Empowering the Emission of Upconversion Nanoparticles for Precise Subcellular Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesizing of Core–Shell UCNPs
2.2. Functionalization of the UCNPs
2.3. Cell Culture
2.4. Differentiation of SH-SY5Y Cells on Gold-Coated TEM Grids
2.5. Cell Imaging by Confocal Microscopy
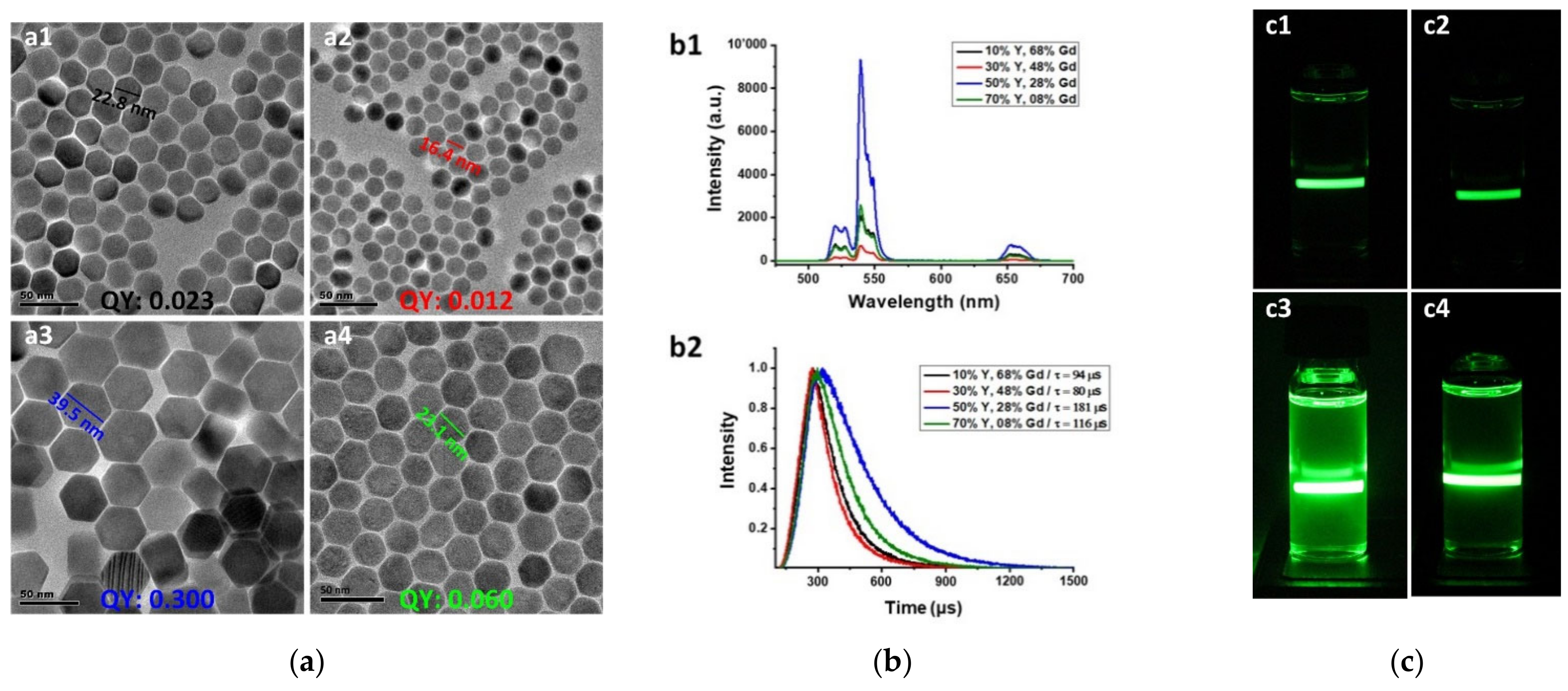
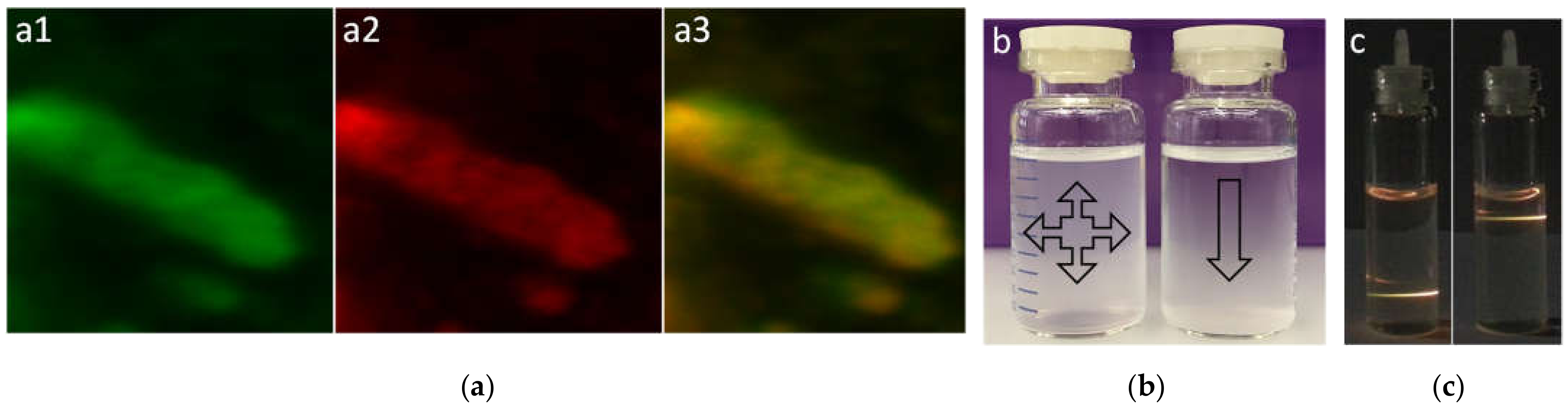
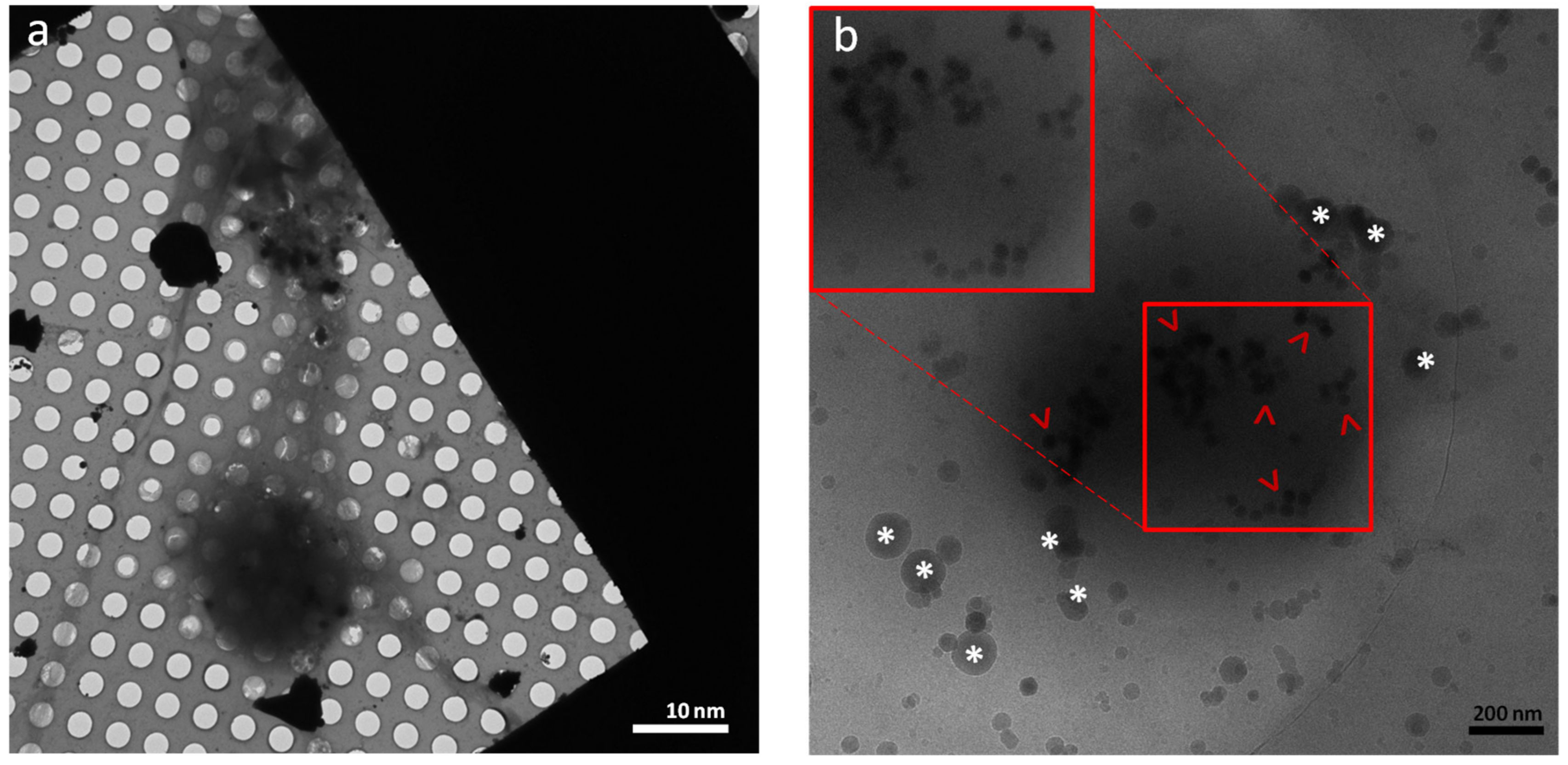
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Martin, J.D.; Cabral, H.; Stylianopoulos, T.; Jain, R.K. Improving cancer immunotherapy using nanomedicines: Progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020, 17, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Kretzmann, J.A.; Norret, M.; Toshniwal, P.; Veder, J.-P.; Jiang, H.; Guagliardo, P.; Munshi, A.M.; Chawla, R.; Evans, C.W.; et al. Intracellular speciation of gold nanorods alters the conformational dynamics of genomic DNA. Nat. Nanotechnol. 2018, 13, 1148–1153. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, B.; Jin, D.; Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 2015, 10, 924. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lin, M.; Lee, A.; Park, Y.I. Lanthanide-Doped Nanoparticles for Diagnostic Sensing. Nanomaterials 2017, 7, 411. [Google Scholar] [CrossRef]
- Samhadaneh, D.M.; Mandl, G.A.; Han, Z.; Mahjoob, M.; Weber, S.C.; Tuznik, M.; Rudko, D.A.; Capobianco, J.A.; Stochaj, U. Evaluation of Lanthanide-Doped Upconverting Nanoparticles for In Vitro and In Vivo Applications. ACS Appl. Bio Mater. 2020, 3, 4358–4369. [Google Scholar] [CrossRef]
- Lyu, L.; Cheong, H.; Ai, X.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Near-infrared light-mediated rare-earth nanocrystals: Recent advances in improving photon conversion and alleviating the thermal effect. NPG Asia Mater. 2018, 10, 685–702. [Google Scholar] [CrossRef]
- Lei, P.; An, R.; Yao, S.; Wang, Q.; Dong, L.; Xu, X.; Du, K.; Feng, J.; Zhang, H. Ultrafast Synthesis of Novel Hexagonal Phase NaBiF4 Upconversion Nanoparticles at Room Temperature. Adv. Mater. 2017, 29, 1700505. [Google Scholar] [CrossRef]
