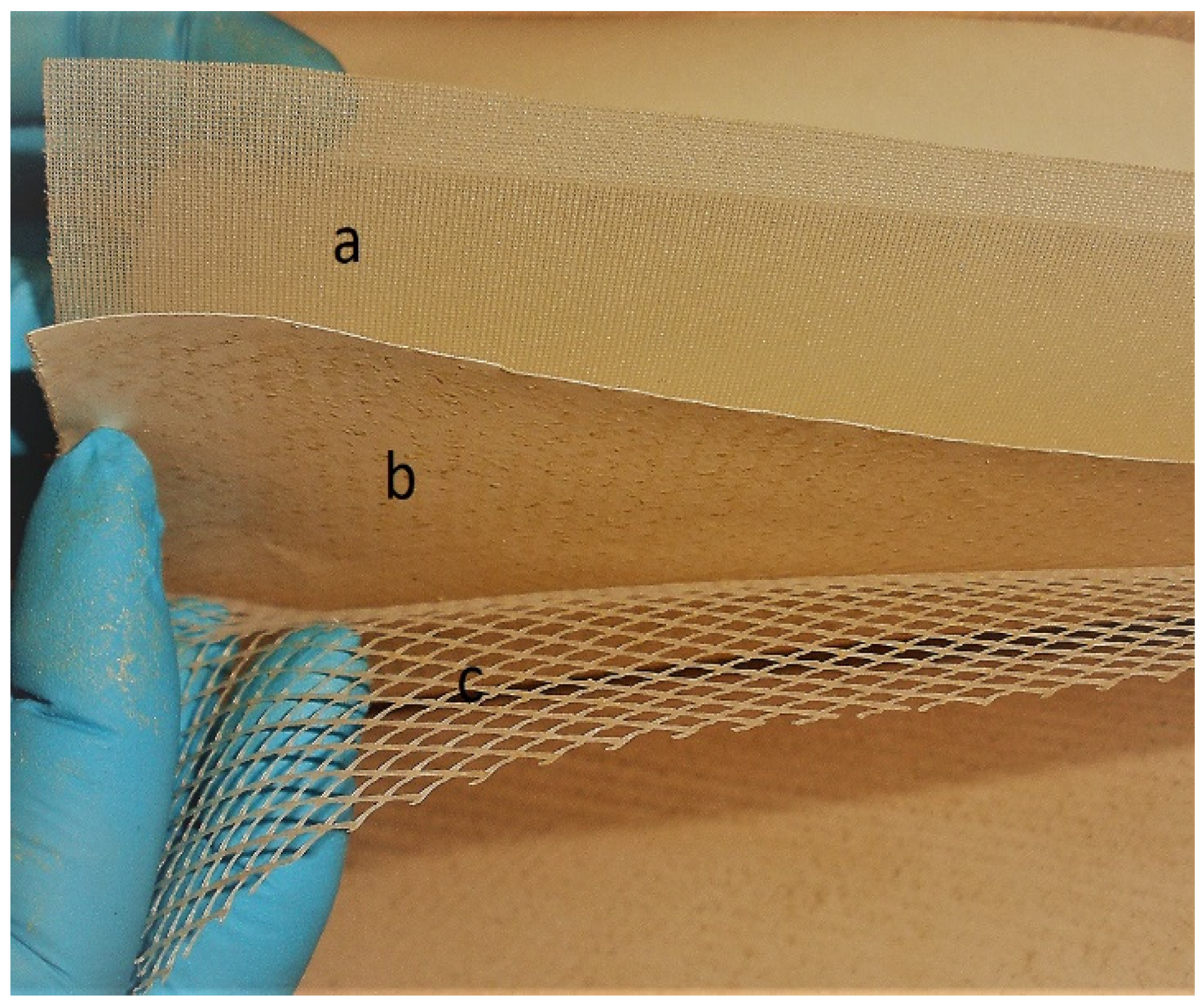
2.1. End-of-Life RO Membrane Module and Hydrophilic Supports for Nanostructured MD Membranes
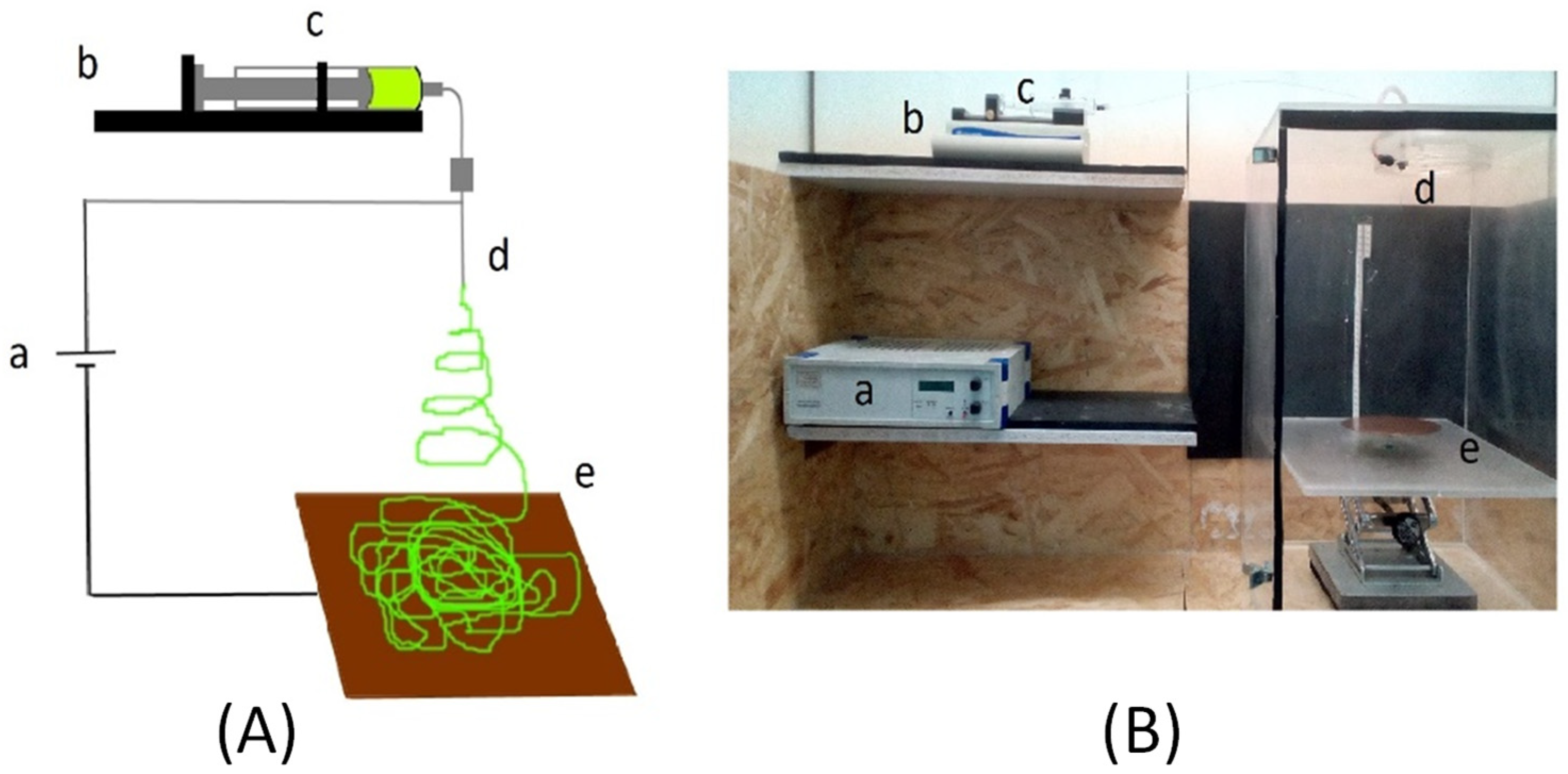
2.2. Preparation of Nanofibrous Supported Hydrophobic/Hydrophilic MD Membranes
2.3. Membrane Characterization
2.4. Long-Term DCMD Desalination Tests
3. Results and Discussion
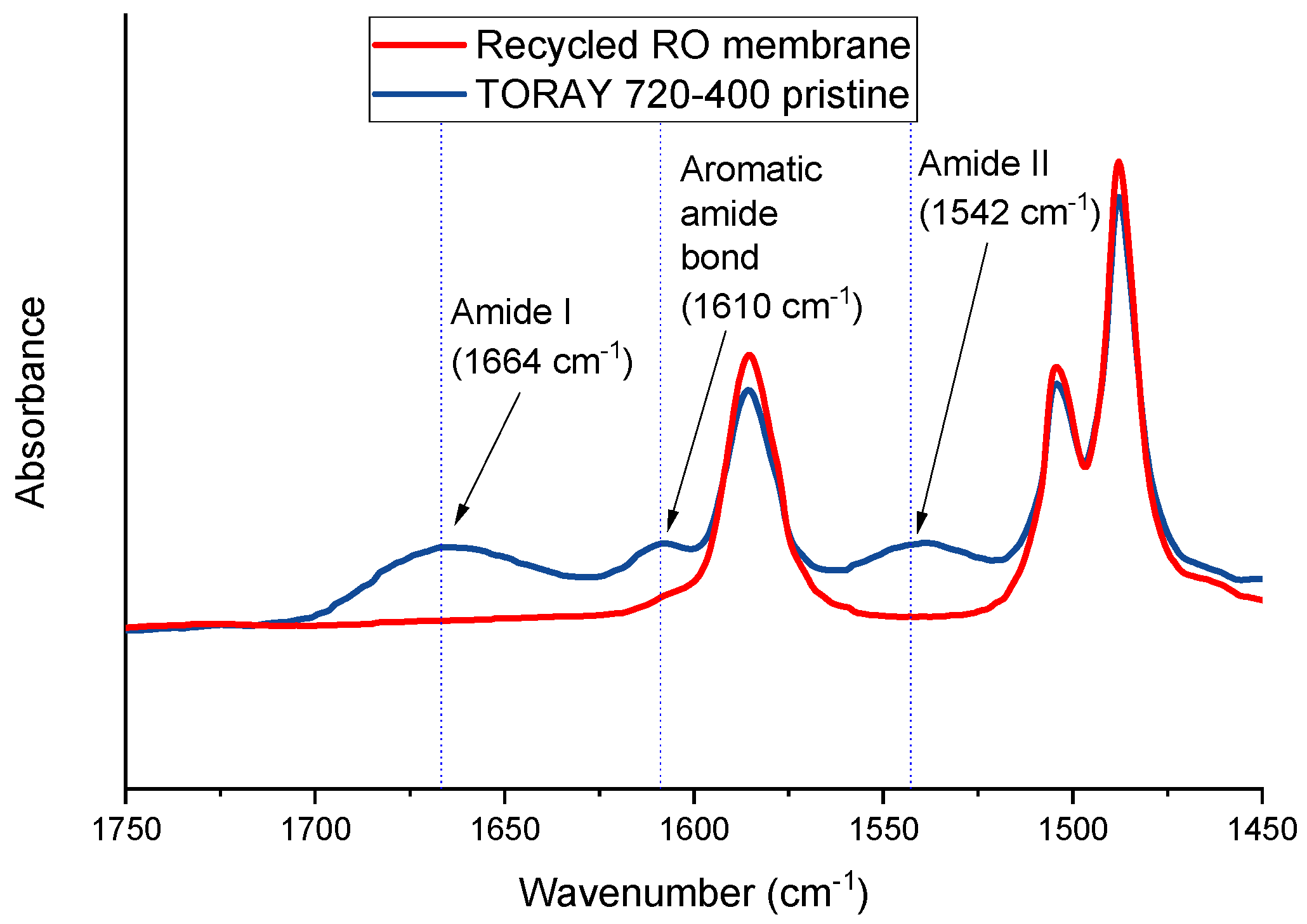
3.1. Membrane Characterization
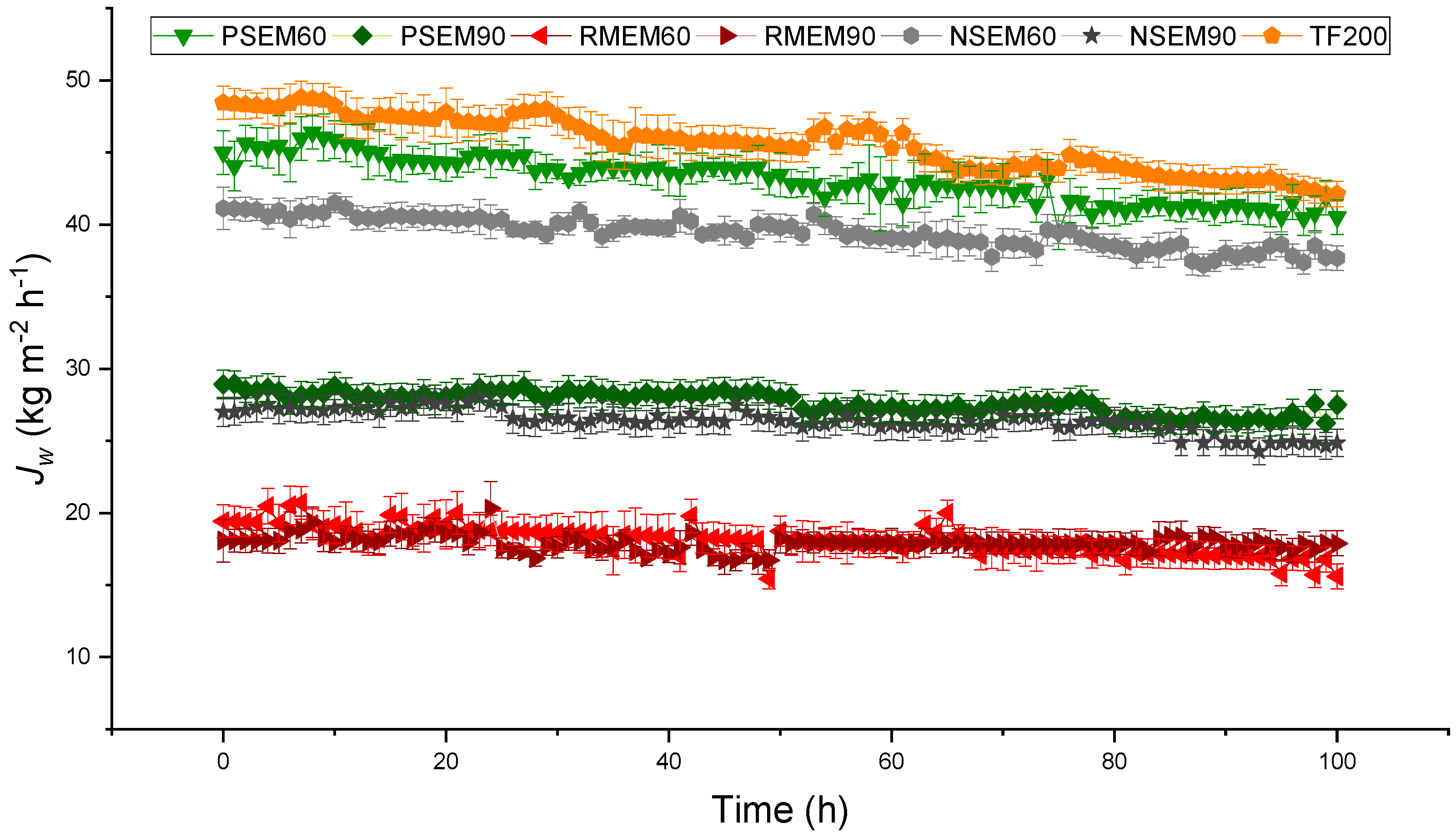
3.2. Long-Term DCMD Desalination Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols: | |
| AC | Activated carbon |
| AGMD | Air gap membrane distillation |
| Am | Effective membrane area (m2) |
| ATR-FTIR | Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy |
| Cf | Feed salt concentration (g L−1) |
| Cf,f | Final feed salt concentration (g L−1) |
| CNT | Carbon nanotubes |
| Cp | Permeate salt concentration (g L−1) |
| DCMD | Direct contact membrane distillation |
| di | Mean inter-fiber space (µm) |
| DSJw | Decay slope of the permeate flux (kg m−2 h−2) |
| DTGS–KBr | Deuterated triglycine sulfate–potassium bromide |
| Eb | Elongation at break (%) |
| ED | Electrodialysis |
| FA | Fluorinated acrylate copolymer |
| FO | Forward osmosis |
| IPA | Isopropyl alcohol |
| Jw | Permeate flux (kg m−2 h−1) |
| Jw, average | Average permeate flux (kg m−2 h−1) |
| LEPw | Liquid entry pressure of water (Pa) |
| MD | Membrane distillation |
| MF | Microfiltration |
| Mn | Number average molecular weight (kg mol−1) |
| Mw | Mass average molecular weight (kg mol-1) |
| NaCl | Sodium chloride |
| NaOCl | Sodium hypochlorite |
| NF | Nanofiltration |
| PA | Polyamide |
| PAN | Polyacrylonitrile |
| PET | Polyester |
| PP | Polypropylene |
| PS | Polystyrene |
| PSf | Polysulfone |
| PVDF | Polyvinylidene fluoride |
| PVDF-HFP | Poly(vinylidene fluoride-co-hexafluoropropylene) |
| RO | Reverse osmosis |
| SBS | Styrene-butadiene-styrene |
| SEM | Scanning electron microscope |
| SGMD | Sweeping gas membrane distillation |
| SiF | Silica fibers |
| SiO2NPs | Silica nanoparticles |
| TBAC | Tetrabutylammonium chloride |
| TFC | Thin film composite |
| Tf | Feed temperature |
| Tp | Permeate temperature |
| Ts | Tensile strength (MPa) |
| UF | Ultrafiltration |
| VMD | Vacuum membrane distillation |
| YM | Young´s modulus (MPa) |
| Greek letters: | |
| α | Rejection factor |
| δ | Thickness (µm) |
| δPVDF | Thickness of the PVDF layer (µm) |
| δS | Thickness of the support layer (µm) |
| δt | Total thickness (µm) |
| ε | Void volume fraction (%) |
| θw | Water contact angle (°) |
