Enhanced Antibacterial Activity of Echinacea angustifolia Extract against Multidrug-Resistant Klebsiella pneumoniae through Niosome Encapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of E. angustifolia Extract
2.3. Optimization of Niosomes-Encapsulated E. angustifolia by Design of Experiments
2.4. Characterization of the Synthesized Niosomes
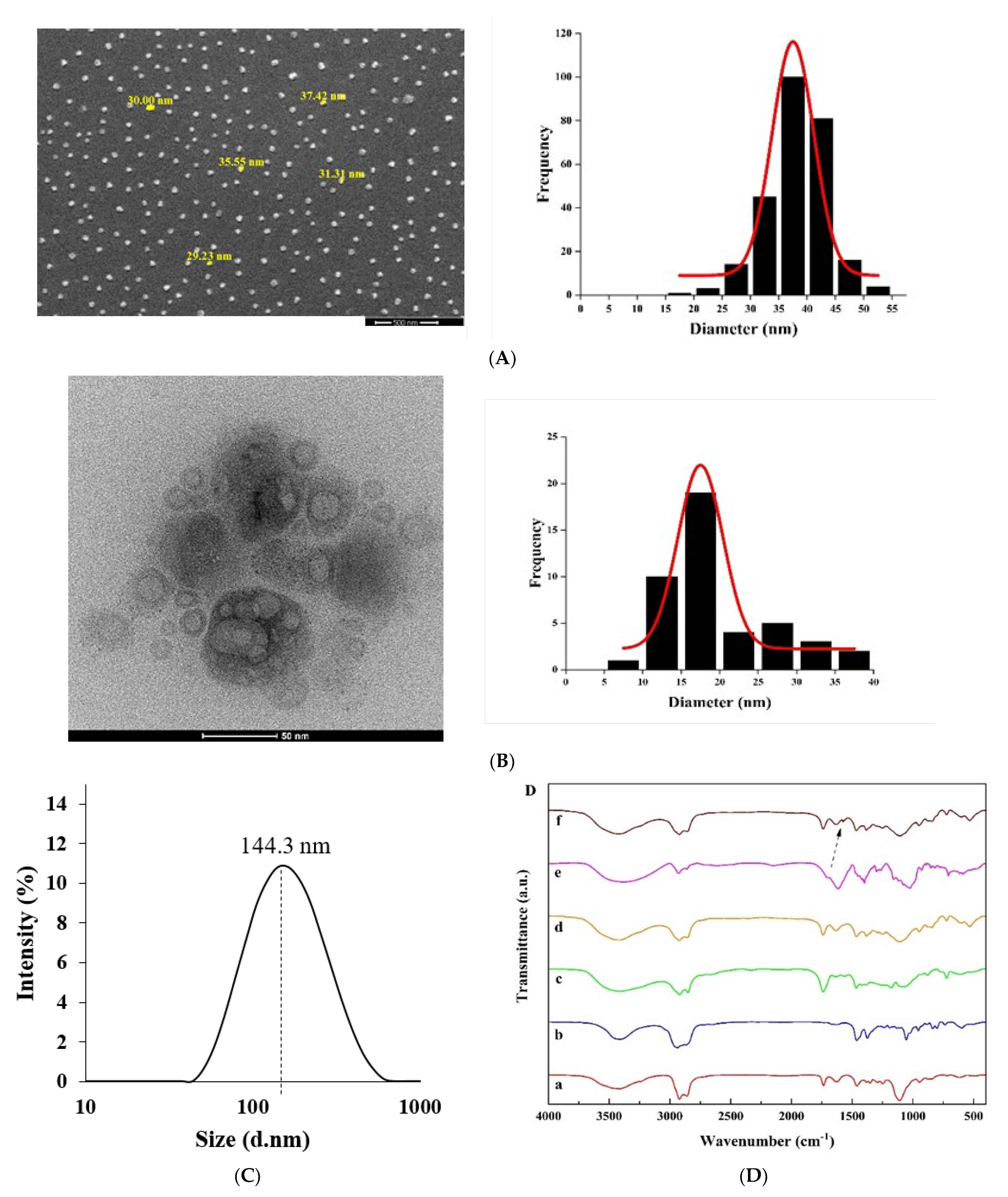
2.4.1. Size, Morphology, and Polydispersity Index (PDI)
2.4.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.3. Entrapment Efficiency
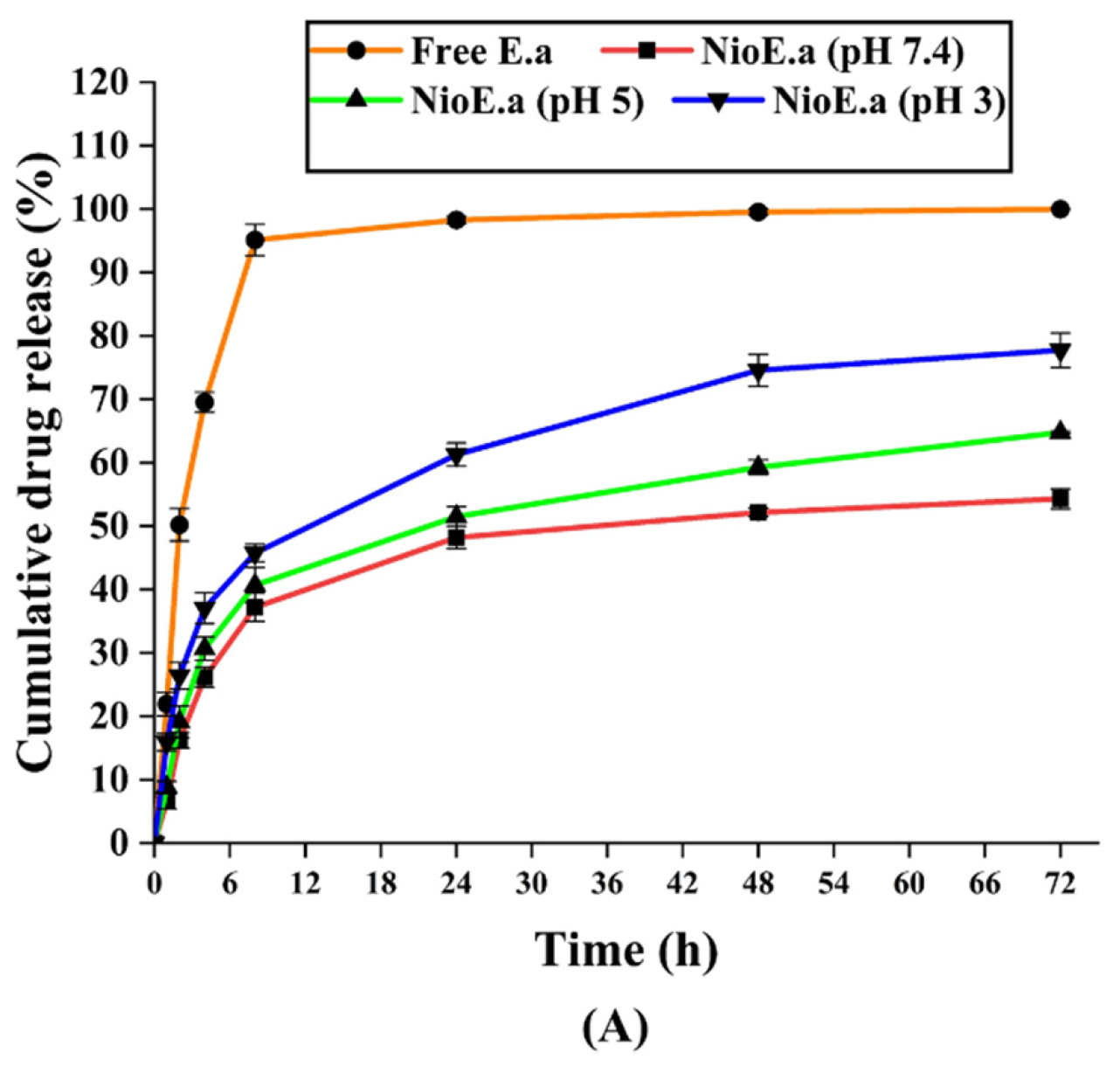
2.4.4. Study of Drug Release
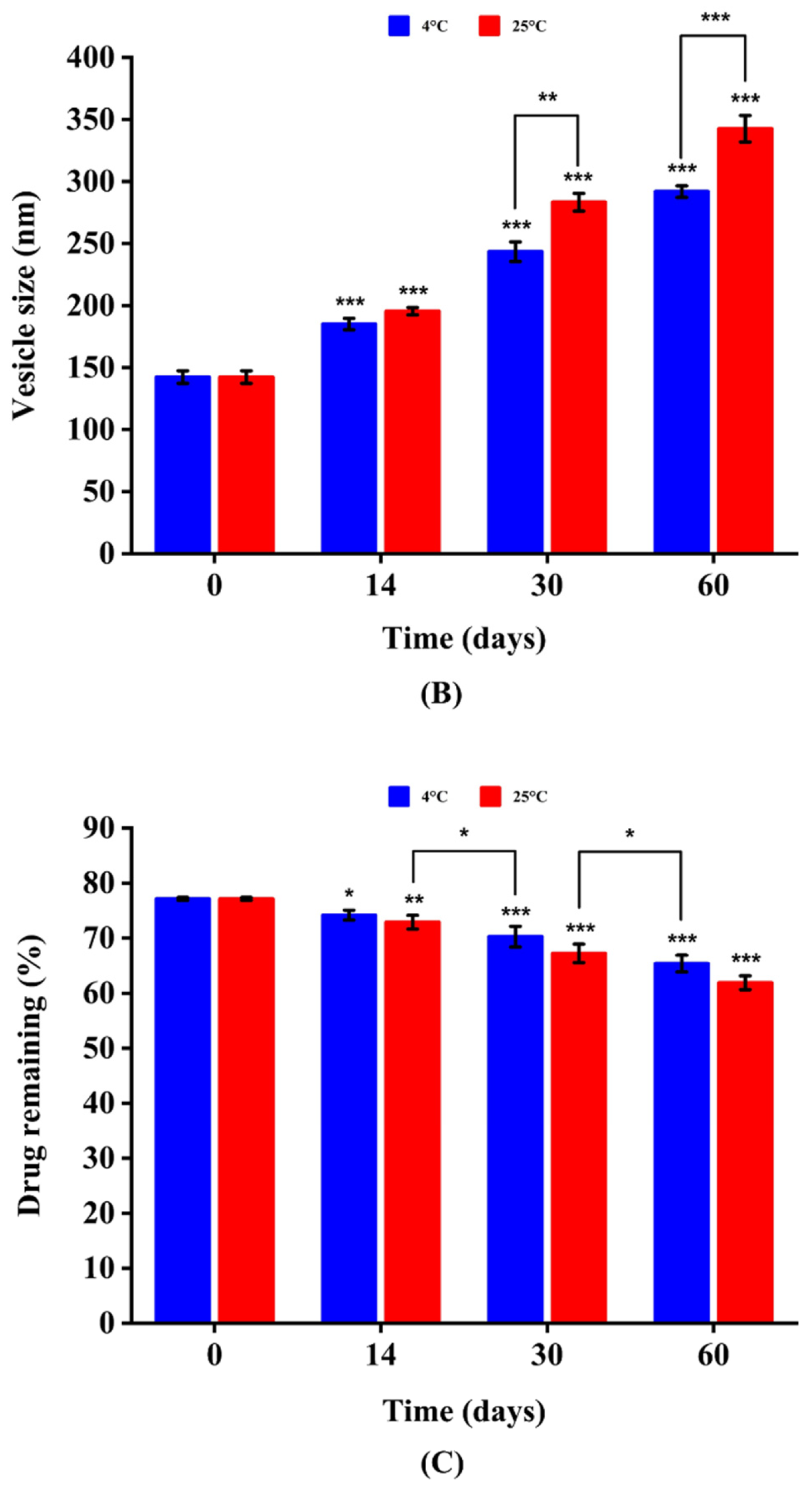
2.4.5. Stability Studies
2.5. Bioactivity of the Synthesized Niosomes
2.5.1. Isolation of K. pneumoniae Strains and Their Antibiotic Susceptibility
2.5.2. Antibacterial Efficacy
Broth Microdilution Assay
Time Kill Assay
2.5.3. Cytotoxicity Study
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Optimization of E. angustifolia-Loaded Niosomes
3.2. Characterization of Niosomes-Encapsulated E. angustifolia
3.2.1. Morphological Studies
3.2.2. Fourier Transform Infrared (FTIR) Analysis
3.2.3. Kinetics and In Vitro Drug Release Studies
3.2.4. Physical Stability of Niosomes-Encapsulated E. angustifolia
3.3. Bioactivity of the Encapsulated Niosomes
3.3.1. Isolation of K. pneumoniae Strains and Their Antibiotic Resistance Profile
3.3.2. Antibacterial Activity
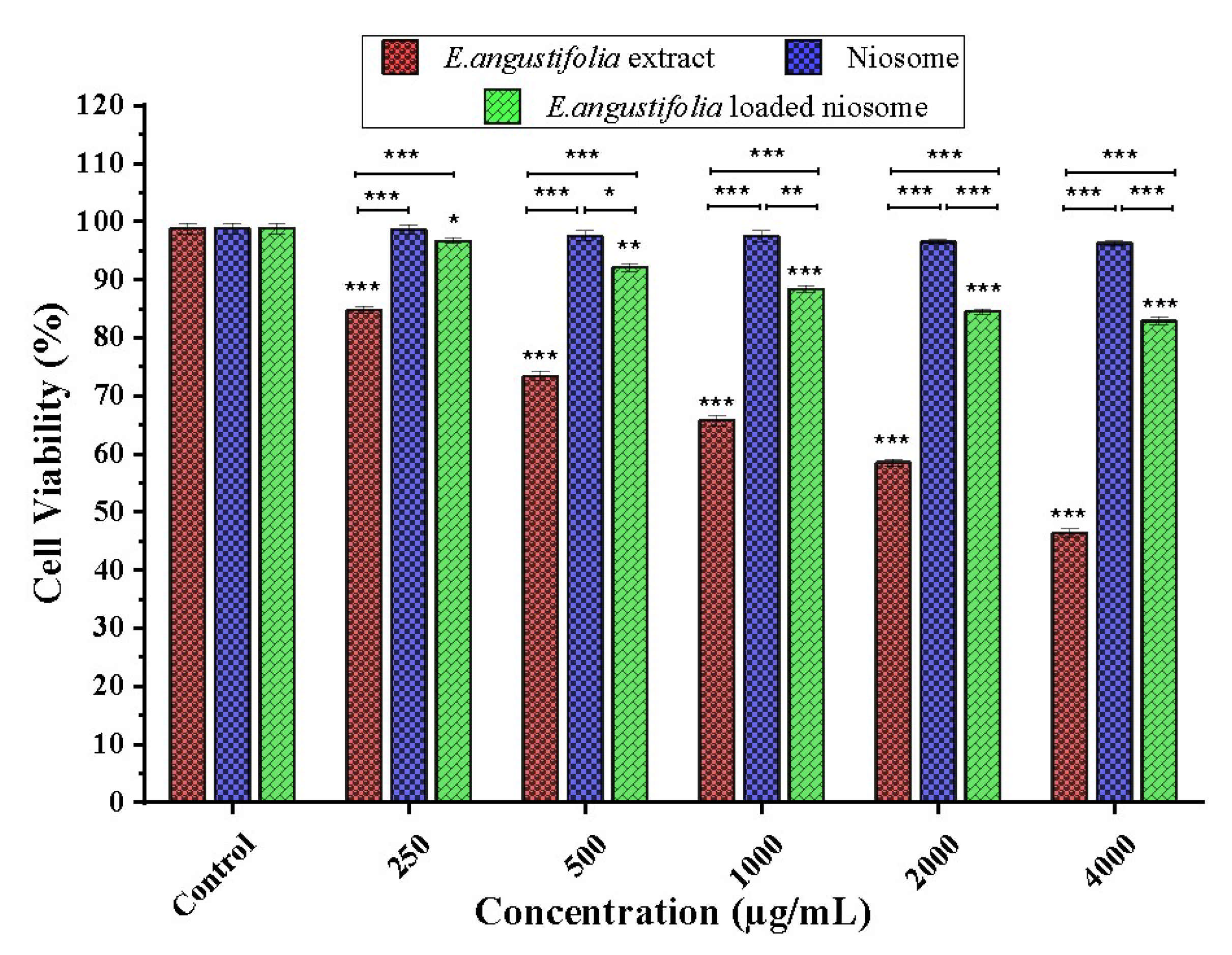
3.3.3. Cell Toxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Ethics Approval and Consent to Participate
Conflicts of Interest
References
- Mahale, N.; Thakkar, P.; Mali, R.; Walunj, D.; Chaudhari, S. Niosomes: Novel sustained release nonionic stable vesicular systems—An overview. Adv. Colloid Interface Sci. 2012, 183, 46–54. [Google Scholar] [CrossRef]
- Kumar, S.; Randhawa, J.K. High melting lipid based approach for drug delivery: Solid lipid nanoparticles. Mater. Sci. Eng. C 2013, 33, 1842–1852. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Buckton, G. Interfacial Phenomena in Drug Delivery and Targeting; CRC Press: Boca Raton, FL, USA, 2000; Volume 5. [Google Scholar]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, G.N.; Parakh, S.; Devraj, R.; Apte, S.; Rao, B.R.; Rambhau, D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J. Colloid Interface Sci. 2002, 251, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ghafelehbashi, R.; Akbarzadeh, I.; Yaraki, M.T.; Lajevardi, A.; Fatemizadeh, M.; Saremi, L.H. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int. J. Pharm. 2019, 569, 118580. [Google Scholar] [CrossRef]
- Khan, D.H.; Bashir, S.; Figueiredo, P.; Santos, H.A.; Khan, M.I.; Peltonen, L. Process optimization of ecological probe sonication technique for production of rifampicin loaded niosomes. J. Drug Deliv. Sci. Technol. 2019, 50, 27–33. [Google Scholar] [CrossRef]
- Ch, M.H.; Targhi, A.A.; Shamsi, F.; Heidari, F.; Moghadam, Z.S.; Mirzaie, A.; Behdad, R.; Moghtaderi, M.; Akbarzadeh, I. Niosome-encapsulated tobramycin reduced antibiotic resistance and enhanced antibacterial activity against multidrug-resistant clinical strains of Pseudomonas aeruginosa. J. Biomed. Mater. Res. Part A 2021, 109, 966–980. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; del Mar Contreras, M.; Soltani-Nejad, A. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Sriya, K.C.; Sai, D.; Sankar, P.R. Phytosomes: A novel approach for herbal phytochemicals for enhancing the bioavailability. Int. J. Pharm. Sci. Rev. Res. 2020, 6, 21–26. [Google Scholar]
- Hu, C.; Kitts, D.D. Studies on the antioxidant activity of Echinacea root extract. J. Agric. Food Chem. 2000, 48, 1466–1472. [Google Scholar] [CrossRef]
- Raeiszadeh, M.; Pardakhty, A.; Sharififar, F.; Farsinejad, A.; Mehrabani, M.; Hosseini-Nave, H.; Mehrabani, M. Development, physicochemical characterization, and antimicrobial evaluation of niosomal myrtle essential oil. Res. Pharm. Sci. 2018, 13, 250. [Google Scholar]
- García-Díaz, M.; Patiño, B.; Vázquez, C.; Gil-Serna, J. A novel niosome-encapsulated essential oil formulation to prevent aspergillus flavus growth and aflatoxin contamination of maize grains during storage. Toxins 2019, 11, 646. [Google Scholar] [CrossRef]
- Zidan, A.S.; Ibrahim, M.M.; El Megrab, N.A. Optimization of methotrexate loaded niosomes by Box-Behnken design: An understanding of solvent effect and formulation variability. Drug Dev. Ind. Pharm. 2017, 43, 1450–1459. [Google Scholar] [CrossRef]
