Nanotoxicity of 2D Molybdenum Disulfide, MoS2, Nanosheets on Beneficial Soil Bacteria, Bacillus cereus and Pseudomonas aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MoS2 Nanosheets
2.2. Bacterial Cultures and Plate Counting
2.3. Characterization of Soil Bacteria
2.4. Characterization of MoS2
2.5. Zeta Potential Measurements
2.6. Analysis of MoS2 Toxicity and Dose–Response Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Soil Bacteria
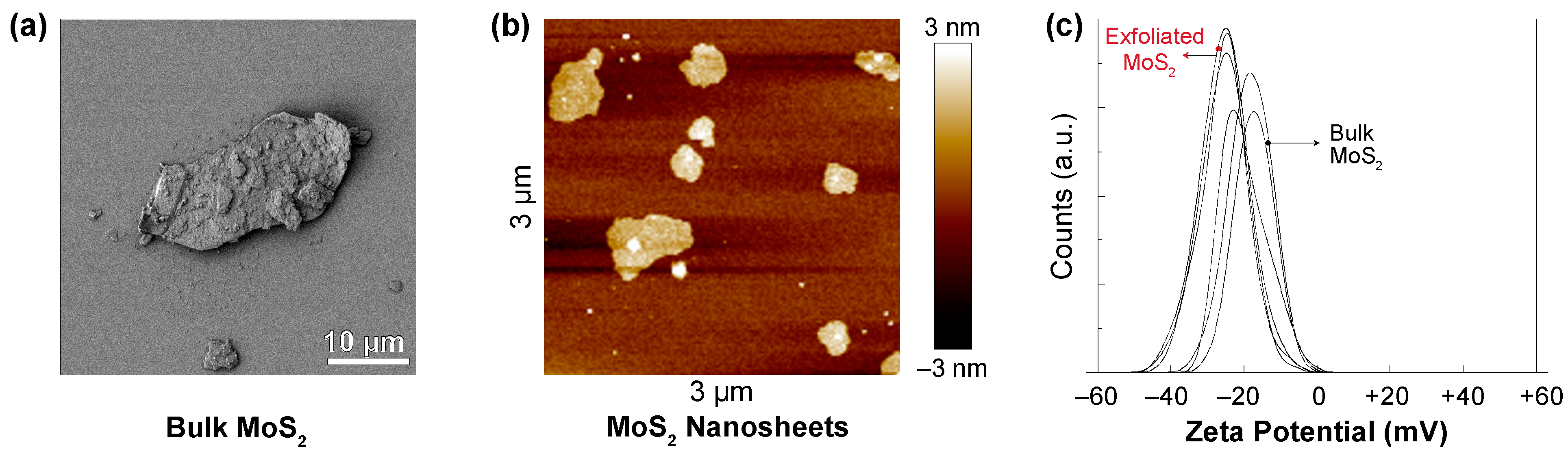
3.2. Characterization of MoS2
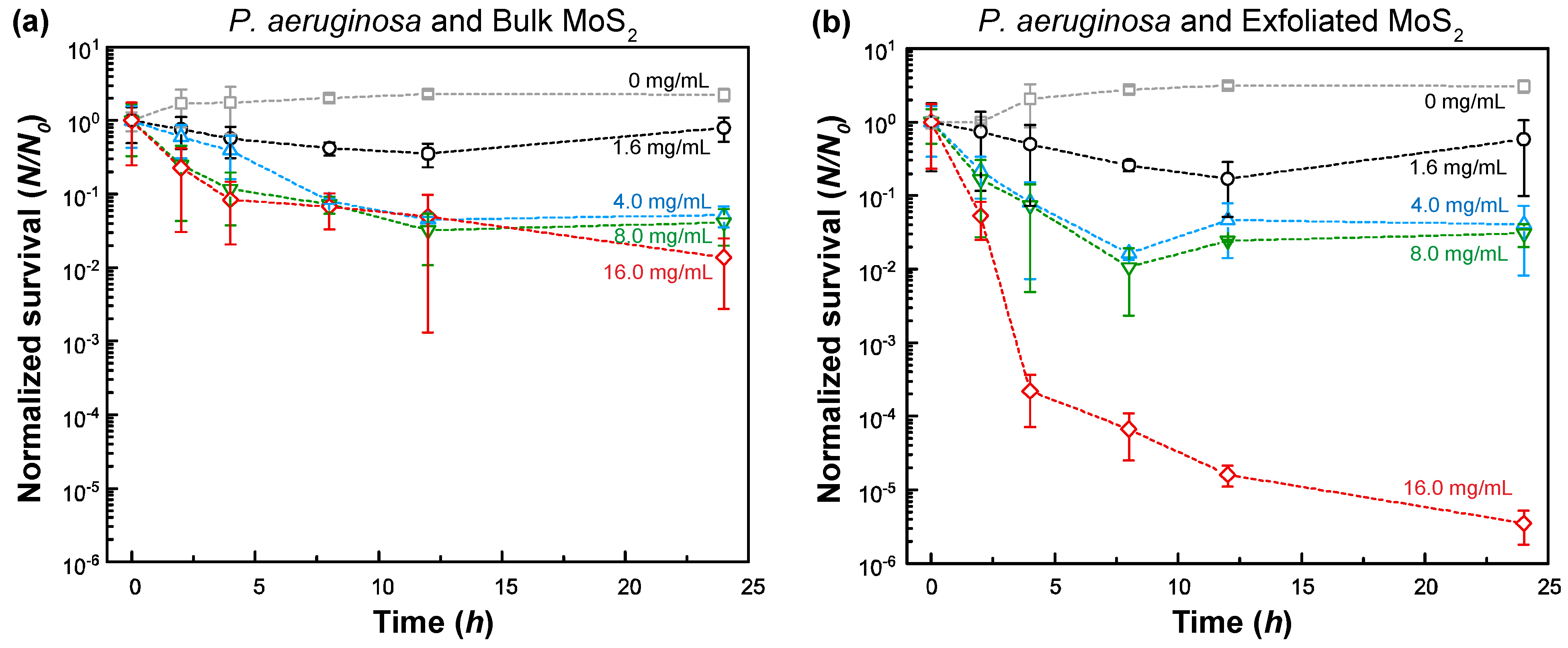
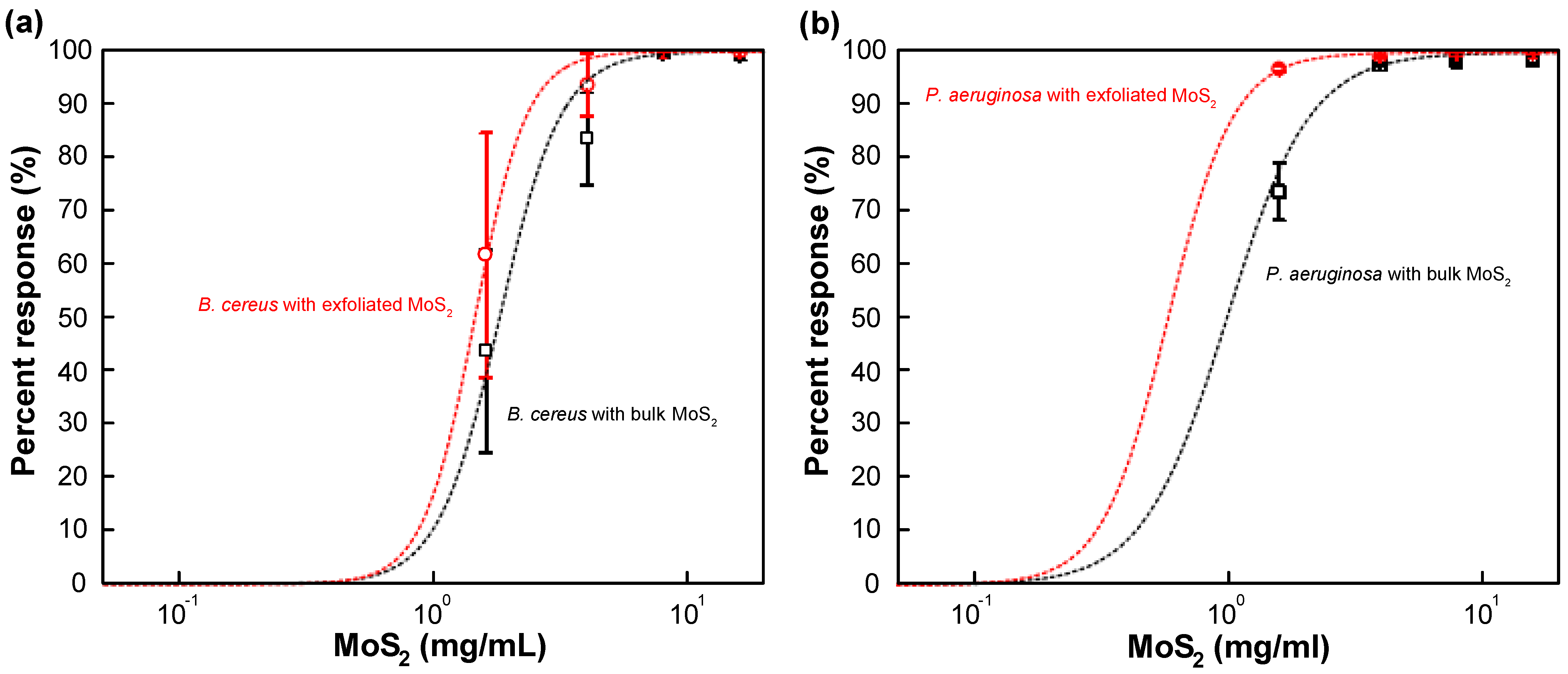
3.3. Survival of B. cereus and P. aeruginosa against MoS2 Exposure
3.4. Mechanism of Interaction between MoS2 and Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arvidsson, R.; Baun, A.; Furberg, A.; Hansen, S.F.; Molander, S. Proxy measures for simplified environmental assessment of manufactured nanomaterials. Environ. Sci. Technol. 2018, 52, 13670–13680. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Servin, A.D.; De La Torre-Roche, R.; Mukherjee, A.; Majumdar, S.; Hawthorne, J.; Marmiroli, M.; Maestri, E.; Marra, R.E.; Isch, S.M. Molecular response of crop plants to engineered nanomaterials. Environ. Sci. Technol. 2016, 50, 7198–7207. [Google Scholar] [CrossRef]
- Oh, J.K.; Liu, S.; Jones, M.; Yegin, Y.; Hao, L.; Tolen, T.N.; Nagabandi, N.; Scholar, E.A.; Castillo, A.; Taylor, T.M.; et al. Modification of aluminum surfaces with superhydrophobic nanotextures for enhanced food safety and hygiene. Food Control 2019, 96, 463–469. [Google Scholar] [CrossRef]
- Sakimoto, K.K.; Kornienko, N.; Cestellos-Blanco, S.; Lim, J.; Liu, C.; Yang, P. Physical biology of the materials–microorganism interface. J. Am. Chem. Soc. 2018, 140, 1978–1985. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Xin, Q.; Rotchell, J.M.; Cheng, J.; Yi, J.; Zhang, Q. Silver nanoparticles affect the neural development of zebrafish embryos. J. Appl. Toxicol. 2015, 35, 1481–1492. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, Y.; Duan, Y.; Yu, X.; Shi, H.; Nakkala, J.R.; Zuo, X.; Hong, L.; Mao, Z.; Gao, C. Surface-Anchored Graphene Oxide Nanosheets on Cell-Scale Micropatterned Poly(d, l-lactide- co-caprolactone) Conduits Promote Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- He, X.; Aker, W.G.; Fu, P.P.; Hwang, H.M. Toxicity of engineered metal oxide nanomaterials mediated by nano-bio-eco-interactions: A review and perspective. Environ. Sci. Nano 2015, 2, 564–582. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bae, M.; Hao, L.; Oh, J.K.; White, A.R.; Min, Y.; Cisneros-Zevallos, L.; Akbulut, M. Bacterial antifouling characteristics of helicene—Graphene films. Nanomaterials 2021, 11, 89. [Google Scholar] [CrossRef]
