Graphene Quantum Dots from Carbonized Coffee Bean Wastes for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
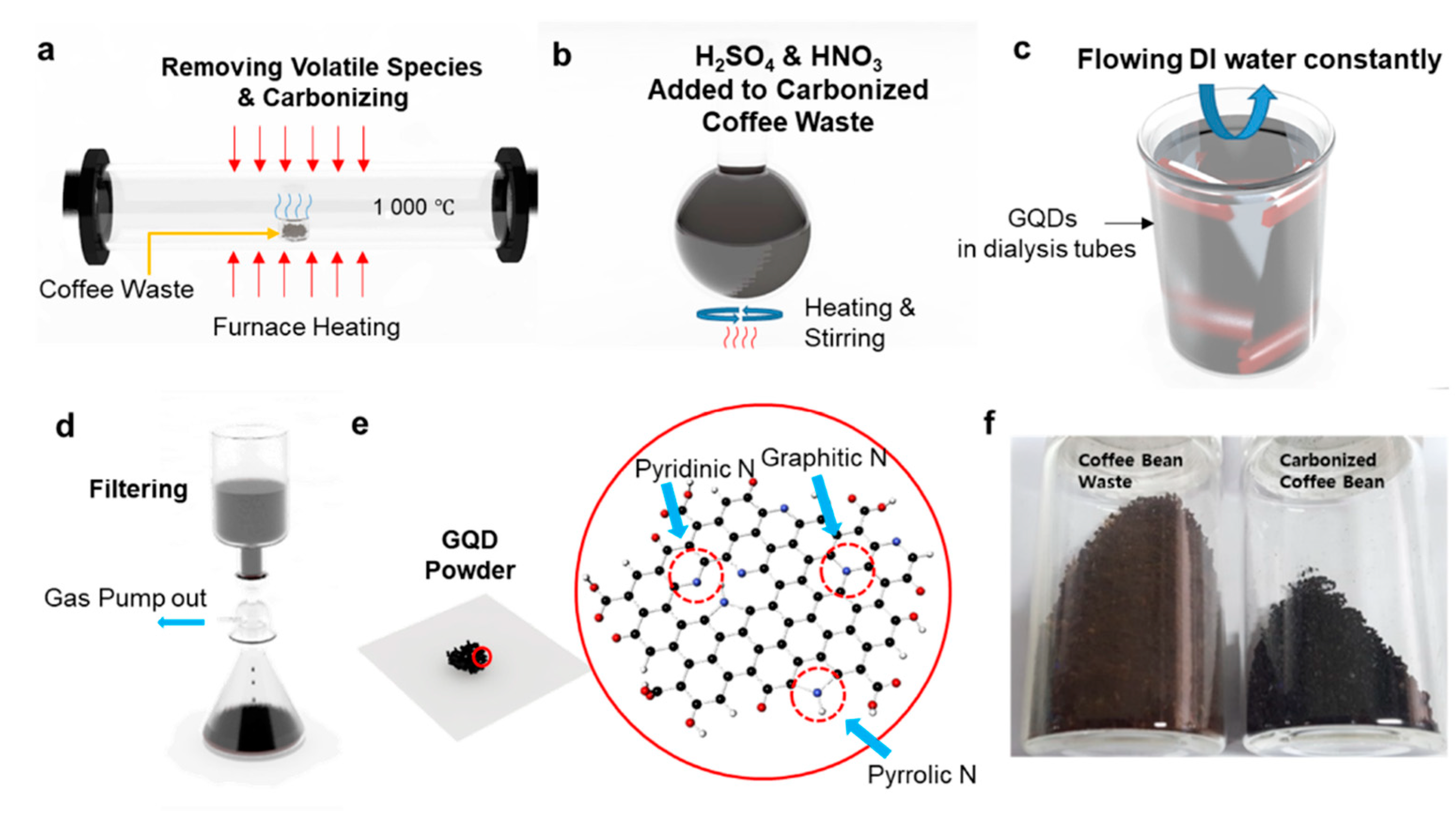
2.1. Synthesis of C-GQDs
2.2. X-ray Photoelectron Spectroscopy (XPS)
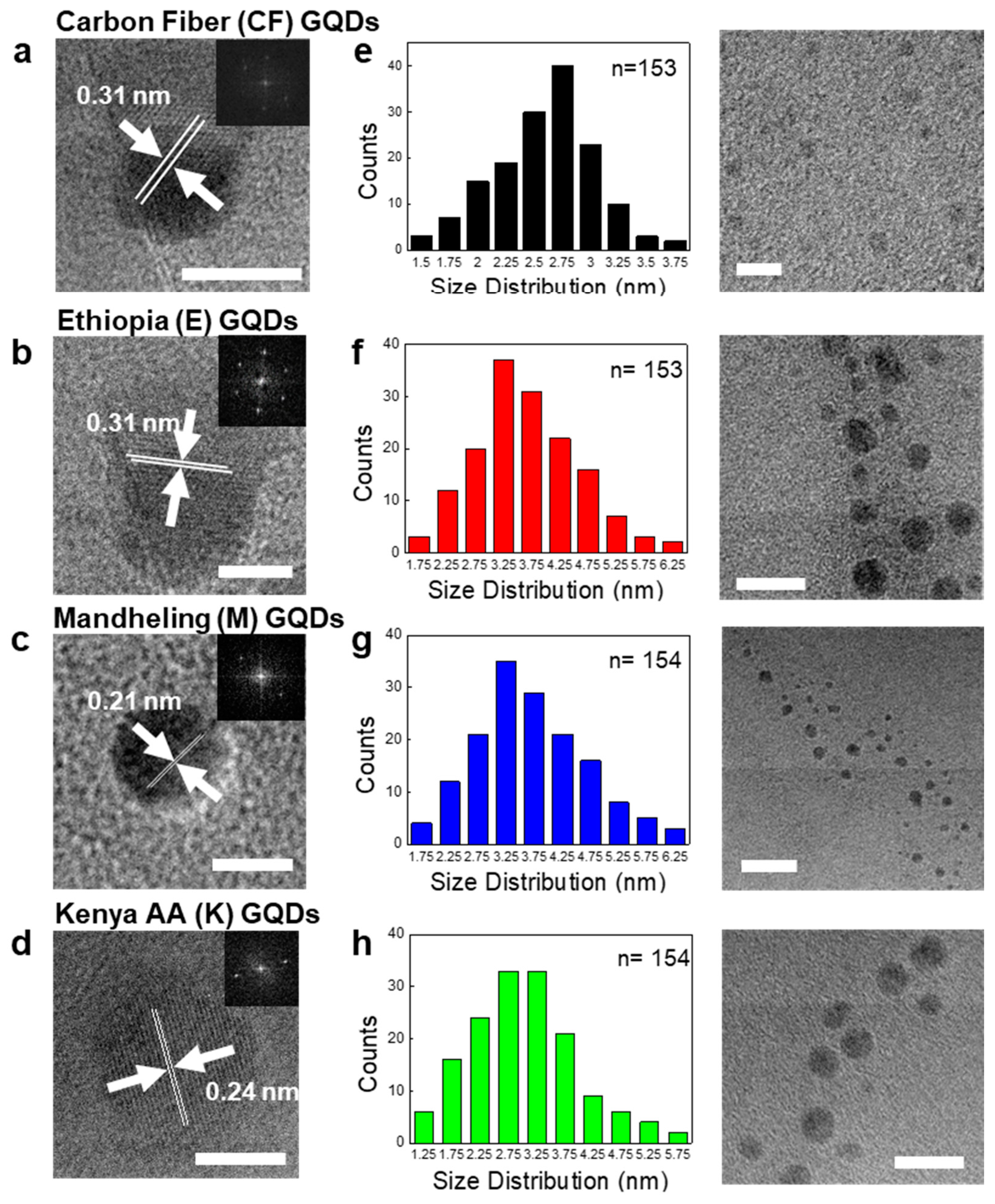
2.3. Transmission Electron Microscopy (TEM)
2.4. Raman Spectroscopy
2.5. Fourier Transform-Infrared Spectroscopy (FT-IR)
2.6. Isolation and Culture of hUCB-MSCs
2.7. Generation of iNSCs through Direct Conversion
2.8. Cell Viability Assay
3. Results and Discussion
3.1. Synthesis of C-GQDs
3.2. Therapeutic Efficacy of C-GQDs for Parkinson’s Disease
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kookos, I. Technoeconomic and environmental assessment of a process for biodiesel production from spent coffee grounds (SCGs). Resour. Conserv. Recycl. 2018, 134, 156–164. [Google Scholar] [CrossRef]
- Leifa, F.; Pandey, A.; Soccol, C.R. Production of Flammulina velutipes on coffee husk and coffee spent-ground. Braz. Arch. Biol. Technol. 2001, 44, 205–212. [Google Scholar] [CrossRef]
- Padmapriya, R.; Tharian, J.A.; Thirunalasundari, T. Coffee waste management-An overview. Int. J. Curr. Sci. 2013, 9, 83–91. [Google Scholar]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- El Achkar, J.H.; Baydoun, A.; Salameh, D.; Louka, N.; Hobaika, Z.; Maroun, R.G. Can coffee grounds be considered as a potential for green energy production? In Proceedings of the 2018 4th International Conference on Renewable Energies for Developing Countries (REDEC), Beirut, Lebanon, 1–2 November 2018; pp. 1–6. [Google Scholar]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotech. 2018, 13, 812–818. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, B.; Li, L.