Bismuth Doping in Nanostructured Tetrahedrite: Scalable Synthesis and Thermoelectric Performance
Abstract
:1. Introduction
2. Materials and Methods
3. Results
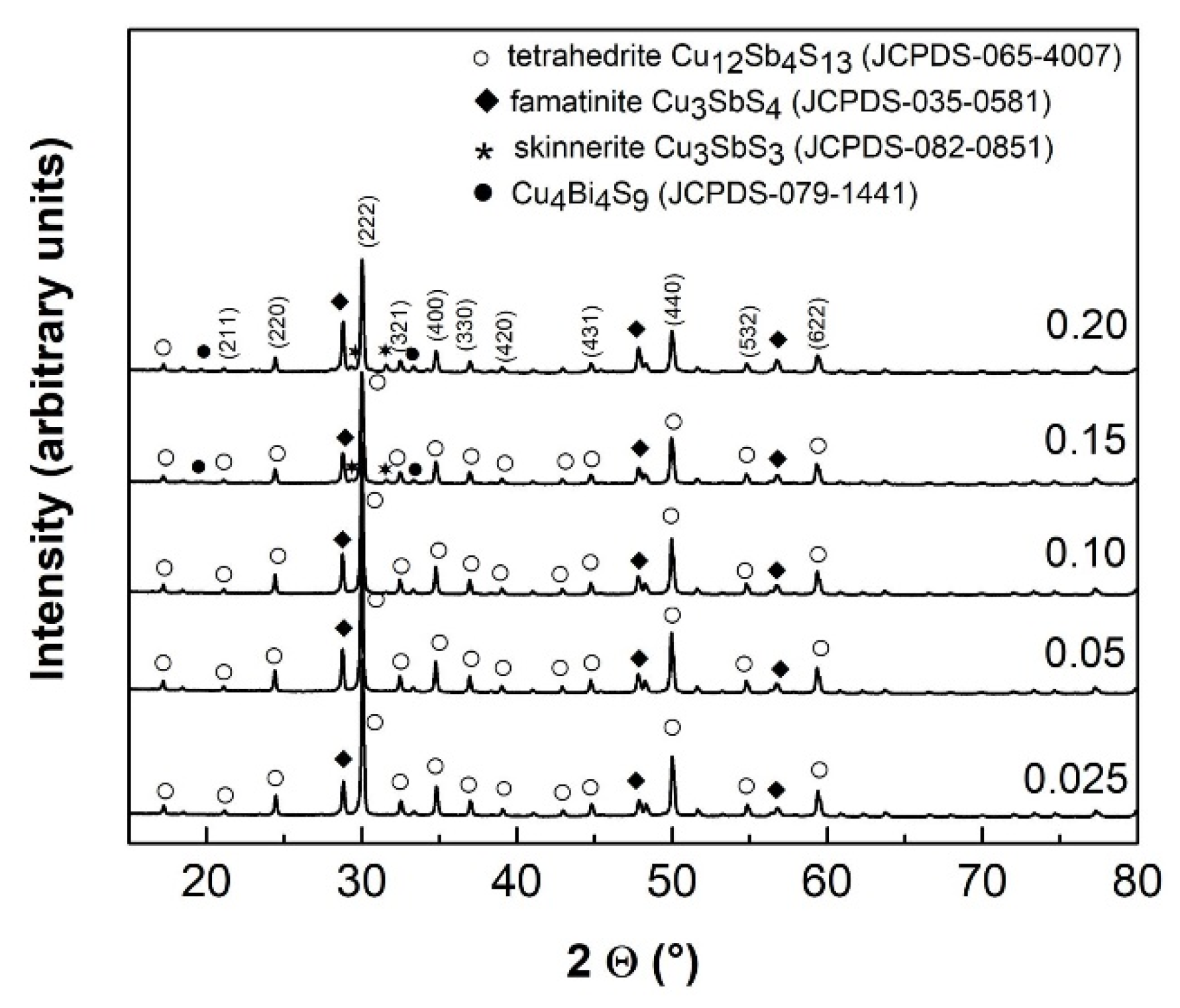
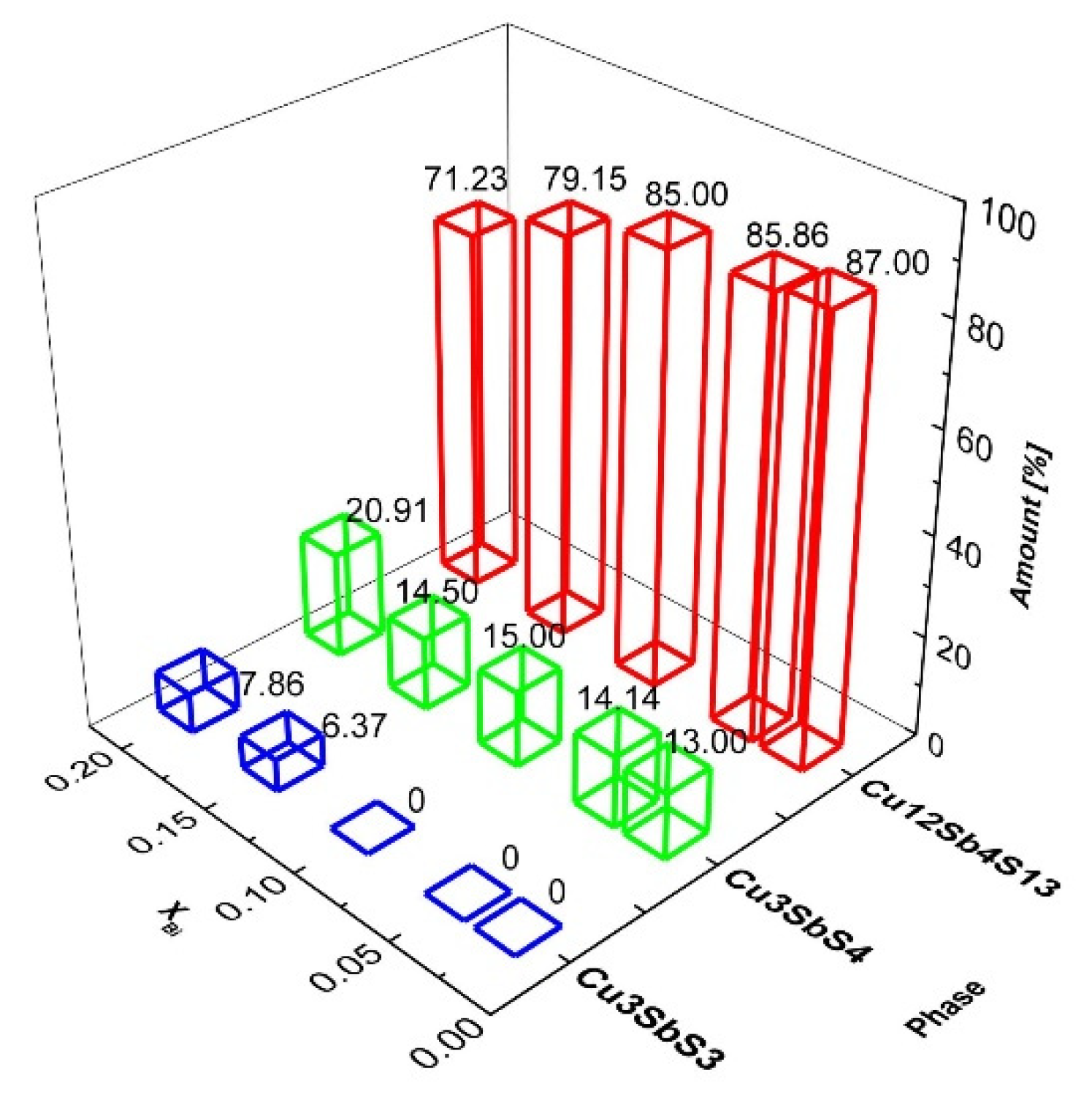
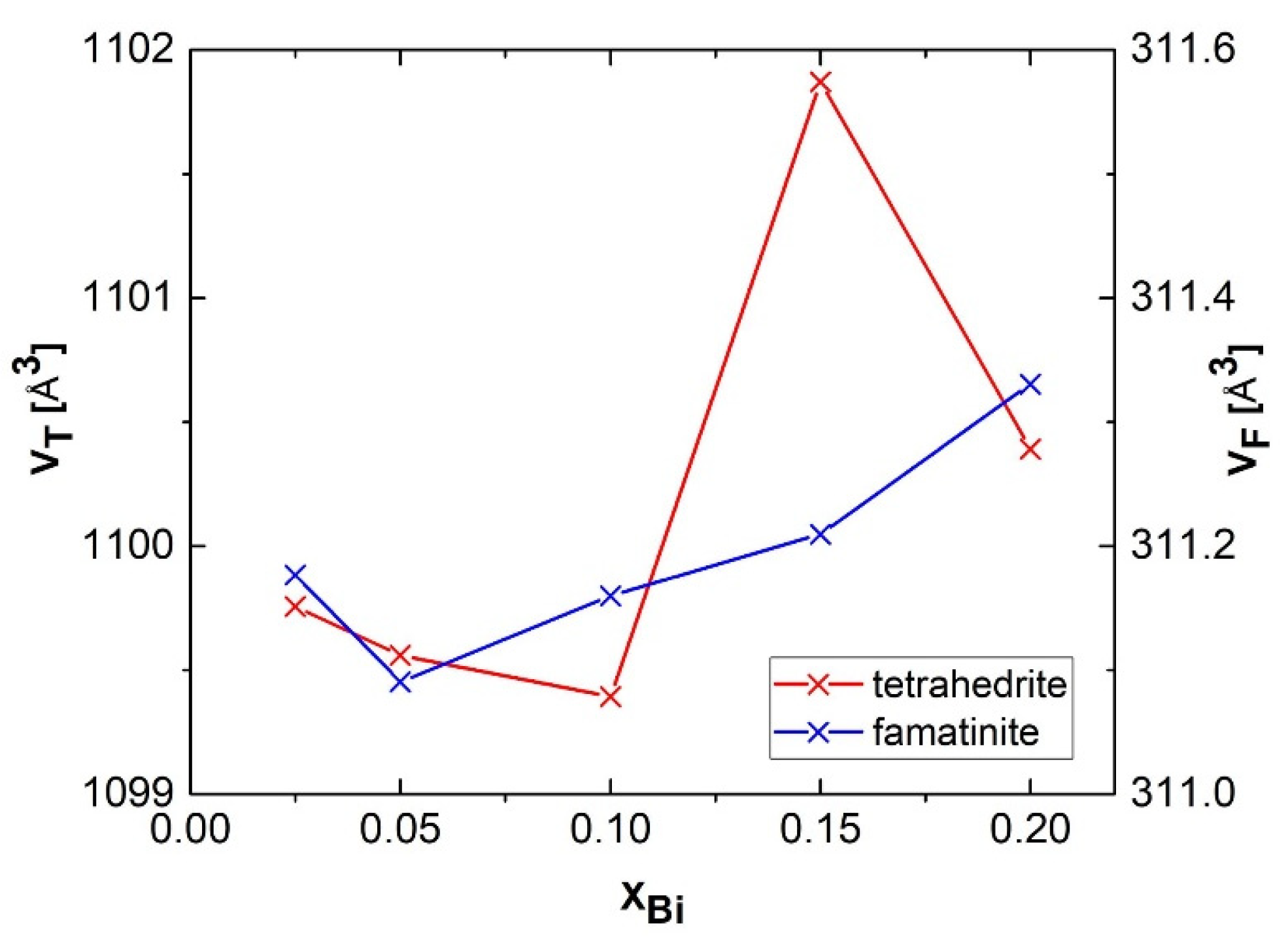
3.1. Phase Analysis and Structural Parameters
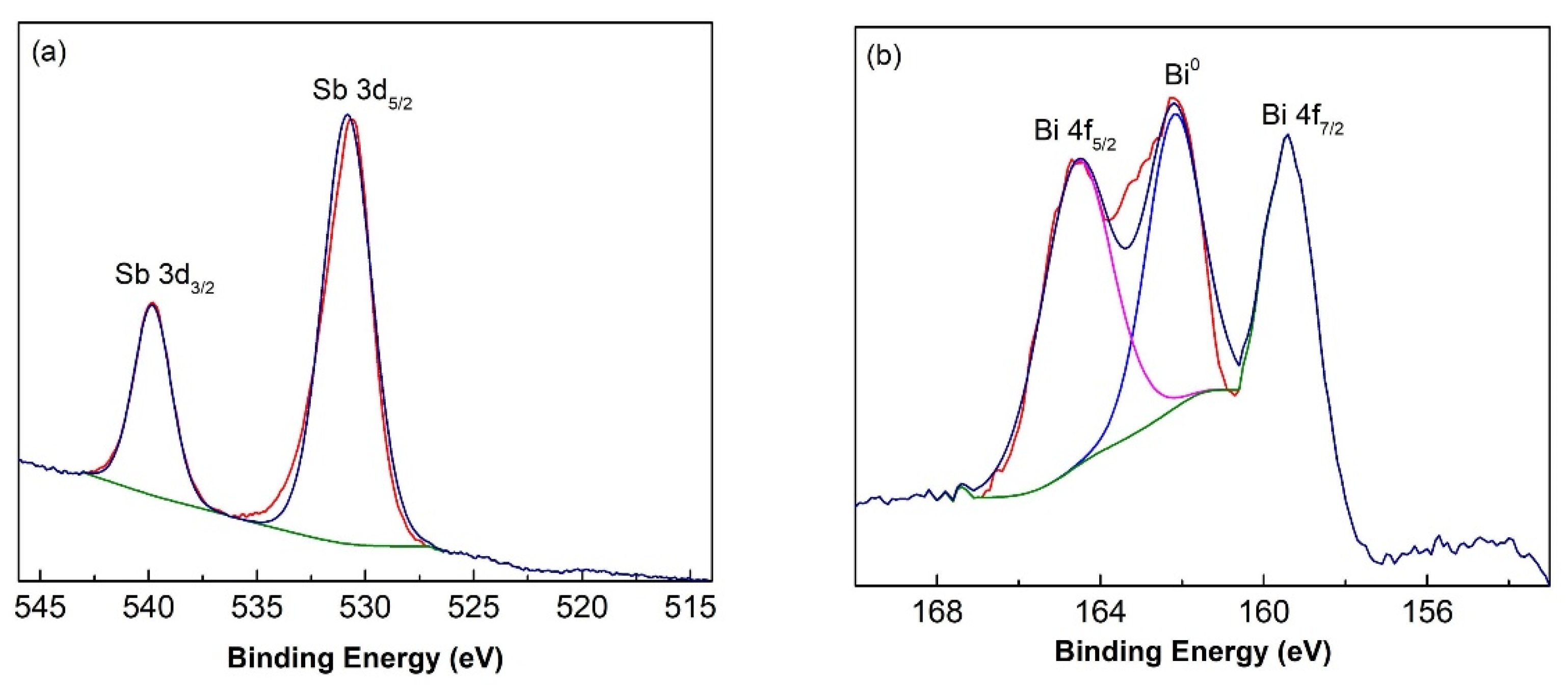
3.2. XPS Analysis
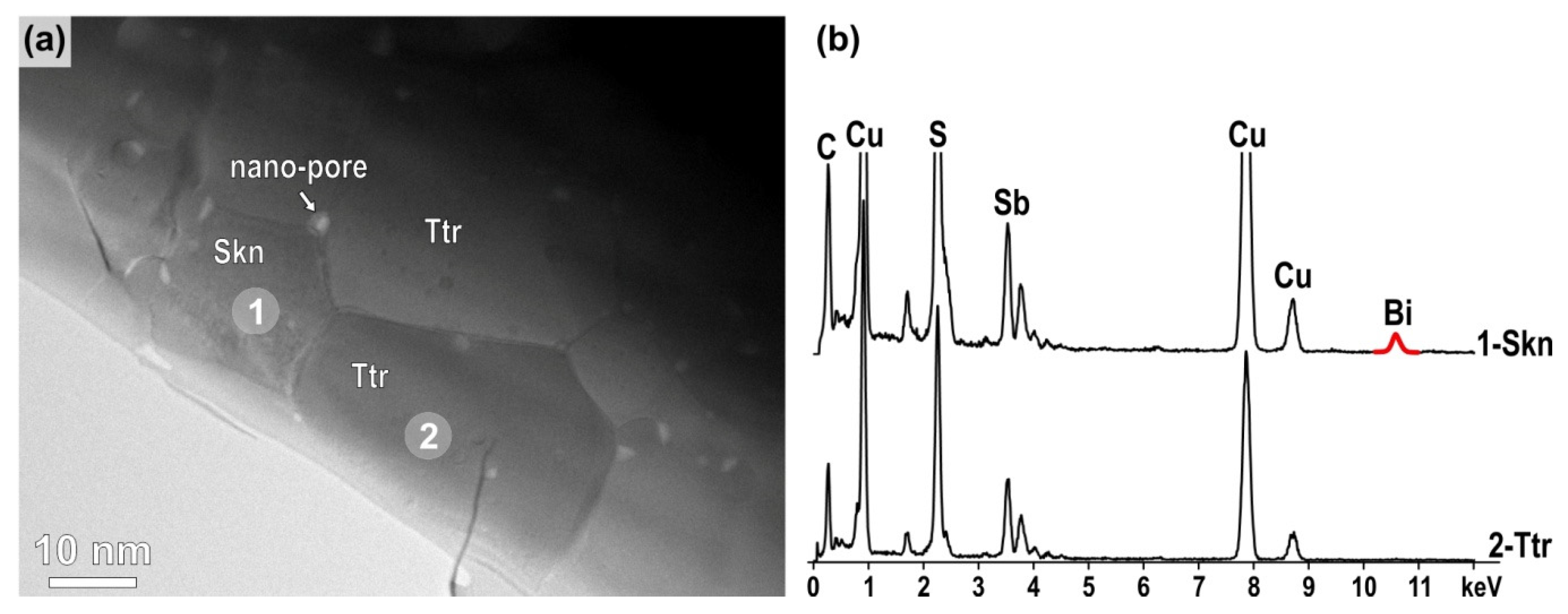
3.3. Microstructural Analyses with SEM and TEM
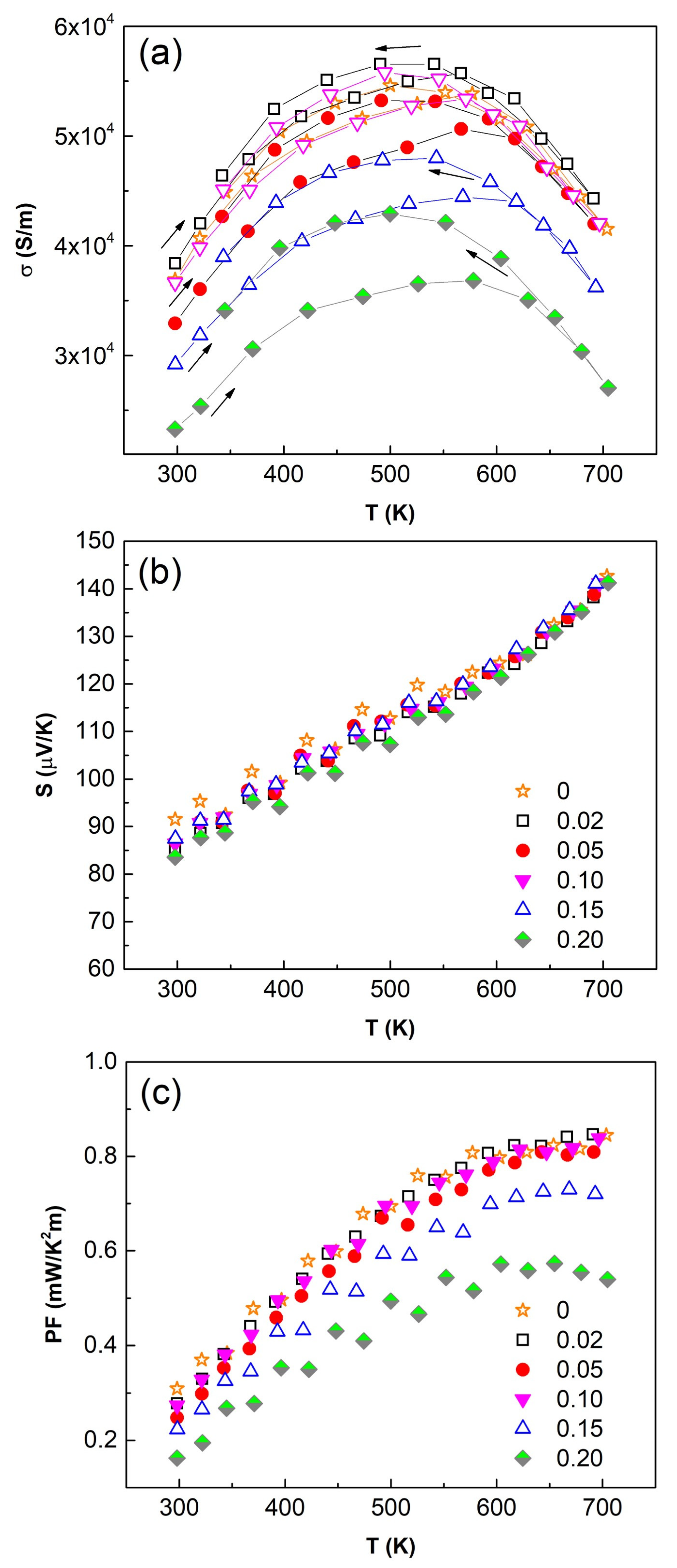
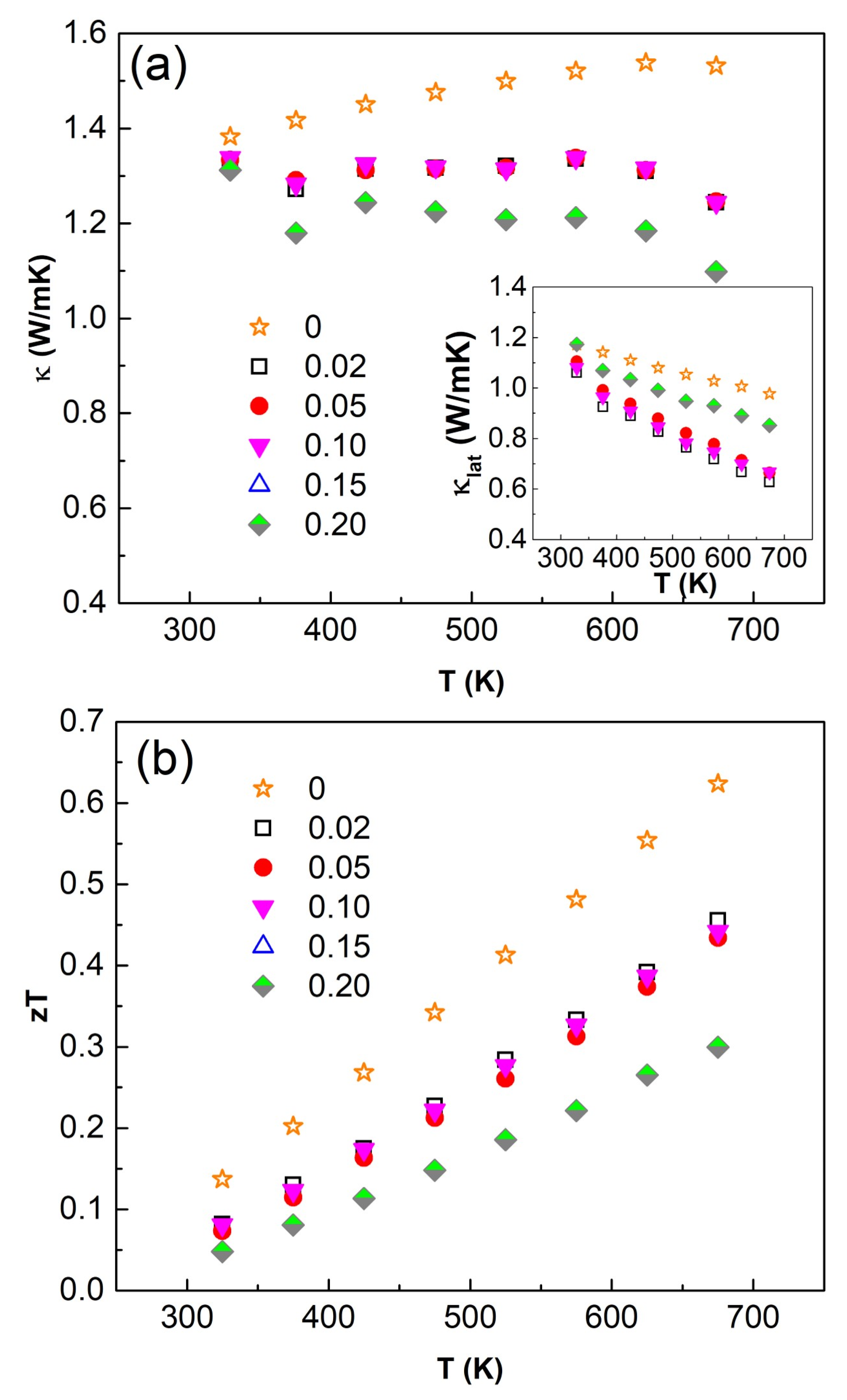
3.4. Thermoelectric Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Skinner, B.; Makovicky, E.; Luce, F. Studies of sulfosalts of copper III. Phase and phase relations in system Cu-Sb-S. Econ. Geol. 1972, 67, 924–938. [Google Scholar] [CrossRef]
- Suekuni, K.; Tsuruta, K.; Ariga, T.; Koyano, M. Thermoelectric properties of mineral tetrahedrites Cu10Tr2Sb4S13 with low thermal conductivity. Appl. Phys. Express 2012, 5, 051201-3. [Google Scholar] [CrossRef]
- Suekuni, K.; Tsuruta, K.; Kunii, M.; Nishiate, H.; Nishibori, E.; Maki, S.; Ohta, M.; Yamamoto, A.; Koyano, M. High-performance thermoelectric mineral Cu12−xNixSb4S13 tetrahedrite. J. Appl. Phys. 2013, 113, 043712. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.; Xia, Y.; Zhou, F.; Ozolins, V.; Chi, H.; Zhou, X.; Uher, C. High performance thermoelectricity in Earth-abundant compounds based on natural mineral tetrahedrites. Adv. Energy Mater. 2013, 3, 342–348. [Google Scholar] [CrossRef]
