α,β-Enone Borylation by Bis(Pinacolato)Diboron Catalyzed by Cu3(BTC)2 Using Cesium Carbonate as a Base
Abstract
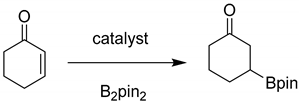
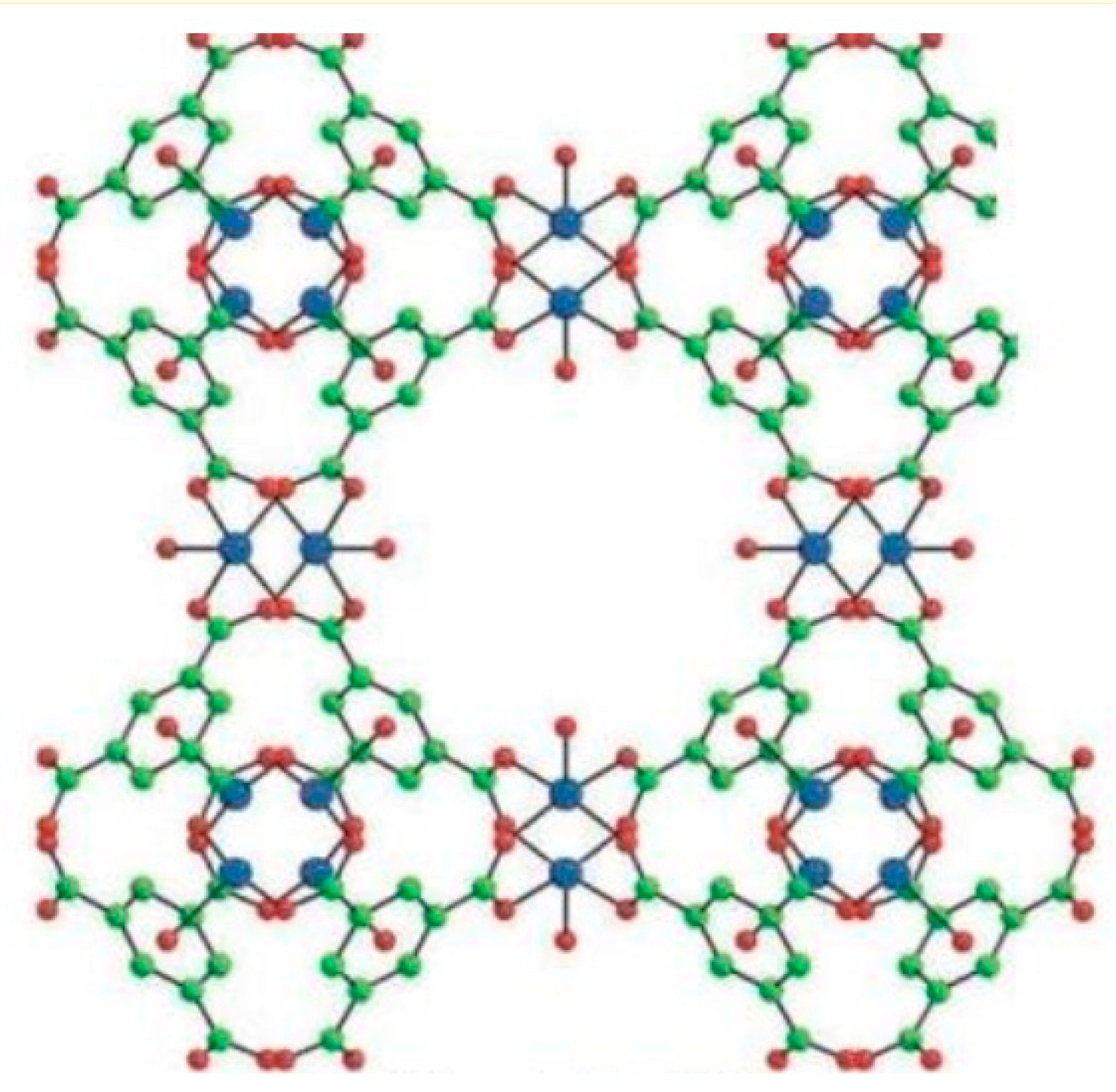
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Product Analysis
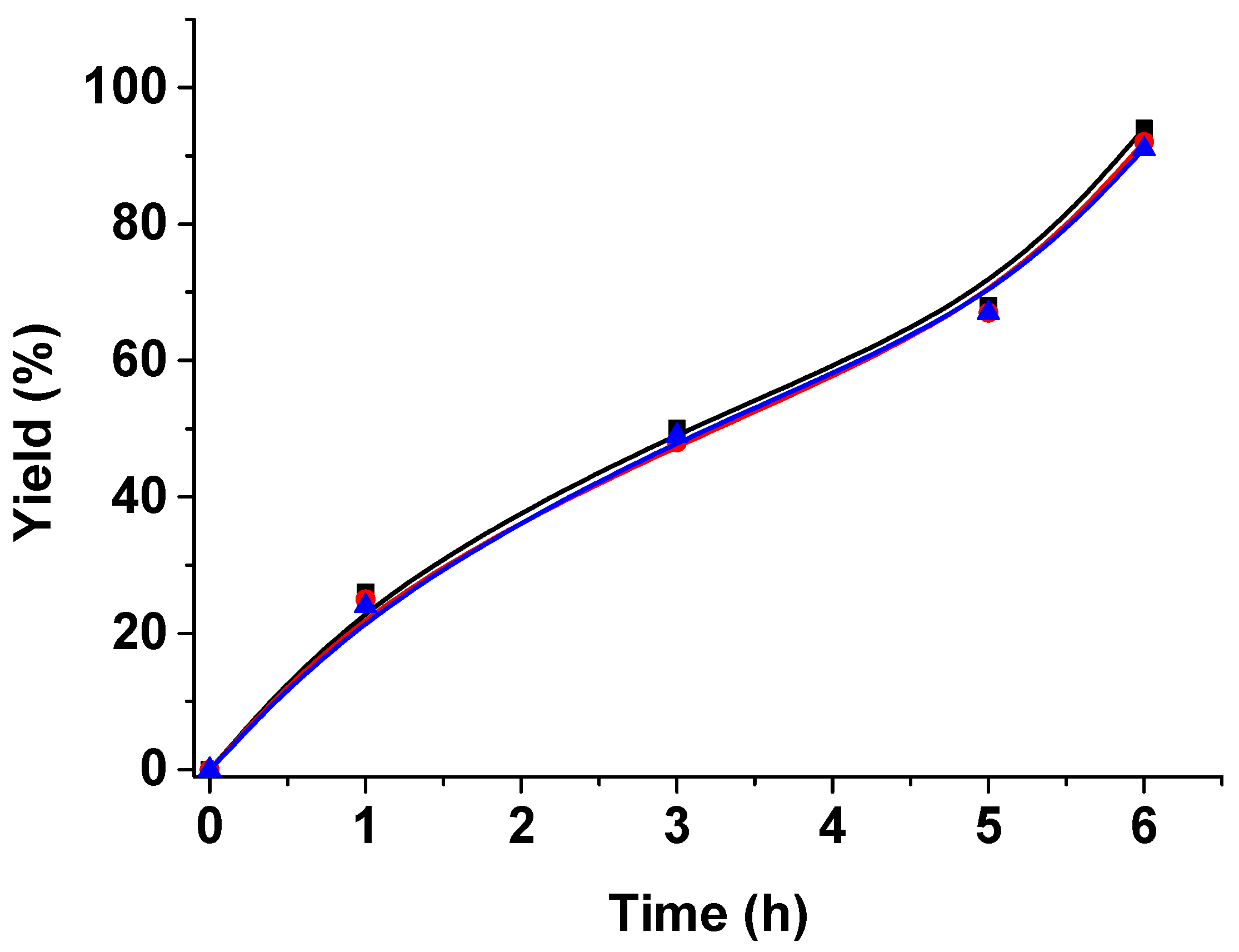
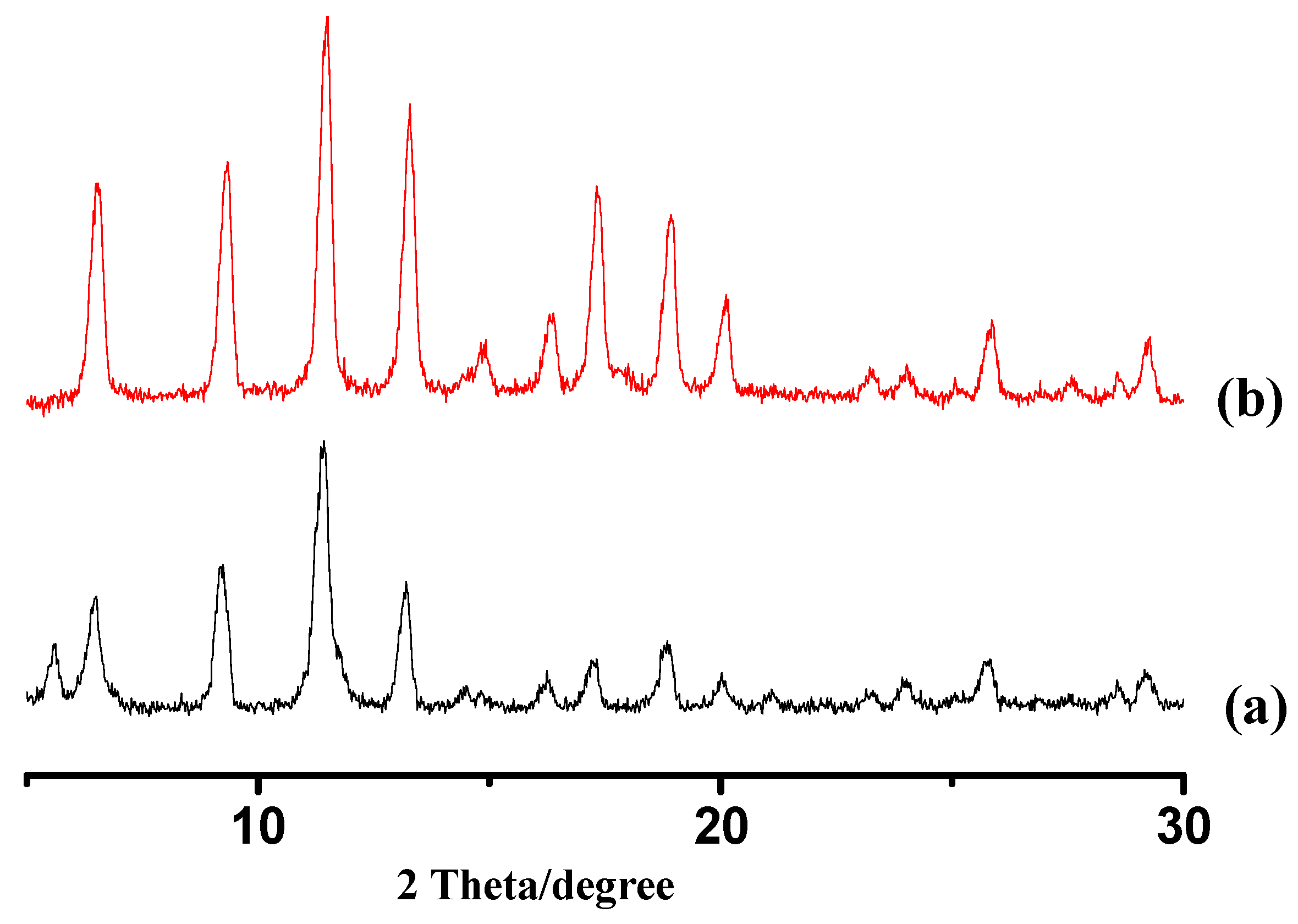
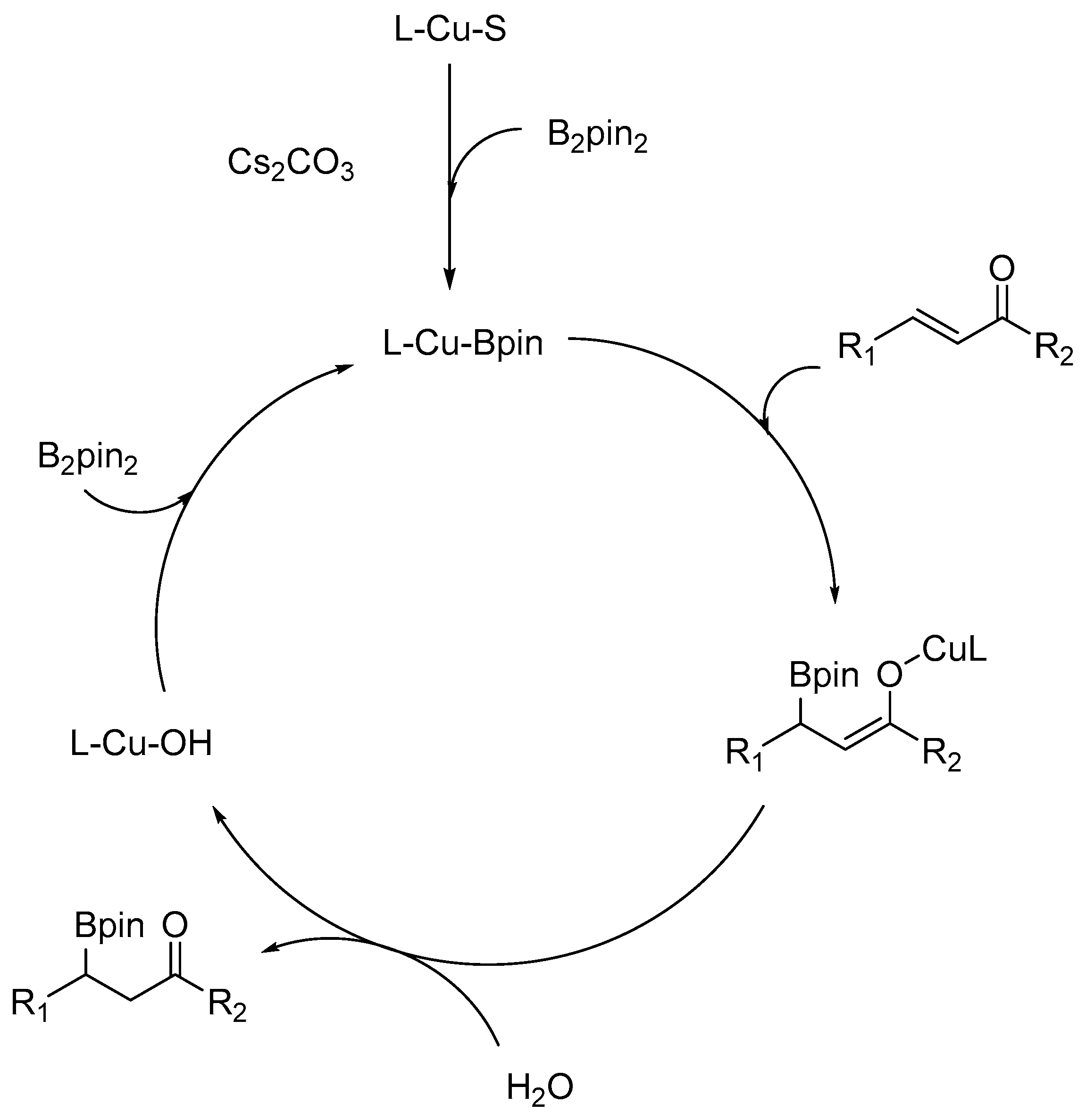
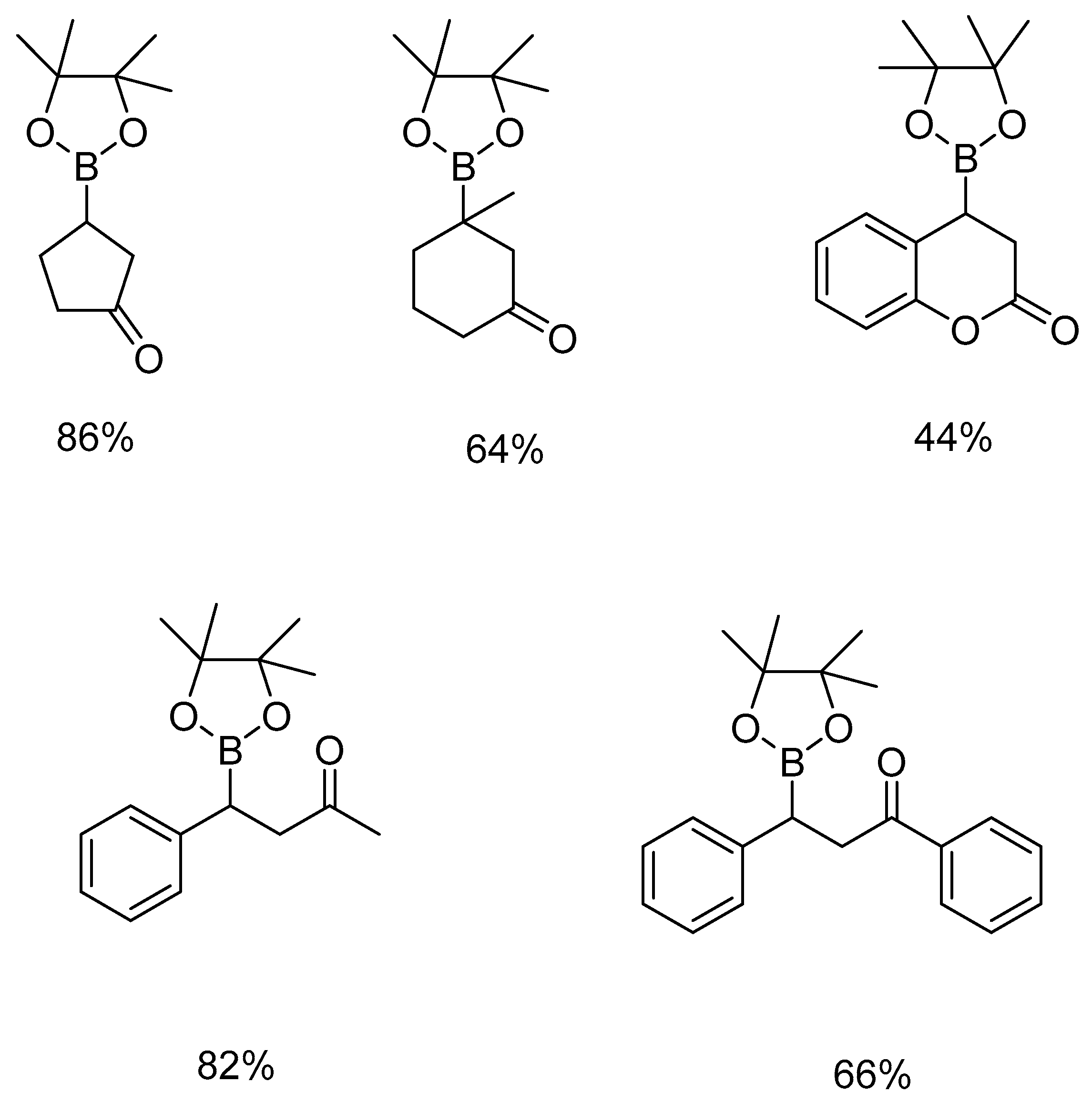
3. Results and Discussion
 | |||||
|---|---|---|---|---|---|
| Run | Catalyst | Base | T (°C) | Time (h) | Yield (%) b |
| 1 | - | Cs2CO3 | RT | 24 | - |
| 2 | Cu3(BTC)2 | - | RT | 24 | - |
| 3 | Cu3(BTC)2 | Cs2CO3 | RT | 24 | 80 |
| 4 | - | Cs2CO3 | 60 | 6 | 3 |
| 5 | Cu3(BTC)2 | - | 60 | 6 | - |
| 6 | Cu3(BTC)2 | Cs2CO3 | 60 | 6 | 94 |
| 7 | Cu3(BTC)2 | Cs2CO3 | 60 | 6 | 92 c |
| 8 | Cu3(BTC)2 | K2CO3 | 60 | 6 | 92 |
| 9 | Cu3(BTC)2 | Na2CO3 | 60 | 6 | 55 |
| 10 | CuCl | Cs2CO3 | 60 | 1, 6 | 41, 41 d |
| 11 | Cu(NO3)2·3H2O | Cs2CO3 | 60 | 6 | - e |
| 12 | MIL-101(Cr) | Cs2CO3 | 60 | 6 | 6 |
| 13 | Al(OH)(BDC) | Cs2CO3 | 60 | 6 | - |
| 14 | ZIF-8 | Cs2CO3 | 60 | 6 | 5 |
| 15 | Cu3(BTC)2 | Cs2CO3 | 60 | 6 | 82 f |
| 16 | Cu3(BTC)2 | Cs2CO3 | 60 | 6 | 88 g |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mkhalid, I.A.I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C−H Activation for the Construction of C−B Bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef]
- Hartwig, J.F. Borylation and Silylation of C–H Bonds: A Platform for Diverse C–H Bond Functionalizations. Acc. Chem. Res. 2012, 45, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, T.; Miyaura, N. Metal-catalyzed reactions of diborons for synthesis of organoboron compounds. Chem. Rec. 2004, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ros, A.; Fernández, R.; Lassaletta, J.M. Functional group directed C–H borylation. Chem. Soc. Rev. 2014, 43, 3229–3243. [Google Scholar] [CrossRef] [PubMed]