- Shao, B.; Zhao, Q.; Jia, Y.; Lv, W.; Jiao, M.; Lü, W.; You, H. A novel synthetic route towards monodisperse β-NaYF4:Ln3+ micro/nanocrystals from layered rare-earth hydroxides at ultra low temperature. Chem. Commun. 2014, 50, 12706–12709. [Google Scholar] [CrossRef]
- Kutsenko, A.B.; Heber, J.; Kapphan, S.E.; Demirbilek, R.; Zakharchenya, R.I. Energy migration and energy transfer processes in RE3+ doped nanocrystalline yttrium oxide. Phys. Status Solidi 2005, 2, 685–688. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.; Wang, J.; Wang, Q.; Han, Y.; Zhu, H.; Chen, X.; Liu, X. Tuning upconversion through energy migration in core–shell nanoparticles. Nat. Mater. 2011, 10, 968–973. [Google Scholar] [CrossRef]
- Abel, K.A.; Boyer, J.-C.; Andrei, C.M.; van Veggel, F.C.J.M. Analysis of the Shell Thickness Distribution on NaYF4/NaGdF4 Core/Shell Nanocrystals by EELS and EDS. J. Phys. Chem. Lett. 2011, 2, 185–189. [Google Scholar] [CrossRef]
- Blasse, G. The physics of new luminescent materials. Mater. Chem. Phys. 1987, 16, 201–236. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- De Vries, A.J.; Smeets, W.J.J.; Blasse, G. The trapping of Gd3+ excitation energy by Cr3+ and rare earth ions in GdAlO3. Mater. Chem. Phys. 1987, 18, 81–92. [Google Scholar] [CrossRef][Green Version]
- Mai, H.-X.; Zhang, Y.-W.; Si, R.; Yan, Z.-G.; Sun, L.; You, L.-P.; Yan, C.-H. High-Quality Sodium Rare-Earth Fluoride Nanocrystals: Controlled Synthesis and Optical Properties. J. Am. Chem. Soc. 2006, 128, 6426–6436. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Rostami, I.; Wang, Z.; Dai, H.; Hu, Z. Energy Migration Engineering of Bright Rare-Earth Upconversion Nanoparticles for Excitation by Light-Emitting Diodes. Adv. Mater. 2015, 27. [Google Scholar] [CrossRef]
- Chen, D.; Lei, L.; Yang, A.; Wang, Z.; Wang, Y. Ultra-broadband near-infrared excitable upconversion core/shell nanocrystals. Chem. Commun. 2012, 48, 5898–5900. [Google Scholar] [CrossRef]
