Carrier Blocking Layer Materials and Application in Organic Photodetectors
Abstract
1. Introduction
1.1. Fundamentals of OPDs
1.2. Performance Metrics of OPDs
1.3. Dark Current in OPDs: Origin and Solutions
1.4. Blocking Layer Engineering
2. Materials for HBLs in OPD
2.1. Small Molecular Organic Materials
2.1.1. BCP
2.1.2. Bphen
2.1.3. C60
2.2. Polymer Organic Material
2.2.1. PEIE
2.2.2. PFN
2.2.3. PEI
2.3. Inorganic Oxide Semiconductor Materials
2.3.1. ZnO
2.3.2. TiO2
2.3.3. SnO2
2.4. Inorganic Salts
3. Materials for EBLs in OPD
3.1. Small Molecular Organic Materials
3.1.1. TFB
3.1.2. TIPS Pentacene
3.2. Polymer Organic Material
3.2.1. PEDOT:PSS
3.2.2. Donor Materials of BHJ
3.3. Inorganic Oxide Semiconductor Materials
3.3.1. NiOx
3.3.2. MoO3
3.3.3. V2O5
3.4. Inorganic Salt
4. Conclusions and Outlook
- (1)
- Organic interface materials in OPDs are expected to be used in future flexible wearable electronic devices, and further research is needed. However, it is necessary to develop new strategies to solve the problem that they are orthogonal to the solvent of the organic active layer according to the device structure.
- (2)
- Inorganic materials have also been studied extensively in OPDs because of their high stability. However, the particle size of the material needs to be further reduced, and there are still relatively few p-type inorganic nanomaterials for EBLs. How to achieve the preparation of high-quality inorganic blocking layers at low temperature or even room temperature is the focus of our attention.
- (3)
- Doping is one of the straightforward ways to modify the carrier blocking layer. The method of inorganic nanoparticle doping in organic interface materials is expected to be used in the future research of OPD because it combines the advantages of the two types of materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, L.; Liang, Q.; Wang, W.; Zhang, Y.; Li, G.; Ji, T.; Hao, Y.; Cui, Y. Research Progress in Organic Photomultiplication Photodetectors. Nanomaterials 2018, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Lei, Y.; Chan, W.K.E.; Chen, S.; Luo, D.; Zhu, F. Near-infrared and visible light dual-mode organic photodetectors. Sci. Adv. 2020, 6, eaaw8065. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.; Choi, W.; Zhu, F. Organic Optical Sensor Based on Monolithic Integration of Organic Electronic Devices. Electronics 2015, 4, 623–632. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Wen, H.; Bao, Q.; Shen, L.; Ding, L. Organic Photodetectors: Materials, Structures, and Challenges. Solar RRL 2020, 4, 2000139. [Google Scholar] [CrossRef]
- Ross, D.; Jansen-van Vuuren, A.A.; Pandey, A.K.; Burn, P.L.; Meredith, P. Organic Photodiodes: The Future of Full Color Detection and Image Sensing. Adv. Mater. 2016, 28, 4766–4802. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, K.; Zhang, J.; Ying, L.; Xie, R.; Yu, G.; Huang, F.; Cao, Y. High-Performance All-Polymer Photodetectors via a Thick Photoactive Layer Strategy. ACS Appl. Mater. Interfaces 2019, 11, 14208–14214. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Brito-Pereira, R.; Gonçalves, B.F.; Etxebarria, I.; Lanceros-Mendez, S. Recent developments on printed photodetectors for large area and flexible applications. Org. Electron. 2019, 66, 216–226. [Google Scholar] [CrossRef]
- Kang, J.; Kim, J.; Ham, H.; Ahn, H.; Lim, S.Y.; Kim, H.M.; Kang, I.N.; Jung, I.H. High-Detectivity Green-Selective All-Polymer p–n Junction Photodetectors. Adv. Opt. Mater. 2020, 8, 2001038. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Ma, Y.; Xia, R.; Li, X.; Wan, F.; Shen, L.; Yip, H.L.; Yuan, Y.; Jiang, Z.Q.; et al. Planar Heterojunction Organic Photodetectors Based on Fullerene and Non-fullerene Acceptor Bilayers for a Tunable Spectral Response. ACS Appl Mater. Interfaces 2020, 12, 55064–55071. [Google Scholar] [CrossRef]
- Nie, R.; Wang, Y.; Deng, X. Aligned nanofibers as an interfacial layer for achieving high-detectivity and fast-response organic photodetectors. ACS Appl. Mater. Interfaces 2014, 6, 7032–7037. [Google Scholar] [CrossRef]
- Brebels, J.; Manca, J.V.; Lutsen, L.; Vanderzande, D.; Maes, W. High dielectric constant conjugated materials for organic photovoltaics. J. Mater. Chem. A 2017, 5, 24037–24050. [Google Scholar] [CrossRef]
- Wadsworth, A.; Hamid, Z.; Kosco, J.; Gasparini, N.; McCulloch, I. The Bulk Heterojunction in Organic Photovoltaic, Photodetector, and Photocatalytic Applications. Adv. Mater. 2020, e2001763. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, C.; Niu, L.; Zhang, X.; Zhang, F. Recent Progress on Broadband Organic Photodetectors and their Applications. Laser Photon. Rev. 2020, 14, 2000262. [Google Scholar] [CrossRef]
- Chow, P.C.Y.; Someya, T. Organic Photodetectors for Next-Generation Wearable Electronics. Adv. Mater. 2020, 32, e1902045. [Google Scholar] [CrossRef]
- Li, N.; Lan, Z.; Cai, L.; Zhu, F. Advances in solution-processable near-infrared phototransistors. J. Mater. Chem. C 2019, 7, 3711–3729. [Google Scholar] [CrossRef]
- Swathi, K.; Narayan, K.S. Image pixel device using integrated organic electronic components. Appl. Phys. Lett. 2016, 109, 193302. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Q. Enhancing the performance of photomultiplication-type organic photodetectors using solution-processed ZnO as an interfacial layer. J. Mater. Chem. C 2019, 7, 1544–1550. [Google Scholar] [CrossRef]
- Kublitski, J.; Hofacker, A.; Boroujeni, B.K.; Benduhn, J.; Nikolis, V.C.; Kaiser, C.; Spoltore, D.; Kleemann, H.; Fischer, A.; Ellinger, F.; et al. Reverse dark current in organic photodetectors and the major role of traps as source of noise. Nat. Commun. 2021, 12, 551. [Google Scholar] [CrossRef]
