Dendritic Forest-Like Ag Nanostructures Prepared Using Fluoride-Assisted Galvanic Replacement Reaction for SERS Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Dendritic Forest-Like Ag Nanostructures on a Si Wafer
2.3. Characterization
2.4. SERS Analysis Procedure
2.5. DNA Probe Modification and Target Hybridization
3. Results and Discussion
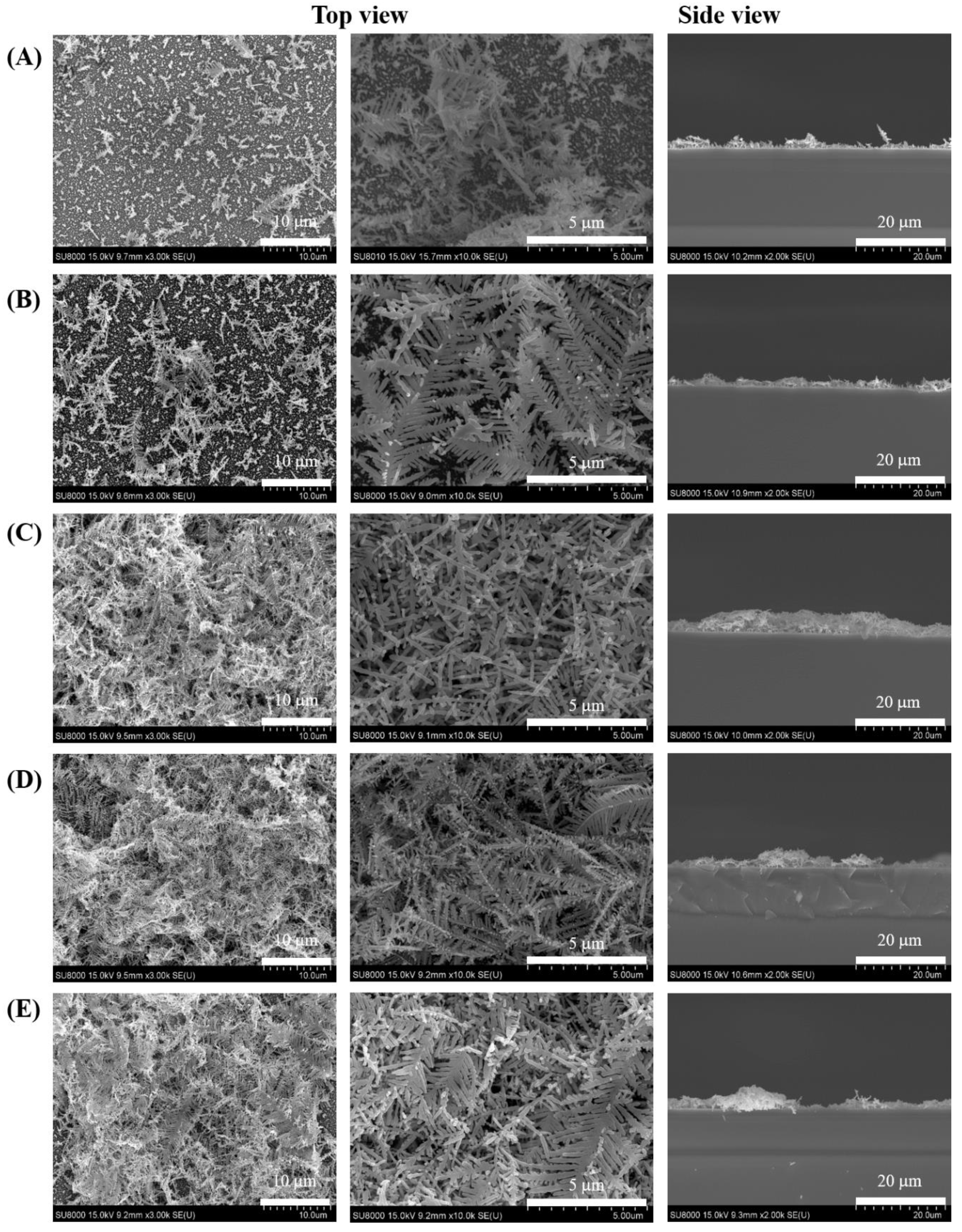
3.1. Characterization of Dendritic Forest-Like Ag Nanostructures
