Impact of Tuning the Surface Charge Distribution on Colloidal Iron Oxide Nanoparticle Toxicity Investigated in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. NPs Synthesis and Characterization
2.1.1. Chemicals and Materials
2.1.2. Synthesis of Poly-Maleic Acid Conjugated with Dodecylamine (PMDA)
2.1.3. Labelling of PMDA Using FITC
2.1.4. Synthesis Iron Oxide Cores
2.1.5. Phase Transfer of NPs Using PMDA
2.1.6. EDBE Functionalization of MYTS
2.1.7. EDBE Conjugation Quantification
2.1.8. PEGylation of MYTS
2.1.9. Synthesis of FITC-MYTS-EDBE and FITC-MYTS-PEG
2.1.10. NPs Characterization
2.2. C. elegans Strain, Maintenance and Synchronization
2.3. Toxicity Evaluation of Different NPs Concentration
2.4. Evaluation of C. elegans NPs Uptake
2.4.1. Confocal Microscopy Analysis
2.4.2. Graphite Furnace Atomic Absorption Spectroscopy (GFAAS) Analysis
2.5. Toxicity Assessment of the Different NPs in C. elegans
2.5.1. Lifespan Assay
2.5.2. Reactive Oxygen Species Measurement
2.5.3. Pumping Rate Assay
2.5.4. Fertility Assay
2.6. Statistical Analyses
3. Results
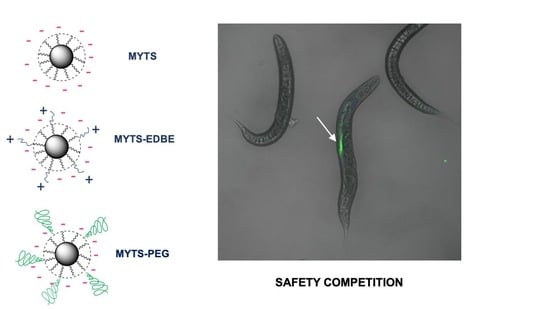
3.1. Synthesis and Characterization of MYTS, MYTS-EDBE and MYTS-PEG
3.2. Evaluation of the Effect of NPs Treatment in C. elegans
3.3. C. elegans Oral Ingestion of the Different NPs
3.4. Toxicity Assessment of the Different NPs in C. elegans
3.4.1. NPs Effects on Lifespan
3.4.2. Effect of NPS on Oxidative Stress in C. elegans
3.4.3. Effects of the NPs on Pumping Rate
3.4.4. Effects on Fertility and Reproduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwaminger, S.P.; Fraga-García, P.; Eigenfeld, M.; Becker, T.M.; Berensmeier, S. Magnetic Separation in Bioprocessing Beyond the Analytical Scale: From Biotechnology to the Food Industry. Front. Bioeng. Biotechnol. 2019, 7, 233. [Google Scholar] [CrossRef]
- Perez, J.M.; Josephson, L.; O’Loughlin, T.; Högemann, D.; Weissleder, R. Magnetic relaxation switches capable of sensing molecular interactions. Nat. Biotechnol. 2002, 20, 816–820. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; Van De Kaa, C.H.; De La Rosette, J.; Weissleder, R. Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. New Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Xu, Y.-J.; Zhang, G.-B.; Ling, D.; Wang, M.-Q.; Zhou, Y.; Wu, Y.-D.; Wu, T.; Hackett, M.J.; Kim, B.H.; et al. Iron oxide nanoclusters for T 1 magnetic resonance imaging of non-human primates. Nat. Biomed. Eng. 2017, 1, 637–643. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Stephen, Z.; Zhang, M. Theranostic Nanoparticles for RNA-Based Cancer Treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Mertz, D.; Harlepp, S.; Goetz, J.; Bégin, D.; Schlatter, G.; Bégin-Colin, S.; Hébraud, A. Nanocomposite Polymer Scaffolds Responding under External Stimuli for Drug Delivery and Tissue Engineering Applications. Adv. Ther. 2019, 3, 1900143. [Google Scholar] [CrossRef]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B: Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef]
- Etemadi, H.; Plieger, P.G. Magnetic Fluid Hyperthermia Based on Magnetic Nanoparticles: Physical Characteristics, Historical Perspective, Clinical Trials, Technological Challenges, and Recent Advances. Adv. Ther. 2020, 3. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Fee, C.J. Size comparison between proteins PEGylated with branched and linear poly(ethylene glycol) molecules. Biotechnol. Bioeng. 2007, 98, 725–731. [Google Scholar] [CrossRef]