- Barbier, T.; Lemoine, P.; Gascoin, S.; Lebedev, O.; Kaltzoglou, A.; Vaqueiro, P.; Powell, A.; Smith, R.; Guilmeau, E. Structural stability of the synthetic thermoelectric ternary and nickel-substituted tetrahedrite phases. J. Alloys Compd. 2015, 634, 253–262. [Google Scholar] [CrossRef]
- Chetty, R.; Bali, A.; Mallik, R. Tetrahedrites as thermoelectric materials: An overview. J. Mater. Chem. C 2015, 3, 12364–12378. [Google Scholar] [CrossRef]
- Qiu, P.; Shi, X.; Chen, L. Cu-based thermoelectric materials. Energy Storage Mater. 2016, 3, 85–97. [Google Scholar] [CrossRef]
- Wei, T.; Qin, Y.; Deng, T.; Song, Q.; Jiang, B.; Liu, R.; Qiu, P.; Shi, X.; Chen, L. Copper chalcogenide thermoelectric materials. Sci. China Mater. 2019, 62, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Powell, A. Recent developments in Earth-abundant copper-sulfide thermoelectric materials. J. Appl. Phys. 2019, 126, 100901-20. [Google Scholar] [CrossRef] [Green Version]
- Baláž, P.; Guilmeau, E.; Daneu, N.; Dobrozhan, O.; Baláž, M.; Hegedüs, M.; Barbier, T.; Achimovičová, M.; Kaňuchová, M.; Briančin, J. Tetrahedrites synthesized via scalable mechanochemical process and spark plasma sintering. J. Eur. Ceram. Soc. 2020, 40, 1922–1930. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, C.; Zhu, H.; Xie, Y. Defect chemistry for thermoelectric materials. J. Am. Chem. Soc. 2016, 138, 14810–14819. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Kanatzidis, M.; Dravid, V. High performance bulk thermoelectrics via a panoscopic approach. Mater. Today 2013, 16, 166–176. [Google Scholar] [CrossRef]