- Liu, X.; Deng, R.; Zhang, Y.; Wang, Y.; Chang, H.; Huang, L.; Liu, X. Probing the nature of upconversion nanocrystals: Instrumentation matters. Chem. Soc. Rev. 2015, 44, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Collins, J.E.; Kang, Y.; Chen, J.; Chen, D.T.N.; Yodh, A.G.; Murray, C.B. Morphologically controlled synthesis of colloidal upconversion nanophosphors and their shape-directed self-assembly. Proc. Natl. Acad. Sci. USA 2010, 107, 22430–22435. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Würth, C.; Muhr, V.; Hirsch, T.; Resch-Genger, U. Particle-size-dependent upconversion luminescence of NaYF4: Yb, Er nanoparticles in organic solvents and water at different excitation power densities. Nano Res. 2018, 11, 6360–6374. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.; Shen, J.; Li, Z.; Zhang, Y.; Han, G. Upconversion Nanoparticles: A Versatile Solution to Multiscale Biological Imaging. Bioconjug. Chem. 2015, 26, 166–175. [Google Scholar] [CrossRef]
- Meng, X.; Li, X. Size Limit and Energy Analysis of Nanoparticles during Wrapping Process by Membrane. Nanomaterials 2018, 8, 899. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Lidke, D.S.; Lidke, K.A. Advances in high-resolution imaging—Techniques for three-dimensional imaging of cellular structures. J. Cell Sci. 2012, 125, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Cheng, Y.; Sliz, P.; Hiroaki, Y.; Fujiyoshi, Y.; Harrison, S.C.; Walz, T. Lipid–protein interactions in double-layered two-dimensional AQP0 crystals. Nature 2005, 438, 633–638. [Google Scholar] [CrossRef]
- Taylor, K.A.; Glaeser, R.M. Retrospective on the early development of cryoelectron microscopy of macromolecules and a prospective on opportunities for the future. J. Struct. Biol. 2008, 163, 214–223. [Google Scholar] [CrossRef]
- Cheng, Y. Single-Particle Cryo-EM at Crystallographic Resolution. Cell 2015, 161, 450–457. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Liu, X. Direct Evidence of a Surface Quenching Effect on Size-Dependent Luminescence of Upconversion Nanoparticles. Angew. Chemie Int. Ed. 2010, 49, 7456–7460. [Google Scholar] [CrossRef]
- Xue, X.; Uechi, S.; Tiwari, R.N.; Duan, Z.; Liao, M.; Yoshimura, M.; Suzuki, T.; Ohishi, Y. Size-dependent upconversion luminescence and quenching mechanism of LiYF4: Er3+/Yb3+ nanocrystals with oleate ligand adsorbed. Opt. Mater. Express 2013, 3, 989–999. [Google Scholar] [CrossRef]