- Edwar Aziz, D.; Abdelbary, A.A.; Elassasy, A.I. Implementing central composite design for developing transdermal diacerein-loaded niosomes: Ex vivo permeation and in vivo deposition. Curr Drug Deliv. 2018, 15, 1330–1342. [Google Scholar] [CrossRef]
- Lionberger, R.A.; Lee, S.L.; Lee, L.; Raw, A.; Lawrence, X.Y. Quality by design: Concepts for ANDAs. Aaps J. 2008, 10, 268–276. [Google Scholar] [CrossRef]
- Qumbar, M.; Imam, S.S.; Ali, J.; Ahmad, J.; Ali, A. Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: In-vitro characterization and in-vivo activity. Biomed. Pharmacother. 2017, 93, 255–266. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; AbouGhaly, M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box-Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015, 485, 235–243. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Shayan, M.; Bourbour, M.; Moghtaderi, M.; Noorbazargan, H.; Eshrati Yeganeh, F.; Saffar, S.; Tahriri, M.J.B. Preparation, optimization and in-vitro evaluation of curcumin-loaded niosome@ calcium alginate nanocarrier as a new approach for breast cancer treatment. Biology 2021, 10, 173. [Google Scholar] [CrossRef]
- Sohrabi, S.; Haeri, A.; Mahboubi, A.; Mortazavi, A.; Dadashzadeh, S. Chitosan gel-embedded moxifloxacin niosomes: An efficient antimicrobial hybrid system for burn infection. Int. J. Biol. Macromol. 2016, 85, 625–633. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef]
- Cowan, S. Cowan and Steel Manual for Identification of Medical Bacteria, 2nd ed.; Cambridge University Press: New York, NY, USA, 1974. [Google Scholar]
- Barakat, H.S.; Kassem, M.A.; El-Khordagui, L.K.; Khalafallah, N.M. Vancomycin-eluting niosomes: A new approach to the inhibition of Staphylococcal biofilm on abiotic surfaces. AAPS PharmSciTech. 2014, 15, 1263–1274. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Yaraki, M.T.; Bourbour, M.; Noorbazargan, H.; Lajevardi, A.; Shilsar, S.M.S.; Heidari, F.; Mousavian, S.M. Optimized doxycycline-loaded niosomal formulation for treatment of infection-associated prostate cancer: An in-vitro investigation. J. Drug Deliv. Sci. Technol. 2020, 57, 101715. [Google Scholar] [CrossRef]