- Lipka, J.; Semmler-Behnke, M.; Sperling, R.A.; Wenk, A.; Takenaka, S.; Schleh, C.; Kissel, T.; Parak, W.J.; Kreyling, W.G. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials 2010, 31, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Karakoti, A.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Pellegrino, T.; Manna, L.; Kudera, S.; Liedl, T.; Koktysh, D.; Rogach, A.L.; Keller, S.; Rädler, J.; Natile, G.; Parak, W.J. Hydrophobic Nanocrystals Coated with an Amphiphilic Polymer Shell: A General Route to Water Soluble Nanocrystals. Nano Lett. 2004, 4, 703–707. [Google Scholar] [CrossRef]
- Yang, L.; Mao, H.; Wang, Y.A.; Cao, Z.; Peng, X.; Wang, X.; Duan, H.; Ni, C.; Yuan, Q.; Adams, G.; et al. Single Chain Epidermal Growth Factor Receptor Antibody Conjugated Nanoparticles for in vivo Tumor Targeting and Imaging. Small 2008, 5, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, L.P.; Luke, C.J.; Perlmutter, D.H.; Silverman, G.A.; Pak, S.C. C. elegans in high-throughput drug discovery. Adv. Drug Deliv. Rev. 2014, 69-70, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valencia, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012, 7, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moragas, L.; Roig, A.; Laromaine, A. C. elegans as a tool for in vivo nanoparticle assessment. Adv. Colloid Interface Sci. 2015, 219, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, A.D.; Hsiao, T.I. The Caenorhabditis elegans epidermis as a model skin. I: Development, patterning, and growth. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 861–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, A.D.; Xu, S. The Caenorhabditis elegans epidermis as a model skin. II: Differentiation and physiological roles. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 879–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgonie, G.; Claeys, M.; Vanfleteren, J.; Dewaele, D.; Coomans, A. Borgonie Claeys M Presence of Peritrophic-like Membranes in the Intestine of 3 Bacteriophagous Nematodes (Nematoda, Rhabditida). Fundam. Appl. Nematol. 1995, 18, 227–233. [Google Scholar]
- Bossinger, O.; Hoffmann, M. Development and Cell Polarity of the C. elegans Intestine. Curr. Front. Perspect. Cell Biol. 2012, 335–360. [Google Scholar] [CrossRef] [Green Version]
- Egberts, H.J.A.; Koninkx, J.F.J.G.; Van Dijk, J.E.; Mouwen, J.M.V.M. Biological and pathobiological aspects of the glycocalyx of the small intestinal epithelium. A review. Vet. Q. 1984, 6, 186–199. [Google Scholar] [CrossRef]
- Balla, K.M.; Troemel, E.R. Caenorhabditis elegans as a model for intracellular pathogen infection. Cell. Microbiol. 2013, 15, 1313–1322. [Google Scholar] [CrossRef] [Green Version]
- Sato, M. C. elegans as a model for membrane traffic. WormBook 2014, 1–47. [Google Scholar] [CrossRef]
- Wang, L.; Audhya, A. In vivo imaging of C. elegans endocytosis. Methods 2014, 68, 518–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wu, Q.; Li, Y.; Wang, D. Translocation, transfer, and in vivo safety evaluation of engineered nanomaterials in the non-mammalian alternative toxicity assay model of nematode Caenorhabditis elegans. RSC Adv. 2013, 3, 5741–5757. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Sommaruga, S.; O’Donnell, M.; Galeffi, P.; Tortora, P.; Prosperi, D.; Colombo, M. Dependence of nanoparticle-cell recognition efficiency on the surface orientation of scFv targeting ligands. Biomater. Sci. 2013, 1, 728–735. [Google Scholar] [CrossRef]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-A.; Sperling, R.A.; Li, J.K.; Yang, T.-Y.; Li, P.-Y.; Zanella, M.; Chang, W.H.; Parak, W.J. Design of an Amphiphilic Polymer for Nanoparticle Coating and Functionalization. Small 2008, 4, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Liab, J.J.; Hartonob, D.; Ongc, C.-N.; Baya, B.-H.; Yung, L.-Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. Biomed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Williams, P.L.; Dusenbery, D.B.; Low, A.K.; Meeks, J.R.; Mackerer, C.R. Using the Nematode Caenorhabditis elegans to Predict Mammalian Acute Lethality to Metallic Salts. Toxicol. Ind. Heal. 1988, 4, 469–478. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Q.; Li, H.; Xia, Q.; Liu, Y. Role of surface charge in determining the biological effects of CdSe/ZnS quantum dots. Int. J. Nanomed. 2015, 10, 7073–7088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kievit, F.M.; Zhang, M. Surface Engineering of Iron Oxide Nanoparticles for Targeted Cancer Therapy. Accounts Chem. Res. 2011, 44, 853–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Baimler, I.V.; Uvarov, O.V.; Smirnova, V.V.; Volkov, M.Y.; Semenova, A.A.; Lisitsyn, A.B. Influence of the Concentration of Fe and Cu Nanoparticles on the Dynamics of the Size Distribution of Nanoparticles. Front. Phys. 2020, 8, 8. [Google Scholar] [CrossRef]
- Orlando, A.; Colombo, M.; Prosperi, D.; Gregori, M.; Panariti, A.; Rivolta, I.; Masserini, M.; Cazzaniga, E. Iron oxide nanoparticles surface coating and cell uptake affect biocompatibility and inflammatory responses of endothelial cells and macrophages. J. Nanoparticle Res. 2015, 17, 1–13. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Aman, A.; Kumar, J.; Kar, M. Evaluation of iron oxide nanoparticles (nps) on aging and age related metabolism and physiological changes in C. elegans. Int. J. Pharm. Sci. Res. 2017, 8, 3813–3816. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Yu, S.-M.; Carenza, E.; Laromaine, A.; Roig, A. Protective Effects of Bovine Serum Albumin on Superparamagnetic Iron Oxide Nanoparticles Evaluated in the Nematode Caenorhabditis elegans. ACS Biomater. Sci. Eng. 2015, 1, 1129–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Moragas, L.; Yu, S.-M.; Benseny-Cases, N.; Stürzenbaum, S.; Roig, A.; Laromaine, A. Toxicogenomics of iron oxide nanoparticles in the nematode C. elegans. Nanotoxicology 2017, 11, 647–657. [Google Scholar] [CrossRef] [Green Version]
- Däwlätşina, G.I.; Minullina, R.T.; Fakhrullin, R.F. Microworms swallow the nanobait: The use of nanocoated microbial cells for the direct delivery of nanoparticles into Caenorhabditis elegans. Nanoscale 2013, 5, 11761–11769. [Google Scholar] [CrossRef]
- Wang, D. Nanotoxicology in Caenorhabditis elegans; Springer Science and Business Media LLC: Berlin, Germany, 2018. [Google Scholar]
- Qiu, Y.; Luo, L.; Yang, Y.; Kong, Y.; Li, Y.; Wang, D. Potential toxicity of nanopolystyrene on lifespan and aging process of nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 705, 135918. [Google Scholar] [CrossRef]
- Avery, L. Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J. Exp. Biol. 1993, 175, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Raizen, D.; Song, B.-M.; Trojanowski, N.; You, Y.-J. Methods for measuring pharyngeal behaviors. WormBook 2012, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.; Candido, E.P. Feeding Is Inhibited by Sublethal Concentrations of Toxicants and by Heat Stress in the Nematode Caenorhabditis elegans: Relationship to the Cellular Stress Response. J. Exp. Zool. 1999, 284, 147–157. [Google Scholar] [CrossRef]
