Low Surface Roughness Graphene Oxide Film Reduced with Aluminum Film Deposited by Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GO Suspension
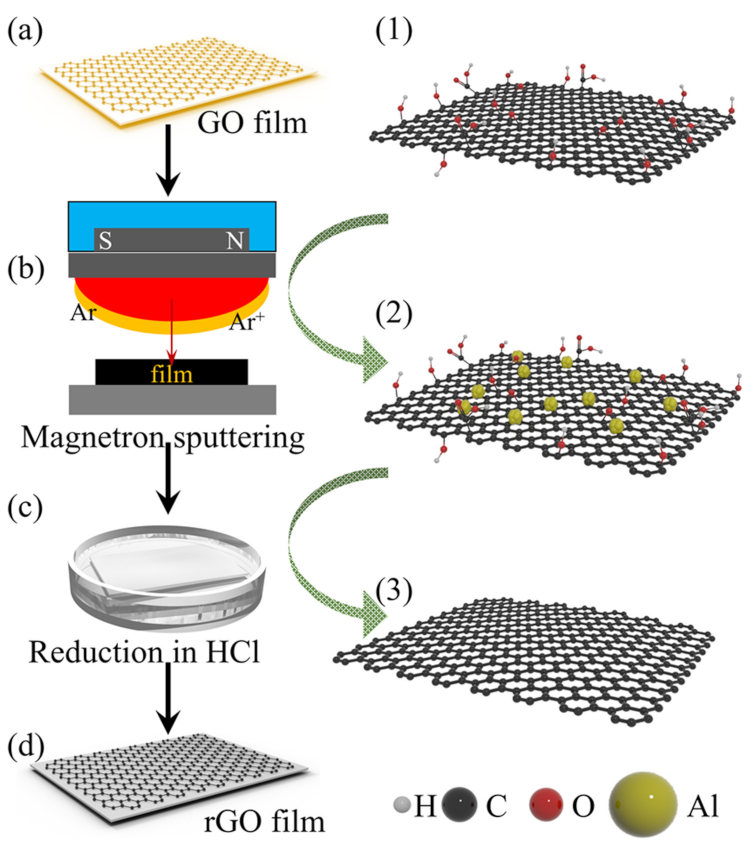
2.2. Preparation of Al-rGO Film
2.3. Characterization
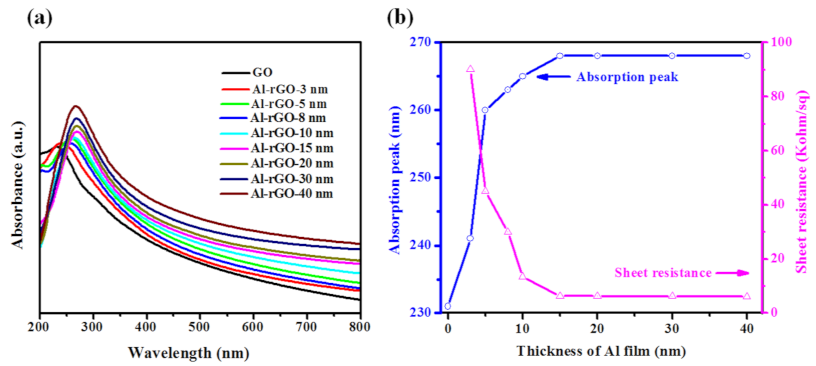
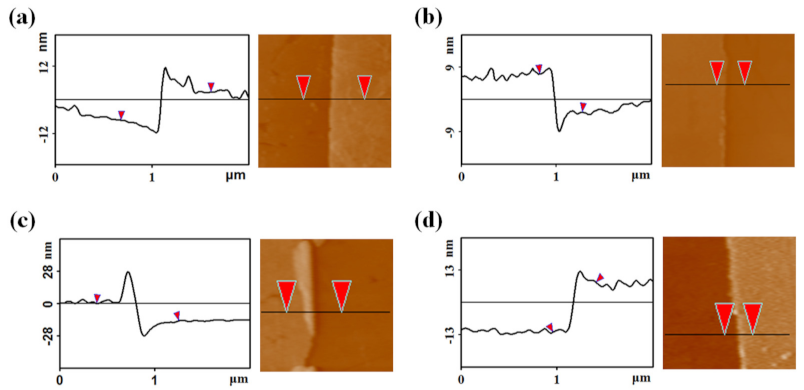
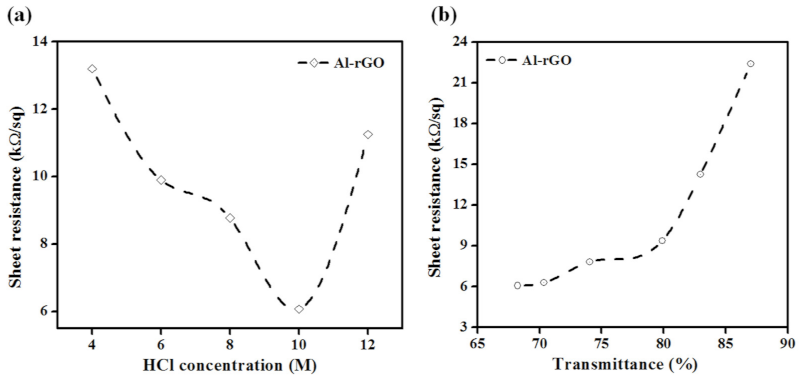
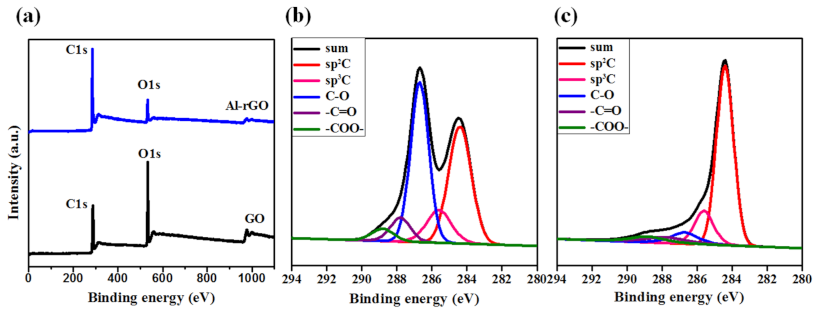
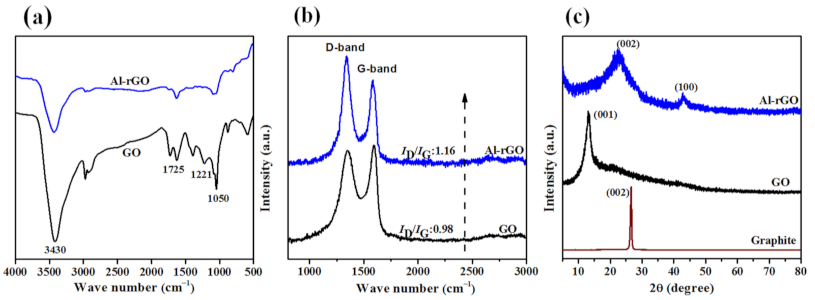
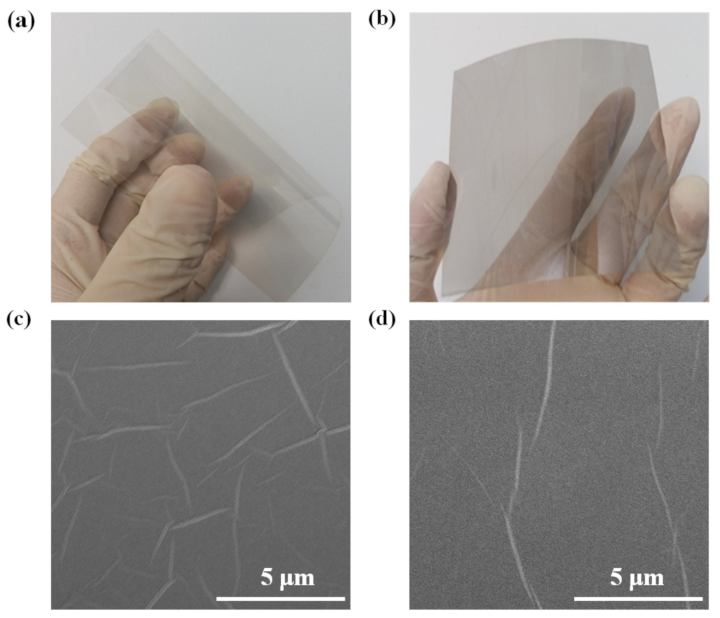
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Wang, T.; Jing, L.-C.; Zhua, Q.; Ethiraj, A.S.; Tian, Y.; Zhao, H.; Yuan, X.-T.; Wen, J.-G.; Li, L.-K.; Geng, H.-Z. Fabrication of architectural structured polydopamine-functionalized reduced graphene oxide/carbon nanotube/PEDOT: PSS nanocomposites as flexible transparent electrodes for OLEDs. Appl. Surf. Sci. 2020, 500, 143997. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jing, L.-C.; Zhu, Q.; Ethiraj, A.S.; Fan, X.; Liu, H.; Tian, Y.; Zhu, Z.; Meng, Z.; Geng, H.-Z. Tannic acid modified graphene/CNT three-dimensional conductive network for preparing high-performance transparent flexible heaters. J. Colloid. Interface Sci. 2020, 577, 300–310. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.-Z.; Jing, L.-C.; Zhu, Q.; Ethiraj, A.S.; Geng, W.; Tian, Y.; Zhu, Z.; Meng, Z.; Geng, H.-Z. Novel biodegradable and ultra-flexible transparent conductive film for green light OLED devices. Carbon 2021, 172, 379–389. [Google Scholar] [CrossRef]
- Wang, J.; Liang, M.; Fang, Y.; Qiu, T.; Zhang, J.; Zhi, L. Rod-coating: Towards large-area fabrication of uniform reduced graphene oxide films for flexible touch screens. Adv. Mater. 2021, 24, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Konios, D.; Petridis, C.; Kakavelakis, G.; Sygletou, M.; Savva, K.; Stratakis, E.; Kymakis, E. Reduced graphene oxide micromesh electrodes for large area, flexible, organic photovoltaic devices. Adv. Funct. Mater. 2015, 25, 2213–2221. [Google Scholar] [CrossRef]
- Indrani, B.; Tsegie, F.; Zlatka, S.; Paul, G.H.; Chen, J.; Ashwani, K.S.; Asim, K.R. Graphene films printable on flexible substrates for sensor applications. 2D Mater. 2017, 4, 015036. [Google Scholar]
