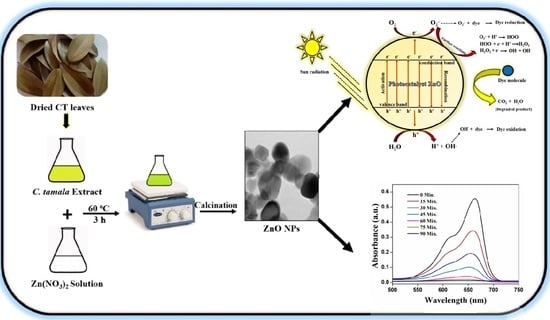
Cinnamomum tamala Leaf Extract Stabilized Zinc Oxide Nanoparticles: A Promising Photocatalyst for Methylene Blue Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of C. tamala Aqueous Extract
2.3. Synthesis of Zinc Oxide Nanoparticles (ZnO NPs)
2.4. Characterization of ZnO NPs
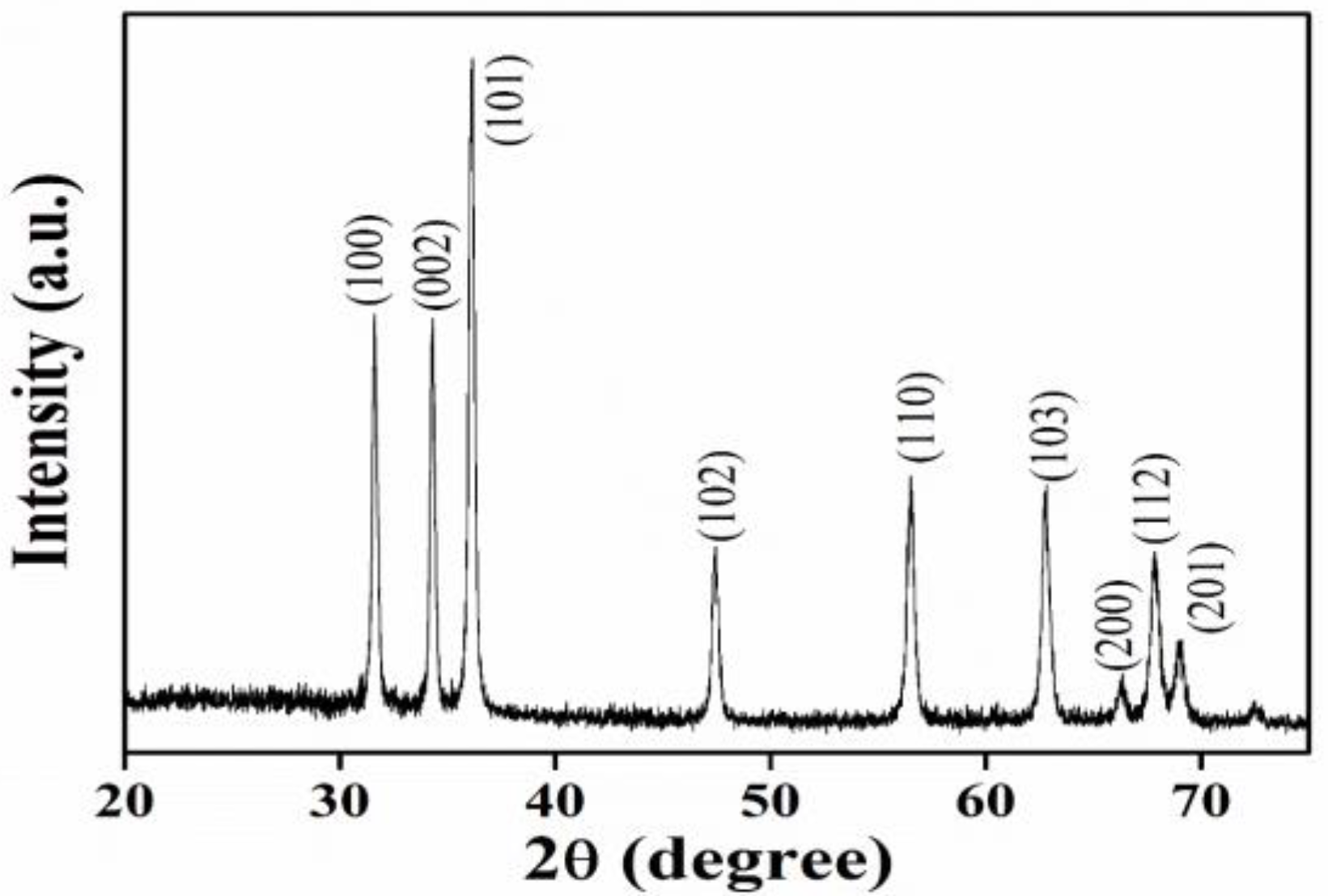
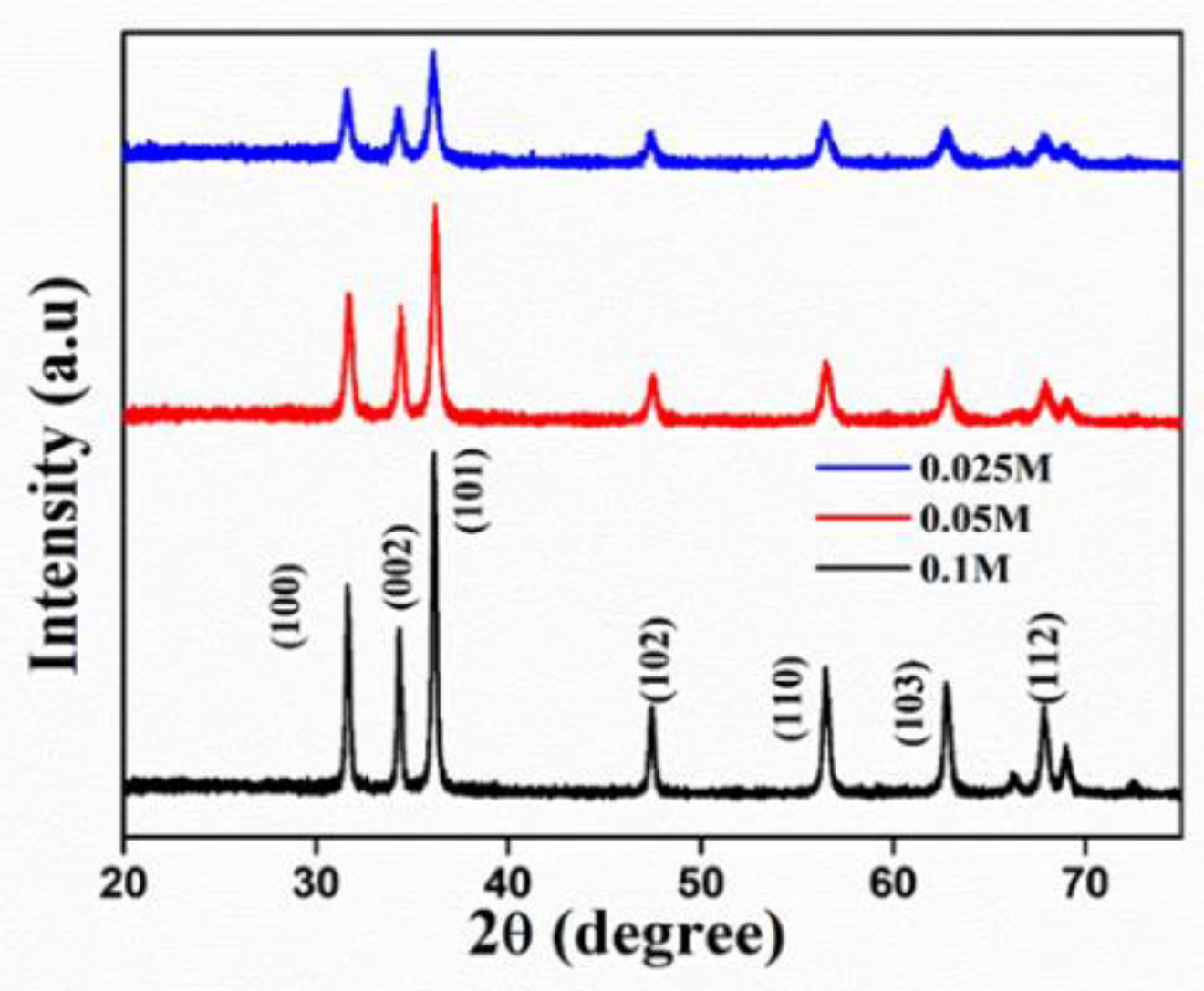
2.4.1. X-ray Diffraction (XRD) Analysis
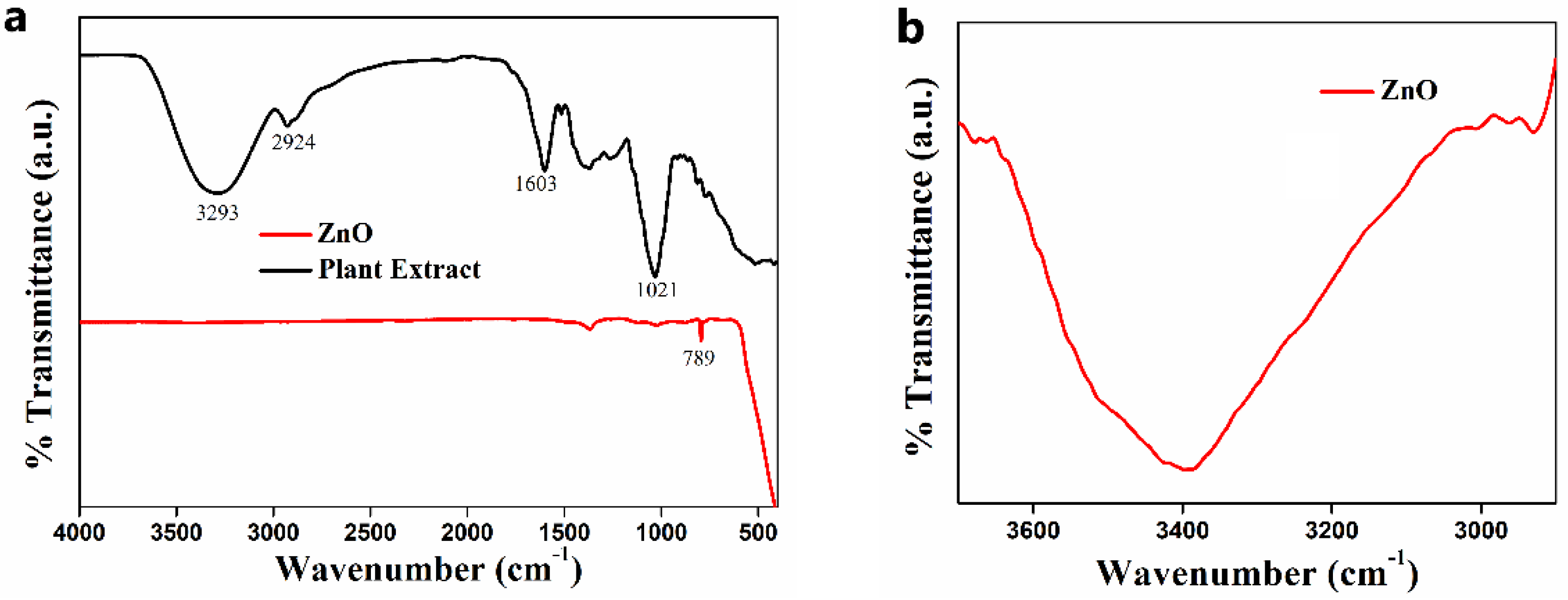
2.4.2. FT-IR Analysis
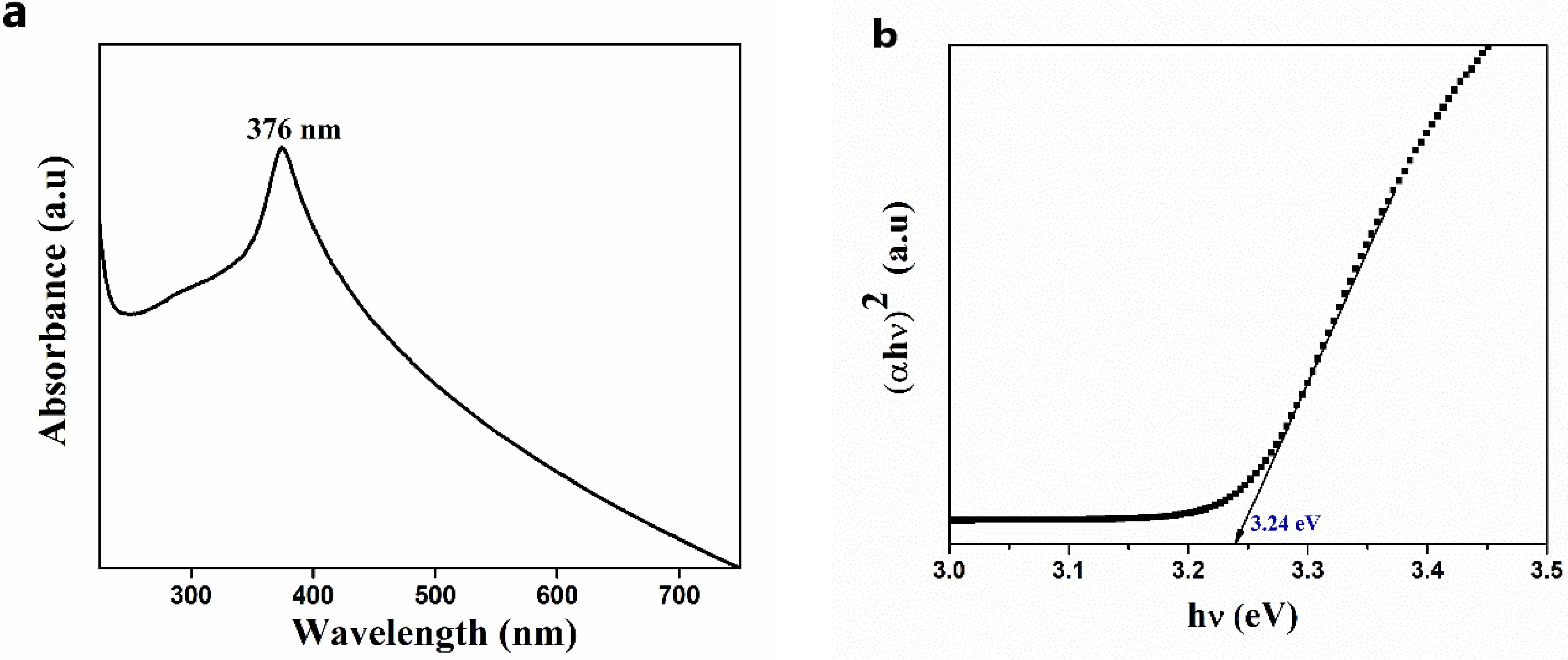
2.4.3. UV-Visible Analysis
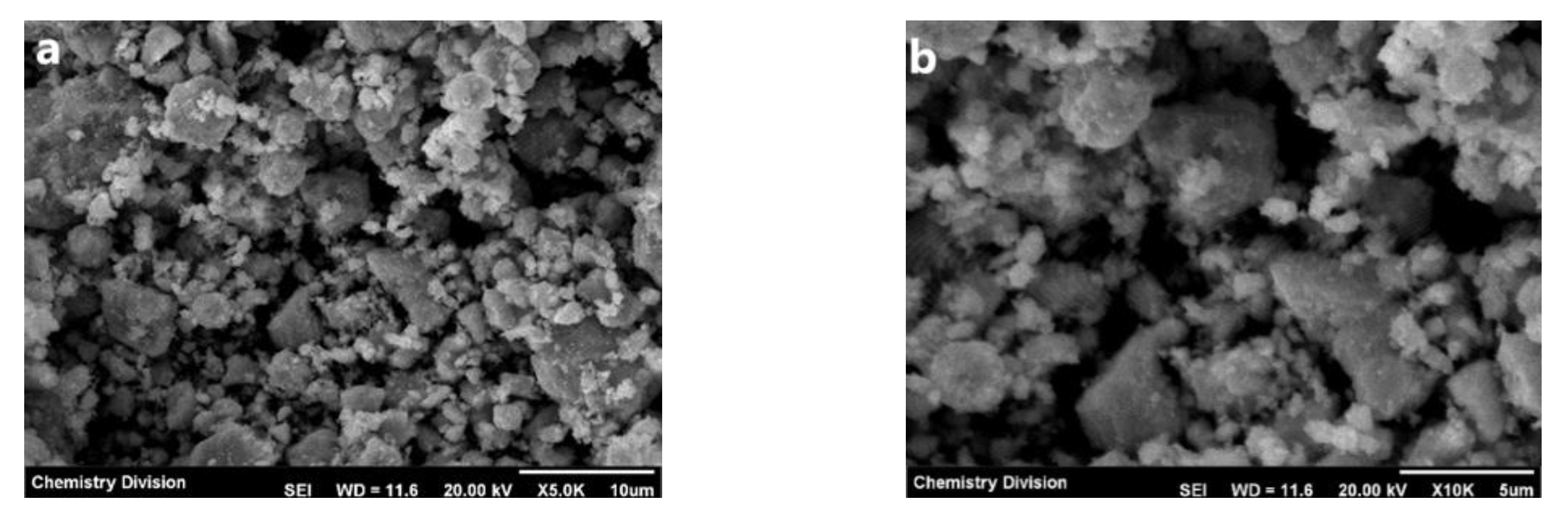
2.4.4. Scanning Electron Microscope-Energy-Dispersive X-ray Spectroscopy (SEM-EDX) Analysis
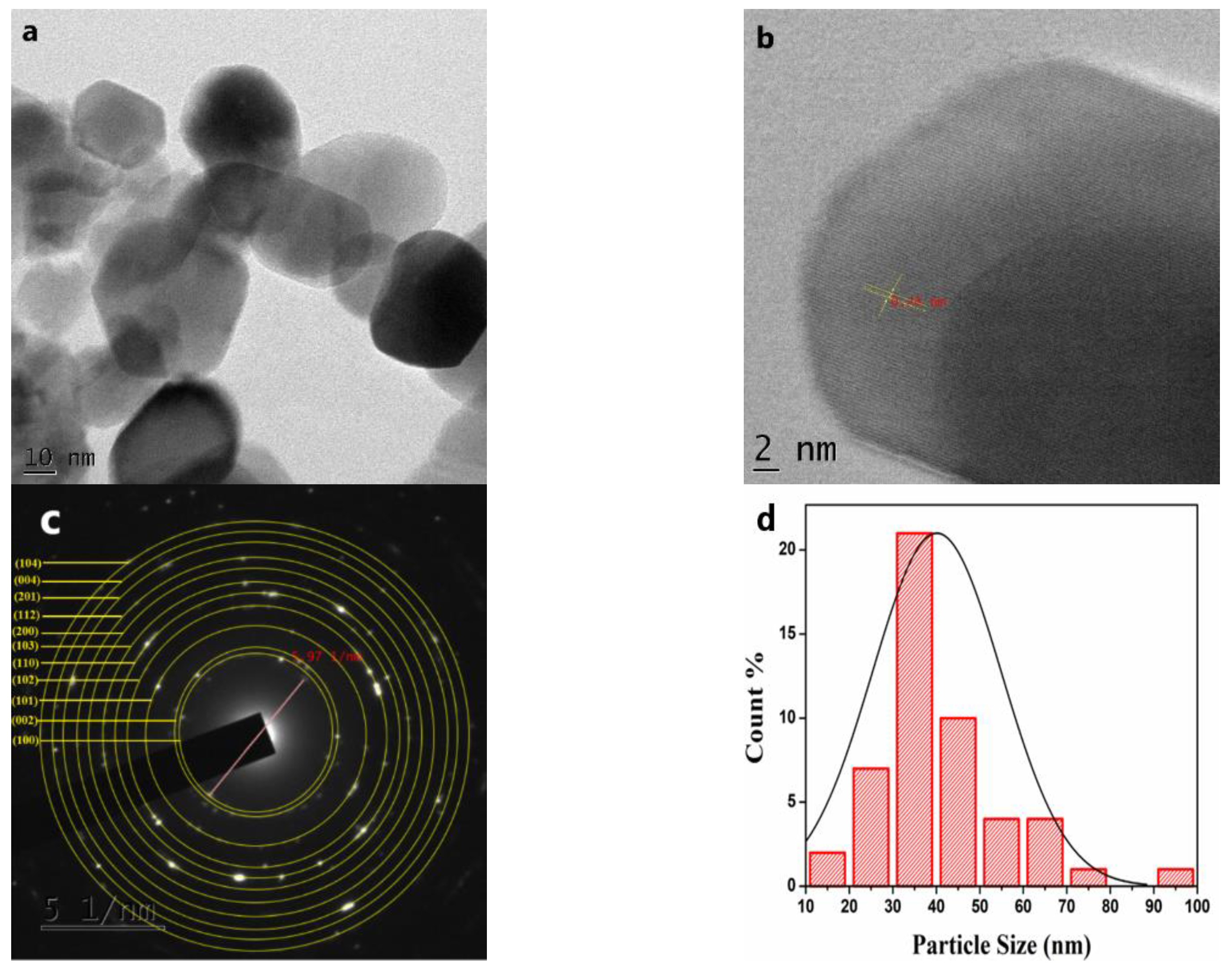
2.4.5. TEM and High-Resolution Transmission Electron Microscopy (HR-TEM) Analysis
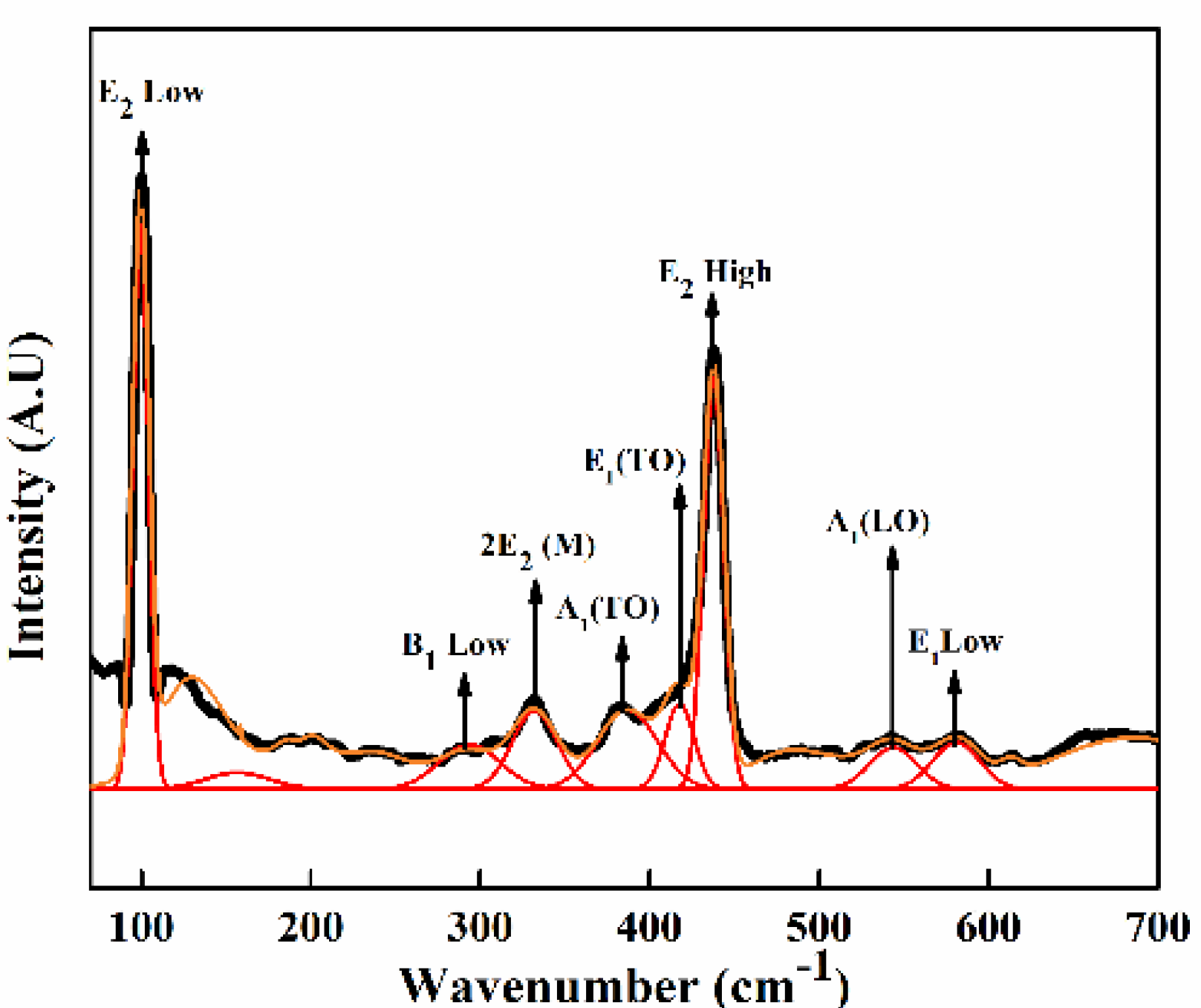
2.4.6. Raman Spectroscopy
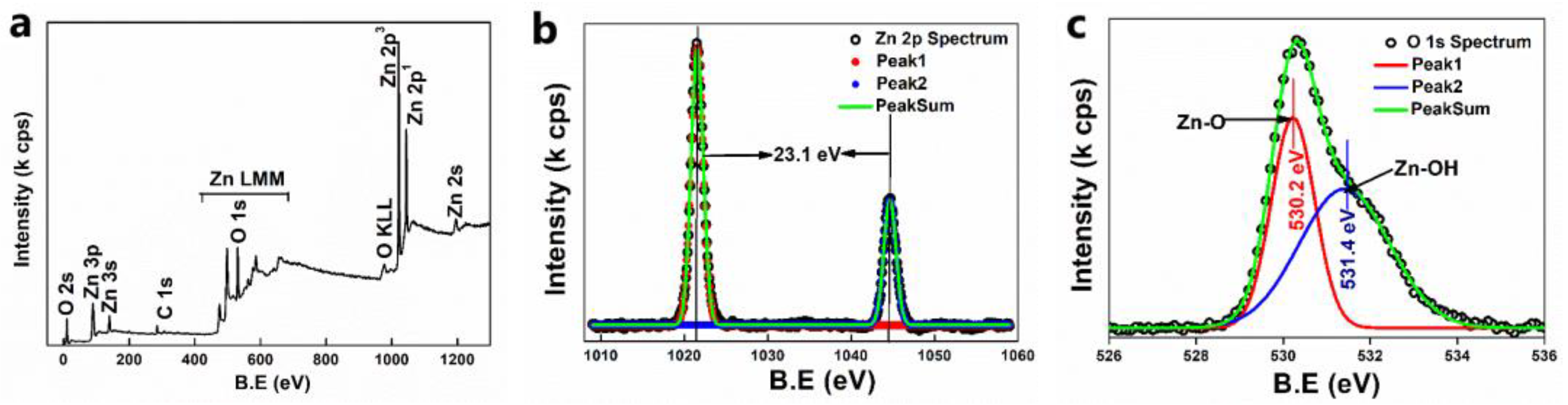
2.4.7. XPS
2.5. Photocatalytic Studies on Methylene Blue Degradation
2.5.1. Effect of Solar Irradiation Time on Photocatalytic Studies
2.5.2. Effect of Amount of Catalyst on Photocatalytic Studies
2.5.3. Effect of Calcination Temperature on Photocatalytic Studies
2.5.4. Effect of Concentration of Zn(NO3)2 on Photocatalytic Studies
2.6. Effect of Solar Irradiation on Photodegradation of MB
2.7. Studies on the Effect of Concentration of Zn(NO3)2 on Crystallite Size of ZnO NPs
3. Results and Discussion
3.1. Mechanism of Formation of ZnO NPs via Biosynthesis
3.2. Characterization of ZnO NPs
3.2.1. X-ray Diffraction Studies
3.2.2. FTIR Analysis
3.2.3. Optical Property Studies
3.2.4. FESEM-EDX Analysis
3.2.5. TEM and HR-TEM Analysis
3.2.6. Raman Spectroscopy
3.2.7. XPS Analysis
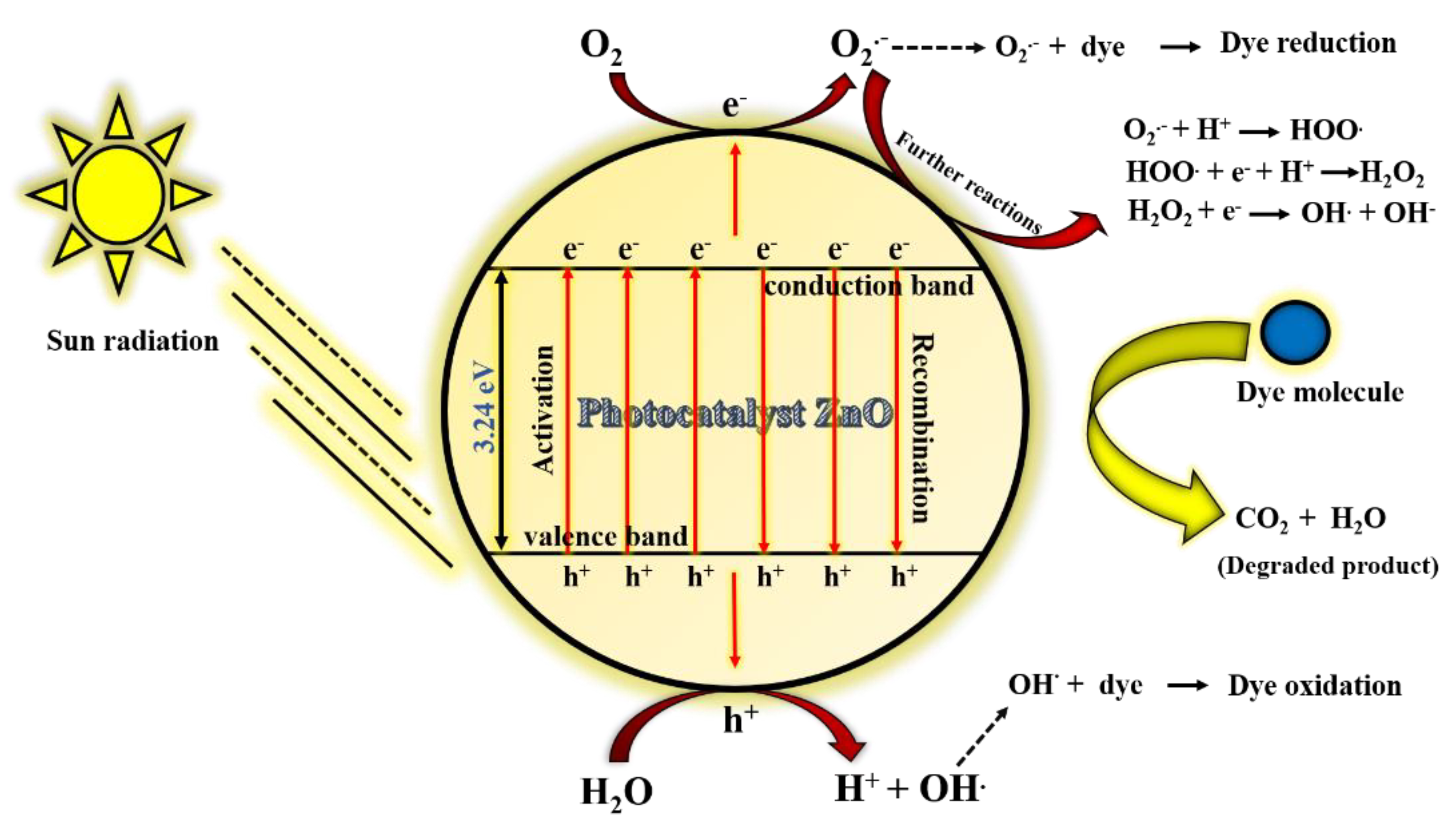
3.3. Mechanism of Photo-Degradability of MB by ZnO Catalyst
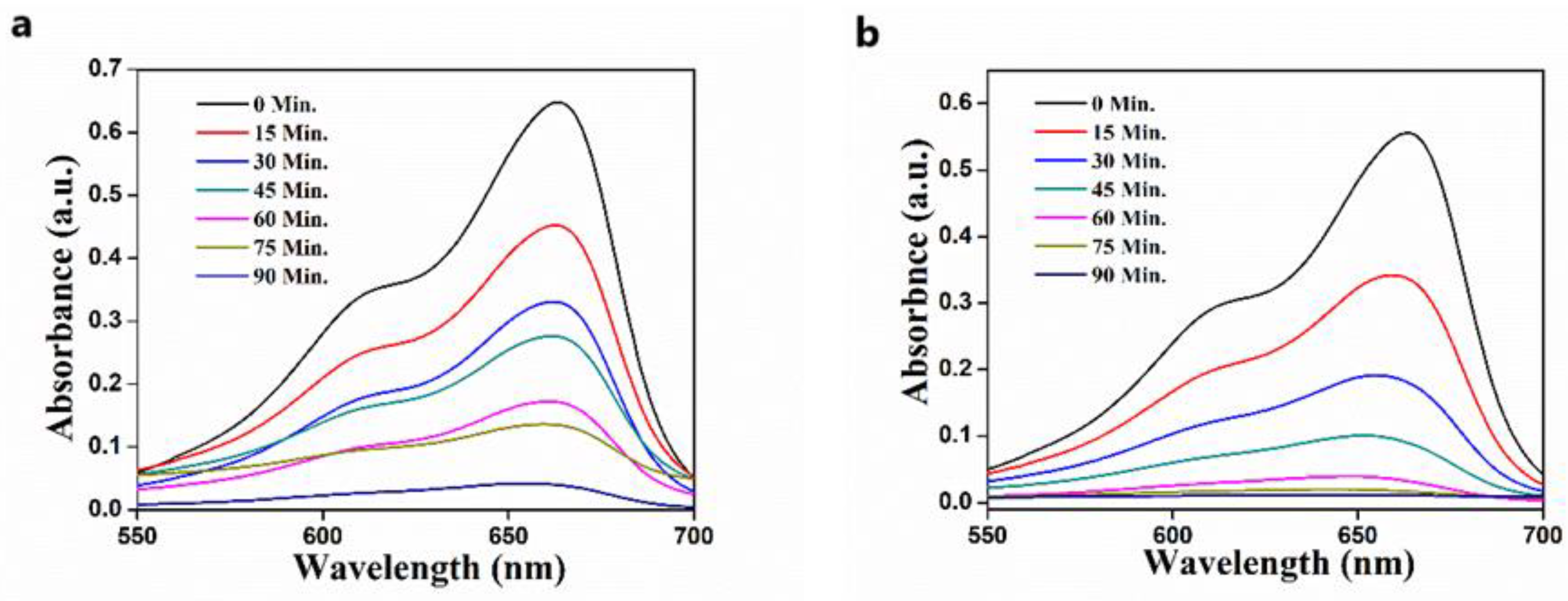
3.4. Effect of Time of Solar Irradiation on Photodegradation of MB
3.5. Effect of ZnO Catalyst Loading on Photodegradation of MB
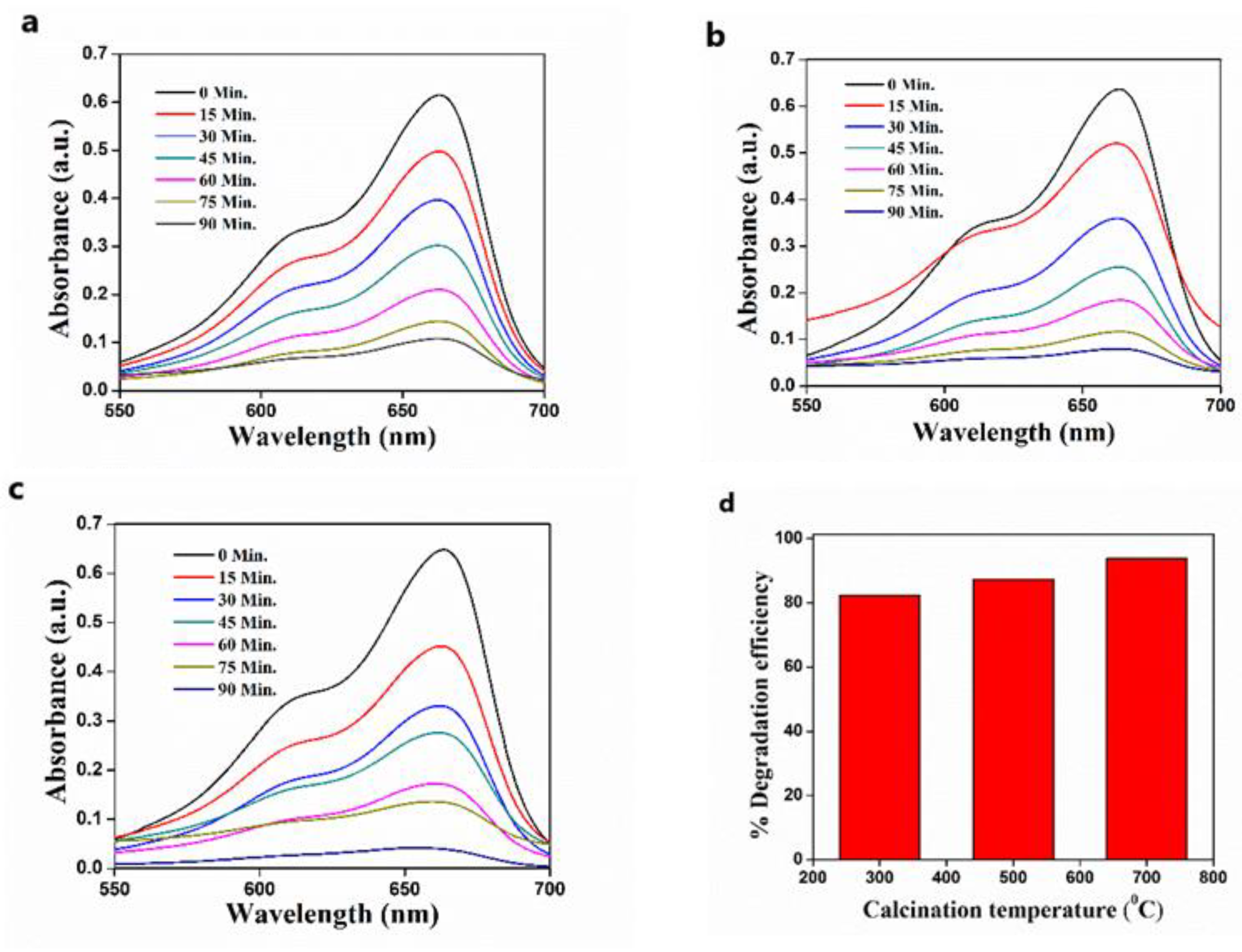