- Zhu, H.L.; Cheng, J.; Zhang, D.; Liang, C.; Reckmeier, C.J.; Huang, H.; Rogach, A.L.; Choy, W.C. Room-Temperature Solution-Processed NiOx:PbI2 Nanocomposite Structures for Realizing High-Performance Perovskite Photodetectors. ACS Nano 2016, 10, 6808–6815. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, Z.; Yang, D.; Ma, D.; Tang, B.; Vadim, A. Structure design and performance of photomultiplication-type organic photodetectors based on an aggregation-induced emission material. Nanoscale 2020, 12, 2648–2656. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, Z.; Peng, F.; Ying, L.; Yu, G.; Huang, F.; Cao, Y. Copper Thiocyanate as an Anode Interfacial Layer for Efficient Near-Infrared Organic Photodetector. ACS Appl. Mater. Interfaces 2021, 13, 1027–1034. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, J. Photodetectors Based on Organic-Inorganic Hybrid Lead Halide Perovskites. Adv. Sci. 2018, 5, 1700256. [Google Scholar] [CrossRef] [PubMed]
- Kasap, S.; Frey, J.B.; Belev, G.; Tousignant, O.; Mani, H.; Greenspan, J.; Laperriere, L.; Bubon, O.; Reznik, A.; DeCrescenzo, G.; et al. Amorphous and polycrystalline photoconductors for direct conversion flat panel X-ray image sensors. Sensors 2011, 11, 5112–5157. [Google Scholar] [CrossRef]
- Loukianova, N.V.; Folkerts, H.O.; Maas, J.P.V.; Verbugt, D.W.E.; Mierop, A.J.; Hoekstra, W.; Roks, E.; Theuwissen, A.J.P. Leakage current modeling of test structures for characterization of dark current in CMOS image sensors. IEEE Trans. Electron. Devices 2003, 50, 77–83. [Google Scholar] [CrossRef]
- Kabir, M.Z.; Imam, S.-A. Transient and steady-state dark current mechanisms in amorphous selenium avalanche radiation detectors. Appl. Phys. Lett. 2013, 102, 153515. [Google Scholar] [CrossRef]
- Simone, G.; Dyson, M.J.; Meskers, S.C.J.; Janssen, R.A.J.; Gelinck, G.H. Organic Photodetectors and their Application in Large Area and Flexible Image Sensors: The Role of Dark Current. Adv. Funct. Mater. 2019, 30, 1904205. [Google Scholar] [CrossRef]
- Baeg, K.J.; Binda, M.; Natali, D.; Caironi, M.; Noh, Y.Y. Organic light detectors: Photodiodes and phototransistors. Adv. Mater. 2013, 25, 4267–4295. [Google Scholar] [CrossRef]
- Zhong, Z.; Bu, L.; Zhu, P.; Xiao, T.; Fan, B.; Ying, L.; Lu, G.; Yu, G.; Huang, F.; Cao, Y. Dark Current Reduction Strategy via a Layer-By-Layer Solution Process for a High-Performance All-Polymer Photodetector. ACS Appl. Mater. Interfaces 2019, 11, 8350–8356. [Google Scholar] [CrossRef] [PubMed]
- Armin, A.; Chen, Z.; Jin, Y.; Zhang, K.; Huang, F.; Shoaee, S. A Shockley-Type Polymer: Fullerene Solar Cell. Adv. Energy Mater. 2018, 8, 1701450. [Google Scholar] [CrossRef]
- Shekhar, H.; Solomeshch, O.; Liraz, D.; Tessler, N. Low dark leakage current in organic planar heterojunction photodiodes. Appl. Phys. Lett. 2017, 111, 223301. [Google Scholar] [CrossRef]
- Jang, M.S.; Yoon, S.; Sim, K.M.; Cho, J.; Chung, D.S. Spatial Confinement of the Optical Sensitizer to Realize a Thin Film Organic Photodetector with High Detectivity and Thermal Stability. J. Phys. Chem. Lett. 2018, 9, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Peng, Y.; Zhong, J.; Luo, X.; Li, Y.; Zheng, T.; Tang, Y.; Du, L.; Peng, L. Near Infrared Sensitive Organic Photodiode Utilizing Exciplex Absorption in NdPC2/C60 Heterojunction. IEEE Photonics Technol. Lett. 2015, 27, 2043–2046. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.; Meng, T.; Yi, C.; Gong, X. Inverted organic photovoltaic cells. Chem. Soc. Rev. 2016, 45, 2937–2975. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.-W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 073508. [Google Scholar] [CrossRef]
- Ma, H.; Yip, H.-L.; Huang, F.; Jen, A.K.Y. Interface Engineering for Organic Electronics. Adv. Funct. Mater. 2010, 20, 1371–1388. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kroger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition metal oxides for organic electronics: Energetics, device physics and applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef]
- Ahn, S.; Jeong, S.-H.; Han, T.-H.; Lee, T.-W. Conducting Polymers as Anode Buffer Materials in Organic and Perovskite Optoelectronics. Adv. Opt. Mater. 2017, 5, 1600512. [Google Scholar] [CrossRef]
- Van Le, Q.; Choi, J.-Y.; Kim, S.Y. Recent advances in the application of two-dimensional materials as charge transport layers in organic and perovskite solar cells. FlatChem 2017, 2, 54–66. [Google Scholar] [CrossRef]
- Ouyang, D.; Huang, Z.; Choy, W.C.H. Solution-Processed Metal Oxide Nanocrystals as Carrier Transport Layers in Organic and Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1804660. [Google Scholar] [CrossRef]
- Li, T.; Chen, Z.; Wang, Y.; Tu, J.; Deng, X.; Li, Q.; Li, Z. Materials for Interfaces in Organic Solar Cells and Photodetectors. ACS Appl Mater. Interfaces 2020, 12, 3301–3326. [Google Scholar] [CrossRef]
- Divayana, Y.; Chen, B.J.; Sun, X.W.; Sarma, K.S. Organic light-emitting devices with a hole-blocking layer inserted between the hole-injection layer and hole-transporting layer. Appl. Phys. Lett. 2006, 88, 083508. [Google Scholar] [CrossRef]
- Han, J.; Yang, D.; Hu, L.; Ma, D.; Qiao, W.; Wang, Z.Y. Low-Bandgap Terpolymers for High-Gain Photodiodes with High Detectivity and Responsivity from 300 nm to 1600 nm. ChemistrySelect 2018, 3, 7385–7393. [Google Scholar] [CrossRef]
- Luo, X.; Lv, W.; Du, L.; Zhao, F.; Peng, Y.; Wang, Y.; Tang, Y. Insight into trap state dynamics for exploiting current multiplication in organic photodetectors. Phys. Status Solidi RRL Rapid Res. Lett. 2016, 10, 485–492. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, X.; Yang, D.; Qiao, W.; Ma, D.; Wang, Z.Y. Optimization of Solubility, Film Morphology and Photodetector Performance by Molecular Side-Chain Engineering of Low-Bandgap Thienothiadiazole-Based Polymers. Adv. Funct. Mater. 2014, 24, 7605–7612. [Google Scholar] [CrossRef]