- Grirrane, A.; Corma, A.; Garcia, H. Stereoselective Single (Copper) or Double (Platinum) Boronation of Alkynes Catalyzed by Magnesia-Supported Copper Oxide or Platinum Nanoparticles. Chem. Eur. J. 2011, 17, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, T.; Miyaura, N. Transition metal-catalyzed borylation of alkanes and arenes via C-H activation. J. Organomet. Chem. 2003, 680, 3–11. [Google Scholar] [CrossRef]
- Lawson, Y.G.; Lesley, M.J.G.; Marder, T.B.; Norman, N.C.; Rice, C.R. Platinum catalysed 1,4-diboration of α,β-unsaturated ketones. Chem. Commun. 1997, 21, 2051–2052. [Google Scholar] [CrossRef]
- Bell, N.J.; Cox, A.J.; Cameron, N.R.; Evans, J.S.O.; Marder, T.B.; Duin, M.A.; Elsevier, C.J.; Baucherel, X.; Tulloch, A.A.D.; Tooze, R.P. Platinum catalysed 3,4- and 1,4-diboration of α,β-unsaturated carbonyl compounds using bis-pinacolatodiboron. Chem. Commun. 2004, 16, 1854–1855. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Das, B.C.; Das, S. Rhodium-catalyzed 1,4-addition reactions of diboron reagents to electron deficient olefins. Tetrahedron Lett. 2002, 43, 2323–2325. [Google Scholar] [CrossRef]
- Mun, S.; Lee, J.-E.; Yun, J. Copper-Catalyzed β-Boration of α,β-Unsaturated Carbonyl Compounds: Rate Acceleration by Alcohol Additives. Org. Lett. 2006, 8, 4887–4889. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kwon, J.; Yun, J. Copper-catalyzed addition of diboron reagents to α,β-acetylenic esters: Efficient synthesis of β-boryl-α,β-ethylenic esters. Chem. Commun. 2008, 6, 733–734. [Google Scholar] [CrossRef] [PubMed]
- Chea, H.; Sim, H.-S.; Yun, J. Copper-Catalyzed Conjugate Addition of Diboron Reagents to α,β-Unsaturated Amides: Highly Reactive Copper-1,2- Bis(diphenylphosphino)benzene Catalyst System. Adv. Synth. Catal. 2009, 351, 855–858. [Google Scholar] [CrossRef]