-S. Large, Solution-Processable Graphene Quantum Dots as Light Absorbers for Photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Luo, Y.; Yang, J.; Hou, J.G. How Graphene Is Cut upon Oxidation? J. Am. Chem. Soc. 2009, 131, 6320–6321. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Luong, J.; Gedanken, A. Green Synthesis of Multifunctional Carbon Dots with Antibacterial Activities. Nanomaterials 2021, 11, 369. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Banerjee, S.; Das, N.C. Graphene based emergent nanolights: A short review on the synthesis, properties and application. Res. Chem. Intermed. 2019, 45, 3823–3853. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Saleh, R.; Bearth, A.; Siegrist, M. “Chemophobia” Today: Consumers’ Knowledge and Perceptions of Chemicals. Risk Anal. 2019, 39, 2668–2682. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, Y.; Li, P.; Wang, Z.; Cao, B.; Bingqiang, C.; Liu, J.; Qiu, Z.; Zhang, W. Ultrafast ammonia-driven, microwave-assisted synthesis of nitrogen-doped graphene quantum dots and their optical properties. Nanophotonics 2017, 6, 259–267. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Bai, G.; Liu, C.P.; Hao, J.; Lin, J.; Jiang, H.; Teng, K.S.; Yang, Z.; et al. Deep Ultraviolet to Near-Infrared Emission and Photoresponse in Layered N-Doped Graphene Quantum Dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R. Trimethylene and the addition of methylene to ethylene. J. Am. Chem. Soc. 1968, 90, 1475–1485. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-C.; You, C.-F.; Chen, C.-Y.; Liu, Y.-C.; Chung, M.-T. Geographic determination of coffee beans using multi-element analysis and isotope ratios of boron and strontium. Food Chem. 2014, 142, 439–445. [Google Scholar] [CrossRef]