- Guo, D.; Yang, D.; Zhao, J.; Vadim, A.; Ma, D. Role of interfaces in controlling charge accumulation and injection in the photodetection performance of photomultiplication-type organic photodetectors. J. Mater. Chem. C 2020, 8, 9024–9031. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, E.; Esopi, M.R.; Cai, C.; Yu, Q. Flexible Narrowband Ultraviolet Photodetectors with Photomultiplication Based on Wide Band Gap Conjugated Polymer and Inorganic Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 24064–24074. [Google Scholar] [CrossRef]
- Liao, L.S.; Klubek, K.P. Power efficiency improvement in a tandem organic light-emitting diode. Appl. Phys. Lett. 2008, 92, 223311. [Google Scholar] [CrossRef]
- Naka, S.; Okada, H.; Onnagawa, H.; Tsutsui, T. High electron mobility in bathophenanthroline. Appl. Phys. Lett. 2000, 76, 197–199. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Su, Z.; Fang, F.; Zhang, G.; Wang, J.; Chu, B.; Fang, X.; Wei, Z.; Li, B.; et al. Efficient organic near-infrared photodetectors based on lead phthalocyanine/C60 heterojunction. Org. Electron. 2014, 15, 2367–2371. [Google Scholar] [CrossRef]
- Nath, D.; Dey, P.; Deb, D.; Rakshit, J.K.; Roy, J.N. Fabrication and characterization of organic semiconductor based photodetector for optical communication. CSI Trans. ICT 2017, 5, 149–160. [Google Scholar] [CrossRef]
- Lee, C.-C.; Biring, S.; Ren, S.-J.; Li, Y.-Z.; Li, M.-Z.; Al Amin, N.R.; Liu, S.-W. Reduction of dark current density in organic ultraviolet photodetector by utilizing an electron blocking layer of TAPC doped with MoO3. Org. Electron. 2018, 65, 150–155. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhao, H.; Jin, H.; Yu, J. Detectivity enhancement of double-layer organic photodetectors consisting of solution-processed interconnecting layers. Mater. Lett. 2019, 243, 81–83. [Google Scholar] [CrossRef]
- Wu, L.; Xu, R.; Yao, G.; Su, D.; Su, Z.; Yang, H. Photomultiplication type near-infrared organic photodetectors with a mixed active layer. Microw. Opt. Technol. Lett. 2020, 63, 714–718. [Google Scholar] [CrossRef]
- Schneidewind, N.; Köpke, M.; Buhl, J.; Murat, Y.; Gerken, M. OLED-OPD Matrix for Fluorescence Sensing on a Single Flexible Substrate. IEEE Sens. J. 2019, 20, 7540–7547. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Z.; Duan, Y.; Zhao, D.; Yu, J. High-Performance Organic Photodetectors by Introducing a Non-Fullerene Acceptor to Broaden Long Wavelength Detective Spectrum. Nanoscale Res. Lett. 2019, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Estrada, R.; Li, Y.Z.; Biring, S.; Amin, N.R.A.; Li, M.Z.; Liu, S.W.; Wong, K.T. Vacuum-Processed Small Molecule Organic Photodetectors with Low Dark Current Density and Strong Response to Near-Infrared Wavelength. Adv. Opt. Mater. 2020, 8, 2000519. [Google Scholar] [CrossRef]
- Song, S.; Hill, R.; Choi, K.; Wojciechowski, K.; Barlow, S.; Leisen, J.; Snaith, H.J.; Marder, S.R.; Park, T. Surface modified fullerene electron transport layers for stable and reproducible flexible perovskite solar cells. Nano Energy 2018, 49, 324–332. [Google Scholar] [CrossRef]
- Kim, I.K.; Jo, J.H.; Lee, J.; Choi, Y.J. Detectivity analysis for organic photodetectors. Org. Electron. 2018, 57, 89–92. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Duan, L.; Chen, H.; Wang, L.; Dong, G.; Xu, Z. Squarylium and rubrene based filterless narrowband photodetectors for an all-organic two-channel visible light communication system. Org. Electron. 2016, 37, 346–351. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, X.; Ma, D. Fast response organic photodetectors with high detectivity based on rubrene and C60. Org. Electron. 2013, 14, 3019–3023. [Google Scholar] [CrossRef]
- Armin, A.; Hambsch, M.; Kim, I.K.; Burn, P.L.; Meredith, P.; Namdas, E.B. Thick junction broadband organic photodiodes. Laser Photon. Rev. 2014, 8, 924–932. [Google Scholar] [CrossRef]
- Kim, I.K.; Pal, B.N.; Ullah, M.; Burn, P.L.; Lo, S.-C.; Meredith, P.; Namdas, E.B. High-Performance, Solution-Processed Non-polymeric Organic Photodiodes. Adv. Opt. Mater. 2015, 3, 50–56. [Google Scholar] [CrossRef]
- Joo, C.W.; Kim, J.; Moon, J.; Lee, K.M.; Pi, J.-E.; Kang, S.-Y.; Ahn, S.-D.; Park, Y.-S.; Chung, D.S. High-performance fab-compatible processed near-infrared organic thin-film photodiode with 3.3×1012 Jones detectivity and 80% external quantum efficiency. Org. Electron. 2019, 70, 101–106. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A Universal Method to Produce Low-Work Function Electrodes for Organic Electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, B.; Lee, K.; Forrest, S.; Kanicki, J. Bilayer Interdiffused Heterojunction Organic Photodiodes Fabricated by Double Transfer Stamping. Adv. Opt. Mater. 2017, 5, 1600784. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Wang, T.; Hu, Y.; Deng, Z.; Cui, Q.; Lou, Z.; Hou, Y.; Teng, F. Fast and sensitive polymer photodetectors with extra high external quantum efficiency and large linear dynamic range at low working voltage bias. Org. Electron. 2018, 62, 448–453. [Google Scholar] [CrossRef]
- Klab, T.; Luszczynska, B.; Ulanski, J.; Wei, Q.; Chen, G.; Zou, Y. Influence of PEIE interlayer on detectivity of red-light sensitive organic non-fullerene photodetectors with reverse structure. Org. Electron. 2020, 77, 105527. [Google Scholar] [CrossRef]
- Salamandra, L.; La Notte, L.; Fazolo, C.; Di Natali, M.; Penna, S.; Mattiello, L.; Cinà, L.; Del Duca, R.; Reale, A. A comparative study of organic photodetectors based on P3HT and PTB7 polymers for visible light communication. Org. Electron. 2020, 81, 105666. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. High sensitivity and fast response solution processed polymer photodetectors with polyethylenimine ethoxylated (PEIE) modified ITO electrode. Opt. Express 2017, 25, 7719–7729. [Google Scholar] [CrossRef]
