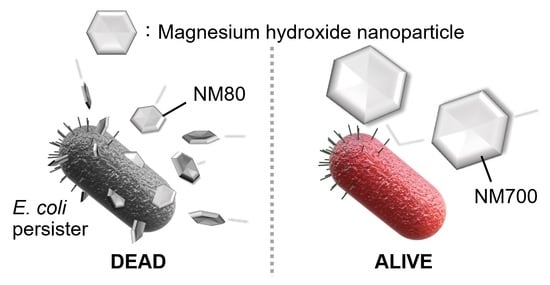
Magnesium Hydroxide Nanoparticles Kill Exponentially Growing and Persister Escherichia coli Cells by Causing Physical Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Magnesium Hydroxide Nanoparticles
2.2. Bacteria Cultivation
2.3. Bactericidal Effect of NM80, NM300, and NM700
2.4. SEM Analysis of E. coli
3. Results
3.1. Characterization of Magnesium Hydroxide Nanoparticles
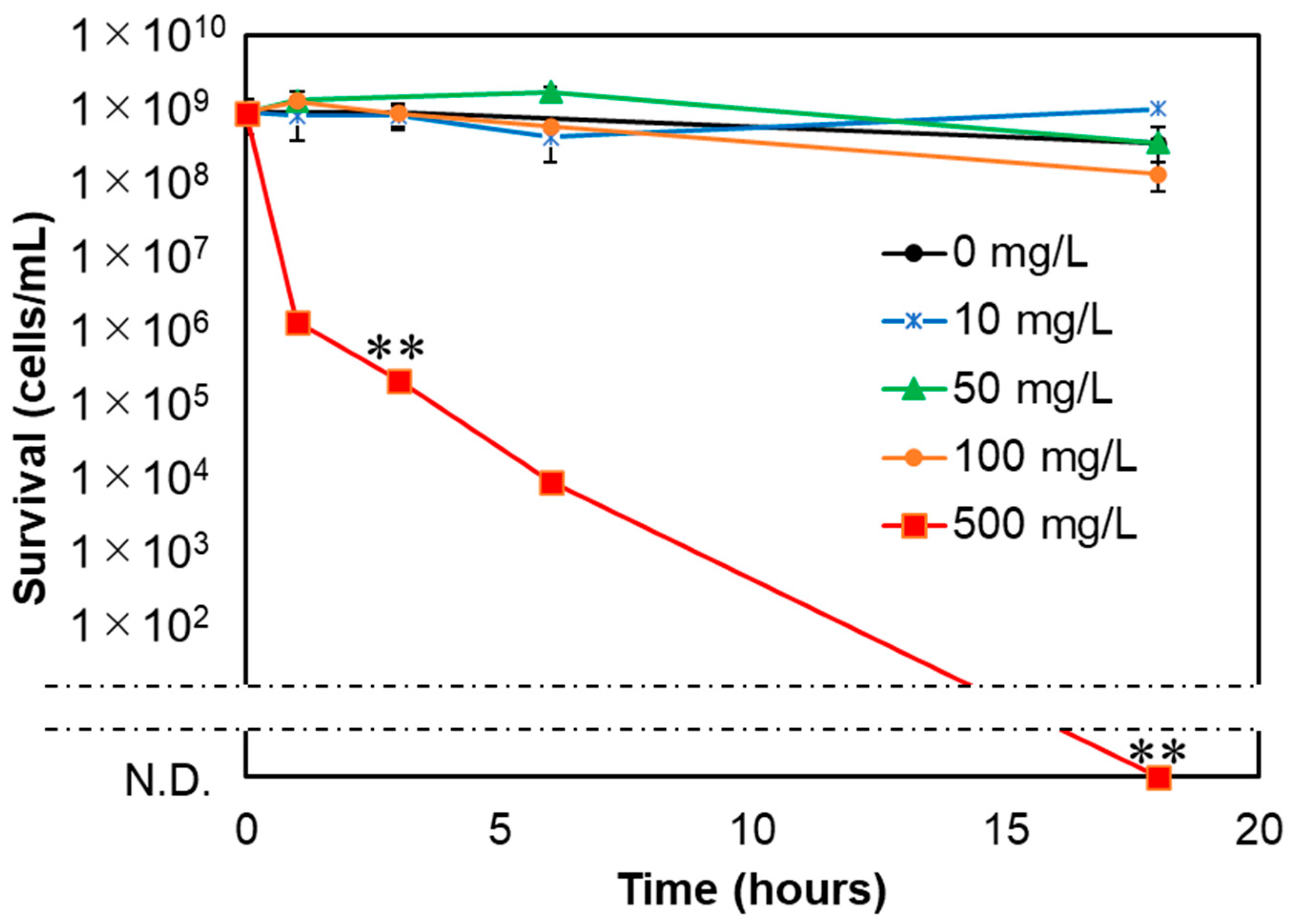
3.2. Bactericidal Effects of NM80, NM300, and NM700 on E. coli
3.3. Magnesium Ions Do Not Affect Bacterial Death
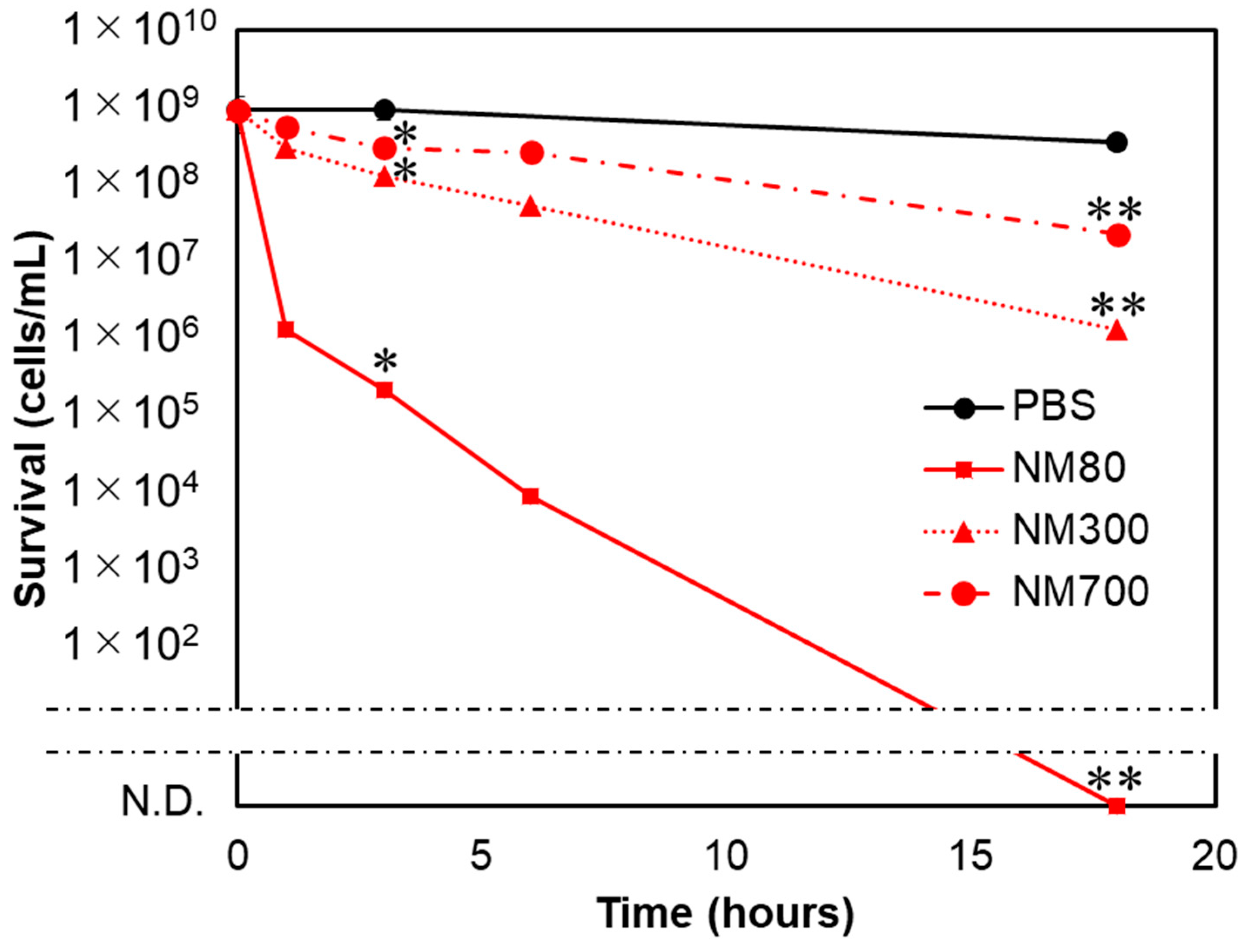
3.4. Smaller Magnesium Hydroxide Nanoparticles Physically Injure Bacteria and Kill Them
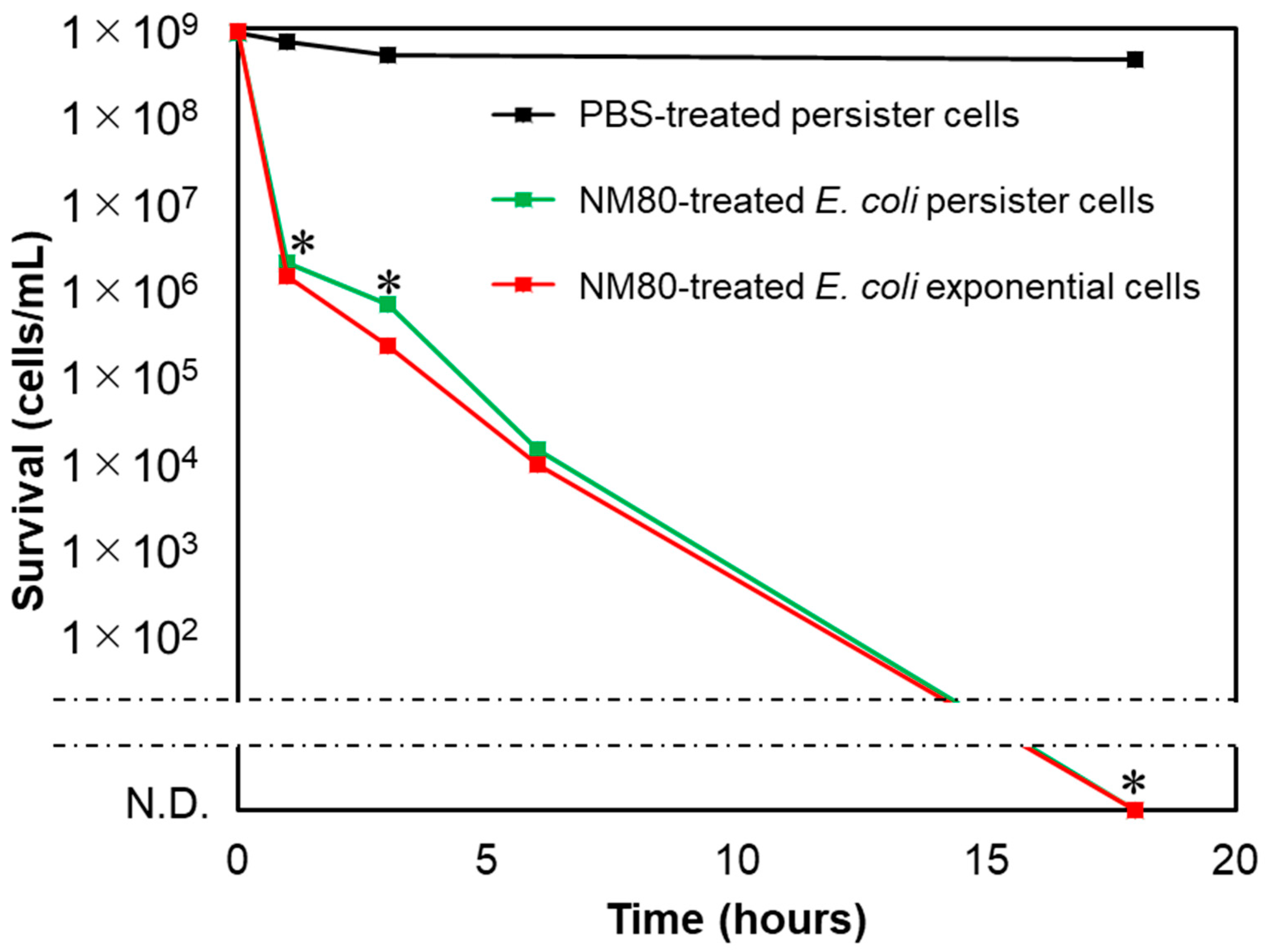
3.5. NM80 Sterilizes even Persister Cells with High Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Decktor, D.L.; Robinson, M.; Maton, P.N.; Lanza, F.L.; Gottlieb, S. Effects of Aluminum/Magnesium Hydroxide and Calcium Carbonate on Esophageal and Gastric pH in Subjects with Heartburn. Am. J. Ther. 1995, 2, 546–552. [Google Scholar] [CrossRef]
- Kinnunen, O.; Salokannel, J. Constipation in elderly long-stay patients: Its treatment by magnesium hydroxide and bulk-laxative. Ann. Clin. Res. 1987, 19, 321–323. [Google Scholar]
- Shinde, S.; Paralikar, P.; Ingle, A.P.; Rai, M. Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from Aspergillus niger. Arab. J. Chem 2020, 13, 3172–3182. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, W.; Zhang, M.; Qu, J.; Shi, L.; Qu, H.; Xu, J. Hydrothermal synthesis of 4ZnO·B2O3·H2O/RGO hybrid material and its flame retardant behavior in flexible PVC and magnesium hydroxide composites. Appl. Surf. Sci. 2017, 425, 896–904. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Wang, M.; Liu, P.; Pan, Y.; Liu, D. Synergistic effect of an aromatic boronic acid derivative and magnesium hydroxide on the flame retardancy of epoxy resin. Polym. Degrad. Stab. 2016, 130, 257–263. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Kawasaki, N.; Nakazawa, Y.; Kanai, K.; Okamoto, N.; Shimomura, Y. Co-instillation of nano-solid magnesium hydroxide enhances corneal permeability of dissolved timolol. Exp. Eye Res. 2017, 165, 118–124. [Google Scholar] [CrossRef]
- Lih, E.; Kum, C.H.; Park, W.; Chun, S.Y.; Cho, Y.; Joung, Y.K.; Park, K.S.; Hong, Y.J.; Ahn, D.J.; Kim, B.S.; et al. Modified Magnesium Hydroxide Nanoparticles Inhibit the Inflammatory Response to Biodegradable Poly(lactide-co-glycolide) Implants. ACS Nano 2018, 12, 6917–6925. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Controlling the Antimicrobial Action of Surface Modified Magnesium Hydroxide Nanoparticles. Biomimetics 2019, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, Y.; Chen, Z.; Pan, D.; Cheng, Y.; Liu, Z.; Lin, Z.; Guan, X. Investigation of antibacterial activity and related mechanism of a series of nano-Mg(OH)2. ACS Appl. Mater. Interfaces 2013, 5, 1137–1142. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, C.; Chen, S. Structural characterization and catalytic sterilization performance of a TiO2 nano-photocatalyst. Food Sci. Nutr. 2020, 8, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Chang, K.-C.; Chen, L.-Y.; Wang, P.-C.; Chou, C.-C.; Wu, Z.