- Newman, S.P. Principles of metered-dose inhaler design. Respir. Care 2005, 50, 1177–1190. [Google Scholar]
- Nowroozi, F.; Almasi, A.; Javidi, J.; Haeri, A.; Dadashzadeh, S. Effect of surfactant type, cholesterol content and various downsizing methods on the particle size of niosomes. Iran. J. Pharm. Res. IJPR 2018, 17, 1. [Google Scholar]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Surfactant effects on lipid-based vesicles properties. J. Pharm. Sci. 2018, 107, 1237–1246. [Google Scholar] [CrossRef]
- Buraphacheep, V.; Teeranachaideekul, V.; Supaperm, T. Effect of charged and non-ionic membrane additives on physicochemical properties and stability of niosomes. AAPS. PharmSciTech. 2008, 9, 851. [Google Scholar]
- Manosroi, A.; Wongtrakul, P.; Manosroi, J.; Sakai, H.; Sugawara, F.; Yuasa, M.; Abe, M. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf. B Biointerfaces 2003, 30, 129–138. [Google Scholar] [CrossRef]
- García-Manriqueab, P.; Machado, N.D. Effect of drug molecular weight on niosomes size and encapsulation efficiency. Colloids and Surfaces B: Biointerfaces 2020, 186, 110711. [Google Scholar] [CrossRef]
- Yeo, L.K.; Chaw, C.H.; Elkordy, A.A. The Effects of hydration parameters and co-surfactants on methylene blue-loaded niosomes prepared by the thin film hydration method. Pharmaceuticals 2019, 12, 46. [Google Scholar] [CrossRef]
- Ruckmani, K.; Sankar, V. Formulation and optimization of Zidovudine niosomes. AAPS PharmSciTech. 2010, 11, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; El-gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS. PharmSciTech. 2008, 9, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Bakshi, V.; Vitta, P.; Raghuram, A.; Pandey, S.; Udupa, N. Effect of cholesterol content and surfactant HLB on vesicle properties of niosomes. Indian J. Pharm. Sci. 2004, 66, 121–123. [Google Scholar]
- Lee, S.-C.; Lee, K.-E.; Kim, J.-J.; Lim, S.-H. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J. Liposome Res. 2005, 15, 157–166. [Google Scholar] [CrossRef]