- Pierre, A.; Deckman, I.; Lechene, P.B.; Arias, A.C. High Detectivity All-Printed Organic Photodiodes. Adv. Mater. 2015, 27, 6411–6417. [Google Scholar] [CrossRef]
- Deckman, I.; Lechêne, P.B.; Pierre, A.; Arias, A.C. All-printed full-color pixel organic photodiode array with a single active layer. Org. Electron. 2018, 56, 139–145. [Google Scholar] [CrossRef]
- London, A.E.; Huang, L.; Zhang, B.A.; Oviedo, M.B.; Tropp, J.; Yao, W.; Wu, Z.; Wong, B.M.; Ng, T.N.; Azoulay, J.D. Donor–acceptor polymers with tunable infrared photoresponse. Polym. Chem. 2017, 8, 2922–2930. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, W.; London, A.E.; Azoulay, J.D.; Ng, T.N. Temperature-Dependent Detectivity of Near-Infrared Organic Bulk Heterojunction Photodiodes. ACS Appl. Mater. Interfaces 2017, 9, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, C.; Xu, X.; Zhang, L.; Huang, L.; Chen, J.; Wu, H.; Cao, Y. Largely enhanced efficiency with a PFN/Al bilayer cathode in high efficiency bulk heterojunction photovoltaic cells with a low bandgap polycarbazole donor. Adv. Mater. 2011, 23, 3086–3089. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Zhu, L.; Lv, L.; Hu, Y.; Deng, Z.; Cui, Q.; Lou, Z.; Hou, Y.; Teng, F. Work-function-controlled operation mode transition between photodiode and photoconductor modes in organic photodetectors. Org. Electron. 2019, 64, 138–145. [Google Scholar] [CrossRef]
- Miao, J.; Zhang, F.; Lin, Y.; Wang, W.; Gao, M.; Li, L.; Zhang, J.; Zhan, X. Highly Sensitive Organic Photodetectors with Tunable Spectral Response under Bi-Directional Bias. Adv. Opt. Mater. 2016, 4, 1711–1717. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Y.; Deng, Z.; Wang, Y.; Lv, L.; Zhu, L.; Lou, Z.; Hou, Y.; Teng, F. High sensitivity, fast response and low operating voltage organic photodetectors by incorporating a water/alcohol soluble conjugated polymer anode buffer layer. RSC Adv. 2017, 7, 1743–1748. [Google Scholar] [CrossRef]
- Xie, B.; Xie, R.; Zhang, K.; Yin, Q.; Hu, Z.; Yu, G.; Huang, F.; Cao, Y. Self-filtering narrowband high performance organic photodetectors enabled by manipulating localized Frenkel exciton dissociation. Nat. Commun. 2020, 11, 2871. [Google Scholar] [CrossRef]
- Jia, X.; Wu, N.; Wei, J.; Zhang, L.; Luo, Q.; Bao, Z.; Li, Y.-Q.; Yang, Y.; Liu, X.; Ma, C.-Q. A low-cost and low-temperature processable zinc oxide-polyethylenimine (ZnO:PEI) nano-composite as cathode buffer layer for organic and perovskite solar cells. Org. Electron. 2016, 38, 150–157. [Google Scholar] [CrossRef]
- Falco, A.; Zaidi, A.M.; Lugli, P.; Abdellah, A. Spray deposition of Polyethylenimine thin films for the fabrication of fully-sprayed organic photodiodes. Org. Electron. 2015, 23, 186–192. [Google Scholar] [CrossRef]
- Grimoldi, A.; Colella, L.; La Monaca, L.; Azzellino, G.; Caironi, M.; Bertarelli, C.; Natali, D.; Sampietro, M. Inkjet printed polymeric electron blocking and surface energy modifying layer for low dark current organic photodetectors. Org. Electron. 2016, 36, 29–34. [Google Scholar] [CrossRef]
- Cesarini, M.; Brigante, B.; Caironi, M.; Natali, D. Reproducible, High Performance Fully Printed Photodiodes on Flexible Substrates through the Use of a Polyethylenimine Interlayer. ACS Appl. Mater. Interfaces 2018, 10, 32380–32386. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Zhu, L.; Lv, L.; Hu, Y.; Deng, Z.; Cui, Q.; Lou, Z.; Hou, Y.; Teng, F. High sensitivity and fast response sol-gel ZnO electrode buffer layer based organic photodetectors with large linear dynamic range at low operating voltage. Org. Electron. 2018, 56, 51–58. [Google Scholar] [CrossRef]
- Liu, J.; Wen, H.; Shen, L. Highly sensitive, broadband, fast response organic photodetectors based on semi-tandem structure. Nanotechnology 2020, 31, 214001. [Google Scholar] [CrossRef]
- Eckstein, R.; Strobel, N.; Rödlmeier, T.; Glaser, K.; Lemmer, U.; Hernandez-Sosa, G. Fully Digitally Printed Image Sensor Based on Organic Photodiodes. Adv. Opt. Mater. 2018, 6, 1701108. [Google Scholar] [CrossRef]
- Jeong, J.; Nam, S.; Kim, H.; Kim, Y. Inverted Organic Photodetectors with ZnO Electron-Collecting Buffer Layers and Polymer Bulk Heterojunction Active Layers. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 130–136. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, M.; Qin, R.; Yu, J. Low dark-current and high-photodetectivity transparent organic ultraviolet photodetector by using polymer-modified ZnO as the electron transfer layer. Opt. Lett. 2018, 43, 3212–3215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, D.; Ma, D.; Vadim, A.; Ahamad, T.; Alshehri, S.M. Ultrahigh Gain Polymer Photodetectors with Spectral Response from UV to Near-Infrared Using ZnO Nanoparticles as Anode Interfacial Layer. Adv. Funct. Mater. 2016, 26, 6619–6626. [Google Scholar] [CrossRef]
- Fan, P.; Zhang, D.; Wu, Y.; Yu, J.; Russell, T.P. Polymer-Modified ZnO Nanoparticles as Electron Transport Layer for Polymer-Based Solar Cells. Adv. Funct. Mater. 2020, 30, 2002932. [Google Scholar] [CrossRef]
- Opoku, H.; Choy, J.-Y.; Kumar, K.A.; Kim, H.-S.; Shrestha, N.K.; Mane, S.D.; Bathula, C. Benzo[1, 2-b: 4, 5-b′]dithiophene-based copolymers as panchromatic light sensors in organic photodiodes application. J. Mater. Res. Technol. 2020, 9, 15632–15637. [Google Scholar] [CrossRef]
- Gielen, S.; Kaiser, C.; Verstraeten, F.; Kublitski, J.; Benduhn, J.; Spoltore, D.; Verstappen, P.; Maes, W.; Meredith, P.; Armin, A.; et al. Intrinsic Detectivity Limits of Organic Near-Infrared Photodetectors. Adv. Mater. 2020, 32, e2003818. [Google Scholar] [CrossRef]
- Xia, Y.; Aguirre, L.E.; Xu, X.; Inganäs, O. All-Polymer High-Performance Photodetector through Lamination. Adv. Electron. Mater. 2020, 6, 1901017. [Google Scholar] [CrossRef]