-B.; Hu, A. Blue light irradiation triggers the antimicrobial potential of ZnO nanoparticles on drug-resistant Acinetobacter baumannii. J. Photochem. Photobiol. B Biol. 2018, 180, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, S.; Zhang, K.; Gao, N.; Zhang, M.; Albasher, G.; Shi, J.; Wang, C. The interaction of Ag2O nanoparticles with Escherichia coli: Inhibition-sterilization process. Sci. Rep. 2021, 11, 1703. [Google Scholar] [CrossRef]
- Wood, T.K.; Song, S.; Yamasaki, R. Ribosome dependence of persister cell formation and resuscitation. J. Microbiol. 2019, 57, 213–219. [Google Scholar] [CrossRef]
- Hobby, G.L.; Meyer, K.; Chaffee, E. Observations on the Mechanism of Action of Penicillin. Proc. Soc. Exp. Biol. Med. 1942, 50, 281–285. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial persistence as a phenotypic switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef]
- Dörr, T.; Vulić, M.; Lewis, K. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol. 2010, 8, e1000317. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yamasaki, R.; Song, S.; Zhang, W.; Wood, T.K. Single cell observations show persister cells wake based on ribosome content. Environ. Microbiol. 2018, 20, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, S.; Zhang, Y.; Zhang, W. DNA adenine methylation is involved in persister formation in E. coli. Microbiol. Res. 2021, 246, 126709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yamasaki, R.; Song, S.; Wood, T.K. Interkingdom signal indole inhibits Pseudomonas aeruginosa persister cell waking. J. Appl. Microbiol. 2019, 127, 1768–1775. [Google Scholar] [CrossRef]