| (θw)PVDF | Thickness of the PVDF layer (°) |
| (θw)s | Water contact angle of the support layer (°) |
| Δm | Mass difference (kg) |
| Δt | Time difference (h) |
| ΔT | Temperature difference between the feed and permeate (K) |
References
- IDA and GWI desalData. IDA Desalination Yearbook 2017–2018; Media Analytics Ltd.: Oxford, UK, 2017; ISBN 978-1-907467-52-3. [Google Scholar]
- IDA and GWI desalData. The IDA Water Security Handbook 2020–2021; Media Analytics Ltd.: Oxford, UK, 2020; ISBN 978-1-907467-62-2. [Google Scholar]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.; Yang, D.R.; Hong, S. Towards a low-energy seawater reverse osmosis desalination plant: A review and theoretical analysis for future directions. J. Membr. Sci. 2020, 595, 117607. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J. Minimal Liquid Discharge (MLD) and Zero Liquid Discharge (ZLD) strategies for wastewater management and resource recovery—Analysis, challenges and prospects. J. Environ. Chem. Eng. 2020, 8, 104418. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- García, N.P.; del Vigo, F.; Chesters, S.; Armstrong, M.; Wilson, R.; Fazel, M. A Study of the Physical and Chemical Damage on Reverse Osmosis Membranes Detected by Autopsies. In Proceedings of the The International Desalination Association World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. [Google Scholar]
- Lawler, W.; Bradford-Hartke, Z.; Cran, M.J.; Duke, M.; Leslie, G.; Ladewig, B.P.; Le-Clech, P. Towards new opportunities for reuse, recycling and disposal of used reverse osmosis membranes. Desalination 2012, 299, 103–112. [Google Scholar] [CrossRef]
- Ould Mohamedou, E.; Penate Suarez, D.B.; Vince, F.; Jaouen, P.; Pontie, M. New lives for old reverse osmosis (RO) membranes. Desalination 2010, 253, 62–70. [Google Scholar] [CrossRef]
- Pontié, M. Old RO membranes: Solutions for reuse. Desalin. Water Treat. 2015, 53, 1492–1498. [Google Scholar] [CrossRef]
- Prince, C.; Cran, M.; Le-Clech, P.; Uwe-Hoehn, K.; Duke, M. Reuse and recycling of used desalination membranes. Proc. OzWater 2011, 11, 1–8. [Google Scholar]
- Hailemariam, R.H.; Woo, Y.C.; Damtie, M.M.; Kim, B.C.; Park, K.-D.; Choi, J.-S. Reverse osmosis membrane fabrication and modification technologies and future trends: A review. Adv. Colloid Interface Sci. 2020, 276, 102100. [Google Scholar] [CrossRef]
- Lawler, W.; Antony, A.; Cran, M.; Duke, M.; Leslie, G.; Le-Clech, P. Production and characterisation of UF membranes by chemical conversion of used RO membranes. J. Membr. Sci. 2013, 447, 203–211. [Google Scholar] [CrossRef]