- Carp, O. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-Phase Synthesis of Titanium Dioxide Nanoparticles and Nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Peng, Q.; Yi, J.X.; Wang, X.; Li, Y. Near monodisperse TiO2 nanoparticles and nanorods. Chemistry 2006, 12, 2383–2391. [Google Scholar] [CrossRef]
- Wang, C.C.; Ying, J.Y. Sol-Gel Synthesis and Hydrothermal Processing of Anatase and Rutile Titania Nanocrystals. Chem. Mater. 1999, 11, 3113–3120. [Google Scholar] [CrossRef]
- Yoldas, B.E. Hydrolysis of titanium alkoxide and effects of hydrolytic polycondensation parameters. J. Mater. Sci. 1986, 21, 1087–1092. [Google Scholar] [CrossRef]
- Jensen, G.V.; Bremholm, M.; Lock, N.; Deen, G.R.; Jensen, T.R.; Iversen, B.B.; Niederberger, M.; Pedersen, J.S.; Birkedal, H. Anisotropic Crystal Growth Kinetics of Anatase TiO2Nanoparticles Synthesized in a Nonaqueous Medium. Chem. Mater. 2010, 22, 6044–6055. [Google Scholar] [CrossRef]
- Zhu, H.L.; Choy, W.C.H.; Sha, W.E.I.; Ren, X. Photovoltaic Mode Ultraviolet Organic Photodetectors with High On/Off Ratio and Fast Response. Adv. Opt. Mater. 2014, 2, 1082–1089. [Google Scholar] [CrossRef]
- Sista, S.; Park, M.H.; Hong, Z.; Wu, Y.; Hou, J.; Kwan, W.L.; Li, G.; Yang, Y. Highly efficient tandem polymer photovoltaic cells. Adv. Mater. 2010, 22, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Furube, A.; Murai, M.; Hara, K.; Katoh, R.; Tachiya, M. Dynamics of efficient electron-hole separation in TiO2 nanoparticles revealed by femtosecond transient absorption spectroscopy under the weak-excitation condition. Phys. Chem. Chem. Phys. 2007, 9, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Jessica, E.; Kroeze, T.J.S.; Warman, J.M. Electrodeless Determination of the Trap Density, Decay Kinetics, and Charge Separation Efficiency of Dye-Sensitized Nanocrystalline TiO2. J. Am. Chem. Soc. 2004, 126, 7608–7618. [Google Scholar]
- Anta, J.A.; Nelson, J.; Quirke, N. Charge transport model for disordered materials: Application to sensitizedTiO2. Phys. Rev. B 2002, 65. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Wang, T.; Hu, Y.; Deng, Z.; Cui, Q.; Lou, Z.; Hou, Y.; Teng, F. Sensitive, fast, stable, and broadband polymer photodetector with introducing TiO2 nanocrystal trap states. Org. Electron. 2018, 59, 63–68. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14. [Google Scholar] [CrossRef]
- Yun, A.J.; Kim, J.; Hwang, T.; Park, B. Origins of Efficient Perovskite Solar Cells with Low-Temperature Processed SnO2 Electron Transport Layer. ACS Appl. Energy Mater. 2019, 2, 3554–3560. [Google Scholar] [CrossRef]
- Deng, R.; Yan, C.; Deng, Y.; Hu, Y.; Deng, Z.; Cui, Q.; Lou, Z.; Hou, Y.; Teng, F. High-Performance Polymer Photodetector Using the Non-Thermal-and-Non-Ultraviolet–Ozone-Treated SnO2 Interfacial Layer. Phys. Status Solidi RRL Rapid Res. Lett. 2019, 14, 1900531. [Google Scholar] [CrossRef]
- Huang, J.; Lee, J.; Nakayama, H.; Schrock, M.; Cao, D.X.; Cho, K.; Bazan, G.C.; Nguyen, T.Q. Understanding and Countering Illumination-Sensitive Dark Current: Toward Organic Photodetectors with Reliable High Detectivity. ACS Nano 2021, 15, 1753–1763. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Wang, R.; Liu, S.; Huang, K.; Michinobu, T.; Dong, G. Poly(dithiazolfluorene-alt-selenadiazolobenzotriazole)-Based Blue-Light Photodetector and Its Application in Visible-Light Communication. ACS Appl. Mater. Interfaces 2019, 11, 16758–16764. [Google Scholar] [CrossRef]
- Zafar, Q.; Fatima, N.; Karimov, K.S.; Ahmed, M.M.; Sulaiman, K. Realizing broad-bandwidth visible wavelength photodiode based on solution-processed ZnPc/PC71BM dyad. Opt. Mater. 2017, 64, 131–136. [Google Scholar] [CrossRef]
- Xia, K.; Li, Y.; Wang, Y.; Portilla, L.; Pecunia, V. Narrowband-Absorption-Type Organic Photodetectors for the Far-Red Range Based on Fullerene-Free Bulk Heterojunctions. Adv. Opt. Mater. 2020, 8, 1902056. [Google Scholar] [CrossRef]
- Alzahrani, H.; Sulaiman, K.; Mahmoud, A.Y.; Bahabry, R.R. Study of organic visible-blind photodetector based on Alq3:NPD blend for application in near-ultraviolet detection. Opt. Mater. 2020, 110, 110490. [Google Scholar] [CrossRef]
- Zafar, Q.; Ahmad, Z.; Sulaiman, K. PFO-DBT:MEH-PPV:PC(7)(1)BM ternary blend assisted platform as a photodetector. Sensors 2015, 15, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Esopi, M.R.; Calcagno, M.; Yu, Q. Organic Ultraviolet Photodetectors Exhibiting Photomultiplication, Low Dark Current, and High Stability. Adv. Mater. Technol. 2017, 2, 1700025. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Xu, X.; Li, Y.; Li, K.; Peng, Q. Polymer Solar Cells Exceeding 10% Efficiency Enabled via a Facile Star-Shaped Molecular Cathode Interlayer with Variable Counterions. Adv. Funct. Mater. 2016, 26, 4643–4652. [Google Scholar] [CrossRef]
- Montenegro Benavides, C.; Rechberger, S.; Spiecker, E.; Berlinghof, M.; Unruh, T.; Biele, M.; Schmidt, O.; Brabec, C.J.; Tedde, S.F. Improving spray coated organic photodetectors performance by using 1,8-diiodooctane as processing additive. Org. Electron. 2018, 54, 21–26. [Google Scholar] [CrossRef]
- Keivanidis, P.E.; Khong, S.-H.; Ho, P.K.H.; Greenham, N.C.; Friend, R.H. All-solution based device engineering of multilayer polymeric photodiodes: Minimizing dark current. Appl. Phys. Lett. 2009, 94, 173303. [Google Scholar] [CrossRef]
- Keivanidis, P.E.; Ho, P.K.H.; Friend, R.H.; Greenham, N.C. The Dependence of Device Dark Current on the Active-Layer Morphology of Solution-Processed Organic Photodetectors. Adv. Funct. Mater. 2010, 20, 3895–3903. [Google Scholar] [CrossRef]
- Biele, M.; Montenegro Benavides, C.; Hürdler, J.; Tedde, S.F.; Brabec, C.J.; Schmidt, O. Spray-Coated Organic Photodetectors and Image Sensors with Silicon-Like Performance. Adv. Mater. Technol. 2019, 4, 1800158. [Google Scholar] [CrossRef]