- Kitanosono, T.; Xu, P.; Kobayashi, S. Heterogeneous versus Homogeneous Copper(II) Catalysis in Enantioselective Conjugate-Addition Reactions of Boron in Water. Chem. Asian J. 2014, 9, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Yorimitsu, H.; Oshima, K. Nickel-Catalyzed β-Boration of α,β-Unsaturated Esters and Amides with Bis(pinacolato)diboron. Org. Lett. 2007, 9, 5031–5033. [Google Scholar] [CrossRef]
- Lee, K.-S.; Zhugralin, A.R.; Hoveyda, A.H. Efficient C-B bond formation promoted by N-heterocyclic carbenes: Synthesis of tertiary and quaternary B-substituted carbons through metal-free catalytic boron conjugate additions to cyclic and acyclic α,β-unsaturated carbonyls. J. Am. Chem. Soc. 2009, 131, 7253–7255. [Google Scholar] [CrossRef]
- Wu, H.; Radomkit, S.; O’Brien, J.M.; Hoveyda, A.H. Metal-Free Catalytic Enantioselective C–B Bond Formation: (Pinacolato)boron Conjugate Additions to α,β-Unsaturated Ketones, Esters, Weinreb Amides, and Aldehydes Promoted by Chiral N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2012, 134, 8277–8285. [Google Scholar] [CrossRef]
- Pubill-Ulldemolins, C.; Bonet, A.; Bo, C.; Gulyas, H.; Fernandez, E. Activation of Diboron Reagents with Brønsted Bases and Alcohols: An Experimental and Theoretical Perspective of the Organocatalytic Boron Conjugate Addition Reaction. Chem. Eur. J. 2012, 18, 1121–1126. [Google Scholar] [CrossRef]
- Feng, X.; Yun, J. Catalytic enantioselective boron conjugate addition to cyclic carbonyl compounds: A new approach to cyclic β-hydroxy carbonyls. Chem. Commun. 2009, 43, 6577–6579. [Google Scholar] [CrossRef]
- Kobayashi, S.; Xu, P.; Endo, T.; Ueno, M.; Kitanosono, T. Chiral Copper(II)-Catalyzed Enantioselective Boron Conjugate Additions to α,β-Unsaturated Carbonyl Compounds in Water. Angew. Chem. 2012, 124, 12935–12938. [Google Scholar] [CrossRef]