- Landaburu-Aguirre, J.; García-Pacheco, R.; Molina, S.; Rodríguez-Sáez, L.; Rabadán, J.; García-Calvo, E. Fouling prevention, preparing for re-use and membrane recycling. Towards circular economy in RO desalination. Desalination 2016, 393, 16–30. [Google Scholar] [CrossRef]
- Molina, S.; Landaburu-Aguirre, J.; Rodríguez-Sáez, L.; García-Pacheco, R.; de la Campa, J.G.; García-Calvo, E. Effect of sodium hypochlorite exposure on polysulfone recycled UF membranes and their surface characterization. Polym. Degrad. Stab. 2018, 150, 46–56. [Google Scholar] [CrossRef]
- Molina, S.; García Pacheco, R.; Rodriguez-Sáez, L.; García-Calvo, E.; Campos, E.; Zarzo Martinez, D.; de la Campa, J.G.; de Abajo, J. Transformation of end-of-life RO membranes into recycled NF and UF membranes, surface characterization. In Proceedings of the International Desalination Asociation Word Congress, IDAWC15, San Diego, CA, USA, 30 August–4 September 2015; Volume 15WC-51551. [Google Scholar]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Terrero-Rodríguez, P.; Campos, E.; Molina-Serrano, F.; Rabadán, J.; Zarzo, D.; García-Calvo, E. Validation of recycled membranes for treating brackish water at pilot scale. Desalination 2018, 433, 199–208. [Google Scholar] [CrossRef]
- Moradi, M.R.; Pihlajamäki, A.; Hesampour, M.; Ahlgren, J.; Mänttäri, M. End-of-life RO membranes recycling: Reuse as NF membranes by polyelectrolyte layer-by-layer deposition. J. Membr. Sci. 2019, 584, 300–308. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular economy in membrane technology: Using end-of-life reverse osmosis modules for preparation of recycled anion exchange membranes and validation in electrodialysis. J. Membr. Sci. 2020, 593, 117423. [Google Scholar] [CrossRef]
- Morón-López, J.; Nieto-Reyes, L.; Senán-Salinas, J.; Molina, S.; El-Shehawy, R. Recycled desalination membranes as a support material for biofilm development: A new approach for microcystin removal during water treatment. Sci. Total Environ. 2019, 647, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Morón-López, J.; Nieto-Reyes, L.; Molina, S.; Lezcano, M.Á. Exploring microcystin-degrading bacteria thriving on recycled membranes during a cyanobacterial bloom. Sci. Total Environ. 2020, 736, 139672. [Google Scholar] [CrossRef] [PubMed]