- Tsai, L.-S.; Hwang, J.-C.; Lee, C.-Y.; Lin, Y.-T.; Tsai, C.-L.; Chang, T.-H.; Chueh, Y.-L.; Meng, H.-F. Solution-based silk fibroin dielectric in n-type C60 organic field-effect transistors: Mobility enhancement by the pentacene interlayer. Appl. Phys. Lett. 2013, 103, 233304. [Google Scholar] [CrossRef]
- Hong, J.-P.; Park, A.-Y.; Lee, S.; Kang, J.; Shin, N.; Yoon, D.Y. Tuning of Ag work functions by self-assembled monolayers of aromatic thiols for an efficient hole injection for solution processed triisopropylsilylethynyl pentacene organic thin film transistors. Appl. Phys. Lett. 2008, 92, 143311. [Google Scholar] [CrossRef]
- Benavides, C.M.; Murto, P.; Chochos, C.L.; Gregoriou, V.G.; Avgeropoulos, A.; Xu, X.; Bini, K.; Sharma, A.; Andersson, M.R.; Schmidt, O.; et al. High-Performance Organic Photodetectors from a High-Bandgap Indacenodithiophene-Based pi-Conjugated Donor-Acceptor Polymer. ACS Appl. Mater. Interfaces 2018, 10, 12937–12946. [Google Scholar] [CrossRef] [PubMed]
- Montenegro Benavides, C.; Biele, M.; Schmidt, O.; Brabec, C.J.; Tedde, S.F. TIPS Pentacene as a Beneficial Interlayer for Organic Photodetectors in Imaging Applications. IEEE Trans. Electron. Devices 2018, 65, 1516–1522. [Google Scholar] [CrossRef]
- Bouthinon, B.; Clerc, R.; Verilhac, J.M.; Racine, B.; De Girolamo, J.; Jacob, S.; Lienhard, P.; Joimel, J.; Dhez, O.; Revaux, A. On the front and back side quantum efficiency differences in semi-transparent organic solar cells and photodiodes. J. Appl. Phys. 2018, 123, 125501. [Google Scholar] [CrossRef]
- Huang, J.; Lee, J.; Schrock, M.; Dixon, A.L.; Lill, A.T.; Cho, K.; Bazan, G.C.; Nguyen, T.-Q. Large-gain low-voltage and wideband organic photodetectors via unbalanced charge transport. Mater. Horizons 2020, 7, 3234–3241. [Google Scholar] [CrossRef]
- Liu, M.; Miao, J.; Wang, J.; Zhao, Z.; Yang, K.; Zhang, X.; Peng, H.; Zhang, F. Broadband organic photodetectors exhibiting photomultiplication with a narrow bandgap non-fullerene acceptor as an electron trap. J. Mater. Chem. C 2020, 8, 9854–9860. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.-C.; Yin, Z.; Zheng, Q. Broadband organic photodetectors based on ternary blend active layers with enhanced and spectrally flat response. J. Mater. Chem. C 2020, 8, 14049–14055. [Google Scholar] [CrossRef]
- Wang, W.; Du, M.; Zhang, M.; Miao, J.; Fang, Y.; Zhang, F. Organic Photodetectors with Gain and Broadband/Narrowband Response under Top/Bottom Illumination Conditions. Adv. Opt. Mater. 2018, 6, 1800249. [Google Scholar] [CrossRef]
- Jovanov, V.; Donfack, P.; Müller, A.; Materny, A.; Knipp, D.; Wagner, V. Standing wave spectrometer with semi-transparent organic detector. J. Mater. Chem. C 2018, 6, 11457–11464. [Google Scholar] [CrossRef]
- Hu, Z.; Li, C.; Nie, R.; Li, Y.-Q.; Tang, J.-X.; Deng, X. Biomaterial functionalized graphene oxides with tunable work function for high sensitive organic photodetectors. RSC Adv. 2015, 5, 99431–99438. [Google Scholar] [CrossRef]
- Abdullah, S.M.; Rafique, S.; Azmer, M.I.; Jilani, A.; Sajith, V.K.; Supangat, A. Modified photo-current response of an organic photodiode by using V2O5 in both hole and electron transport layers. Sens. Actuator A Phys. 2018, 272, 334–340. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, Q.; Mao, P.; Li, H.; Wang, J. All-solution-processed PIN architecture for ultra-sensitive and ultra-flexible organic thin film photodetectors. Sci. China Chem. 2016, 59, 1258–1263. [Google Scholar] [CrossRef]
- Xiong, S.; Li, L.; Qin, F.; Mao, L.; Luo, B.; Jiang, Y.; Li, Z.; Huang, J.; Zhou, Y. Universal Strategy To Reduce Noise Current for Sensitive Organic Photodetectors. ACS Appl. Mater. Interfaces 2017, 9, 9176–9183. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, X.; Zhou, K.; Xia, Y.; Ma, W.; Inganäs, O. Large-Area, Semitransparent, and Flexible All-Polymer Photodetectors. Adv. Funct. Mater. 2018, 28, 1805570. [Google Scholar] [CrossRef]
- Seiberlich, M.; Strobel, N.; Ruiz-Preciado, L.A.; Ruscello, M.; Lemmer, U.; Hernandez-Sosa, G. Aerosol-Jet-Printed Donor-Blocking Layer for Organic Photodiodes. Adv. Electron. Mater. 2020, 7, 2000811. [Google Scholar] [CrossRef]
- Yin, X.; Guo, Y.; Xie, H.; Que, W.; Kong, L.B. Nickel Oxide as Efficient Hole Transport Materials for Perovskite Solar Cells. Solar RRL 2019, 1900001. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Meyer, J.; Steirer, K.X.; Armstrong, N.R.; Olson, D.; Kahn, A. Energy level alignment in PCDTBT:PC70BM solar cells: Solution processed NiOx for improved hole collection and efficiency. Org. Electron. 2012, 13, 744–749. [Google Scholar] [CrossRef]
- Lim, S.B.; Ji, C.H.; Oh, I.S.; Oh, S.Y. Reduced leakage current and improved performance of an organic photodetector using an ytterbium cathode interlayer. J. Mater. Chem. C 2016, 4, 4920–4926. [Google Scholar] [CrossRef]
- Manders, J.R.; Lai, T.-H.; An, Y.; Xu, W.; Lee, J.; Kim, D.Y.; Bosman, G.; So, F. Low-Noise Multispectral Photodetectors Made from All Solution-Processed Inorganic Semiconductors. Adv. Funct. Mater. 2014. [Google Scholar] [CrossRef]
- Kim, K.T.; Ji, C.H.; Song, D.H.; Kim, H.S.; Oh, S.Y. Studies on the fabrication and characteristics of organic photodiode using novel Ga doped NiOx as an electron blocking layer. Mol. Cryst. Liq. Cryst. 2018, 91–95. [Google Scholar] [CrossRef]
- Li, Y.; Mao, L.; Yu, L.; Li, X.; Zhang, J. NiOx nanoparticles obtained from hydrothermally treated NiC2O4 as an electron blocking layer for organic photodetectors. Nanotechnology 2020, 31, 505601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, L.; Peng, C.; Mao, L.; Li, X.; Zhang, J. Top-Illuminated Flexible Organic Photodetectors Integrated With Hole Extraction Layers Synthesized With Solution-Processed NiOx Films at Room Temperature. IEEE Trans. Electron. Devices 2020, 67, 4308–4312. [Google Scholar] [CrossRef]