- He, J.; Tritt, T. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, 1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioffe, A.F. Semiconductor Thermoelements and Thermoelectric Cooling; Infosearch Ltd.: London, UK, 1957; 184p. [Google Scholar]
- Dresselhaus, M.; Chen, G.; Tang, M.; Yang, R.; Lee, H.; Wang, D.; Ren, Z.; Fleurial, J.; Gogna, P. New directions for low-dimensional thermoelectric materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Hicks, L.; Dresselhaus, M. Effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. B 1993, 47, 12727–12731. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.; Dresselhaus, M. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B 1993, 47, 16631–16634. [Google Scholar] [CrossRef]
- Vaqueiro, P.; Powell, A. Recent developments in nanostructured materials for high-performance thermoelectrics. J. Mater. Chem. 2010, 20, 9577–9584. [Google Scholar] [CrossRef] [Green Version]
- Kanatzidis, M.G. Nanostructured thermoelectrics: The new paradigm? Chem. Mater. 2010, 22, 648–659. [Google Scholar] [CrossRef]
- Lan, Y.; Minnich, A.; Chen, G.; Ren, Z. Enhancement of thermoelectric figure-of-merit by a bulk nanostructuring approach. Adv. Funct. Mater. 2010, 20, 357–376. [Google Scholar] [CrossRef]