- Feng, J.; Dong, H.; Yu, L.; Dong, L. The optical and electronic properties of graphene quantum dots with oxygen-containing groups: A density functional theory study. J. Mater. Chem. C 2017, 5, 5984–5993. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef] [PubMed]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The Role of Oxygen during Thermal Reduction of Graphene Oxide Studied by Infrared Absorption Spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Dawson, T.M.; Dawson, V.L. Molecular Pathways of Neurodegeneration in Parkinson’s Disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α -synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M.-Y. Exogenous α-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; Trojanowski, J.Q. Pathological α -Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 2012, 338, 6. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.J.; Yoo, J.M.; Suh, Y.; Kim, D.; Kang, I.; Moon, J.; Park, M.; Kim, J.; Kang, K.-S.; Hong, B.H. Graphene Quantum Dots from Carbonized Coffee Bean Wastes for Biomedical Applications. Nanomaterials 2021, 11, 1423. https://doi.org/10.3390/nano11061423
Kim DJ, Yoo JM, Suh Y, Kim D, Kang I, Moon J, Park M, Kim J, Kang K-S, Hong BH. Graphene Quantum Dots from Carbonized Coffee Bean Wastes for Biomedical Applications. Nanomaterials. 2021; 11(6):1423. https://doi.org/10.3390/nano11061423
Chicago/Turabian StyleKim, Dong Jin, Je Min Yoo, Yeonjoon Suh, Donghoon Kim, Insung Kang, Joonhee Moon, Mina Park, Juhee Kim, Kyung-Sun Kang, and Byung Hee Hong. 2021. "Graphene Quantum Dots from Carbonized Coffee Bean Wastes for Biomedical Applications" Nanomaterials 11, no. 6: 1423. https://doi.org/10.3390/nano11061423
APA StyleKim, D. J., Yoo, J. M., Suh, Y., Kim, D., Kang, I., Moon, J., Park, M., Kim, J., Kang, K.-S., & Hong, B. H. (2021). Graphene Quantum Dots from Carbonized Coffee Bean Wastes for Biomedical Applications. Nanomaterials, 11(6), 1423. https://doi.org/10.3390/nano11061423