- Tokito, S.; Noda, K.; Taga, Y. Metal oxides as a hole-injecting layer for an organic electroluminescent device. J. Phys. D Appl. Phys. 1996, 29, 2750–2753. [Google Scholar] [CrossRef]
- Yu, X.-G.; Yu, J.-S.; Huang, W.; Zeng, H.-J. Enhanced charge carrier injection in heterojunction organic field-effect transistor by inserting an MoO3 buffer layer. Chin. Phys. B 2012, 21, 117307. [Google Scholar] [CrossRef]
- Chen, L.-M.; Hong, Z.; Li, G.; Yang, Y. Recent Progress in Polymer Solar Cells: Manipulation of Polymer:Fullerene Morphology and the Formation of Efficient Inverted Polymer Solar Cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Sato, Y.; Kajii, H.; Ohmori, Y. Improved performance of polymer photodetectors using indium–tin-oxide modified by phosphonic acid-based self-assembled monolayer treatment. Org. Electron. 2014, 15, 1753–1758. [Google Scholar] [CrossRef]
- Yoon, S.; Koh, C.W.; Woo, H.Y.; Chung, D.S. Systematic Optical Design of Constituting Layers to Realize High-Performance Red-Selective Thin-Film Organic Photodiodes. Adv. Opt. Mater. 2018, 6, 1701085. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.-T.; Zhao, C.; Guo, L.J.; Kanicki, J. Top illuminated organic photodetectors with dielectric/metal/dielectric transparent anode. Org. Electron. 2015, 20, 103–111. [Google Scholar] [CrossRef]
- Shekhar, H.; Fenigstein, A.; Leitner, T.; Lavi, B.; Veinger, D.; Tessler, N. Hybrid image sensor of small molecule organic photodiode on CMOS-Integration and characterization. Sci. Rep. 2020, 10, 7594. [Google Scholar] [CrossRef]
- Fu, W.B.; Shang, G.L.; Gong, X.X.; De Zhang, L.; Fei, G.T. Preparation of large scale and highly ordered vanadium pentoxide (V2O5) nanowire arrays towards high performance photodetectors. J. Mater. Chem. C 2017, 5, 1471–1478. [Google Scholar] [CrossRef]
- Terán-Escobar, G.; Pampel, J.; Caicedo, J.M.; Lira-Cantú, M. Low-temperature, solution-processed, layered V2O5 hydrate as the hole-transport layer for stable organic solar cells. Energy Environ. Sci. 2013, 6, 3088. [Google Scholar] [CrossRef]
- Li, G.; Chu, C.W.; Shrotriya, V.; Huang, J.; Yang, Y. Efficient inverted polymer solar cells. Appl. Phys. Lett. 2006, 88, 253503. [Google Scholar] [CrossRef]
- Back, H.; Kong, J.; Kang, H.; Kim, J.; Kim, J.-R.; Lee, K. Flexible polymer solar cell modules with patterned vanadium suboxide layers deposited by an electro-spray printing method. Sol. Energy Mater. Sol. Cells 2014, 130, 555–560. [Google Scholar] [CrossRef]
- Cho, S.-P.; Yeo, J.-S.; Kim, D.-Y.; Na, S.-I.; Kim, S.-S. Brush painted V2O5 hole transport layer for efficient and air-stable polymer solar cells. Sol. Energy Mater. Sol. Cells 2015, 132, 196–203. [Google Scholar] [CrossRef]
- Beliatis, M.J.; Helgesen, M.; García-Valverde, R.; Corazza, M.; Roth, B.; Carlé, J.E.; Jørgensen, M.; Krebs, F.C.; Gevorgyan, S.A. Slot-Die-Coated V2O5as Hole Transport Layer for Flexible Organic Solar Cells and Optoelectronic Devices. Adv. Eng. Mater. 2016, 18, 1494–1503. [Google Scholar] [CrossRef]
- Yaacobi-Gross, N.; Treat, N.D.; Pattanasattayavong, P.; Faber, H.; Perumal, A.K.; Stingelin, N.; Bradley, D.D.C.; Stavrinou, P.N.; Heeney, M.; Anthopoulos, T.D. High-Efficiency Organic Photovoltaic Cells Based on the Solution-Processable Hole Transporting Interlayer Copper Thiocyanate (CuSCN) as a Replacement for PEDOT:PSS. Adv. Energy Mater. 2015, 5, 1401529. [Google Scholar] [CrossRef]
- Mishra, A.; Rana, T.; Looser, A.; Stolte, M.; Würthner, F.; Bäuerle, P.; Sharma, G.D. High performance A–D–A oligothiophene-based organic solar cells employing two-step annealing and solution-processable copper thiocyanate (CuSCN) as an interfacial hole transporting layer. J. Mater. Chem. A 2016, 4, 17344–17353. [Google Scholar] [CrossRef]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef]
- Shekhar, H.; Lami, V.; Solomeshch, O.; Fenigstein, A.; Tomer, L.; Becky, L.; Vaynzof, Y.; Tessler, N. Doping induced performance enhancement in inverted small molecule organic photodiodes operating below 1V reverse bias—Towards compatibility with CMOS for imaging applications. Org. Electron. 2019, 67, 1–9. [Google Scholar] [CrossRef]






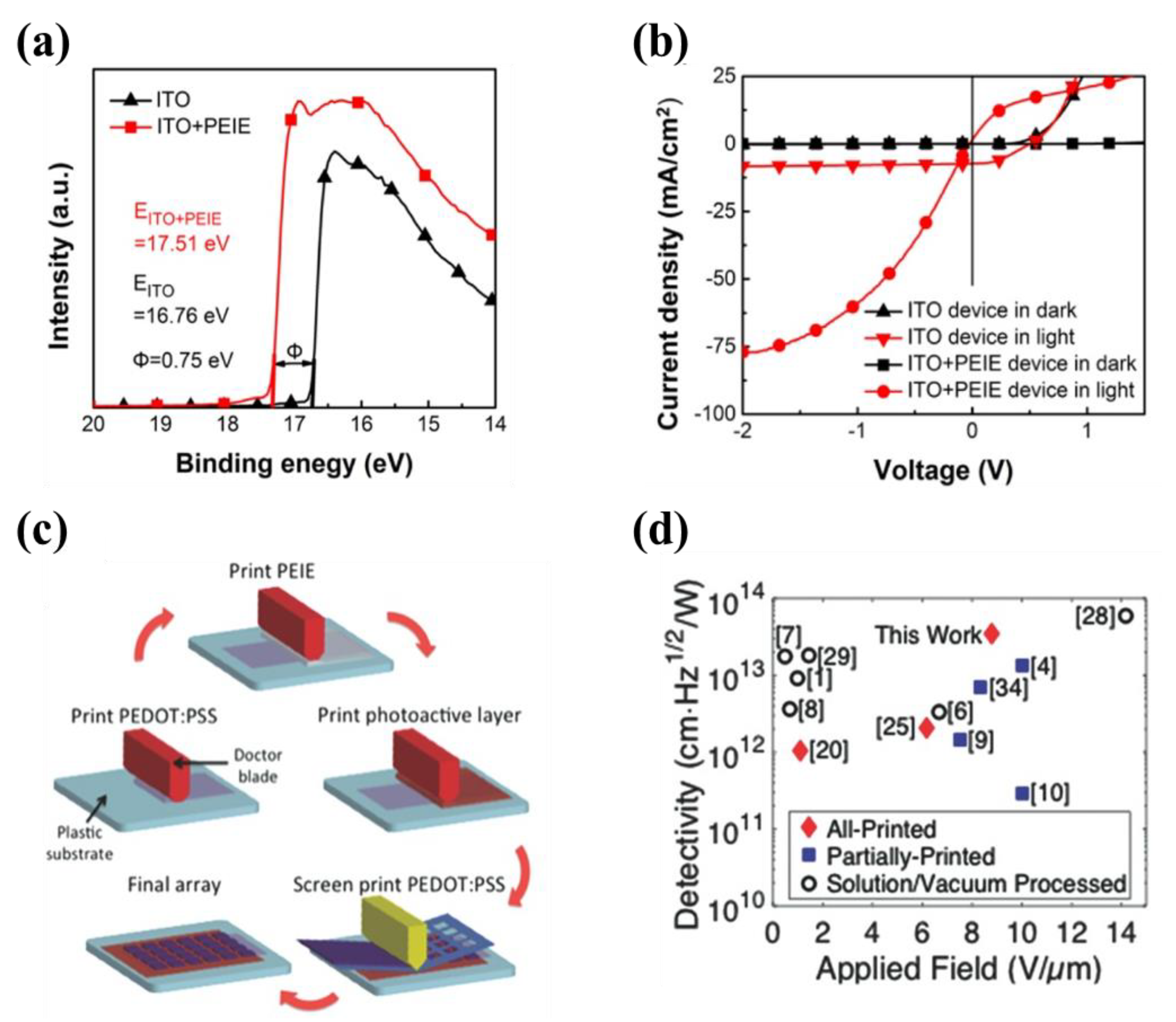


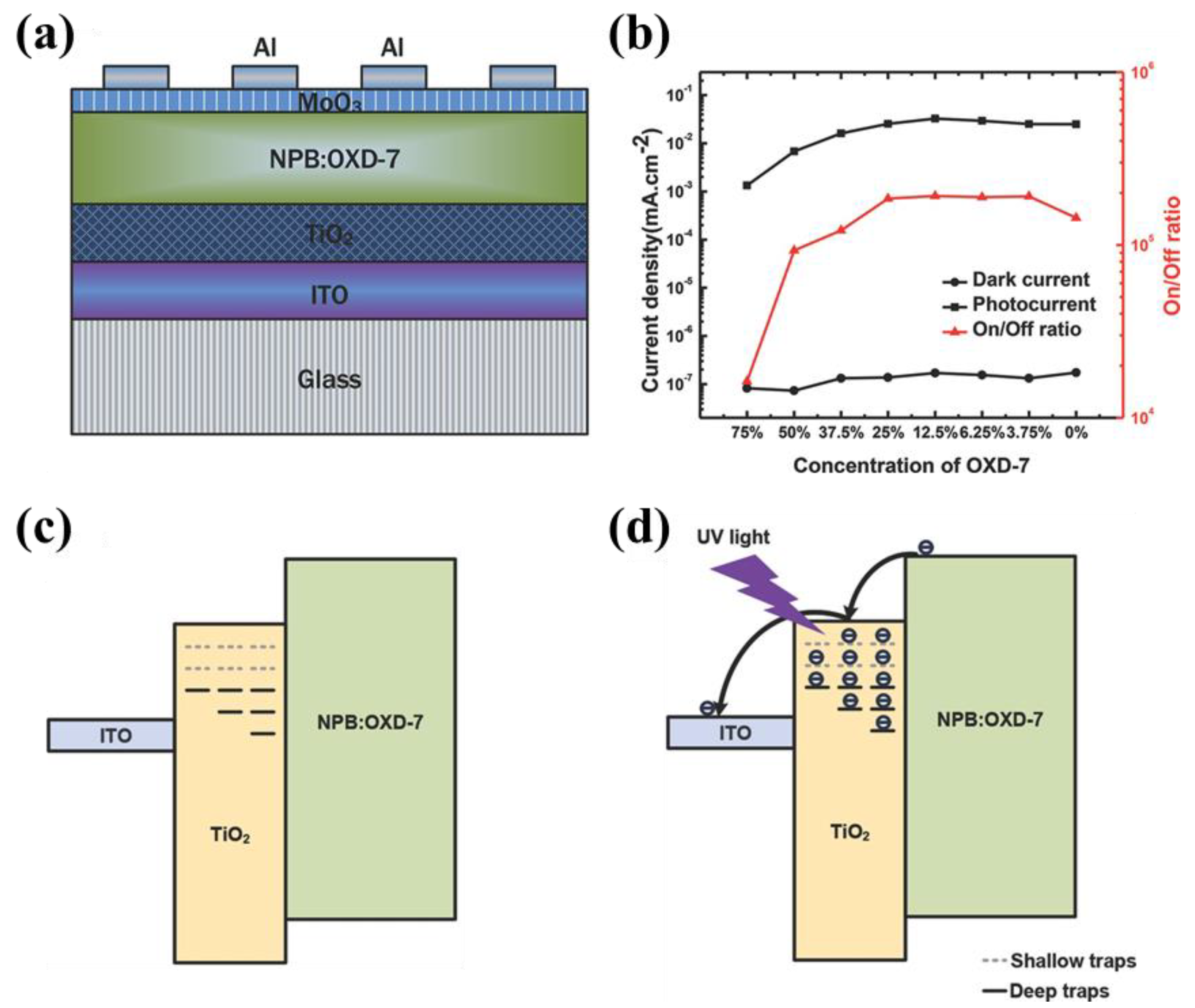
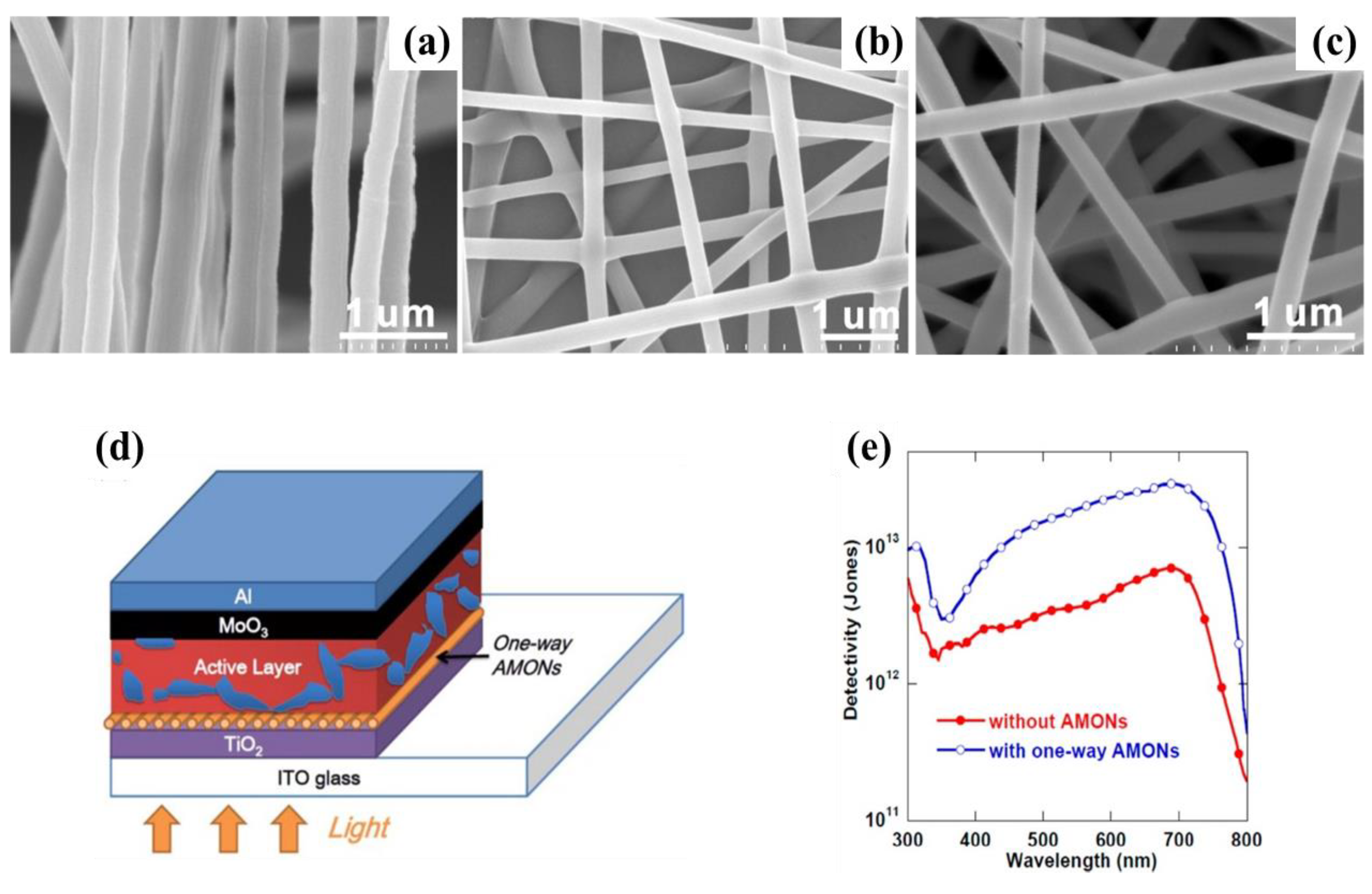



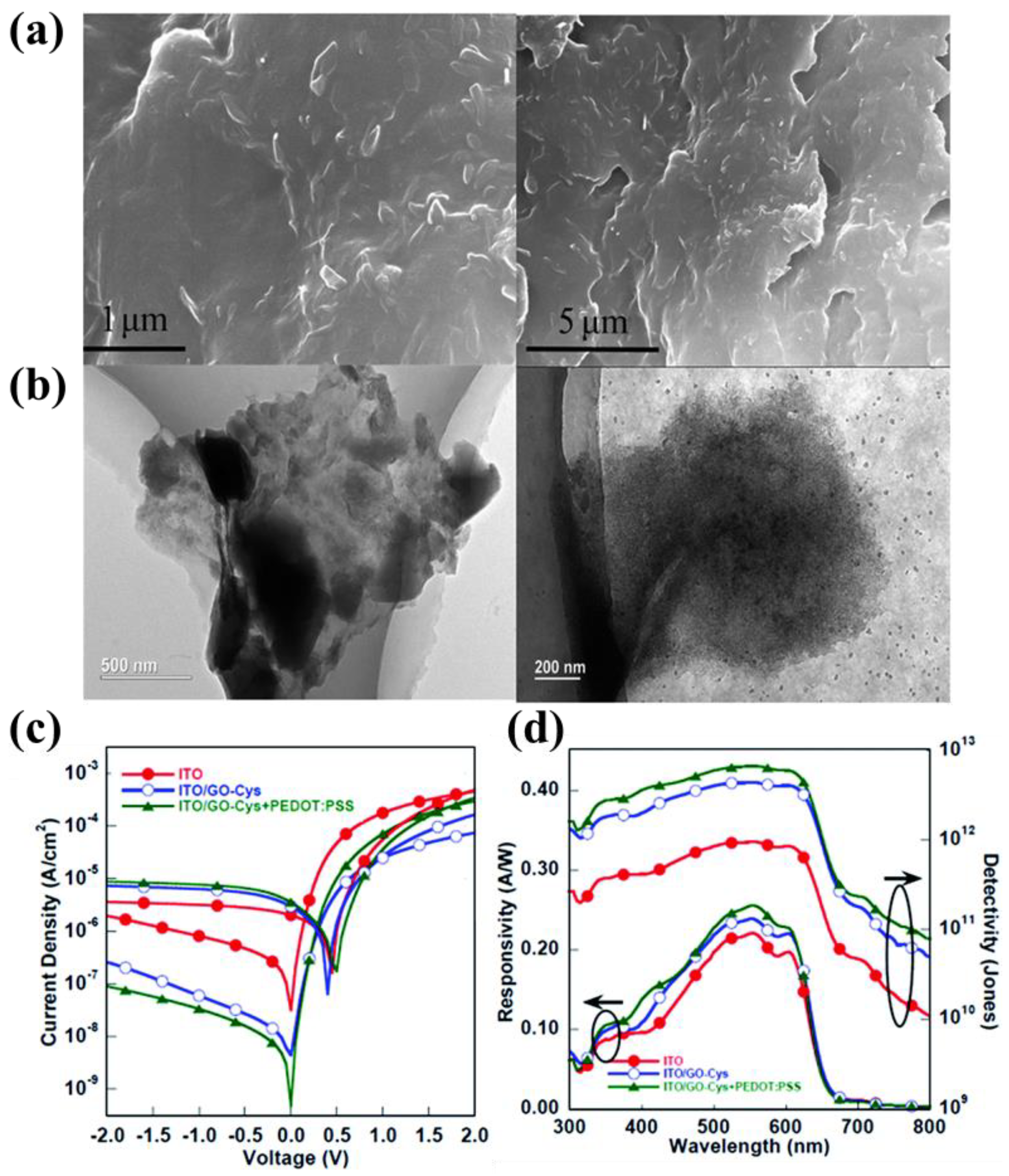
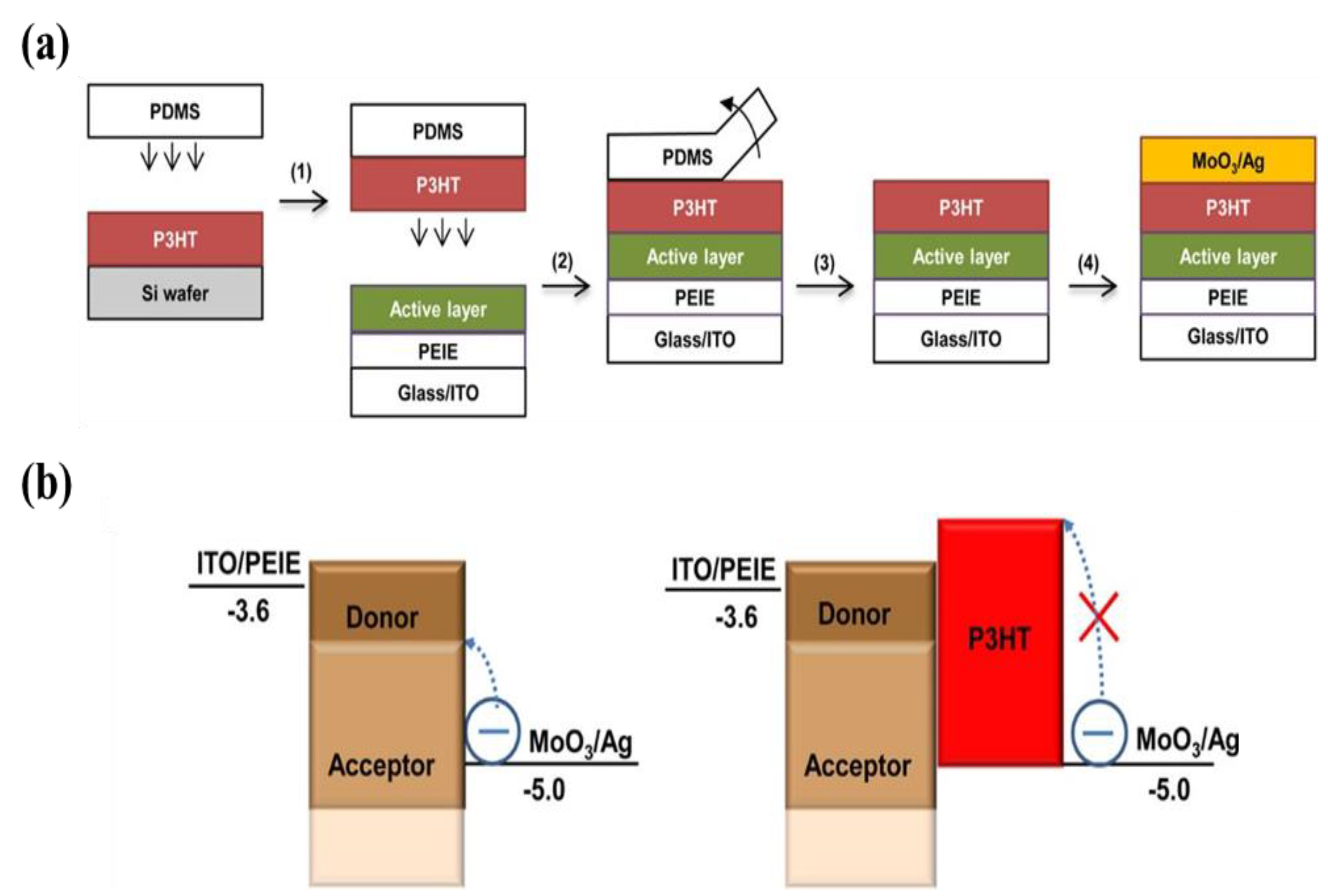
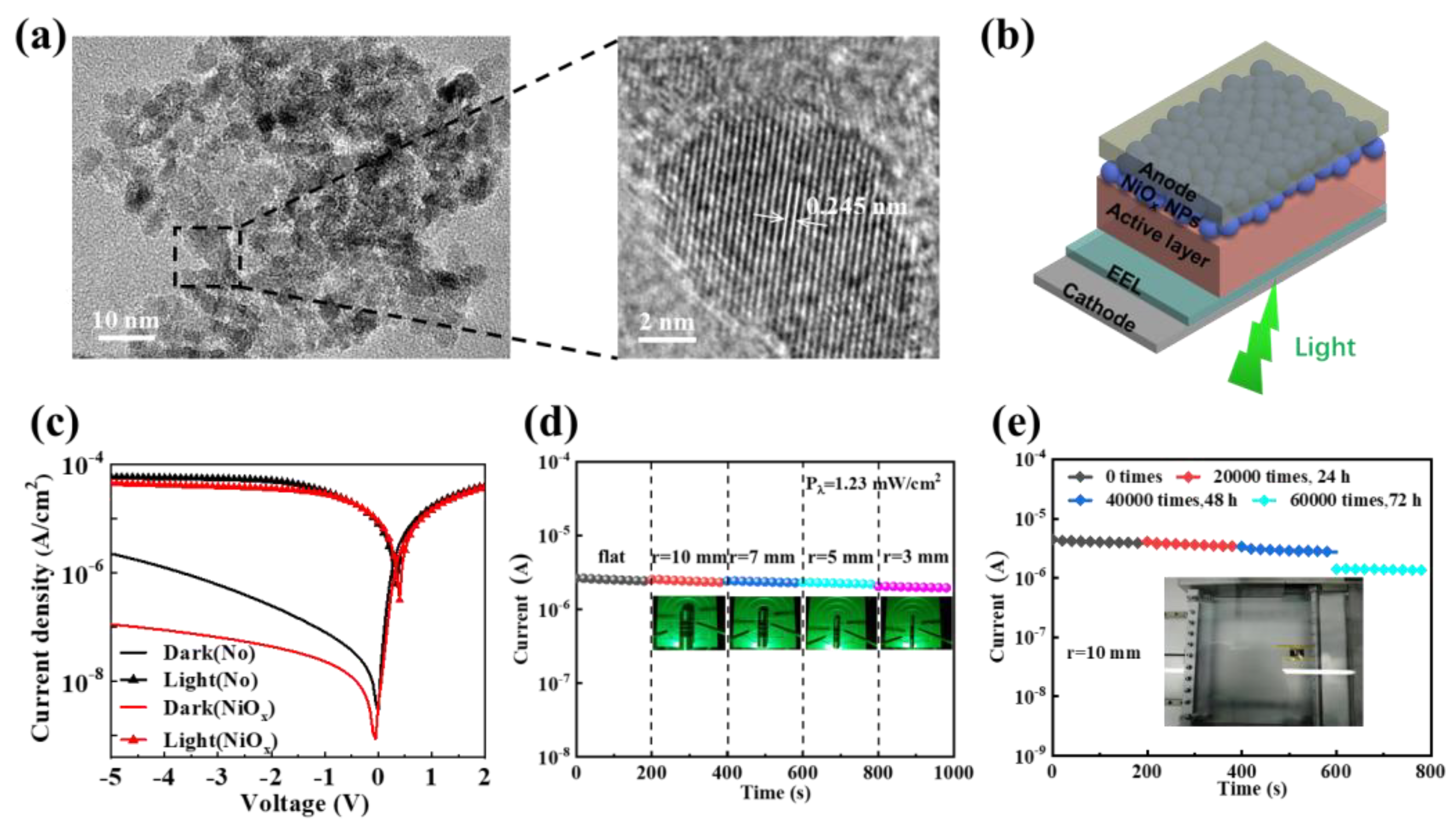
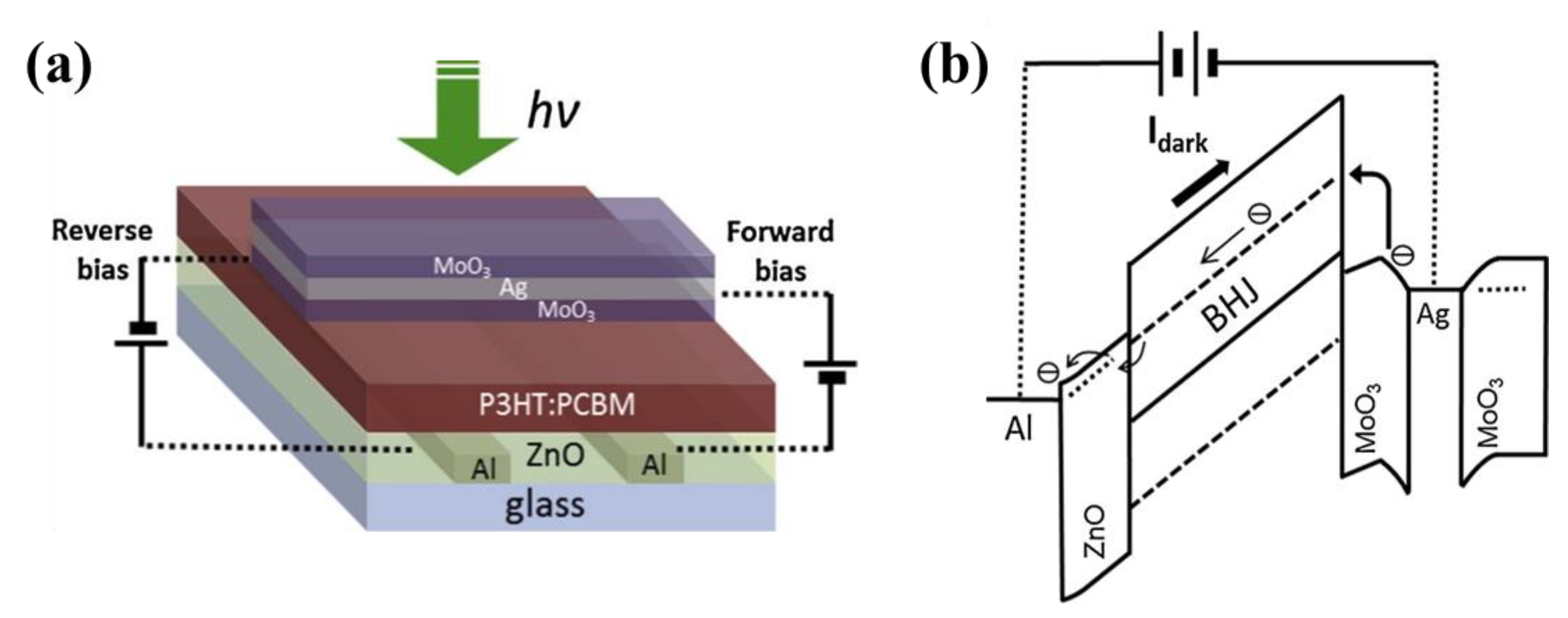
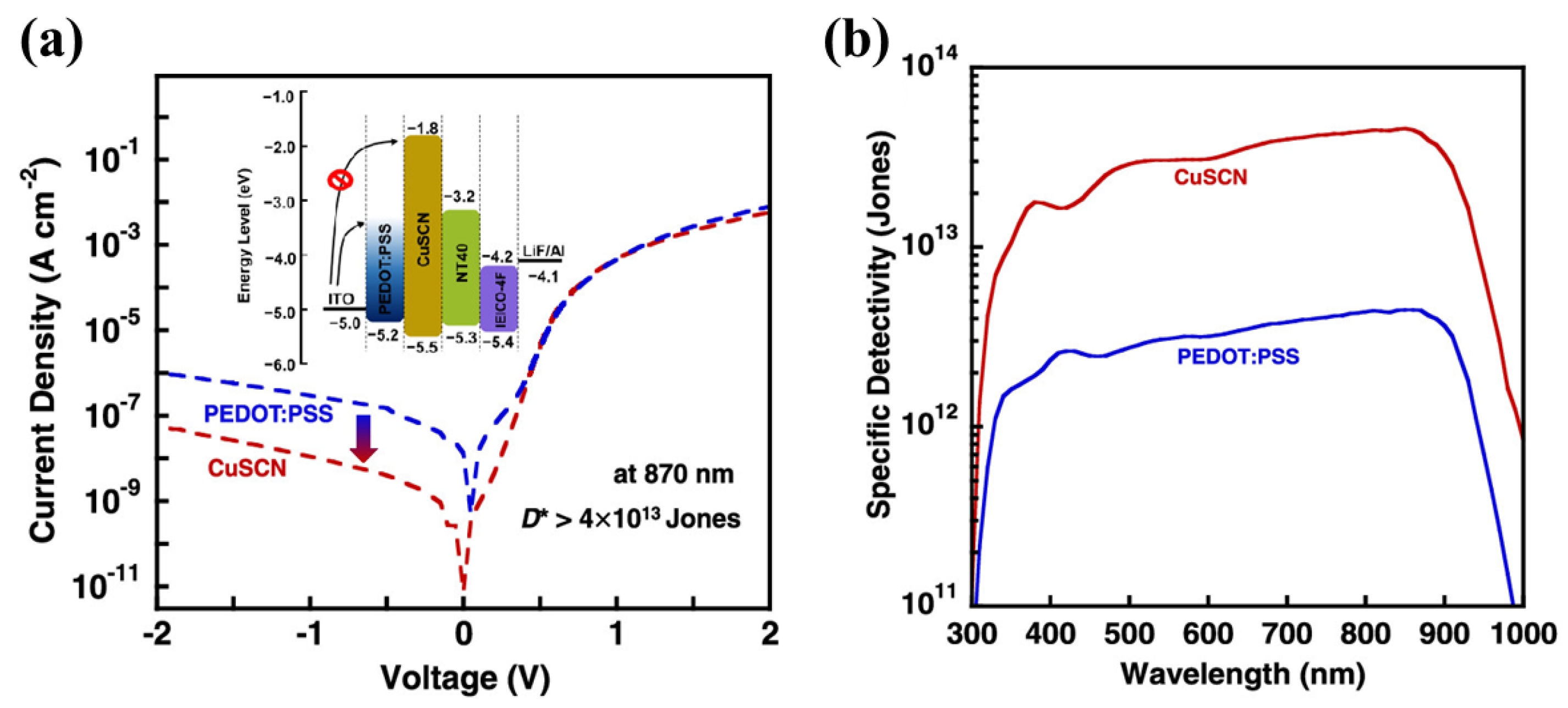
| Materials | Device Structure | Jd (A cm−2) | EQE (%) | D* (Jones) | R (A W−1) | Measurement Conditions | Ref |
|---|---|---|---|---|---|---|---|
| BCP | ITO/PEDOT:PSS/active layer/BCP/Al | 1.1 × 10−9 | - | 1.4 × 1012 | 0.068 | −2 V, @ 800 nm | [44] |
| ITO/TPBi/MoO3/C70/active layer/BCP/Al | 2.2 × 10−2 | 68,927 1 | 2.2 × 1012 | 188 | −6 V, @ 345 nm | [45] | |
| ITO/PEDOT:PSS/active layer/BCP/Al | 1.3 × 10−5 | 2170 | 8.3 × 1011 | 6.39 | −15 V, @ 360 nm | [46] | |
| Bphen | ITO/TAPC:MoO3/active layer/Bphen/Ag | 1.11 × 10−9 | 41.8 | 6.43 × 1012 | 0.121 | −3 V, @ 360 nm | [51] |
| ITO/PEDOT:PSS/active layer/BCP/Ag | 4.82 × 10−4 | - | 3.7 × 1011 | - | −1.5 V, @ 350 nm, 0.5 mW cm−2 | [52] | |
| ITO/MoO3/CuI/active layer/Bphen/Al | ~10−2 | 400 | 1012 | - | −8 V, @ 870 nm | [53] | |
| ITO/PEDOT:PSS/active layer/BCP/Ag | ~10−5 | 43.78 | 2.67 × 1012 | 0.25 | @ 710 nm | [55] | |
| ITO/TAPC/active layer/BCP/Ag | 1.15 × 10−9 | 74.6 | 4.14 × 1013 | 0.439 | −2 V, @ 730 nm | [56] | |
| PEIE | ITO/PEIE/active layer/MoO3/Ag | 7.7 × 10−9 | 60 | 4.8 × 1012 | 0.24 | −1.5 V, @ 546 nm | [65] |
| ITO/PEIE/active layer/Al | 4 × 10−5 | 12,000 | 2.27 × 1012 | 54 | −0.8 V, @ 530 nm | [66] | |