- Agarwal, V.; Chatterjee, K. Recent advances in the field of transition metal dichalcogenides for biomedical applications. Nanoscale 2018, 10, 16365–16397. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, 1–30. [Google Scholar] [CrossRef]
- Chen, I.C.; Zhang, M.; Min, Y.; Akbulut, M. Deposition Kinetics of Graphene Oxide on Charged Self-Assembled Monolayers. J. Phys. Chem. C 2016, 120, 8333–8342. [Google Scholar] [CrossRef]
- Yegin, C.; Nagabandi, N.; Feng, X.; King, C.; Catalano, M.; Oh, J.K.; Talib, A.J.; Scholar, E.A.; Verkhoturov, S.V.; Cagin, T.; et al. Metal-Organic-Inorganic Nanocomposite Thermal Interface Materials with Ultralow Thermal Resistances. ACS Appl. Mater. Interfaces 2017, 9. [Google Scholar] [CrossRef]
- Wu, D.; Bai, Y.; Wang, W.; Xia, H.; Tan, F.; Zhang, S.; Su, B.; Wang, X.; Qiao, X.; Wong, P.K. Highly pure MgO2 nanoparticles as robust solid oxidant for enhanced Fenton-like degradation of organic contaminants. J. Hazard. Mater. 2019, 374, 319–328. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Singh, G. MoS2/graphene composite paper for sodium-ion battery electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.F.; Asti, M.; Gili, F.; Deorsola, F.A.; Bensaid, S.; Fino, D.; Kraft, G.; Garcia, I.; Dassenoy, F. Engine bench and road testing of an engine oil containing MoS2 particles as nano-additive for friction reduction. Tribol. Int. 2017, 105, 317–325. [Google Scholar] [CrossRef]
- Li, W.; Geng, X.; Guo, Y.; Rong, J.; Gong, Y.; Wu, L.; Zhang, X.; Li, P.; Xu, J.; Cheng, G. Reduced graphene oxide electrically contacted graphene sensor for highly sensitive nitric oxide detection. ACS Nano 2011, 5, 6955–6961. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Mahurin, S.M.; Dai, S.; Jiang, D. Ion-gated gas separation through porous graphene. Nano Lett. 2017, 17, 1802–1807. [Google Scholar] [CrossRef]
- Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanostructures for water purification: Graphene and beyond. Nanoscale 2016, 8, 15115–15131. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, M.; Zhao, Q.; Gao, W.; Xie, T.; Bai, H. Superstretchable nacre-mimetic graphene/poly (vinyl alcohol) composite film based on interfacial architectural engineering. ACS Nano 2017, 11, 4777–4784. [Google Scholar] [CrossRef]