- Mokhtar, M.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int. J. Pharm. 2008, 361, 104–111. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Khorrami, A.; Arami, S. Nonionic surfactant-based vesicular system for transdermaldrug delivery. Drug. Deliv. 2015, 22, 1071–1077. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Pardakhty, A.; Varshosaz, J.; Rouholamini, A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int. J. Pharm. 2007, 328, 130–141. [Google Scholar] [CrossRef]
- Varshosaz, J.; Pardakhty, A.; Hajhashemi, V.-I.; Najafabadi, A.R. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv. 2003, 10, 251–262. [Google Scholar] [CrossRef]
- Agarwal, R.; Katare, O.; Vyas, S. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int. J. Pharm. 2001, 228, 43–52. [Google Scholar] [CrossRef]
- Mirzaie, A.; Peirovi, N.; Akbarzadeh, I.; Moghtaderi, M.; Heidari, F.; Yeganeh, F.E.; Noorbazargan, H.; Mirzazadeh, S.; Bakhtiari, R. Preparation and optimization of ciprofloxacin encapsulated niosomes: A new approach for enhanced antibacterial activity, biofilm inhibition and reduced antibiotic resistance in ciprofloxacin-resistant methicillin-resistance Staphylococcus aureus. Bioorg. Chem. 2020, 103, 104231. [Google Scholar] [CrossRef]
- Agarwal, S.; Mohamed, M.S.; Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Formulation, characterization and evaluation of morusin loaded niosomes for potentiation of anticancer therapy. RSC Adv. 2018, 8, 32621–32636. [Google Scholar] [CrossRef]
- Nasseri, B. Effect of cholesterol and temperature on the elastic properties of niosomal membranes. Int. J. Pharm. 2005, 300, 95–101. [Google Scholar] [CrossRef]
- Alemi, A.; Reza, J.Z.; Haghiralsadat, F.; Jaliani, H.Z.; Karamallah, M.H.; Hosseini, S.A.; Karamallah, S.H. Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy. J. Nanobiotechnol. 2018, 16, 28. [Google Scholar] [CrossRef]
- Khazaeli, P.; Pardakhty, A.; Shoorabi, H. Caffeine-loaded niosomes: Characterization and in vitro release studies. Drug Deliv. 2007, 14, 447–452. [Google Scholar] [CrossRef]
- Namdeo, A.; Jain, N. Niosomal delivery of 5-fluorouracil. J. Microencapsul. 1999, 16, 731–740. [Google Scholar]
- Kishore, R.S.; Pappenberger, A.; Dauphin, I.B.; Ross, A.; Buergi, B.; Staempfli, A.; Mahler, H.-C. Degradation of polysorbates 20 and 80: Studies on thermal autoxidation and hydrolysis. J. Pharm. Sci. 2011, 100, 721–731. [Google Scholar] [CrossRef]