- Baleeswaraiah, M.; Narayanan, T.N.; Kaushik, B.; Pulickel, M.A.; Saikat, T. Temperature dependent electrical transport of disordered reduced graphene oxide. 2D Mater. 2014, 1, 011008. [Google Scholar]
- Ning, J.; Wang, J.; Li, X.; Qiu, T.; Luo, B.; Hao, L.; Liang, M.; Wang, B.; Zhi, L. A fast room-temperature strategy for direct reduction of graphene oxide films towards flexible transparent conductive films. J. Mater. Chem. A 2014, 2, 10969–10973. [Google Scholar] [CrossRef]
- Pham, V.H.; Cuong, T.V.; Hur, S.H.; Shin, E.W.; Kim, J.S.; Chung, J.S.; Kim, E.J. Fast and simple fabrication of a large transparent chemically-converted graphene film by spray-coating. Carbon 2010, 48, 1945–1951. [Google Scholar] [CrossRef]
- Shi, L.; Yang, J.; Huang, Z.; Li, J.; Tang, Z.; Li, Y.; Zheng, Q. Fabrication of transparent, flexible conducing graphene thin films via soft transfer printing method. Appl. Surf. Sci. 2013, 276, 437–446. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Pei, S.; Ren, W.; Tang, D.; Gao, L.; Liu, B.; Li, F.; Liu, C.; Cheng, H.-M. Field emission of single-layer graphene films prepared by electrophoretic deposition. Adv. Mater. 2009, 21, 1756–1760. [Google Scholar] [CrossRef]
- Shao, J.-J.; Lv, W.; Yang, Q.-H. Self-assembly of graphene oxide at interfaces. Adv. Mater. 2014, 26, 5586–5612. [Google Scholar] [CrossRef]
- Donarelli, M.; Prezioso, S.; Perrozzi, F.; Giancaterini, L.; Cantalini, C.; Treossi, E.; Palermo, V.; Santucci, S.; Ottaviano, L. Graphene oxide for gas detection under standard humidity conditions. 2D Mater. 2015, 2, 035018. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Tkachev, S.V.; Buslaeva, E.Y.; Naumkin, A.V.; Kotova, S.L.; Laure, I.V.; Gubin, S.P. Reduced graphene oxide. Inorg. Mater. 2012, 48, 796–802. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Mater. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Func. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-J.; Kai, W.; Yan, J.; Wei, T.; Zhi, L.-J.; Feng, J.; Ren, Y.-M.; Song, L.-P.; Wei, F. Facile synthesis of graphene nanosheets via Fe reduction of exfoliated graphite oxide. ACS Nano 2011, 5, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Zhong, M.; Yang, Y.; Yuefang, W.; Wang, M. A green and ultrafast approach to the synthesis of scalable graphene nanosheets with Zn powder for electrochemical energy storage. J. Mater. Chem. 2011, 21, 15449–15455. [Google Scholar] [CrossRef]