3.6. The Effect of Calcination Temperature on the Photodegradation of MB
3.7. Effect of Precursor Concentration on Photocatalytic Efficiency
3.8. The Effect of Concentration of Zn(NO3)2 on Crystallite Size of ZnO NPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zare, E.N.; Padil, V.V.T.; Mokhtari, B.; Venkateshaiah, A.; Wacławek, S.; Černík, M.; Tay, F.R.; Varma, R.S.; Makvandi, P. Advances in Biogenically Synthesized Shaped Metal- and Carbon-Based Nanoarchitectures and Their Medicinal Applications. Adv. Colloid Interface Sci. 2020, 283, 1–17. [Google Scholar] [CrossRef]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.N.; Varma, R.S. Greener Techniques for the Synthesis of Silver Nanoparticles Using Plant Extracts, Enzymes, Bacteria, Biodegradable Polymers, and Microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-Derived Nanostructures: Types and Applications. Green Chem. 2015, 18, 20–52. [Google Scholar] [CrossRef]
- Kim, C.S.; Moon, B.K.; Park, J.H.; Choi, B.C.; Seo, H.J. Solvothermal Synthesis of Nanocrystalline TiO2 in Toluene with Surfactant. J. Cryst. Growth 2003, 257, 309–315. [Google Scholar] [CrossRef]
- Gotić, M.; Musić, S. Synthesis of Nanocrystalline Iron Oxide Particles in the Iron(III) Acetate/Alcohol/Acetic Acid System. Eur. J. Inorg. Chem. 2008, 6, 966–973. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef]
- Yadav, A.; Burak, G.; Ahmadivand, A.; Kaushik, A.; Cheng, G.J.; Ouyang, Z.; Wang, Q.; Yadav, V.S.; Mishra, Y.K.; Wu, Y.; et al. Controlled Self-Assembly of Plasmon-Based Photonic Nanocrystals for High Performance Photonic Technologies. Nano Today 2021, 37, 101072. [Google Scholar] [CrossRef]
- Vabbina, P.K.; Sinha, R.; Ahmadivand, A.; Karabiyik, M.; Gerislioglu, B.; Awadallah, O.; Pala, N. Sonochemical Synthesis of a Zinc Oxide Core-Shell Nanorod Radial p-n Homojunction Ultraviolet Photodetector. ACS Appl. Mater. Interfaces 2017, 9, 19791–19799. [Google Scholar] [CrossRef] [PubMed]
- Devatha, C.P.; Thalla, A.K. Green Synthesis of Nanomaterials. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–184. [Google Scholar]
- Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Tree Gum-Based Renewable Materials: Sustainable Applications in Nanotechnology, Biomedical and Environmental Fields. Biotechnol. Adv. 2018, 36, 1984–2016. [Google Scholar] [CrossRef]
- Machado, S.; Pinto, S.L.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green Production of Zero-Valent Iron Nanoparticles Using Tree Leaf Extracts. Sci. Total Environ. 2013, 445–446, 1–8. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesis of Iron Nanoparticles by Various Tea Extracts: Comparative Study of the Reactivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 295–301. [Google Scholar] [CrossRef]
- Kumar, M.D.K.R.A. Green Chemistry and Engineering, 1st ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Yadav, S.; Khurana, J.M. Cinnamomum Tamala Leaf Extract-Mediated Green Synthesis of Ag Nanoparticles and Their Use in Pyranopyrazles Synthesis. Cuihua Xuebao/Chin. J. Catal. 2015, 36, 1042–1046. [Google Scholar] [CrossRef]
- Hassan, W.; Zainab Kazmi, S.N. Antimicrobial Activity of Cinnamomum Tamala Leaves. J. Nutr. Disord. Ther. 2015, 6. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles toward Organosulfur Pollutants. Sci. Rep. 2019. [Google Scholar] [CrossRef]
- Widiyandari, H.; Ketut Umiati, N.A.; Dwi Herdianti, R. Synthesis and Photocatalytic Property of Zinc Oxide (ZnO) Fine Particle Using Flame Spray Pyrolysis Method. J. Phys. Conf. Ser. 2018, 1025. [Google Scholar] [CrossRef]
- Akshay Kumar, K.P.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Makvandi, P.; Černík, M.; Padil, V.V.T.; Varma, R.S. Electrospun Fibers Based on Botanical, Seaweed, Microbial, and Animal Sourced Biomacromolecules and Their Multidimensional Applications. Int. J. Biol. Macromol. 2021, 171, 130–149. [Google Scholar] [CrossRef]