- Ilie, O.; van Loosdrecht, M.C.; Picioreanu, C. Mathematical modelling of tooth demineralisation and pH profiles in dental plaque. J. Theor. Biol. 2012, 309, 159–175. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Kamboj, S.; Saini, V.; Bala, S. Formulation and characterization of drug loaded nonionic surfactant vesicles (Niosomes) for oral bioavailability enhancement. Scientific. World. Journal. 2014, 2014, 959741. [Google Scholar] [CrossRef]
- Machado, N.D.; Fernández, M.A.; Häring, M.; Saldías, C.; Díaz, D.D. Niosomes encapsulated in biohydrogels for tunable delivery of phytoalexin resveratrol. RSC Adv. 2019, 9, 7601–7609. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Balasubramaniam, A.; Anil Kumar, V.; Sadasivan Pillai, K. Formulation and in vivo evaluation of niosome-encapsulated daunorubicin hydrochloride. Drug Dev. Ind. Pharm. 2002, 28, 1181–1193. [Google Scholar] [CrossRef]
- Seras-Cansell, M.; Ollivon, M.; Lesieur, S. Generation of non-ionic monoalkyl amphiphile-cholesterol vesicles: Evidence of membrane impermeability to octyl glucoside. STP Pharma Sci. 1996, 6, 12–20. [Google Scholar]
- Tanaka, M.; Fukuda, H.; Horiuchi, T. Properties of the aqueous vesicle dispersion formed with poly (oxyethylene) hydrogenated castor oil. J. Am. Oil Chem. Soc. 1990, 67, 55–60. [Google Scholar] [CrossRef]
- Omri, A.; Ravaoarinoro, M. Comparison of the bactericidal action of amikacin, netilmicin and tobramycin in free and liposomal formulation against Pseudomonas aeruginosa. Chemotherapy 1996, 42, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-M.; Chen, C.-H.; Pornpattananangkul, D.; Zhang, L.; Chan, M.; Hsieh, M.-F.; Zhang, L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Ketkar, S.; Patil, S.; Fearnley, J.; Mahadik, K.R.; Paradkar, A.R. Potentiating antimicrobial efficacy of propolis through niosomal-based system for administration. Integr. Med. Res. 2015, 4, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Adeli-Sardou, M. Evaluation of varum-loaded niosomes on breast cancer cells: Physicochemical properties, in vitro cytotoxicity, flow cytometric, DNA fragmentation and cell migration assay. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
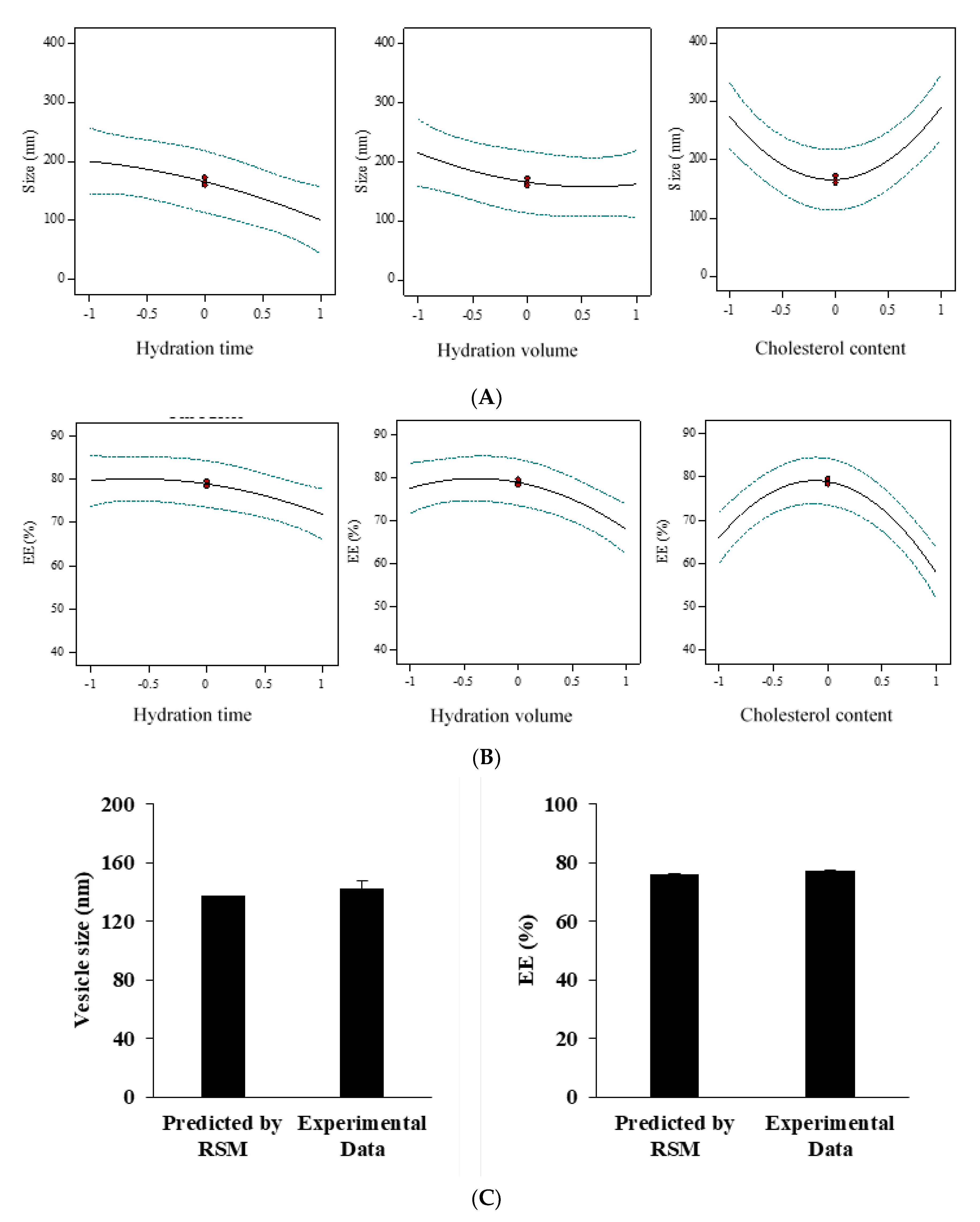
| Level | −1 | 0 | +1 |