- Beyer, M.; Clausen-Schaumann, H. Mechanochemistry: The mechanical activation of covalent bonds. Chem. Rev. 2005, 105, 2921–2948. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; 413p. [Google Scholar] [CrossRef]
- Friscic, T. New opportunities for materials synthesis using mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- Šepelák, V.; Becker, K. Mechanochemistry: From mechanical degradation to novel materials properties. J. Korean Ceram. Soc. 2012, 49, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billík, P.; Cherkezova-Zheleva, Z.; Criado, J.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [Green Version]
- Do, J.; Friscic, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baláž, P.; Baláž, M.; Achimovičová, M.; Bujňáková, Z.; Dutková, E. Chalcogenide mechanochemistry in materials science: Insight into synthesis and applications (a review). J. Mater. Sci. 2017, 52, 11851–11890. [Google Scholar] [CrossRef]
- Mucsi, G. A review on mechanical activation and mechanical alloying in stirred media mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Friscic, T.; Mottillo, C.; Titi, H. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef]
- Baláž, P.; Sekula, F.; Jakabský, Š.; Kammel, R. Application of attrition grinding in alkaline leaching of tetrahedrite. Miner. Eng. 1995, 8, 1299–1308. [Google Scholar] [CrossRef]
- Golebiowska, B.; Pieczka, A.; Parafiniuk, J. Substitution of Bi for Sb and As in minerals of the tetrahedrite series from Redziny, Lower Silesia, Southwestern Poland. Can. Mineral. 2012, 50, 267–279. [Google Scholar] [CrossRef]