- Morón-López, J.; Molina, S. Optimization of Recycled-Membrane Biofilm Reactor (R-MBfR) as a sustainable biological treatment for microcystins removal. Biochem. Eng. J. 2020, 153, 107422. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Molina, S.; Rodríguez-Sáez, L.; Teli, S.B.; García-Calvo, E. Transformation of end-of-life RO membranes into NF and UF membranes: Evaluation of membrane performance. J. Membr. Sci. 2015, 495, 305–315. [Google Scholar] [CrossRef]
- Morón-López, J.; Nieto-Reyes, L.; Aguado, S.; El-Shehawy, R.; Molina, S. Recycling of end-of-life reverse osmosis membranes for membrane biofilms reactors (MBfRs). Effect of chlorination on the membrane surface and gas permeability. Chemosphere 2019, 231, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Khayet, M.; Matsuura, T. Chapter 1—Introduction to Membrane Distillation. In Membrane Distillation; Khayet, M., Matsuura, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–16. ISBN 978-0-444-53126-1. [Google Scholar]
- Topuz, F.; Holtzl, T.; Szekely, G. Scavenging organic micropollutants from water with nanofibrous hypercrosslinked cyclodextrin membranes derived from green resources. Chem. Eng. J. 2021, 419, 129443. [Google Scholar] [CrossRef]
- Cseri, L.; Topuz, F.; Abdulhamid, M.A.; Alammar, A.; Budd, P.M.; Szekely, G. Electrospun Adsorptive Nanofibrous Membranes from Ion Exchange Polymers to Snare Textile Dyes from Wastewater. Adv. Mater. Technol. 2021, 2000955. [Google Scholar] [CrossRef]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy consumption and environmental impact assessment of desalination plants and brine disposal strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Alrehaili, O.; Perreault, F.; Sinha, S.; Westerhoff, P. Increasing net water recovery of reverse osmosis with membrane distillation using natural thermal differentials between brine and co-located water sources: Impacts at large reclamation facilities. Water Res. 2020, 184, 116134. [Google Scholar] [CrossRef]
- Bindels, M.; Carvalho, J.; Gonzalez, C.B.; Brand, N.; Nelemans, B. Techno-economic assessment of seawater reverse osmosis (SWRO) brine treatment with air gap membrane distillation (AGMD). Desalination 2020, 489, 114532. [Google Scholar] [CrossRef]
- Khayet, M.; García-Payo, M.C.; García-Fernández, L.; Contreras-Martínez, J. Dual-layered electrospun nanofibrous membranes for membrane distillation. Desalination 2018, 426, 174–184. [Google Scholar] [CrossRef]
- Khayet, M.; Mengual, J.I.; Matsuura, T. Porous hydrophobic/hydrophilic composite membranes: Application in desalination using direct contact membrane distillation. J. Membr. Sci. 2005, 252, 101–113. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Rabadán, F.J.; Terrero, P.; Molina Martínez, S.; Martínez, D.; Campos, E.; Molina, F.; Rodríguez, L.; Ortíz de Lejarazu, A.; Landaburu-Aguirre, J.; et al. Life+13 transfomem: A recycling example within the desalination world. In Proceedings of the XI AEDYR International Congress, Valencia, Spain, 19–21 October 2016; p. VAL-112-16. [Google Scholar]