|---|---|---|---|
| A (Hydration time, min) | 30 | 45 | 60 |
| B (Hydration volume, mL) | 6 | 8 | 10 |
| B (Hydration volume, mL) | 6 | 8 | 10 |
| Run | Levels of Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| Hydration Time (min) | Hydration Volume (mL) | Cholesterol Content (µmol) | Average Size (nm) | Polydispersity Index (PDI) | Entrapment Efficiency (EE) (%) | |
| 1 | 0 | −1 | −1 | 327.1 | 0.37 | 67.2 |
| 2 | 0 | 0 | 0 | 285.4 | 0.31 | 49.6 |
| 3 | 0 | 1 | −1 | 259.6 | 0.32 | 52.2 |
| 4 | −1 | 0 | −1 | 350.1 | 0.38 | 54.2 |
| 5 | 0 | 1 | 1 | 291.3 | 0.34 | 69.7 |
| 6 | 1 | 1 | 0 | 119.1 | 0.23 | 64.0 |
| 7 | 1 | 0 | 1 | 181.9 | 0.18 | 73.3 |
| 8 | 0 | −1 | 1 | 395.4 | 0.39 | 61.3 |
| 9 | −1 | 1 | 0 | 172.5 | 0.26 | 79.6 |
| 10 | −1 | −1 | 0 | 235.8 | 0.30 | 56.4 |
| 11 | 0 | 0 | 0 | 188.7 | 0.23 | 66.1 |
| 12 | 1 | 0 | −1 | 164.8 | 0.26 | 78.8 |
| 13 | 1 | −1 | 0 | 145.7 | 0.24 | 47.6 |
| 14 | −1 | 0 | 1 | 160.2 | 0.27 | 78.3 |
| 15 | 0 | 0 | 0 | 205.7 | 0.28 | 75.4 |
| Number | A (Hydration Time, min) | B (Hydration Volume, mL) | C (Cholesterol Content, µmol) | Desirability |
|---|---|---|---|---|
| 1 | 53 | 8 | 150 | 0.933 |
| Release Model | Zero-Order | Korsmeyer–Peppas | First-Order | Higuchi | |
|---|---|---|---|---|---|
| R2 | R2 | n * | R2 | R2 | |
| FreeEa **—pH 7.4 | 0.8109 | 0.8425 | 0.5944 | 0.9254 | 0.7468 |
| NioEa ***—pH 7.4 | 0.5981 | 0.9925 | 0.4160 | 0.7548 | 0.9322 |
| NioEa—pH 5 | 0.6541 | 0.9739 | 0.4675 | 0.7628 | 0.9691 |
| NioEa—pH 3 | 0.7739 | 0.9785 | 0.5146 | 0.8035 | 0.9475 |
| Strain No. | MIC of Ea Extract (µg mL−1) | MIC of Extract-Loaded Niosome (µg mL−1) | Increased Efficacy of Niosome (Fold) | MBC of Ea Extract (µg mL−1) | MBC of Extract-Loaded Niosome (µg mL−1) | Increased Efficacy of Niosome (Fold) |
|---|---|---|---|---|---|---|
| 4 | 2000 | 125 | 16.0 | 4000 | 250 | 16.0 |
| 6 | 4000 | 500 | 8.0 | 4000 | 500 | 8.0 |
| 10 | 2000 | 125 | 16.0 | 2000 | 125 | 16.0 |
| 13 | 2000 | 500 | 4.0 | 2000 | 500 | 4.0 |
| 16 | 4000 | 250 | 16.0 | 4000 | 250 | 16.0 |
| 24 | 2000 | 250 | 8.0 | 2000 | 250 | 8.0 |
| 29 | 1000 | 125 | 8.0 | 2000 | 125 | 16.0 |
| 33 | 2000 | 125 | 16.0 | 2000 | 250 | 8.0 |
| 37 | 4000 | 1000 | 4.0 | 4000 | 2000 | 2.0 |