- Shahbazi, M.; Faghfouri, L.; Ferreira, M.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, A.; Lopes, E.; Villeroy, B.; Monnier, J.; Godart, C.; Lenoir, B. Effect of Ni, Bi and Se on the tetrahedrite formation. RSC Adv. 2016, 6, 102359–102367. [Google Scholar] [CrossRef]
- Goncalves, A.; Lopes, E.; Monnier, J.; Bourgon, J.; Vaney, J.; Piarristeguy, A.; Pradel, A.; Lenoir, B.; Delaizir, G.; Pereira, M.; et al. Fast and scalable preparation of tetrahedrite for thermoelectrics via glass crystallization. J. Alloys Compd. 2016, 664, 209–217. [Google Scholar] [CrossRef]
- Goncalves, A.; Lopes, E.; Montemor, M.; Monnier, J.; Lenoir, B. Oxidation studies of Cu12Sb3.9Bi0.1S10Se3 tetrahedrite. J. Electron. Mater. 2018, 47, 2880–2889. [Google Scholar] [CrossRef]
- Goncalves, A.; Lopes, E. Towards the use of Cu-S based synthetic minerals for thermoelectric applications. Semicond 2019, 53, 1817–1824. [Google Scholar] [CrossRef]
- Lee, G.; Kim, I. Effects of Zn/Bi double doping on the charge transport and thermoelectric properties of tetrahedrites Cu12−xZnxSb4−yBiyS13. J. Electron. Mater. 2020, 49, 2768–2774. [Google Scholar] [CrossRef]
- Kwak, S.; Pi, J.; Lee, G.; Kim, I. Solid-state synthesis and thermoelectric properties of tetrahedrites Cu12Sb4−yBiyS13. Korean J. Met. Mater. 2020, 58, 272–277. [Google Scholar] [CrossRef]