- Saeed, K.; Khan, I.; Shah, T.; Park, S.Y. Synthesis, Characterization and Photocatalytic Activity of Silver Nanoparticles/Amidoxime-Modified Polyacrylonitrile Nanofibers. Fibers Polym. 2015, 16, 1870–1875. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Cheong, J.Y.; Shin, S.H.; Akshaykumar, K.P.; Yun, T.G.; Bae, J.; Wacławek, S.; Černík, M.; Agarwal, S.; Greiner, A.; et al. Recycling Non-Food-Grade Tree Gum Wastes into Nanoporous Carbon for Sustainable Energy Harvesting. Green Chem. 2020, 22, 1198–1208. [Google Scholar] [CrossRef]
- Stoyanova, M.; Christoskova, S. Catalytic Degradation of Methylene Blue in Aqueous Solutions over Ni- and Co-Oxide Systems. Cent. Eur. J. Chem. 2011, 9, 1000–1007. [Google Scholar] [CrossRef]
- Ali, R.; Ooi, B.S. Photodegradation of New Methylene Blue N in Aqueous Solution Using Zinc Oxide and Titanium Dioxide as Catalyst. J. Teknol. 2012, 45, 31–41. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Solecka, M.; Zylla, R. Biodegradation, Decolourisation and Detoxification of Textile Wastewater Enhanced by Advanced Oxidation Processes. J. Biotechnol. 2001, 89, 175–184. [Google Scholar] [CrossRef]
- Saranya, K.S.; Vellora, V.; Padil, T.; Senan, C.; Pilankatta, R.; Saranya, K.; George, B.; Wacławek, S.; Černík, M. Green Synthesis of High Temperature Stable Anatase Titanium Dioxide Nanoparticles Using Gum Kondagogu: Characterization and Solar Driven Photocatalytic Degradation of Organic Dye. Nanomaterials 2018, 8, 1002. [Google Scholar] [CrossRef] [PubMed]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. ISRN Nanotechnol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Varma, R.S. Journey on Greener Pathways: From the Use of Alternate Energy Inputs and Benign Reaction Media to Sustainable Applications of Nano-Catalysts in Synthesis and Environmental Remediation. Green Chem. 2014, 16, 2027–2041. [Google Scholar] [CrossRef]
- Reddy, G.B.; Madhusudhan, A.; Ramakrishna, D.; Ayodhya, D.; Venkatesham, M.; Veerabhadram, G. Green Chemistry Approach for the Synthesis of Gold Nanoparticles with Gum Kondagogu: Characterization, Catalytic and Antibacterial Activity. J. Nanostruct. Chem. 2015, 5, 185–193. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, I.; Szczygieł, B. Characterization of Selected Synthetic Corrosion Products of Zinc Expected in Neutral Environment Containing Chloride Ions. J. Spectrosc. 2018. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Saleemi, F.; Batool, I. Synthesis and Characterization of ZnO Nanoparticles. Mater. Today Proc. 2015, 2, 5619–5621. [Google Scholar] [CrossRef]