- Kitanosono, T.; Kobayashi, S. Asymmetric Boron Conjugate Additions to Enones in Water Catalyzed by Copper(0). Asian J. Org. Chem. 2013, 2, 961–966. [Google Scholar] [CrossRef]
- Calow, A.D.J.; Sol, C.; Whiting, A.; Fernandez, E. Base-Free β-Boration of α,β-Unsaturated Imines Catalysed by Cu2O with Concurrent Enhancement of Asymmetric Induction. ChemCatChem 2013, 5, 2233–2239. [Google Scholar] [CrossRef]
- Cano, R.; Ramon, D.J.; Yus, M. Impregnated Copper on Magnetite as Recyclable Catalyst for the Addition of Alkoxy Diboron Reagents to C−C Double Bonds. J. Org. Chem. 2010, 75, 3458–3460. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.B.; Calderone, J.A.; Santos, W.L. Unexpected Copper(II) Catalysis: Catalytic Amine Base Promoted β-Borylation of α,β-Unsaturated Carbonyl Compounds in Water. Org. Lett. 2012, 14, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, L.; Séguin, E.; Poelman, H.; Thibault-Starzyk, F.; Jacobs, P.A.; De Vos, D.E. Probing the Lewis Acidity and Catalytic Activity of the Metal-Organic Framework [Cu3(btc)2] (BTC = Benzene-1,3,5-tricarboxylate). Chem. Eur. J. 2006, 12, 7353–7363. [Google Scholar] [CrossRef] [PubMed]
- Shamzhy, M.V.; Opanasenko, M.V.; Garcia, H.; Čejka, J. Annulation of phenols with methylbutenol over MOFs: The role of catalyst structure and acid strength in producing 2,2-dimethylbenzopyran derivatives. Microporous Mesoporous Mater. 2015, 202, 297–302. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Formation of C–C and C–Heteroatom Bonds by C–H Activation by Metal Organic Frameworks as Catalysts or Supports. ACS Catal. 2019, 9, 1081–1102. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Catalysis in Confined Spaces of Metal Organic Frameworks. ChemCatChem 2020, 12, 4732–4753. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Matrosova, M.M.; Jeon, J.; Jun, J.W.; Timofeeva, M.N.; Jhung, S.H. Catalytic behavior of metal-organic frameworks in the Knoevenagel condensation reaction. J. Catal. 2014, 316, 251–259. [Google Scholar] [CrossRef]
- Anbu, N.; Dhakshinamoorthy, A. Regioselective ring opening of styrene oxide by carbon nucleophiles catalyzed by metal-organic frameworks under solvent-free conditions. J. Ind. Eng. Chem. 2018, 58, 9–17. [Google Scholar] [CrossRef]
- Nikseresht, A.; Ghasemi, S.; Parak, S. [Cu3(BTC)2]: A metal-organic framework as an environment-friendly and economically catalyst for the synthesis of tacrine analogues by Friedländer reaction under conventional and ultrasound irradiation. Polyhedron 2018, 151, 112–117. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, B.R.; Lee, C.Y.; Kim, J. N-Sulfonyl amidine synthesis via three-component coupling reaction using heterogeneous copper catalyst derived from metal-organic frameworks. Tetrahedron Lett. 2016, 57, 4070–4073. [Google Scholar] [CrossRef]
- Perez-Mayoral, E.; Cejka, J. [Cu3(BTC)2]: A Metal-Organic Framework Catalyst for the Friedländer Reaction. ChemCatChem 2011, 3, 157–159. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, T.; Li, M.; He, C.; Duan, C. Highly shape- and regio-selective peroxy-trifluoromethylation of styrene by metal-organic framework Cu3(BTC)2. Catal. Sci. Technol. 2017, 7, 5872–5881. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Concepcion, P.; Garcia, H. Synthesis of borasiloxanes by oxidative hydrolysis of silanes and pinacolborane using Cu3(BTC)2 as a solid catalyst. Chem. Commun. 2017, 53, 9998–10001. [Google Scholar] [CrossRef]