- Hao, L.; Chen, I.-C.; Oh, J.K.; Nagabandi, N.; Bassan, F.; Liu, S.; Scholar, E.; Zhang, L.; Akbulut, M.; Jiang, B. Nanocomposite Foam Involving Boron Nitride Nanoplatelets and Polycaprolactone: Porous Structures with Multiple Length Scales for Oil Spill Cleanup. Ind. Eng. Chem. Res. 2017, 56. [Google Scholar] [CrossRef]
- Su, Y.; Tong, X.; Huang, C.; Chen, J.; Liu, S.; Gao, S.; Mao, L.; Xing, B. Green algae as carriers enhance the bioavailability of 14C-labeled few-layer graphene to freshwater snails. Environ. Sci. Technol. 2018, 52, 1591–1601. [Google Scholar] [CrossRef]
- Kang, W.; Li, X.; Sun, A.; Yu, F.; Hu, X. Study of the persistence of the phytotoxicity induced by graphene oxide quantum dots and of the specific molecular mechanisms by integrating omics and regular analyses. Environ. Sci. Technol. 2019, 53, 3791–3801. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, Q.; Yang, D.; Cui, J. Molybdenum sulfide induce growth enhancement effect of rice (Oryza sativa L.) through regulating the synthesis of chlorophyll and the expression of aquaporin gene. J. Agric. Food Chem. 2018, 66, 4013–4021. [Google Scholar] [CrossRef] [PubMed]
- Ataca, C.; Ciraci, S. Functionalization of single-layer Mos2 honeycomb structures. J. Phys. Chem. C 2011, 115, 13303–13311. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Yin, Z.; Li, H.; Liu, J.; Cao, X.; Zhang, Q.; Zhang, H. Layer thinning and etching of mechanically exfoliated MoS2 nanosheets by thermal annealing in air. Small 2013, 9, 3314–3319. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Smith, G.C.; Sawtell, D.A.G.; Kelly, P.J.; Banks, C.E. 2D nanosheet molybdenum disulphide (MoS2) modified electrodes explored towards the hydrogen evolution reaction. Nanoscale 2015, 7, 18152–18168. [Google Scholar] [CrossRef]
- Paul, J.F.; Payen, E. Vacancy formation on MoS2 hydrodesulfurization catalyst: DFT study of the mechanism. J. Phys. Chem. B 2003, 107, 4057–4064. [Google Scholar] [CrossRef]
- Garadkar, K.M.; Patil, A.A.; Hankare, P.P.; Chate, P.A.; Sathe, D.J.; Delekar, S.D. MoS2: Preparation and their characterization. J. Alloy. Compd. 2009, 487, 786–789. [Google Scholar] [CrossRef]
- Conley, H.J.; Wang, B.; Ziegler, J.I.; Haglund, R.F.; Pantelides, S.T.; Bolotin, K.I. Bandgap engineering of strained monolayer and bilayer MoS2. Nano Lett. 2013, 13, 3626–3630. [Google Scholar] [CrossRef]