- Wang, J.; Deng, R.; MacDonald, M.A.; Chen, B.; Yuan, J.; Wang, F.; Chi, D.; Andy Hor, T.S.; Zhang, P.; Liu, G.; et al. Enhancing multiphoton upconversion through energy clustering at sublattice level. Nat. Mater. 2013, 13, 157. [Google Scholar] [CrossRef]
- Xu, D.; Li, A.; Yao, L.; Lin, H.; Yang, S.; Zhang, Y. Lanthanide-Doped KLu2F7 Nanoparticles with High Upconversion Luminescence Performance: A Comparative Study by Judd-Ofelt Analysis and Energy Transfer Mechanistic Investigation. Sci. Rep. 2017, 7, 43189. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. JoVE 2016, e53193. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, X.; Fang, S.; Gao, J.; Yang, G.; Zhao, H. Systemic toxicity and toxicokinetics of a high dose of polyethylene glycol 400 in dogs following intravenous injection. Drug Chem. Toxicol. 2011, 34, 208–212. [Google Scholar] [CrossRef]
- Rostami, I.; Zhao, Z.J.; Wang, Z.H.; Zhang, W.K.; Zhong, Y.; Zeng, Q.; Jia, X.R.; Hu, Z.Y. Peptide-conjugated PEGylated PAMAM as a highly affinitive nanocarrier towards HER2-overexpressing cancer cells. RSC Adv. 2016, 6, 107337–107343. [Google Scholar] [CrossRef]
- Kavosi, B.; Navaee, A.; Salimi, A. Amplified fluorescence resonance energy transfer sensing of prostate specific antigen based on aggregation of CdTe QDs/antibody and aptamer decorated of AuNPs-PAMAM dendrimer. J. Lumin. 2018, 204, 368–374. [Google Scholar] [CrossRef]
- Oh, E.; Hong, M.-Y.; Lee, D.; Nam, S.-H.; Yoon, H.C.; Kim, H.-S. Inhibition Assay of Biomolecules based on Fluorescence Resonance Energy Transfer (FRET) between Quantum Dots and Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 3270–3271. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Energy transfer in lanthanide upconversion studies for extended optical applications. Chem. Soc. Rev. 2015, 44, 1608–1634. [Google Scholar] [CrossRef] [PubMed]