- 3Kumar, D.; Chetty, R.; Femi, O.; Chattopadhyay, K.; Malar, P.; Mallik, R. Thermoelectric properties of Bi doped tetrahedrite. J. Electron. Mater. 2017, 46, 2616–2622. [Google Scholar] [CrossRef]
- Kumar, D.; Ren, M.; Osipowicz, T.; Mallik, R.; Malar, P. Tetrahedrite (Cu12Sb4S13) thin films for photovoltaic and thermoelectric applications. Sol. Energy 2018, 174, 422–430. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction. Powder Diffraction File (PDF); International Centre for Diffraction Data: Newtown Square, PA, USA, 2004. [Google Scholar]
- Kim, H.; Gibbs, Z.; Tang, Y.; Wang, H.; Snyder, G. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Alleno, E.; Berardan, D.; Byl, C.; Candolfi, C.; Daou, R.; Decourt, R.; Guilmeau, E.; Hebert, S.; Hejtmanek, J.; Lenoir, B.; et al. A round robin test of the uncertainty on the measurement of the thermoelectric dimensionless figure of merit of Co0.97Ni0.03Sb3. Rev. Sci. Instr. 2015, 86. [Google Scholar] [CrossRef]
- Chen, K. Synthesis and Thermoelectric Properties of Cu-Sb-S Compounds. Ph.D. Thesis, Queen Mary University of London, London, UK, 2016. [Google Scholar]
- Barbier, T.; Rollin-Martinet, S.; Lemoine, P.; Gascoin, F.; Kaltzoglou, A.; Vaqueiro, P.; Powell, A.; Guilmeau, E. Thermoelectric materials: A new rapid synthesis process for nontoxic and high-performance tetrahedrite compounds. J. Am. Ceram. Soc. 2016, 99, 51–56. [Google Scholar] [CrossRef]