| 46 | 1000 | 62.5 | 16.0 | 1000 | 125 | 8.0 |
| 51 | 2000 | 500 | 4.0 | 4000 | 1000 | 4.0 |
| 56 | 1000 | 62.5 | 16.0 | 2000 | 125 | 16.0 |
| 61 | 1000 | 125 | 8.0 | 1000 | 125 | 8.0 |
| 66 | 1000 | 250 | 4.0 | 1000 | 250 | 4.0 |
| 71 | 500 | 62.5 | 8.0 | 500 | 125 | 4.0 |
| 73 | 1000 | 62.5 | 16.0 | 1000 | 125 | 8.0 |
| 77 | 2000 | 125 | 16.0 | 2000 | 125 | 16.0 |
| 82 | 2000 | 250 | 8.0 | 2000 | 250 | 8.0 |
| 84 | 1000 | 125 | 8.0 | 1000 | 250 | 4.0 |
| 87 | 2000 | 250 | 8.0 | 2000 | 250 | 8.0 |
| 91 | 1000 | 62.5 | 16.0 | 1000 | 125 | 8.0 |
| 94 | 2000 | 250 | 8.0 | 2000 | 250 | 8.0 |
| 96 | 1000 | 250 | 4.0 | 1000 | 250 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghtaderi, M.; Mirzaie, A.; Zabet, N.; Moammeri, A.; Mansoori-Kermani, A.; Akbarzadeh, I.; Eshrati Yeganeh, F.; Chitgarzadeh, A.; Bagheri Kashtali, A.; Ren, Q. Enhanced Antibacterial Activity of Echinacea angustifolia Extract against Multidrug-Resistant Klebsiella pneumoniae through Niosome Encapsulation. Nanomaterials 2021, 11, 1573. https://doi.org/10.3390/nano11061573
Moghtaderi M, Mirzaie A, Zabet N, Moammeri A, Mansoori-Kermani A, Akbarzadeh I, Eshrati Yeganeh F, Chitgarzadeh A, Bagheri Kashtali A, Ren Q. Enhanced Antibacterial Activity of Echinacea angustifolia Extract against Multidrug-Resistant Klebsiella pneumoniae through Niosome Encapsulation. Nanomaterials. 2021; 11(6):1573. https://doi.org/10.3390/nano11061573
Chicago/Turabian StyleMoghtaderi, Maryam, Amir Mirzaie, Negar Zabet, Ali Moammeri, Amirreza Mansoori-Kermani, Iman Akbarzadeh, Faten Eshrati Yeganeh, Arman Chitgarzadeh, Aliasghar Bagheri Kashtali, and Qun Ren. 2021. "Enhanced Antibacterial Activity of Echinacea angustifolia Extract against Multidrug-Resistant Klebsiella pneumoniae through Niosome Encapsulation" Nanomaterials 11, no. 6: 1573. https://doi.org/10.3390/nano11061573
APA StyleMoghtaderi, M., Mirzaie, A., Zabet, N., Moammeri, A., Mansoori-Kermani, A., Akbarzadeh, I., Eshrati Yeganeh, F., Chitgarzadeh, A., Bagheri Kashtali, A., & Ren, Q. (2021). Enhanced Antibacterial Activity of Echinacea angustifolia Extract against Multidrug-Resistant Klebsiella pneumoniae through Niosome Encapsulation. Nanomaterials, 11(6), 1573. https://doi.org/10.3390/nano11061573