- Liu, C.; Kong, D.; Hsu, P.C.; Yuan, H.; Lee, H.W.; Liu, Y.; Wang, H.; Wang, S.; Yan, K.; Lin, D.; et al. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat. Nanotechnol. 2016, 11, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Rowley-Neale, S.; Shalamanova, L.; Lynch, S.; Wilson-Nieuwenhuis, J.T.; El Mohtadi, M.; Banks, C.E.; Whitehead, K.A. Molybdenum Disulfide Surfaces to Reduce Staphylococcus aureus and Pseudomonas aeruginosa Biofilm Formation. ACS Appl. Mater. Interfaces 2020, 12, 21057–21069. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Levenfors, J.J.; Hedman, R.; Thaning, C.; Gerhardson, B.; Welch, C.J. Broad-spectrum antifungal metabolites produced by the soil bacterium Serratia plymuthica A 153. Soil Biol. Biochem. 2004, 36, 677–685. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Loukidou, M.X.; Matis, K.A. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem. 2004, 39, 909–916. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Hwang, G.; Ahn, I.-S.; Mhin, B.J.; Kim, J.-Y. Adhesion of nano-sized particles to the surface of bacteria: Mechanistic study with the extended DLVO theory. Colloids Surf. B Biointerfaces 2012, 97, 138–144. [Google Scholar] [CrossRef]
- Rousk, J.; Ackermann, K.; Curling, S.F.; Jones, D.L. Comparative toxicity of nanoparticulate CuO and ZnO to soil bacterial communities. PLoS ONE 2012, 7, e34197. [Google Scholar] [CrossRef]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Andrews, J.C.; Cotte, M.; Rico, C.; Peralta-Videa, J.R.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max). ACS Nano 2013, 7, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, M.D.; Treseder, K.K.; Atsatt, P.R. The brighter side of soils: Quantum dots track organic nitrogen through fungi and plants. Ecology 2009, 90, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Moll, J.; Gogos, A.; Bucheli, T.D.; Widmer, F.; Heijden, M.G.A. Effect of nanoparticles on red clover and its symbiotic microorganisms. J. Nanobiotechnol. 2016, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Huang, F.; Wang, Z.-H.; Cai, Y.-X.; Chen, S.-H.; Tian, J.-H.; Cai, K.-Z. Heavy metal bioaccumulation and cation release by growing Bacillus cereus RC-1 under culture conditions. Ecotoxicol. Environ. Saf. 2018, 157, 216–226. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. Genomic analysis of Bacillus cereus NWUAB01 and its heavy metal removal from polluted soil. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Rowe, J.J. Oxygen regulation of nitrate uptake in denitrifying Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1987, 53, 745–750. [Google Scholar] [CrossRef]