- Craig, J.; Barton, P. Thermochemical approximations for sulfosalts. Econ. Geol. 1973, 68, 493–506. [Google Scholar] [CrossRef]
- Tatsuka, K.; Morimoto, N. Tetrahedrite stability relations in Cu-Sb-S system. Econ. Geol. 1977, 72, 258–270. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Craig, J.R. Mineral Chemistry of Metal Sulfides; Cambridge University Press: Cambridge, UK, 1978; 493p. [Google Scholar]
- Chen, K.; Du, B.; Bonini, N.; Weber, C.; Yan, H.; Reece, M. Theory-guided synthesis of an eco-friendly and low-cost copper based sulfide thermoelectric material. J. Phys. Chem. C 2016, 120, 27135–27140. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, Y. Lattice expansion and grain boundary excess volume of nanocrystalline Se prepared by mechanical milling. Rev. Adv. Mater. Sci. 2017, 48, 52–61. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics: A Ready-Reference of Chemical and Physical Data, 85th ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; p. 2712. [Google Scholar]
- Wang, Y.; Chen, J.; Jiang, L.; Sun, K.; Liu, F.; Lai, Y. Photoelectrochemical properties of Bi2S3 thin films deposited by successive ionic layer adsorption and reaction (SILAR) method. J. Alloys Compd. 2016, 686, 684–692. [Google Scholar] [CrossRef]
- An, C.; Jin, Y.; Tang, K.; Qian, Y. Selective synthesis and characterization of famatinite nanofibers and tetrahedrite nanoflakes. J. Mater. Chem. 2003, 13, 301–303. [Google Scholar] [CrossRef]