- Bakkeren, E.; Huisman, J.S.; Fattinger, S.A.; Hausmann, A.; Furter, M.; Egli, A.; Slack, E.; Sellin, M.E.; Bonhoeffer, S.; Regoes, R.R. Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature 2019, 573, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Rycroft, J.A.; Gollan, B.; Grabe, G.J.; Hall, A.; Cheverton, A.M.; Larrouy-Maumus, G.; Hare, S.A.; Helaine, S. Activity of acetyltransferase toxins involved in Salmonella persister formation during macrophage infection. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Kim, J.S.; Chowdhury, N.; Yamasaki, R.; Wood, T.K. Viable but non-culturable and persistence describe the same bacterial stress state. Environ. Microbiol. 2018, 20, 2038–2048. [Google Scholar] [CrossRef]
- Kwan, B.W.; Valenta, J.A.; Benedik, M.J.; Wood, T.K. Arrested protein synthesis increases persister-like cell formation. Antimicrob. Agents Chemother. 2013, 57, 1468–1473. [Google Scholar] [CrossRef]
- Cui, P.; Niu, H.; Shi, W.; Zhang, S.; Zhang, W.; Zhang, Y. Identification of Genes Involved in Bacteriostatic Antibiotic-Induced Persister Formation. Front. Microbiol. 2018, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of persister cells of Pseudomonas aeruginosa and Staphylococcus aureus by chemical treatment and evaluation of their susceptibility to membrane-targeting agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Narayanaswamy, V.P.; Keagy, L.L.; Duris, K.; Wiesmann, W.; Loughran, A.J.; Townsend, S.M.; Baker, S. Novel glycopolymer eradicates antibiotic-and CCCP-induced persister cells in Pseudomonas aeruginosa. Front. Microbiol. 2018, 9, 1724. [Google Scholar] [CrossRef]