- Shi, S.; Xu, J.; Li, L. Preparation and Photocatalytic Activity of ZnO Nanorods and ZnO/Cu2O Nanocomposites. Main Group Chem. 2017, 16, 47–55. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, Y.; Chen, S.; Ren, Q. Facile Synthesis of ZnO/Ag Composites with the Enhanced Absorption and Degradation of MB and MO under Visible Light. Ferroelectrics 2018, 526, 152–160. [Google Scholar] [CrossRef]
- Chikkanna, M.M.; Neelagund, S.E.; Rajashekarappa, K.K. Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) and Their Biological Activity. SN Appl. Sci. 2019. [CrossRef]
- Yang, H.Y.; Lee, H.; Kim, W. Effect of Zinc Nitrate Concentration on the Structural and the Optical Properties of ZnO Nanostructures. Appl. Surf. Sci. 2010, 256, 6117–6120. [Google Scholar] [CrossRef]
- Alami, Z.Y.; Salem, M.; Gaidi, M. Effect of Zn Concentration On Structural and Optical Proprieties of ZNO Thin Films Deposited By Spray Pyrolysis. Adv. Energy Int. J. 2015, 2, 11–24. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective Photocatalytic Degradation of Rhodamine B Dye by ZnO Nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Al-Kordy, H.M.H.; Sabry, S.A.; Mabrouk, M.E.M. Statistical Optimization of Experimental Parameters for Extracellular Synthesis of Zinc Oxide Nanoparticles by a Novel Haloalaliphilic Alkalibacillus Sp.W7. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Santhiya, S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, Characterization, and Antibacterial Activity of Zinc Oxide Nanoparticles Derived from Bauhinia Tomentosa Leaf Extract. J. Nanostruct. Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef]
- Yang, R.D.; Tripathy, S.; Li, Y.; Sue, H.J. Photoluminescence and Micro-Raman Scattering in ZnO Nanoparticles: The Influence of Acetate Adsorption. Chem. Phys. Lett. 2005, 411, 150–154. [Google Scholar] [CrossRef]