- Ueno, A.; Hasanuzzaman, M.; Yumoto, I.; Okuyama, H. Verification of degradation of n-alkanes in diesel oil by Pseudomonas aeruginosa strain WatG in soil microcosms. Curr. Microbiol. 2006, 52, 182–185. [Google Scholar] [CrossRef][Green Version]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Předota, M.; Machesky, M.L.; Wesolowski, D.J. Molecular origins of the zeta potential. Langmuir 2016, 32, 10189–10198. [Google Scholar] [CrossRef]
- Decho, A.W.; Gutierrez, T. Microbial extracellular polymeric substances (EPSs) in ocean systems. Front. Microbiol. 2017, 8, 1–28. [Google Scholar] [CrossRef]
- Wingender, J. Microbial Extracellular Polymeric Substances; Springer: Berlin/Heidelberg, Germany, 1999; Volume 66, ISBN 9783642642777. [Google Scholar]
- Boks, N.P.; Norde, W.; van der Mei, H.C.; Busscher, H.J. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 2008, 154, 3122–3133. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, M.; Dell‘Aversana, C.; Benedetti, R.; Giardina, P.; Rossi, M.; Valadan, M.; Vergara, A.; Cutarelli, A.; Montone, A.M.I.; et al. Biological interactions of biocompatible and water-dispersed MoS2 nanosheets with bacteria and human cells. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Swoboda, J.G.; Campbell, J.; Meredith, T.C.; Walker, S. Wall teichoic acid function, biosynthesis, and inhibition. ChemBioChem 2010, 11, 35–45. [Google Scholar] [CrossRef]
- Xia, G.; Kohler, T.; Peschel, A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int. J. Med. Microbiol. 2010, 300, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Da Re, S.; Henry, M.; Fontaine, T.; Balestrino, D.; Latour-Lambert, P.; Ghigo, J.M. Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide. Proc. Natl. Acad. Sci. USA 2006, 103, 12558–12563. [Google Scholar] [CrossRef] [PubMed]
- Foschiatti, M.; Cescutti, P.; Tossi, A.; Rizzo, R. Inhibition of cathelicidin activity by bacterial exopolysaccharides. Mol. Microbiol. 2009, 72, 1137–1146. [Google Scholar] [CrossRef]
- Zhu, C.; Mu, X.; Van Aken, P.A.; Maier, J.; Yu, Y. Fast Li storage in MoS2-graphene-carbon nanotube nanocomposites: Advantageous functional integration of 0D, 1D, and 2D nanostructures. Adv. Energy Mater. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Weroński, P. Application of the DLVO theory for particle deposition problems. Adv. Colloid Interface Sci. 1999, 83, 137–226. [Google Scholar] [CrossRef]