- Lawler, W.; Alvarez-Gaitan, J.; Leslie, G.; Le-Clech, P. Comparative life cycle assessment of end-of-life options for reverse osmosis membranes. Desalination 2015, 357, 45–54. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nano-fibers: Effects of polymer concentration and desalination by direct contact membrane distillation. J. Membr. Sci. 2014, 454, 133–143. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M.; Cojocaru, C.; García-Payo, M.C.; Arribas, P. Response surface modeling and optimization of electrospun nanofiber membranes. Open Nanosci. J. 2013, 7, 8–17. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Chapter 8—MD Membrane Characterization. In Membrane Distillation; Khayet, M., Matsuura, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 189–225. ISBN 978-0-444-53126-1. [Google Scholar]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nanofibers at different electrospinning times: 1. Desalination by direct contact membrane distillation. J. Membr. Sci. 2013, 433, 167–179. [Google Scholar] [CrossRef]
- Arribas, P.; García-Payo, M.C.; Khayet, M.; Gil, L. Heat-treated optimized polysulfone electrospun nanofibrous membranes for high performance wastewater microfiltration. Sep. Purif. Technol. 2019, 226, 323–336. [Google Scholar] [CrossRef]
- Li, X.; García-Payo, M.C.; Khayet, M.; Wang, M.; Wang, X. Superhydrophobic polysulfone/polydimethylsiloxane electrospun nanofibrous membranes for water desalination by direct contact membrane distillation. J. Membr. Sci. 2017, 542, 308–319. [Google Scholar] [CrossRef]
- Godino, P.; Peña, L.; Mengual, J.I. Membrane distillation: Theory and experiments. J. Membr. Sci. 1996, 121, 83–93. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nanofibers at different electrospinning times: 2. Theoretical analysis, polarization effects and thermal efficiency. J. Membr. Sci. 2013, 433, 180–191. [Google Scholar] [CrossRef]
- Su, C.-I.; Shih, J.-H.; Huang, M.-S.; Wang, C.-M.; Shih, W.-C.; Liu, Y. A study of hydrophobic electrospun membrane applied in seawater desalination by membrane distillation. Fibers Polym. 2012, 13, 698–702. [Google Scholar] [CrossRef]