- Vandichel, M.; Vermoortele, F.; Cottenie, S.; De Vos, D.E.; Waroquier, M.; Van Speybroeck, V. Insight in the activity and diastereoselectivity of various Lewis acid catalysts for the citronellal cyclization. J. Catal. 2013, 305, 118–129. [Google Scholar] [CrossRef][Green Version]
- Guo, C.; Zhang, Y.; Zhang, L.; Guo, Y.; Akram, N.; Wang, J. 2-Methylimidazole-Assisted Synthesis of Nanosized Cu3(BTC)2 for Controlling the Selectivity of the Catalytic Oxidation of Styrene. ACS Appl. Nano Mater. 2018, 1, 5289–5296. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Wang, Y.; Luan, Y.; Li, X.; Du, X. Growth of Cu-BTC MOFs on dendrimer-like porous silica nanospheres for the catalytic aerobic epoxidation of olefins. New J. Chem. 2020, 44, 14350–14357. [Google Scholar] [CrossRef]
- Ye, J.-Y.; Liu, C.-J. Cu3(BTC)2: CO oxidation over MOF based catalysts. Chem. Commun. 2011, 47, 2167–2169. [Google Scholar] [CrossRef]
- Latha, G.; Devarajan, N.; Suresh, P. Framework Copper Catalyzed Oxidative Synthesis of Quinazolinones: A Benign Approach Using Cu3(BTC)2 MOF as an Efficient and Reusable Catalyst. ChemistrySelect 2020, 5, 10041–10047. [Google Scholar] [CrossRef]
- Kal-Koshvandi, A.T.; Maleki, A.; Tarlani, A.; Soroush, M.R. Synthesis and Characterization of Ultrapure HKUST-1 MOFs as Reusable Heterogeneous Catalysts for the Green Synthesis of Tetrazole Derivatives. ChemistrySelect 2020, 5, 3164–3172. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; García, C.V.; Concepcion, P.; Garcia, H. Arene borylation through CH activation using Cu3(BTC)2 as heterogeneous catalyst. Catal. Today 2021, 366, 212–217. [Google Scholar] [CrossRef]
- Anbu, N.; Dhakshinamoorthy, A. Cu3(BTC)2 as a viable heterogeneous solid catalyst for Friedel-Crafts alkylation of indoles with nitroalkenes. J. Colloid Interface Sci. 2017, 494, 282–289. [Google Scholar]