- Steffy, K.; Shanthi, G.; Maroky, A.S.; Selvakumar, S. Synthesis and Characterization of ZnO Phytonanocomposite Using Strychnos Nux-Vomica L. (Loganiaceae) and Antimicrobial Activity against Multidrug-Resistant Bacterial Strains from Diabetic Foot Ulcer. J. Adv. Res. 2018, 9, 69–77. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Vujancevic, J.D.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.B.; Stojadinovic, S.; Brankovic, G.O.; Nikolic, M.V. Research Photocatalytic Degradation of Methylene Blue under Natural Sunlight Using Iron Titanate Nanoparticles Prepared by a Modified Sol-Gel Method. R. Soc. Open Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ae, K.; Ae, N.K.; Singh, S. Photocatalytic Degradation of Two Commercial Reactive Dyes in Aqueous Phase Using Nanophotocatalysts. Nanoscale Res. Lett. 2009. [Google Scholar] [CrossRef]
- Chijioke-Okere, M.O.; Okorocha, N.J.; Anukam, B.N.; Oguzie, E.E. Photocatalytic Degradation of a Basic Dye Using Zinc Oxide Nanocatalyst. Int. Lett. Chem. Phys. Astron. 2019, 81, 18–26. [Google Scholar] [CrossRef]
- Ram, C.; Pareek, R.K.; Singh, V. Photocatalytic Degradation of Textile Dye by Using Titanium Dioxide Nanocatalyst. Int. J. Theor. Appl. Sci. 2012, 4, 82–88. [Google Scholar]
- Bansal, P.; Sud, D. Photodegradation of Commercial Dye, Procion Blue HERD from Real Textile Wastewater Using Nanocatalysts. Desalination 2011, 267, 244–249. [Google Scholar] [CrossRef]
- Nadarajan, R.; Wan Abu Bakar, W.A.; Ali, R. Effect of Calcination Temperature on Metal Oxides and Their Photocatalytic Activity. Adv. Mater. Res. 2015, 1107, 73–78. [Google Scholar] [CrossRef]
- Lv, K.; Xiang, Q.; Yu, J. Effect of Calcination Temperature on Morphology and Photocatalytic Activity. Appl. Catal. B Environ. 2011, 104, 275–281. [Google Scholar] [CrossRef]