- Luo, Z.-J.; Geng, H.-Z.; Zhang, X.; Du, B.; Ding, E.-X.; Wang, J.; Lu, Z.; Sun, B.; Wang, J.; Liu, J. A timesaving, low-cost, high-yield method for the synthesis of ultrasmall uniform graphene oxide nanosheets and their application in surfactants. Nanotechnology 2016, 27, 055601. [Google Scholar] [CrossRef]
- Ning, Y.-J.; Zhu, Z.-R.; Cao, W.-W.; Wu, L.; Jing, L.-C.; Wang, T.; Yuan, X.-T.; Teng, L.-H.; Bin, P.-S.; Geng, H.-Z. Anti-corrosion reinforcement of waterborne polyurethane coating with polymerized graphene oxide by the one-pot method. J. Mater. Sci. 2021, 56, 337–350. [Google Scholar] [CrossRef]
- Jasim, D.A.; Lozano, N.; Kostarelos, K. Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology. 2D Mater. 2016, 3, 014006. [Google Scholar] [CrossRef]
- Pham, V.H.; Pham, H.D.; Dang, T.T.; Hur, S.H.; Kim, E.J.; Kong, B.S.; Kim, S.; Chung, J.S. Chemical reduction of an aqueous suspension of graphene oxide by nascent hydrogen. J. Mater. Chem. 2012, 22, 10530–10536. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing sugar: New functional molecules for the green synthesis of graphene nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Ouyang, J. Ultrasonication-assisted ultrafast reduction of graphene oxide by zinc powder at room temperature. Carbon 2011, 49, 5389–5397. [Google Scholar] [CrossRef]
- Dey, R.S.; Hajra, S.; Sahu, R.K.; Raj, C.R.; Panigrahi, M.K. A rapid room temperature chemical route for the synthesis of graphene: Metal-mediated reduction of graphene oxide. Chem. Commun. 2012, 48, 1787–1789. [Google Scholar] [CrossRef]
- Yuan, X.-T.; Xu, C.-X.; Geng, H.-Z.; Ji, Q.; Wang, L.; He, B.; Jiang, Y.; Kong, J.; Li, J. Multifunctional PVDF/CNT/GO mixed matrix membranes for ultrafiltrationand fouling detection. J. Hazard. Mater. 2020, 384, 120978. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via l-ascorbic acid. Chem. Commun 2010, 46, 1112–1114. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Khanra, P.; Kuila, T.; Jung, D.; Lee, J.H. Efficient reduction of graphene oxide using Tin-powder and its electrochemical performances for use as an energy storage electrode material. J. Phys. Chem. A 2013, 1, 11320–11328. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, K.; Wei, T.; Yan, J.; Song, L.; Shao, B. An environmentally friendly and efficient route for the reduction of graphene oxide by aluminum powder. Carbon 2010, 48, 1686–1689. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, S.; Chen, J.-T.; Hu, X.-P.; Du, Z.-F.; Qiu, Y.-X.; Zhao, D.-L. Reduction of graphene oxide at room temperature with vitamin C for RGO-TiO2 photoanodes in dye-sensitized solar cell. Thin Solid Film. 2015, 584, 29–36. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Ding, Y.H.; Zhang, P.; Zhuo, Q.; Ren, H.M.; Yang, Z.M.; Jiang, Y. A green approach to the synthesis of reduced graphene oxide nanosheets under UV irradiation. Nanotechnology 2011, 22, 215601. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.-M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Zhang, J.; Chen, P.; Yang, G.; Zhou, X.; Zhang, Q.; Yan, Q.; Zhang, H. Comparative studies on single-layer reduced graphene oxide films obtained by electrochemical reduction and hydrazine vapor reduction. Nanoscale Res. Lett. 2012, 7, 1–7. [Google Scholar] [CrossRef]
- Sofer, Z.; Jankovsky, O.; Simek, P.; Soferova, L.; Sedmidubsky, D.; Pumera, M. Highly hydrogenated graphene via active hydrogen reduction of graphene oxide in the aqueous phase at room temperature. Nanoscale 2014, 6, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Domingues, S.H.; Kholmanov, I.N.; Kim, T.; Kim, J.; Tan, C.; Chou, H.; Alieva, Z.A.; Piner, R.; Zarbin, A.J.G.; Ruoff, R.S. Reduction of graphene oxide films on Al foil for hybrid transparent conductive film applications. Carbon 2013, 63, 454–459. [Google Scholar] [CrossRef]
- Kymakis, E.; Savva, K.; Stylianakis, M.M.; Fotakis, C.; Stratakis, E. Flexible organic photovoltaic cells with in situ nonthermal photoreduction of spin-coated graphene oxide electrodes. Adv. Funct. Mater. 2013, 23, 2742–2749. [Google Scholar] [CrossRef]