- Rabouw, F.T.; Prins, P.T.; Villanueva-Delgado, P.; Castelijns, M.; Geitenbeek, R.G.; Meijerink, A. Quenching Pathways in NaYF4:Er3+, Yb3+ Upconversion Nanocrystals. ACS Nano 2018, 12, 4812–4823. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-H.; Tang, D.-W.; Hsieh, H.-Y.; Wu, W.-S.; Lin, B.-X.; Chuang, E.-Y.; Sung, H.-W.; Mi, F.-L. Nanoparticle-induced tight-junction opening for the transport of an anti-angiogenic sulfated polysaccharide across Caco-2 cell monolayers. Acta Biomater. 2013, 9, 7449–7459. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41. [Google Scholar] [CrossRef]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. Update on in vitro cytotoxicity assays for drug development. Expert Opin. Drug Discov. 2008, 3, 655–669. [Google Scholar] [CrossRef]
- Morris, M.C.; Deshayes, S.; Heitz, F.; Divita, G. Cell-penetrating peptides: From molecular mechanisms to therapeutics. Biol. Cell 2008, 100, 201–217. [Google Scholar] [CrossRef]
- Fu, X.; Shi, Y.; Qi, T.; Qiu, S.; Huang, Y.; Zhao, X.; Sun, Q.; Lin, G. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal. Transduct. Target. Ther. 2020, 5, 262. [Google Scholar] [CrossRef]
- Nag, O.K.; Delehanty, J.B. Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery. Pharmaceutics 2019, 11, 543. [Google Scholar] [CrossRef]
- Shahmoradian, S.H.; Galiano, M.R.; Wu, C.; Chen, S.; Rasband, M.N.; Mobley, W.C.; Chiu, W. Preparation of Primary Neurons for Visualizing Neurites in a Frozen-hydrated State Using Cryo-Electron Tomography. JoVE 2014, e50783. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostami, I. Empowering the Emission of Upconversion Nanoparticles for Precise Subcellular Imaging. Nanomaterials 2021, 11, 1541. https://doi.org/10.3390/nano11061541
Rostami I. Empowering the Emission of Upconversion Nanoparticles for Precise Subcellular Imaging. Nanomaterials. 2021; 11(6):1541. https://doi.org/10.3390/nano11061541
Chicago/Turabian StyleRostami, Iman. 2021. "Empowering the Emission of Upconversion Nanoparticles for Precise Subcellular Imaging" Nanomaterials 11, no. 6: 1541. https://doi.org/10.3390/nano11061541
APA StyleRostami, I. (2021). Empowering the Emission of Upconversion Nanoparticles for Precise Subcellular Imaging. Nanomaterials, 11(6), 1541. https://doi.org/10.3390/nano11061541