- Li, K.; Hou, D.; Fu, C.; Wang, K.; Wang, J. Fabrication of PVDF nanofibrous hydrophobic composite membranes reinforced with fabric substrates via electrospinning for membrane distillation desalination. J. Environ. Sci. 2018, 75, 277–288. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Yan, J.; Wang, K.; Xie, B.; Hu, Y.; Kang, W.; Cheng, B. Electrospun polyvinylidene fluoride/fluorinated acrylate copolymer tree-like nanofiber membrane with high flux and salt rejection ratio for direct contact membrane distillation. Desalination 2019, 466, 68–76. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Verliefde, A.R.; Mhlanga, S.D. Enhanced flux in direct contact membrane distillation using superhydrophobic PVDF nanofibre membranes embedded with organically modified SiO2 nanoparticles. J. Chem. Technol. Biotechnol. 2019, 94, 2826–2837. [Google Scholar] [CrossRef]
- Lalia, B.S.; Guillen-Burrieza, E.; Arafat, H.A.; Hashaikeh, R. Fabrication and characterization of polyvinylidenefluoride-co-hexafluoropropylene (PVDF-HFP) electrospun membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 428, 104–115. [Google Scholar] [CrossRef]
- Kyoungjin An, A.; Lee, E.-J.; Guo, J.; Jeong, S.; Lee, J.-G.; Ghaffour, N. Enhanced vapor transport in membrane distillation via functionalized carbon nanotubes anchored into electrospun nanofibres. Sci. Rep. 2017, 7, 41562. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, C.; Lu, X.; Ng, D.; Truong, Y.B.; Xie, Z. Activated carbon enhanced hydrophobic/hydrophilic dual-layer nanofiber composite membranes for high-performance direct contact membrane distillation. Desalination 2018, 446, 59–69. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Johir, M.A.H.; Choi, J.S.; Shon, H.K. A novel dual-layer bicomponent electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Chem. Eng. J. 2014, 256, 155–159. [Google Scholar] [CrossRef]
- Makanjuola, O.; Anis, S.F.; Hashaikeh, R. Enhancing DCMD vapor flux of PVDF-HFP membrane with hydrophilic silica fibers. Sep. Purif. Technol. 2021, 263, 118361. [Google Scholar] [CrossRef]

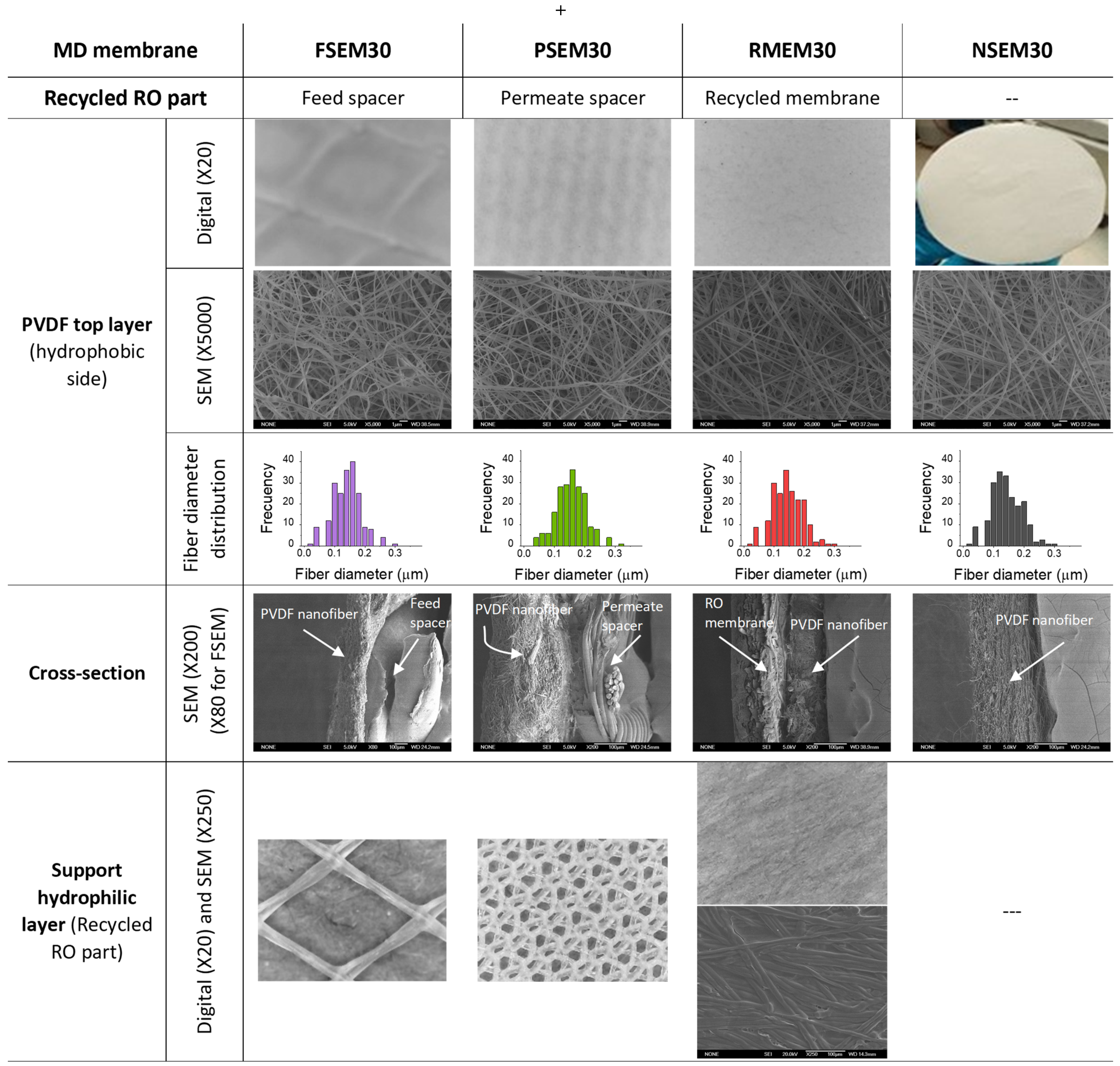

| Membrane Code | Bottom Layer (Hydrophilic Support) | Top Layer (PVDF Hydrophobic Membrane) | δt (μm) | di (μm) | ε (%) | LEPw (103 Pa) | YM (MPa) | Ts (MPa) | Eb (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| δS (μm) | (θw)S (°) | δPVDF (μm) | (θw)PVDF (°) | ||||||||
| NSEM30 | - | - | 28 ± 5 | 139 ± 5 | 28 ± 5 | 2.02 ± 0.01 | 82 ± 1 | 14 ± 2 | 17 ± 1 | 4 ± 1 | 77± 15 |
| RMEM30 | 98 ± 3 | 65 ± 7 | 31 ± 7 | 140 ± 5 | 128 ± 4 | <0.1 c | 64 ± 1 | 65 ± 4 | 3721 ± 86 | 93 ± 3 | 8 ± 1 |