- Pu, Y.; Li, Y.; Jin, X.; Tian, T.; Ma, Q.; Zhao, Z.; Lin, S.-y.; Chen, Z.; Li, B.; Yao, G. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol. Cell 2019, 73, 143–156.e4. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Lombardi, L.; Klatt, A.-B.; Trampuz, A.; Di Luca, M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, R.; Song, S.; Benedik, M.J.; Wood, T.K. Persister Cells Resuscitate Using Membrane Sensors that Activate Chemotaxis, Lower cAMP Levels, and Revive Ribosomes. iScience 2020, 23, 100792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Wang, Z. Thymine Sensitizes Gram-Negative Pathogens to Antibiotic Killing. Front. Microbiol. 2021, 12, 622798. [Google Scholar] [CrossRef]
- Kwan, B.W.; Chowdhury, N.; Wood, T.K. Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 2015, 17, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Moreira Martins, P.M.; Gong, T.; de Souza, A.A.; Wood, T.K. Copper Kills Escherichia coli Persister Cells. Antibiotics 2020, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Nachr Ges wiss goettingen. Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 1989. [Google Scholar]
- Dulbecco, R.; Vogt, M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J. Exp. Med. 1954, 99, 167–182. [Google Scholar] [CrossRef]
- Kawano, A.; Yamasaki, R.; Sakakura, T.; Takatsuji, Y.; Haruyama, T.; Yoshioka, Y.; Ariyoshi, W. Reactive Oxygen Species Penetrate Persister Cell Membranes of Escherichia coli for Effective Cell Killing. Front. Cell. Infect. Microbiol. 2020, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Wong, B.B.; Lusk, J.E. Mutants in three genes affecting transport of magnesium in Escherichia coli: Genetics and physiology. J. Bacteriol. 