- Helal, A.; Harraz, F.; Ismail, A.; Sami, T.; Ibrahim, I. Controlled synthesis of bismuth sulfide nanorods by hydrothermal method and their photocatalytic activity. Mater. Des. 2016, 102, 202–212. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D. Natural mineral tetrahedrite as a direct source of thermoelectric materials. Phys. Chem. Chem. Phys. 2013, 15, 5762–5766. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baláž, P.; Guilmeau, E.; Achimovičová, M.; Baláž, M.; Daneu, N.; Dobrozhan, O.; Kaňuchová, M. Bismuth Doping in Nanostructured Tetrahedrite: Scalable Synthesis and Thermoelectric Performance. Nanomaterials 2021, 11, 1386. https://doi.org/10.3390/nano11061386
Baláž P, Guilmeau E, Achimovičová M, Baláž M, Daneu N, Dobrozhan O, Kaňuchová M. Bismuth Doping in Nanostructured Tetrahedrite: Scalable Synthesis and Thermoelectric Performance. Nanomaterials. 2021; 11(6):1386. https://doi.org/10.3390/nano11061386
Chicago/Turabian StyleBaláž, Peter, Emmanuel Guilmeau, Marcela Achimovičová, Matej Baláž, Nina Daneu, Oleksandr Dobrozhan, and Mária Kaňuchová. 2021. "Bismuth Doping in Nanostructured Tetrahedrite: Scalable Synthesis and Thermoelectric Performance" Nanomaterials 11, no. 6: 1386. https://doi.org/10.3390/nano11061386
APA StyleBaláž, P., Guilmeau, E., Achimovičová, M., Baláž, M., Daneu, N., Dobrozhan, O., & Kaňuchová, M. (2021). Bismuth Doping in Nanostructured Tetrahedrite: Scalable Synthesis and Thermoelectric Performance. Nanomaterials, 11(6), 1386. https://doi.org/10.3390/nano11061386