- Perni, S.; Preedy, E.C.; Prokopovich, P. Success and failure of colloidal approaches in adhesion of microorganisms to surfaces. Adv. Colloid Interface Sci. 2014, 206, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Putz, K.W.; Compton, O.C.; Segar, C.; An, Z.; Nguyen, S.T.; Brinson, L.C. Evolution of order during vacuum-assisted self-assembly of graphene oxide paper and associated polymer nanocomposites. ACS Nano 2011, 5, 6601–6609. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, W.; Fu, G.; Mou, X.; Hou, R.; Zhu, Y.; Cai, K. In situ self-assembly of graphene oxide/polydopamine/Sr2+ nanosheets on titanium surfaces for enhanced osteogenic differentiation of mesenchymal stem cells. Carbon N. Y. 2019, 142, 567–579. [Google Scholar] [CrossRef]
- Gigantelli, J.W.; Torres Gomez, J.; Osato, M.S. In vitro susceptibilities of ocular Bacillus cereus isolates to clindamycin, gentamicin, and vancomycin alone or in combinaton. Antimicrob. Agents Chemother. 1991, 35, 201–202. [Google Scholar] [CrossRef][Green Version]
- Field, T.R.; White, A.; Elborn, J.S.; Tunney, M.M. Effect of oxygen limitation on the in vitro antimicrobial susceptibility of clinical isolates of Pseudomonas aeruginosa grown planktonically and as biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, F.; Yang, B.; Song, S. Oxidation of Molybdenum Disulfide Sheet in Water under in Situ Atomic Force Microscopy Observation. J. Phys. Chem. C 2017, 121, 9938–9943. [Google Scholar] [CrossRef]
- Hakobyan, K.Y.; Sohn, H.Y.; Hakobyan, A.K.; Bryukvin, V.A.; Leontiev, V.G.; Tsibin, O.I. The oxidation of molybdenum sulphide concentrate with water vapour Part 1—Thermodynamic aspects. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2013, 116, 152–154. [Google Scholar] [CrossRef]
- Pandit, S.; Karunakaran, S.; Boda, S.K.; Basu, B.; De, M. High antibacterial activity of functionalized chemically exfoliated MoS2. ACS Appl. Mater. Interfaces 2016, 8, 31567–31573. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef]
- Desriac, N.; Broussolle, V.; Postollec, F.; Mathot, A.G.; Sohier, D.; Coroller, L.; Leguerinel, I. Bacillus cereus cell response upon exposure to acid environment: Toward the identification of potential biomarkers. Front. Microbiol. 2013, 4, 1–13. [Google Scholar] [CrossRef]
- Duport, C.; Jobin, M.; Schmitt, P. Adaptation in Bacillus cereus: From stress to disease. Front. Microbiol. 2016, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mols, M.; Abee, T. Bacillus cereus responses to acid stress. Environ. Microbiol. 2011, 13, 2835–2843. [Google Scholar] [CrossRef]
- Goepfert, J.M.; Spira, W.M.; Kim, H.U. Bacillus cereus: Food poisoning organism. A review. J. Milk Food Technol. 1972, 35, 213–227. [Google Scholar] [CrossRef]