- Anbu, N.; Dhakshinamoorthy, A. Cu3(BTC)2 catalyzed dehydrogenative coupling of dimethylphenylsilane with phenol and homocoupling of dimethylphenylsilane to disiloxane. J. Colloid Interface Sci. 2017, 490, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, C.G.; Schwarzer, M.; Herrmann, M.; Affini, A.; Pelagatti, P.; Maestri, G.; Maggi, R.; Loebbecke, S. Batch versus Flow Acetalization of Benzaldehyde with HKUST-1: Diffusion Pathways and Performance Comparison. ChemCatChem 2016, 8, 1293–1297. [Google Scholar] [CrossRef]
- Pathan, N.B.; Rahatgaonkar, A.M.; Chorghade, M.S. Metal-organic framework Cu3(BTC)2(H2O)3 catalyzed Aldol synthesis of pyrimidine-chalcone hybrids. Catal. Commun. 2011, 12, 1170–1176. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. HKUST-1 catalyzed room temperature hydrogenation of acetophenone by silanes. Catal. Commun. 2017, 97, 74–78. [Google Scholar] [CrossRef]
- Anbu, N.; Dhakshinamoorthy, A. Cu3(BTC)2 catalyzed oxidation of silane to silanol using TBHP or water as oxidants. Appl. Catal. A Gen. 2017, 544, 145–153. [Google Scholar] [CrossRef]
- Anbu, N.; Dhakshinamoorthy, A. Cu3(BTC)2 metal-organic framework catalyzed N-arylation of benzimidazoles and imidazoles with phenylboronic acid. J. Ind. Eng. Chem. 2018, 65, 120–126. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Cu3(BTC)2 as heterogeneous catalyst for the room temperature oxidative hydroxylation of arylboronic acids. Tetrahedron Lett. 2016, 72, 2895–2899. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Tuneable nature of metal organic frameworks as heterogeneous solid catalysts for alcohol oxidation. Chem. Commun. 2017, 53, 10851–10869. [Google Scholar] [CrossRef]