| PSEM30 | 221 ± 5 | - b | 33 ± 10 | 140 ± 2 | 255 ± 4 | 2.43 ± 0.08 | 80 ± 4 | 15 ± 1 | 881 ± 42 | 32 ± 1 | 15 ± 1 |
| FSEM30 | 774 ± 7 a | - b | 32 ± 16 | 132 ± 5 | 806 ± 9 a | 3.58 ± 0.17 | 78 ± 4 | 7 ± 2 | 11 ± 1 | 3 ± 1 | 88 ± 8 |
| NaCl Concentration (g L−1) | NSEM30 | RMEM30 | PSEM30 | FSEM30 | TF200 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Jw (kg m−2 h−1) | α (%) | Jw (kg m−2 h−1) | α (%) | Jw (kg m−2 h−1) | α (%) | Jw (kg m−2 h−1) | α (%) | Jw (kg m−2 h−1) | α (%) | |
| 0 | 72.5 ± 0.6 | - | 29.2 ± 0.2 | - | 74.7 ± 0.8 | - | 63.4 ± 0.5 | - | 55.9 ± 0.1 | - |
| 6 | 69.1 ± 0.4 | 99.97 | 25.7 ± 0.3 | 99.98 | 70.0 ± 0.2 | 99.98 | 58.3 ± 0.1 | 99.78 | 52.4 ± 0.1 | 99.99 |
| 12 | 66.6 ± 0.4 | 99.94 | 24.7 ± 0.2 | 99.97 | 68.5 ± 0.2 | 99.93 | 54.9 ± 0.2 | 99.74 | 51.6 ± 0.1 | 99.99 |
| 30 | 64.8 ± 1.1 | 99.94 | 23.6 ± 0.2 | 99.97 | 66.5 ± 0.3 | 99.93 | 51.3 ± 0.2 | 99.78 | 51.0 ± 0.3 | 99.99 |
| 60 | 61.9 ± 0.2 | 99.95 | 22.1 ± 0.2 | 99.99 | 63.6 ± 0.1 | 99.95 | 49.3 ± 0.4 | 99.71 | 50.0 ± 0.1 | 99.99 |
| 90 | 59.3 ± 0.3 | 99.95 | 20.9 ± 0.2 | 99.99 | 59.7 ± 0.2 | 99.95 | 46.5 ± 0.3 | 99.79 | 48.6 ± 0.1 | 99.99 |
| 120 | 56.6 ± 0.2 | 99.96 | 19.8 ± 0.5 | 99.99 | 57.7 ± 0.1 | 99.95 | 45.8 ± 0.3 | 99.77 | 46.3 ± 0.1 | 99.99 |
| 150 | 54.8 ± 0.3 | 99.97 | 17.3 ± 0.2 | 99.99 | 55.2 ± 0.1 | 99.95 | 44.0 ± 0.3 | 99.66 | 43.8 ± 0.1 | 99.99 |
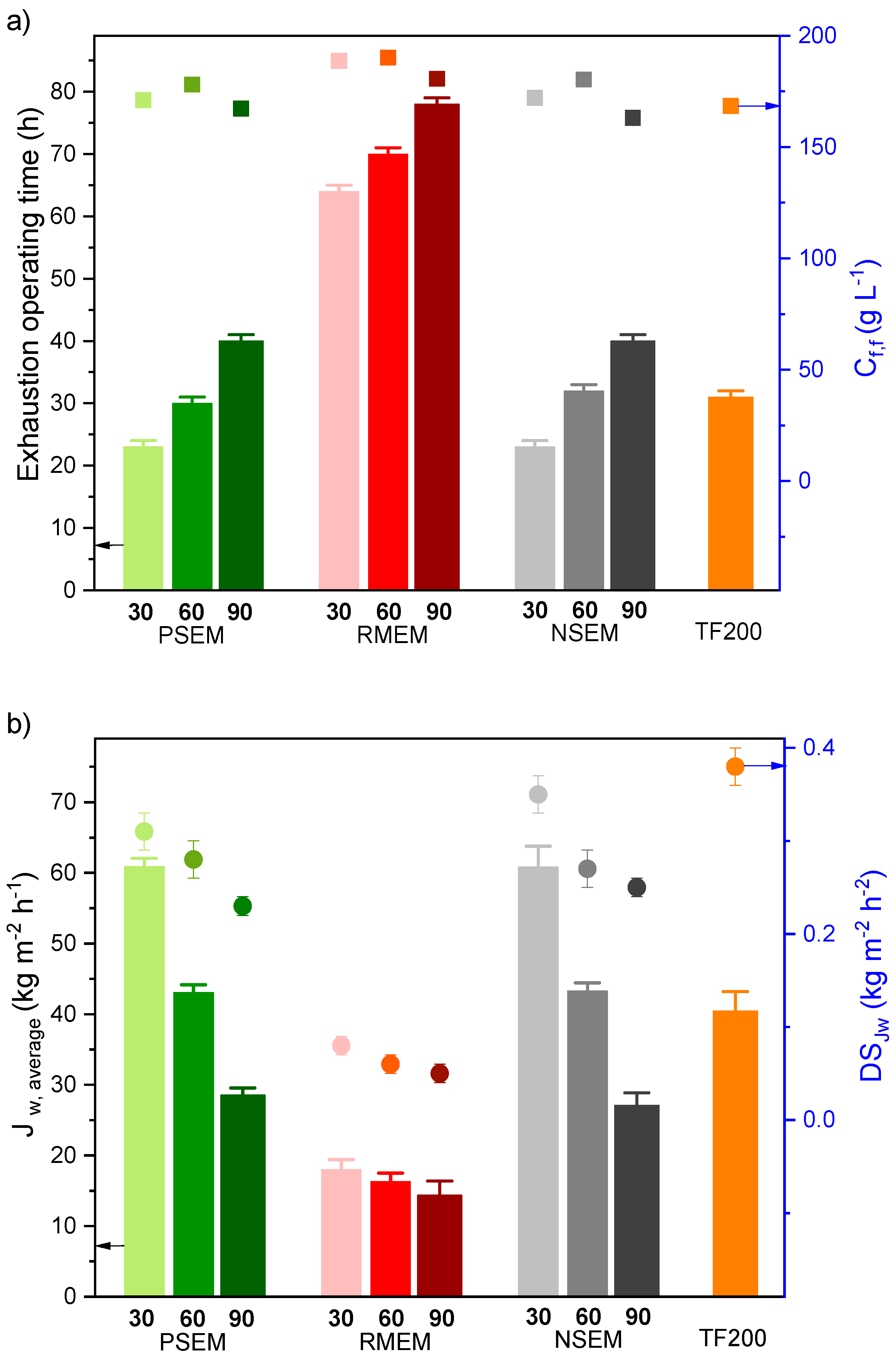
| Membrane Code | Bottom Layer (Hydrophilic Support) | Top Layer (PVDF Hydrophobic Membrane) | δt (μm) | di (μm) | ε (%) | LEPw (103 Pa) | ||
|---|---|---|---|---|---|---|---|---|
| δS (μm) | (θw)s (°) | δPVDF (μm) | (θw)PVDF (°) | |||||
| NSEM60 | - | - | 68 ± 5 | 140 ± 2 | 68 ± 5 | 1.49 ± 0.28 | 87 ± 1 | 44 ± 3 |
| NSEM90 | - | - | 125 ± 6 | 139 ± 4 | 125 ± 6 | 1.14 ± 0,35 | 84 ± 1 | 56 ± 2 |
| RMEM60 | 98 ± 3 | 65 ± 7 | 74 ± 9 | 137 ± 2 | 171 ± 6 | <0.1 b | 69 ± 2 | 71 ± 2 |
| RMEM90 | 98 ± 3 | 65 ± 7 | 111 ± 10 | 139 ± 2 | 209 ± 7 | <0.1 b | 65 ± 4 | 81 ± 3 |
| PSEM60 | 221 ± 5 | - a | 84 ± 19 | 137 ± 3 | 306 ± 14 | 2.14 ± 0.78 | 77 ± 3 | 49 ± 1 |
| PSEM90 | 221 ± 5 | - a | 142 ± 20 | 138 ± 6 | 363 ± 15 | 1.46 ± 0.23 | 82 ± 3 | 60 ± 3 |
| Material | Support | Membrane Characteristics | DCMD Conditions | Permeate Flux (kg m−2 h−1) | Salt Rejection Factor (%) | Ref. |
|---|---|---|---|---|---|---|
| PVDF | Recycled RO permeate spacer (PSEM60) | ε: 77%; δ: 306 μm; LEPw: 0.49 bar; θw: 137°. | cf: 65 g/L; ΔT = 60 K | 43.2 ± 1.5 | 99.99 | This study |
| PVDF | Recycled RO membrane (RMEM60) | ε: 69%; δ: 171 μm; LEPw: 0.71 bar; θw: 137°. | cf: 65 g/L; ΔT = 60 K | 18.1 ± 0.9 | 99.99 | This study |