1976, 126, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yin, X.; Wu Orr, M.; Dambach, M.; Curtis, R.; Storz, G. Increasing intracellular magnesium levels with the 31-amino acid MgtS protein. Proc. Natl. Acad. Sci. USA 2017, 114, 5689–5694. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.M.; Bagga, D.A.; Miller, C.G.; Maguire, M.E. Magnesium transport in Salmonella typhimurium: The influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol. Microbiol. 1991, 5, 2753–2762. [Google Scholar] [CrossRef]
- van Veen, H.W.; Abee, T.; Kortstee, G.J.; Konings, W.N.; Zehnder, A.J. Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli. Biochemistry 1994, 33, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Leipe, D.D.; Koonin, E.V.; Aravind, L. Evolutionary history and higher order classification of AAA+ ATPases. J. Struct. Biol. 2004, 146, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Nepal, S.; Kumar, P. Growth, Cell Division, and Gene Expression of Escherichia coli at Elevated Concentrations of Magnesium Sulfate: Implications for Habitability of Europa and Mars. Microorganisms 2020, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Toxicological risks of selected flame-retardant chemicals. In National Research Council (US) Subcommittee on Flame-Retardant Chemicals; National Academy Press: Washington, DC, USA, 2000; pp. 131–146. [Google Scholar]
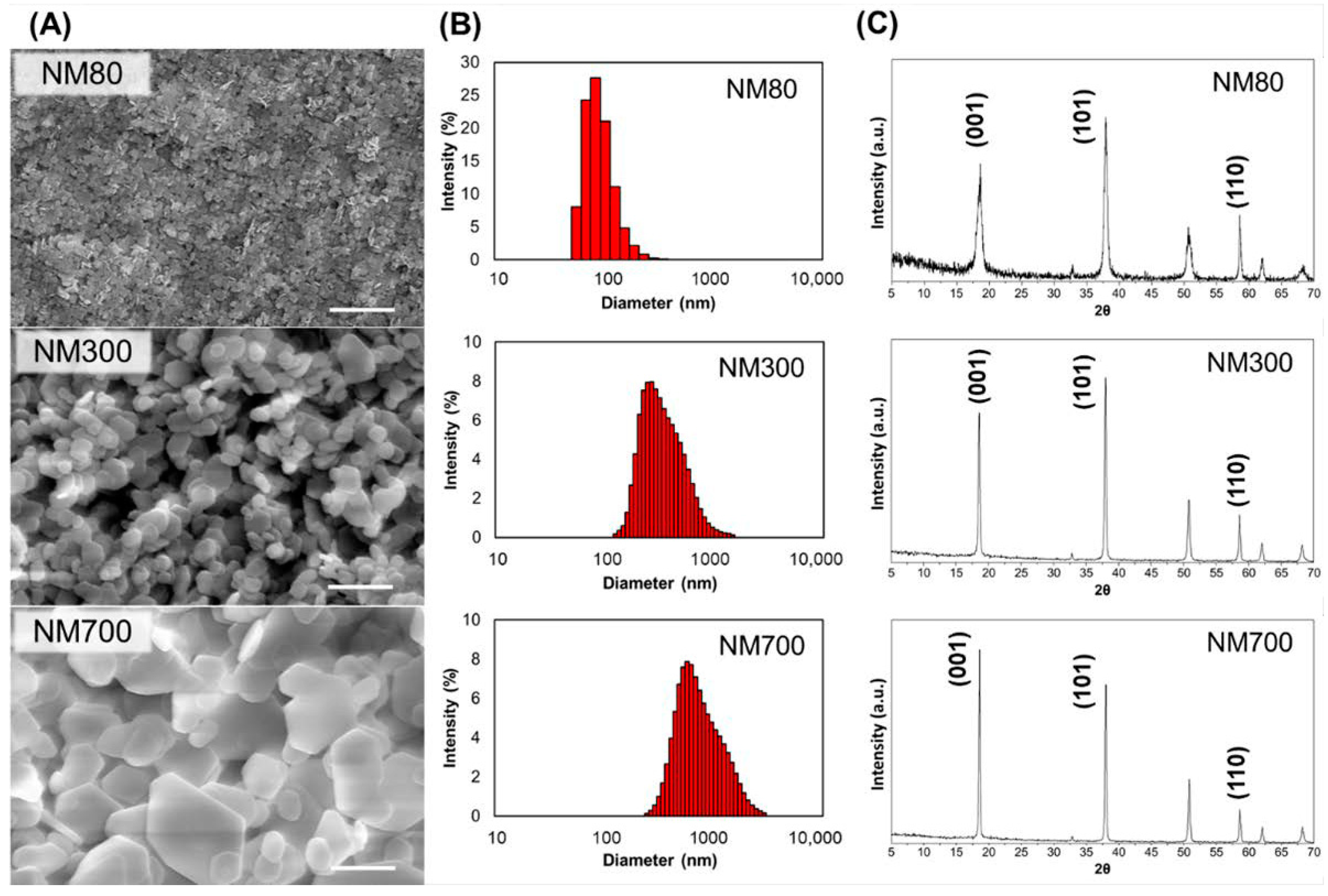



| Strains | Features | Reference |
|---|---|---|
| E. coli BW25113 | rrnB3 ΔlacZ4787 hsdR514 Δ(araBAD)567 Δ(rhaBAD)568 rph-1 | [34] |
| E. coli BW25113 ΔcorA | ΔcorA, KmR | [34] |
| E. coli BW25113 ΔmgtA | ΔmgtA, KmR | [34] |
| E. coli BW25113 ΔpitA | ΔpitA, KmR | [34] |