- Balcha, A.; Prakash Yadav, O.; Dey, T. Photocatalytic Degradation of Methylene Blue Dye by Zinc Oxide Nanoparticles Obtained from Precipitation and Sol-Gel Methods. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Isai, K.A.; Shrivastava, V.S. Photocatalytic Degradation of Methylene Blue Using ZnO and 2%Fe–ZnO Semiconductor Nanomaterials Synthesized by Sol–Gel Method: A Comparative Study. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef]
- Kahsay, M.H. Synthesis and Characterization of ZnO Nanoparticles Using Aqueous Extract of Becium Grandiflorum for Antimicrobial Activity and Adsorption of Methylene Blue. Appl. Water Sci. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Ravichandran, V.; Sumitha, S.; Ning, C.Y.; Xian, O.Y.; Kiew Yu, U.; Paliwal, N.; Shah, S.A.A.; Tripathy, M. Durian Waste Mediated Green Synthesis of Zinc Oxide Nanoparticles and Evaluation of Their Antibacterial, Antioxidant, Cytotoxicity and Photocatalytic Activity. Green Chem. Lett. Rev. 2020, 13, 102–116. [Google Scholar] [CrossRef]
- Khan, M.; Ware, P.; Shimpi, N. Synthesis of ZnO Nanoparticles Using Peels of Passiflora Foetida and Study of Its Activity as an Efficient Catalyst for the Degradation of Hazardous Organic Dye. SN Appl. Sci. 2021, 3. [Google Scholar] [CrossRef]
- Lu, J.; Batjikh, I.; Hurh, J.; Han, Y.; Ali, H.; Mathiyalagan, R.; Ling, C.; Ahn, J.C.; Yang, D.C. Photocatalytic Degradation of Methylene Blue Using Biosynthesized Zinc Oxide Nanoparticles from Bark Extract of Kalopanax Septemlobus. Optik 2019, 182, 980–985. [Google Scholar] [CrossRef]
- Alamdari, S.; Ghamsari, M.S.; Lee, C.; Han, W.; Park, H.; Tafreshi, M.J.; Afarideh, H. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
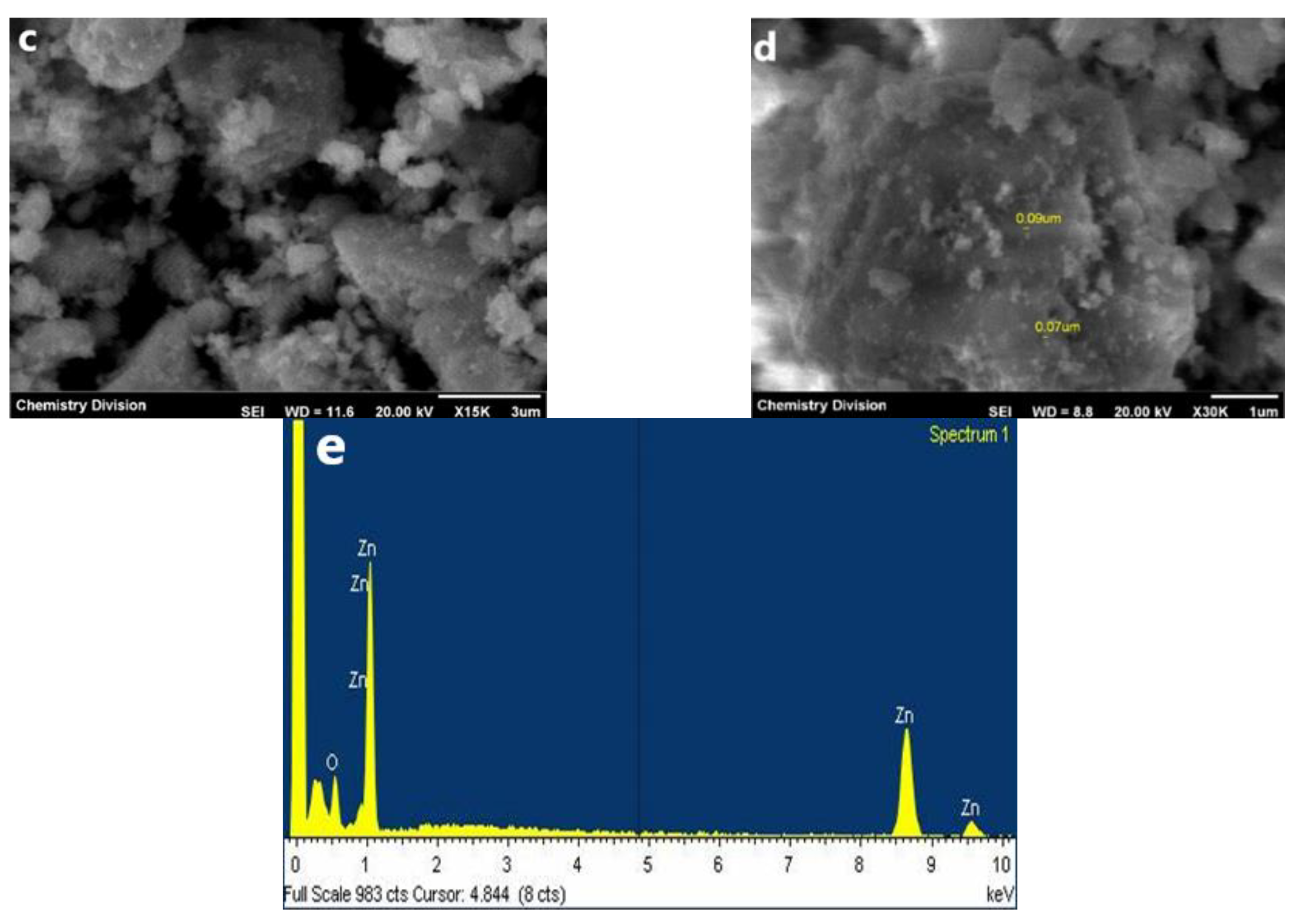


| Element | Weight% | Atomic% |
|---|---|---|
| Zn | 82.87 | 54.21 |
| O | 17.13 | 45.79 |
| Total | 100 | 100 |
| Sl. No. | Concentration (M) | 2Ɵ (Degree) | FWHM | Crystallite Size (nm) |
|---|---|---|---|---|
| 1 | 0.025 | 36.28 | 0.56569 | 25.51 |
| 2 | 0.05 | 36.20 | 0.47367 | 30.44 |
| 3 | 0.1 | 36.15 | 0.28872 | 49.95 |
| Sl. No. | ZnO Synthesis Method | % Efficiency (Time) | Light Source | MB Concentration | Reference |
|---|---|---|---|---|---|