- Wu, T.; Xu, H.; Liang, X.; Tang, M. Caenorhabditis elegans as a complete model organism for biosafety assessments of nanoparticles. Chemosphere 2019, 221, 708–726. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Chen, P.-J.; Liao, V.H.-C. Nanoscale zerovalent iron (nZVI) at environmentally relevant concentrations induced multigenerational reproductive toxicity in Caenorhabditis elegans. Chemosphere 2016, 150, 615–623. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Lin, Y.-J.; Liao, C.-M. Toxicity-based toxicokinetic/toxicodynamic assessment of bioaccumulation and nanotoxicity of zerovalent iron nanoparticles in Caenorhabditis elegans. Int. J. Nanomed. 2017, 12, 4607–4621. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.-M.; Gonzalez-Moragas, L.; Milla, M.; Kolovou, A.; Santarella-Mellwig, R.; Schwab, Y.; Laromaine, A.; Roig, A. Bio-identity and fate of albumin-coated SPIONs evaluated in cells and by the C. elegans model. Acta Biomater. 2016, 43, 348–357. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; Wu, J.; Zhang, L.; Huang, J.; Zhang, Y.; Zhou, X.; Liang, B. Iron Overload Coordinately Promotes Ferritin Expression and Fat Accumulation in Caenorhabditis elegans. Genetics 2016, 203, 241–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.; Leibold, E.A. Mechanisms of iron metabolism in Caenorhabditis elegans. Front. Pharmacol. 2014, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Chege, P.M.; McColl, G. Caenorhabditis elegans: A model to investigate oxidative stress and metal dyshomeostasis in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Marzetti, E.; Seo, A.Y.; Kim, J.-S.; Prolla, T.A.; Leeuwenburgh, C. The emerging role of iron dyshomeostasis in the mitochondrial decay of aging. Mech. Ageing Dev. 2010, 131, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Hydrodynamic Diameter (nm) | Polydispersity Index (PDI) * | ζ-Potential (mV) | |
|---|---|---|---|
| MYTS | 30.3 ± 0.9 | 0.152 ± 0.009 | −41.2 ± 0.8 |
| MYTS-PEG | 35.1 ± 0.8 | 0.133 ± 0.007 | −37.1 ± 3.8 |
| MYTS-EDBE | 35.0 ±1.4 | 0.143 ± 0.010 | −35.7 ± 0.3 |
| Concentration (µg/mL) | Number of Worms | Mean Lifespan (day) (1) | Maximum Lifespan (day) (2) | p-Value (3) | |
|---|---|---|---|---|---|
| MYTS | 0 | 70 | 17.0 ± 0.40 | 30 ± 0.33 | |
| 50 | 70 | 9.5 ± 0.70 | 30 ± 0.58 | 1.67 × 10−5 | |
| 100 | 56 | 3.3 ± 0.45 | 23 ± 0.33 | 1.61 × 10−10 | |
| MYTS-PEG | 0 | 70 | 16.4 ± 0.60 | 29 ± 0.57 | |
| 50 | 65 | 16.0 ± 0.43 | 29 ± 0.33 | 0.823 | |
| 100 | 68 | 6.5 ± 0.65 | 29 ± 0.34 | 8.45 × 10−5 | |
| MYTS-EDBE | 0 | 55 | 16.6 ± 0.62 | 31 ± 0.57 | |
| 50 | 53 | 15.9 ± 0.60 | 29 ± 0.57 | 0.748 | |
| 100 | 42 | 15.3 ± 0.50 | 29 ± 0.33 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amigoni, L.; Salvioni, L.; Sciandrone, B.; Giustra, M.; Pacini, C.; Tortora, P.; Prosperi, D.; Colombo, M.; Regonesi, M.E. Impact of Tuning the Surface Charge Distribution on Colloidal Iron Oxide Nanoparticle Toxicity Investigated in Caenorhabditis elegans. Nanomaterials 2021, 11, 1551. https://doi.org/10.3390/nano11061551
Amigoni L, Salvioni L, Sciandrone B, Giustra M, Pacini C, Tortora P, Prosperi D, Colombo M, Regonesi ME. Impact of Tuning the Surface Charge Distribution on Colloidal Iron Oxide Nanoparticle Toxicity Investigated in Caenorhabditis elegans. Nanomaterials. 2021; 11(6):1551. https://doi.org/10.3390/nano11061551
Chicago/Turabian StyleAmigoni, Loredana, Lucia Salvioni, Barbara Sciandrone, Marco Giustra, Chiara Pacini, Paolo Tortora, Davide Prosperi, Miriam Colombo, and Maria Elena Regonesi. 2021. "Impact of Tuning the Surface Charge Distribution on Colloidal Iron Oxide Nanoparticle Toxicity Investigated in Caenorhabditis elegans" Nanomaterials 11, no. 6: 1551. https://doi.org/10.3390/nano11061551
APA StyleAmigoni, L., Salvioni, L., Sciandrone, B., Giustra, M., Pacini, C., Tortora, P., Prosperi, D., Colombo, M., & Regonesi, M. E. (2021). Impact of Tuning the Surface Charge Distribution on Colloidal Iron Oxide Nanoparticle Toxicity Investigated in Caenorhabditis elegans. Nanomaterials, 11(6), 1551. https://doi.org/10.3390/nano11061551