| PVDF | Unsupported (NSEM60) | ε: 87%; δ: 68 μm; LEPw: 0.44 bar; θw: 140°. | cf: 65 g/L; ΔT = 60 K | 39.4 ± 1.1 | 99.99 | This study |
| PVDF | Unsupported | ε: 86%; δ: 144.4 μm; LEPw: 0.63 bar; θw: 139.7°. | cf: 12 g/L; ΔT = 60 K | 15.2 | 99.7 | [41] |
| PVDF-HFP | Unsupported | ε: 90%; δ: 55 μm; θw: 128°. | cf: 30 g/L; ΔT = 55 K | 13.3 | 99.99 | [46] |
| PVDF | PSf nanofibers | ε: 92%; δ: 407 μm; LEPw: 0.79 bar; θw: 140.5°. | cf: 30 g/L; ΔT = 60 K | 47.7 | 99.99 | [31] |
| PVDF | Polyester mesh (big square pores) | ε: 88.6%; δ: 190 μm; LEPw: 0.45 bar; θw: 145°. | cf: 35 g/L; ΔT = 60 K | 49.3 | 99.99 | [47] |
| PVDF/TBAC/FA | Unsupported | ε: 71.28%; δ: 60 μm; LEPw:2.1 bar; θw: 137°. | cf: 35 g/L; ΔT = 40 K | 54.4 | 99.5 | [48] |
| PVDF/SiO2NPs | Unsupported | ε: 80%; δ: 129 μm; LEPw: 0.842 bar; θw: 156.4°. | cf: 30 g/L; ΔT = 60 K | 30.7 | 99.9 | [49] |
| PVDF-HFP | Unsupported | ε: 58%; δ: 65 μm; LEPw: 1.3 bar; θw: 120°. | cf: 35 g/L; ΔT = 41 K | 22 | 98 | [50] |
| PVDF-HFP/CNT | Unsupported | ε: 89%; δ: 83 μm; LEPw: 0.4 bar; θw: 150.4°. | cf: 35 g/L; ΔT = 40 K | 48.1 | 99.99 | [51] |
| PVDF-HFP/AC | Nylon flat sheet | ε: 90%; δ: 200 μm; LEPw: 1.36 bar; θw: 142.7°. | cf: 35 g/L; ΔT = 45 K | 45.6 | 99.99 | [52] |
| PVDF-HFP | PAN nanofibers | ε: 90%; δ: 82 μm; LEPw: 0.94 bar; θw: 150°. | cf: 35 g/L; ΔT = 40 K | 30 | 98.5 | [53] |
| PVDF-HFP/SiF | Unsupported | ε: 71%; δ: 130 μm; LEPw: 2.7 bar; θw: 100°. | cf: 35 g/L; ΔT = 50 K | 26 | 99.99 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Martínez, J.; García-Payo, C.; Khayet, M. Electrospun Nanostructured Membrane Engineering Using Reverse Osmosis Recycled Modules: Membrane Distillation Application. Nanomaterials 2021, 11, 1601. https://doi.org/10.3390/nano11061601
Contreras-Martínez J, García-Payo C, Khayet M. Electrospun Nanostructured Membrane Engineering Using Reverse Osmosis Recycled Modules: Membrane Distillation Application. Nanomaterials. 2021; 11(6):1601. https://doi.org/10.3390/nano11061601
Chicago/Turabian StyleContreras-Martínez, Jorge, Carmen García-Payo, and Mohamed Khayet. 2021. "Electrospun Nanostructured Membrane Engineering Using Reverse Osmosis Recycled Modules: Membrane Distillation Application" Nanomaterials 11, no. 6: 1601. https://doi.org/10.3390/nano11061601
APA StyleContreras-Martínez, J., García-Payo, C., & Khayet, M. (2021). Electrospun Nanostructured Membrane Engineering Using Reverse Osmosis Recycled Modules: Membrane Distillation Application. Nanomaterials, 11(6), 1601. https://doi.org/10.3390/nano11061601