- Kitanosono, T.; Xu, P.; Kobayashi, S. Heterogeneous and Homogeneous Chiral Cu(II) Catalysis in Water: Enantioselective Boron Conjugate Additions to Dienones and Dienoesters. Chem. Commun. 2013, 49, 8184–8186. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, D.; Jin, X.; Mizuno, N.; Yamaguchi, K. Selective Dehydrogenative Mono- or Diborylation of Styrenes by Supported Copper Catalysts. ACS Catal. 2019, 9, 3011–3016. [Google Scholar] [CrossRef]
- Jiao, Z.-F.; Tian, Y.-M.; Guo, X.-N.; Radius, U.; Braunschweig, H.; Marder, T.B.; Guo, X.-Y. Visible-light-driven graphene supported Cu/Pd alloy nanoparticle-catalyzed borylation of alkyl bromides and chlorides in air. J. Catal. 2021, 395, 258–265. [Google Scholar] [CrossRef]
- Yao, Q.; Lu, X.; Liu, K.; Ma, C.; Su, J.; Lin, C.; Li, D.; Dou, J.; Sun, J.; Duan, W. An NHC-CuCl functionalized metal–organic framework for catalyzing β-boration of α,β-unsaturated carbonyl compounds. Dalton Trans. 2019, 48, 5144–5148. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakshinamoorthy, A.; Alvaro, M.; Asiri, A.M.; Garcia, H. α,β-Enone Borylation by Bis(Pinacolato)Diboron Catalyzed by Cu3(BTC)2 Using Cesium Carbonate as a Base. Nanomaterials 2021, 11, 1396. https://doi.org/10.3390/nano11061396
Dhakshinamoorthy A, Alvaro M, Asiri AM, Garcia H. α,β-Enone Borylation by Bis(Pinacolato)Diboron Catalyzed by Cu3(BTC)2 Using Cesium Carbonate as a Base. Nanomaterials. 2021; 11(6):1396. https://doi.org/10.3390/nano11061396
Chicago/Turabian StyleDhakshinamoorthy, Amarajothi, Mercedes Alvaro, Abdullah M. Asiri, and Hermenegildo Garcia. 2021. "α,β-Enone Borylation by Bis(Pinacolato)Diboron Catalyzed by Cu3(BTC)2 Using Cesium Carbonate as a Base" Nanomaterials 11, no. 6: 1396. https://doi.org/10.3390/nano11061396
APA StyleDhakshinamoorthy, A., Alvaro, M., Asiri, A. M., & Garcia, H. (2021). α,β-Enone Borylation by Bis(Pinacolato)Diboron Catalyzed by Cu3(BTC)2 Using Cesium Carbonate as a Base. Nanomaterials, 11(6), 1396. https://doi.org/10.3390/nano11061396