- Zhao, C.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Sai, H.; Li, Z.; Li, F. Formation of uniform reduced graphene oxide films on modified PET substrates using drop-casting method. Particuology 2014, 17, 66–73. [Google Scholar] [CrossRef]
- Ko, Y.U.; Cho, S.-R.; Choi, K.S.; Park, Y.; Kim, S.T.; Kim, N.H.; Kim, S.Y.; Chang, S.T. Microlitre scale solution processing for controlled, rapid fabrication of chemically derived graphene thin films. J. Mater. Chem. 2012, 22, 3606–3613. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Sinha-Ray, S.; Park, J.-J.; Lee, J.-G.; Cha, Y.-H.; Bae, S.-H.; Ahn, J.-H.; Jung, Y.C.; Kim, S.M.; Yarin, A.L.; et al. Self-healing reduced graphene oxide films by supersonic kinetic spraying. Adv. Funct. Mater. 2014, 24, 4986–4995. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Shen, J.; Wei, L.; Liu, A.; Chan-Park, M.B.; Chen, Y. Ethanol-assisted graphene oxide-based thin film formation at pentane-water interface. Langmuir 2011, 27, 9174–9181. [Google Scholar] [CrossRef]
- Shin, K.-Y.; Hong, J.-Y.; Jang, J. Flexible and transparent graphene films as acoustic actuator electrodes using inkjet printing. Chem. Commun. 2011, 47, 8527–8529. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Huai, X.; Wang, J.; Jing, L.-C.; Wang, T.; Liu, J.; Geng, H.-Z. Low Surface Roughness Graphene Oxide Film Reduced with Aluminum Film Deposited by Magnetron Sputtering. Nanomaterials 2021, 11, 1428. https://doi.org/10.3390/nano11061428
Fan X, Huai X, Wang J, Jing L-C, Wang T, Liu J, Geng H-Z. Low Surface Roughness Graphene Oxide Film Reduced with Aluminum Film Deposited by Magnetron Sputtering. Nanomaterials. 2021; 11(6):1428. https://doi.org/10.3390/nano11061428
Chicago/Turabian StyleFan, Xiaowei, Xuguo Huai, Jie Wang, Li-Chao Jing, Tao Wang, Juncheng Liu, and Hong-Zhang Geng. 2021. "Low Surface Roughness Graphene Oxide Film Reduced with Aluminum Film Deposited by Magnetron Sputtering" Nanomaterials 11, no. 6: 1428. https://doi.org/10.3390/nano11061428
APA StyleFan, X., Huai, X., Wang, J., Jing, L.-C., Wang, T., Liu, J., & Geng, H.-Z. (2021). Low Surface Roughness Graphene Oxide Film Reduced with Aluminum Film Deposited by Magnetron Sputtering. Nanomaterials, 11(6), 1428. https://doi.org/10.3390/nano11061428