- Bushell, F.M.L.; Tonner, P.D.; Jabbari, S.; Schmid, A.K.; Lund, P.A. Synergistic impacts of organic acids and pH on growth of Pseudomonas aeruginosa: A comparison of parametric and Bayesian non-parametric methods to model growth. Front. Microbiol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Titley, S.R. Some behavioral aspects of molybdenum in the supergene environment. Trans. AIME 1963, 226, 199–204. [Google Scholar]
- Wagman, D.D. Selected Values of Chemical Thermodynamic Properties; Institute for Materials Research, National Bureau of Standards: Gaithersburg, MA, USA, 1965; Volume 270. [Google Scholar]
- Liu, X.; Cheng, J.; Sprik, M.; Lu, X. Solution structures and acidity constants of molybdic acid. J. Phys. Chem. Lett. 2013, 4, 2926–2930. [Google Scholar] [CrossRef]
- Bae, M. Molybdenum Disulfide 2-D Nanosheets Toxicology to the Environmental Surfaces: Soil Bacteria Survivability Analysis. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2017. [Google Scholar]



| Bacteria | B. cereus | P. aeruginosa |
|---|---|---|
| Type | Gram-positive | Gram-negative |
| Dimensions (μm) | W: 0.79 ± 0.10 L: 2.86 ± 0.83 | W: 0.63 ± 0.18 L: 2.31 ± 0.41 |
| Zeta potential (mV) | −33.3 ± 1.1 | −44.3 ± 1.2 |
| MoS2 | Bulk Form | Exfoliated Form |
|---|---|---|
| Planar diameter (μm) | 12.0 ± 7.6 | 0.9 ± 0.8 |
| Thickness (nm) | 520 ± 364 | 3.1 ± 0.7 |
| Zeta potential (mV) | −18.4 ± 1.5 | −25.4 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.; Oh, J.K.; Liu, S.; Nagabandi, N.; Yegin, Y.; DeFlorio, W.; Cisneros-Zevallos, L.; Scholar, E.M.A. Nanotoxicity of 2D Molybdenum Disulfide, MoS2, Nanosheets on Beneficial Soil Bacteria, Bacillus cereus and Pseudomonas aeruginosa. Nanomaterials 2021, 11, 1453. https://doi.org/10.3390/nano11061453
Bae M, Oh JK, Liu S, Nagabandi N, Yegin Y, DeFlorio W, Cisneros-Zevallos L, Scholar EMA. Nanotoxicity of 2D Molybdenum Disulfide, MoS2, Nanosheets on Beneficial Soil Bacteria, Bacillus cereus and Pseudomonas aeruginosa. Nanomaterials. 2021; 11(6):1453. https://doi.org/10.3390/nano11061453
Chicago/Turabian StyleBae, Michael, Jun Kyun Oh, Shuhao Liu, Nirup Nagabandi, Yagmur Yegin, William DeFlorio, Luis Cisneros-Zevallos, and Ethan M. A. Scholar. 2021. "Nanotoxicity of 2D Molybdenum Disulfide, MoS2, Nanosheets on Beneficial Soil Bacteria, Bacillus cereus and Pseudomonas aeruginosa" Nanomaterials 11, no. 6: 1453. https://doi.org/10.3390/nano11061453
APA StyleBae, M., Oh, J. K., Liu, S., Nagabandi, N., Yegin, Y., DeFlorio, W., Cisneros-Zevallos, L., & Scholar, E. M. A. (2021). Nanotoxicity of 2D Molybdenum Disulfide, MoS2, Nanosheets on Beneficial Soil Bacteria, Bacillus cereus and Pseudomonas aeruginosa. Nanomaterials, 11(6), 1453. https://doi.org/10.3390/nano11061453