| E. coli BW25113 ΔpitB | ΔpitB, KmR | [34] |
| E. coli BW25113 ΔyifB | ΔyifB, KmR | [34] |
| Size (nm) | Mg2+ | |||||
|---|---|---|---|---|---|---|
| D10 | D50 | D90 | (ppm) | pH | (°C) | |
| NM80 | 54.3 | 75.2 | 118.8 | 5.78 | 10.32 | 23.7 |
| NM300 | 206 | 328 | 626 | 4.85 | 10.26 | 23.5 |
| NM700 | 443 | 726 | 1494 | 5.20 | 10.21 | 23.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, Y.; Okita, K.; Kudo, D.; Phuong, D.N.D.; Iwamoto, Y.; Yoshioka, Y.; Ariyoshi, W.; Yamasaki, R. Magnesium Hydroxide Nanoparticles Kill Exponentially Growing and Persister Escherichia coli Cells by Causing Physical Damage. Nanomaterials 2021, 11, 1584. https://doi.org/10.3390/nano11061584
Nakamura Y, Okita K, Kudo D, Phuong DND, Iwamoto Y, Yoshioka Y, Ariyoshi W, Yamasaki R. Magnesium Hydroxide Nanoparticles Kill Exponentially Growing and Persister Escherichia coli Cells by Causing Physical Damage. Nanomaterials. 2021; 11(6):1584. https://doi.org/10.3390/nano11061584
Chicago/Turabian StyleNakamura, Yohei, Kaede Okita, Daisuke Kudo, Dao Nguyen Duy Phuong, Yoshihito Iwamoto, Yoshie Yoshioka, Wataru Ariyoshi, and Ryota Yamasaki. 2021. "Magnesium Hydroxide Nanoparticles Kill Exponentially Growing and Persister Escherichia coli Cells by Causing Physical Damage" Nanomaterials 11, no. 6: 1584. https://doi.org/10.3390/nano11061584
APA StyleNakamura, Y., Okita, K., Kudo, D., Phuong, D. N. D., Iwamoto, Y., Yoshioka, Y., Ariyoshi, W., & Yamasaki, R. (2021). Magnesium Hydroxide Nanoparticles Kill Exponentially Growing and Persister Escherichia coli Cells by Causing Physical Damage. Nanomaterials, 11(6), 1584. https://doi.org/10.3390/nano11061584