| 1 | Precipitation Sol-gel Method | 81% (180 min) 92.5% (180 min) | UV light | 20 mg/L | [49] |
| 2 | Sol-gel | 86% (140 min) | UV light | 10 mg/L | [50] |
| 3 | Green synthesis-Becium grandiflorum aqueous leaf extract | 69% (200 min) | UV light | 100 ppm | [51] |
| 4 | Green synthesis-Durian waste | 84% (40 min) | Solar light | 10mg/L | [52] |
| 5 | Ultra sound assisted green synthesis | 93.25% (70 min) | Solar light | 1 mg/mL | [53] |
| 6 | Green synthesis-Kalopanax septemlobus | 97.5% (30 min) | UV light | 50 µM | [54] |
| 7 | Green synthesis-Sambucus ebulus | 80% (200 min) | UV light | 50 ppm | [55] |
| 8 | Green synthesis-Cinnamomum tamala aqueous leaf extract | 98% (90 min) | Solar light | 10 µM | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narath, S.; Koroth, S.K.; Shankar, S.S.; George, B.; Mutta, V.; Wacławek, S.; Černík, M.; Padil, V.V.T.; Varma, R.S. Cinnamomum tamala Leaf Extract Stabilized Zinc Oxide Nanoparticles: A Promising Photocatalyst for Methylene Blue Degradation. Nanomaterials 2021, 11, 1558. https://doi.org/10.3390/nano11061558
Narath S, Koroth SK, Shankar SS, George B, Mutta V, Wacławek S, Černík M, Padil VVT, Varma RS. Cinnamomum tamala Leaf Extract Stabilized Zinc Oxide Nanoparticles: A Promising Photocatalyst for Methylene Blue Degradation. Nanomaterials. 2021; 11(6):1558. https://doi.org/10.3390/nano11061558
Chicago/Turabian StyleNarath, Sajina, Supin Karonnan Koroth, Sarojini Sharath Shankar, Bini George, Vasundhara Mutta, Stanisław Wacławek, Miroslav Černík, Vinod Vellora Thekkae Padil, and Rajender S. Varma. 2021. "Cinnamomum tamala Leaf Extract Stabilized Zinc Oxide Nanoparticles: A Promising Photocatalyst for Methylene Blue Degradation" Nanomaterials 11, no. 6: 1558. https://doi.org/10.3390/nano11061558
APA StyleNarath, S., Koroth, S. K., Shankar, S. S., George, B., Mutta, V., Wacławek, S., Černík, M., Padil, V. V. T., & Varma, R. S. (2021). Cinnamomum tamala Leaf Extract Stabilized Zinc Oxide Nanoparticles: A Promising Photocatalyst for Methylene Blue Degradation. Nanomaterials, 11(6), 1558. https://doi.org/10.3390/nano11061558