| ITO/PEIE/active layer/Au | 7.7 × 10−10 | - | 2.2 × 1013 | 0.32 | −2 V, @ 629 nm | [67] | |
| ITO/PEIE/active layer/Al | 2.77 × 10−6 | 3200 | 1.04 × 1012 | 14.25 | −1 V, @ 550 nm | [69] | |
| PEDOT:PSS/PEIE/active layer/PEDOT:PSS | 1.5 × 10−10 | 55 | 3.45 × 1013 | - | −5 V, @ 530 nm | [70] | |
| PFN | ITO/PFN/active layer/Ag | 5.10 × 10−4 | 67.09 | 2.47 × 1012 | 0.37 | −0.5 V, @ 680 nm | [75] |
| ITO/PFN/active layer/Al | ~10−3 | 650 | 1.76 × 1012 | 8.7 | −15 V, @ 520 nm | [76] | |
| ITO/PFN/active layer/Al | 1.92 × 10−8 | 208.11 | 9.1 × 1012 | 0.921 | −0.5 V, @ 550 nm | [77] | |
| ITO/PEDOT:PSS/active layer/PFN-Br/Ag | ~10−8 | 65 | 1013 | - | −10 V, @ 860 nm | [78] | |
| ITO/PEDOT:PSS/active layer/PFN-Br/Al | 4.85 × 10−10 | 56 | 2.61 × 1013 | 0.33 | −0.1 V, 720 nm | [6] | |
| PEI | PEDOT:PSS/PEI/active layer/PEDOT:PSS/PEDOT:PSS | ~10−7 | 65 | - | - | −4 V | [80] |
| PEDOT:PSS/PEI/active layer/Poly-PT/PEDOT:PSS | ~10−7 | 65 | 2.2 × 1012 | - | −1 V, @ 505 nm | [81] | |
| PEDOT:PSS/PEI/active layer/PEDOT:PSS | 5.7 × 10−8 | 46 | 3.35 × 1012 | - | −1 V, @ 525 nm | [82] | |
| C60 | ITO/PEDOT:PSS/active layer/C60/Al | 8 × 10−10 | 60 | ~1013 | - | −1 V, @ 532 nm | [61] |
| ITO/PEDOT:PSS/active layer/C60/Al | 1.1 × 10−10 | 67.1 | 9.2 × 1012 | 0.38 | −1 V, @ 700 nm | [62] | |
| ITO/ZnO/active layer/MoO3/Ag | 6.51 × 10−8 | ~70 | 2.58 × 1011 | 0.48 | −0.1 V, @ 700 nm | [84] | |
| Ag/ZnO/active layer/PEDOT:PSS | ~10−10 | - | 2 × 1012 | 0.3 | −1 V, @ 532 nm | [85] | |
| ITO/ZnO/active layer/MoO3/Al | 3.9 × 10−9 | 140,000 | 6.3 × 1012 | - | −0.5 V, @ 680 nm | [88] | |
| ITO/ZnO/PEIE/active layer/MoO3/Ag | 8.075 × 10−6 | 62 | 3.749 × 1012 | 0.281 | 2 V, @ 680 nm | [90] | |
| PEDOT:PSS/ZnO/PEIE/active layer/PEDOT:PSS | 2.11 × 10−8 | ~40 | ~1011 | ~0.17 | 0 V, @ 700 nm | [92] | |
| TiO2 | ITO/TiO2/active layer/MoO3/Ag | ~10−7 | - | ~1012 | 0.022 | 0 V, @ 350 nm | [99] |
| ITO/TiO2/active layer/Al | 1.09 × 10−7 | 113 | 1.9 × 1012 | 0.5 | −1 V, @ 550 nm | [104] | |
| ITO/TiO2/active layer/MoO3/Al | ~10−9 | 94.22 | 2.93 × 1013 | - | 0 V, @ 690 nm | [10] | |
| SnO2 | ITO/SnO2/active layer/Al | 2.89 × 10−4 | 1430 | 2.29 × 1013 | 6.97 | −1 V, @ 625 nm | [107] |
| ITO/SnO2/active layer/MoO3/Ag | ~10−9 | ~70 | 5.82 × 1012 | - | −1 V, @ 900 nm | [108] | |
| Cs2CO3 | ITO/PEDOT:PSS/active layer/Cs2CO3/Al | 2.1 × 10−8 | >100 | 3 × 1012 | - | −3 V, @ 450 nm | [109] |
| LiF | ITO/PEDOT:PSS/active layer/LiF/Al | 1.7 × 10−8 | - | - | 0.004 | −3 V, @ solar simulator | [113] |
| ITO/PEDOT:PSS/active layer/LiF/Al | 2.7 × 10−7 (−1 V) | 5600 | - | 15.9 | −40 V, @ 360 nm | [114] |
| Materials | Device Structure | Jd (A cm−2) | EQE (%) | D* (Jones) | R (A W−1) | Measurement Conditions | Ref |
|---|---|---|---|---|---|---|---|
| TFB | ITO/TFB/active layer/Al | 2 × 10−8 | 35 | 3.34 × 1012 | - | −5 V, @ 900 nm | [116] |
| PEDOT:PSS/TFB/active layer/F8TBT/Al | 4 × 10−9 | 20 | - | - | −0.5 V, @ 650 nm | [117] | |
| ITO/PEDOT:PSS/TFB/active layer/Al | 8 × 10−9 | 9 | - | - | −0.5 V, @ 530 nm | [118] | |
| ITO/TFB/active layer/Al | 3.4 × 10−11 | 82 | 2.19 × 1013 | 0.44 | −5 V, @ 660 nm | [119] | |
| TIPS pentacene | ITO/TIPS pentacene/active layer/Al | 1 × 10−9 | 52 | 1.44 × 1013 | - | −5 V, @ 610 nm | [122] |
| ITO/TIPS pentacene/active layer/Al | 9 × 10−10 | 80 | 1.63 × 1013 | - | −5 V, @ 530 nm | [123] | |
| PEDOT:PSS | ITO/PEDOT:PSS/active layer/Al | ~10−3 | ~250 | ~6 × 1011 | 0.93 | −10 V, @ 400 nm | [126] |
| ITO/PEDOT:PSS/active layer/Al | 2.68 × 10−6 | 3.92 × 105 | 1.4 × 1011 | 46.1 | −25 V, @ 510 nm | [127] | |
| ITO/PEDOT:PSS/active layer/Al | ~10−7 | 2000 | ~1011 | ~0.4 | −50 V, @ 510 nm | [128] | |
| ITO/PEDOT:PSS+ GO–Cys 1/active layer/Al | ~10−10 | - | 5.7 × 1012 | ~0.15 | −0.1 V, @ 620 nm | [130] | |
| P3HT | ITO/PEIE/active layer/P3HT/MoO3/Al | ~1.5 × 10−9 | - | 4.15 × 1012 | 0.19 | −0.1 V, @ 525 nm | [133] |
| ITO/ZnO/active layer/P3HT/MoO3/Al | 1.1 × 10−8 | 70 | 3.0 × 1012 | 0.34 | −3 V, @ 630 nm | [134] | |
| ITO/ZnO/active layer/P3HT/Ag | 9 × 10−5 | - | 6.59 × 1010 | 0.214 | −2 V, @ 630 nm | [135] | |
| NiOx | ITO/NiOx/active layer/Yb/Ag | ~10−8 | 53.39 | 2.15 × 1012 | 0.253 | −1 V, @ 525 nm | [138] |
| ITO/NiOx/active layer/ZnO/Ag | 3.4 × 10−8 | ~52 | 1.2 × 1012 | 0.25 | −1 V, @ 600 nm | [139] | |
| ITO/ZnO:Al/active layer/NiOx/Ag | 1.13 × 10−7 | - | 3.86 × 1012 | 0.74 | −5 V, @ 525 nm | [141] | |
| ITO/ZnO:Al/active layer/NiOx/Ag | 8.09 × 10−8 | 81.93 | 2.15 × 1012 | 0.35 | −5 V, @ 525 nm | [142] | |
| MoO3 | ITO/ZnO/active layer/MoO3/Ag | 5.9 × 10−9 | - | 3.04 × 1012 | - | −3 V, @ 650 nm | [147] |
| Al/ZnO/active layer/MoO3/Ag/MoO3 | 2.95 × 10−7 | 31 | 4.49 × 1011 | 0.14 | −1.5 V, @ 650 nm | [148] | |
| Al/PCBM/active layer/MoO3/Ag/MoO3 | 1 × 10−9 | 30 | 6 × 1012 | - | −1 V, @ 500 nm | [149] | |
| V2O5 | ITO/PEDOT:PSS + V2O5/active layer/V2O5/Al | 5.53 × 10−3 | - | - | - | −1 V, @ (AM 1.5 G) solar simulator | [155] |
| CuSCN | ITO/CuSCN/active layer/LiF/Al | 2.7 × 10−10 | 57.2 | 4.4 × 1013 | 0.4 | −0.1 V, @ 870 nm | [21] |
| ITO/CuSCN/active layer/BCP/Mg:Ag | 1 × 10−8 | - | - | - | −1 V | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, H.; Zhang, J. Carrier Blocking Layer Materials and Application in Organic Photodetectors. Nanomaterials 2021, 11, 1404. https://doi.org/10.3390/nano11061404
Li Y, Chen H, Zhang J. Carrier Blocking Layer Materials and Application in Organic Photodetectors. Nanomaterials. 2021; 11(6):1404. https://doi.org/10.3390/nano11061404
Chicago/Turabian StyleLi, Yi, Hu Chen, and Jianhua Zhang. 2021. "Carrier Blocking Layer Materials and Application in Organic Photodetectors" Nanomaterials 11, no. 6: 1404. https://doi.org/10.3390/nano11061404
APA StyleLi, Y., Chen, H., & Zhang, J. (2021). Carrier Blocking Layer Materials and Application in Organic Photodetectors. Nanomaterials, 11(6), 1404. https://doi.org/10.3390/nano11061404