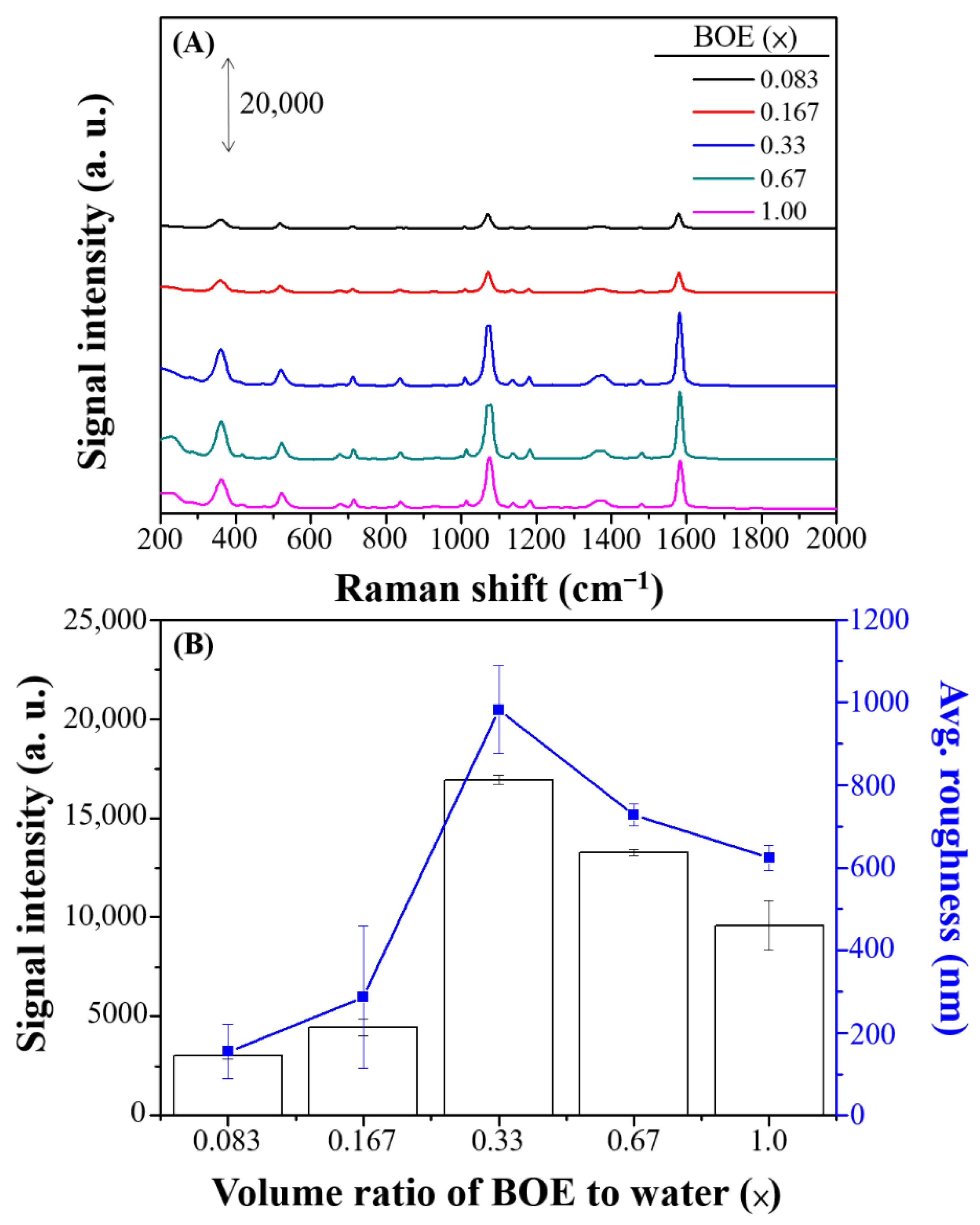
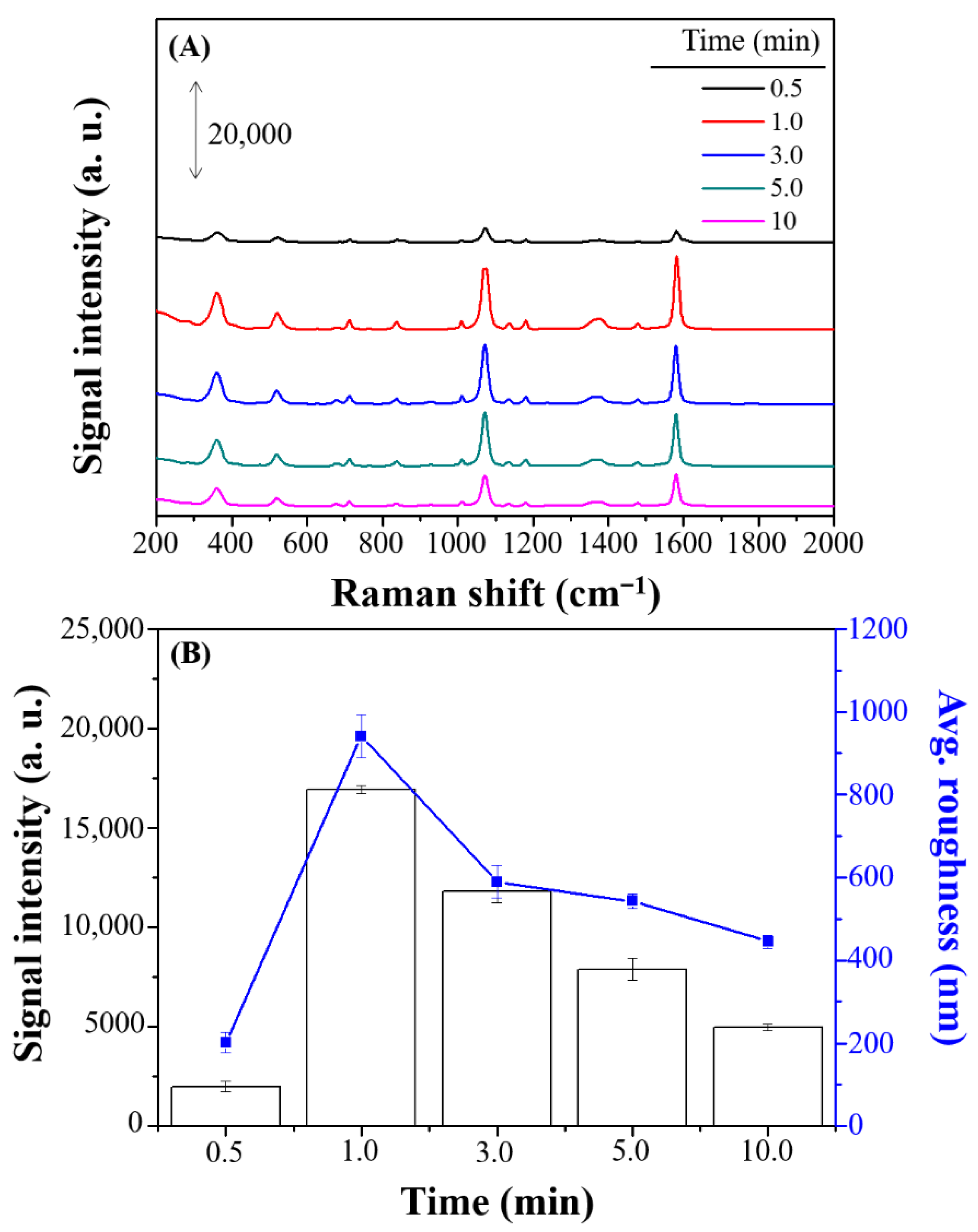
3.2. Optimal Combination of SERS Performance
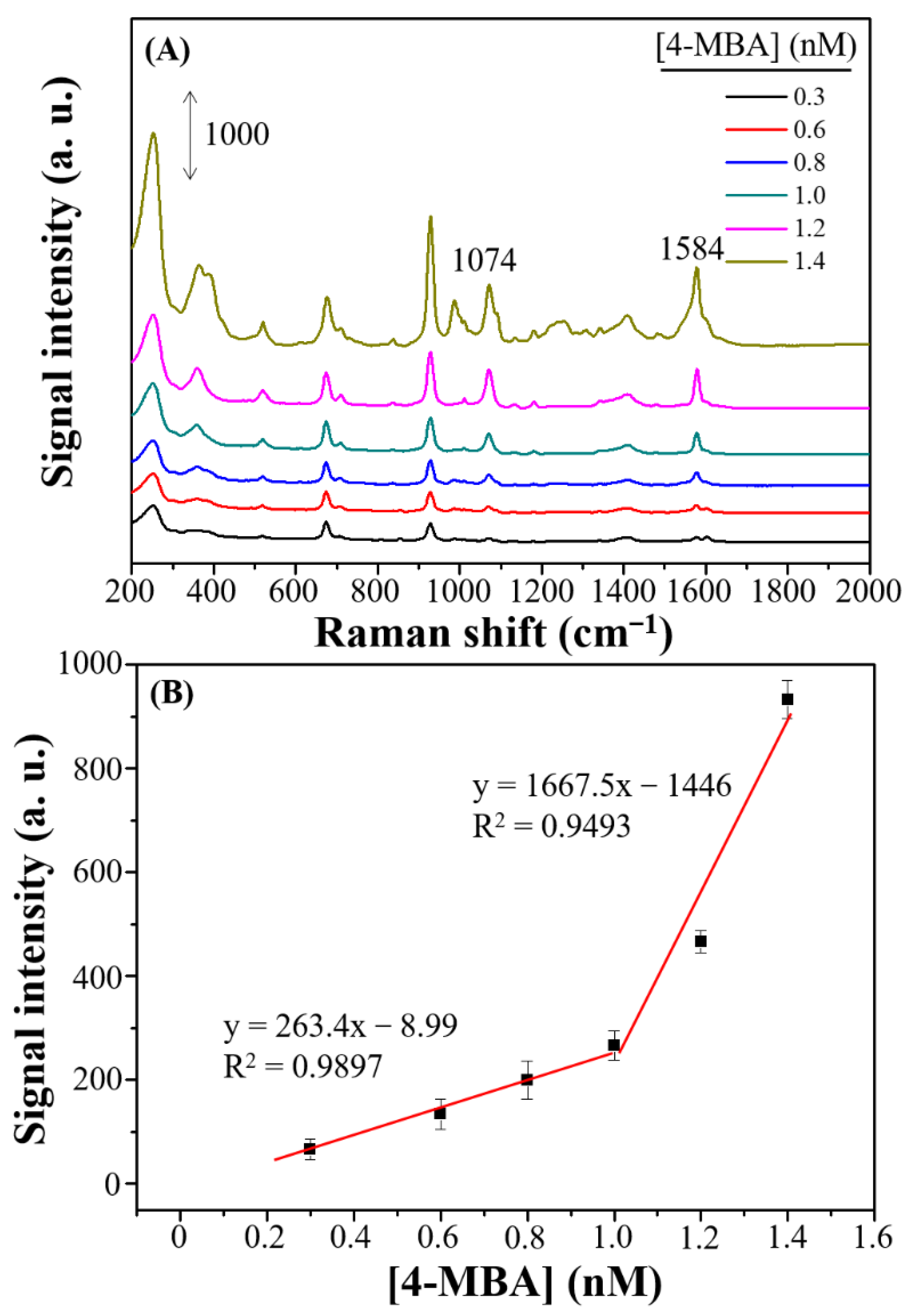
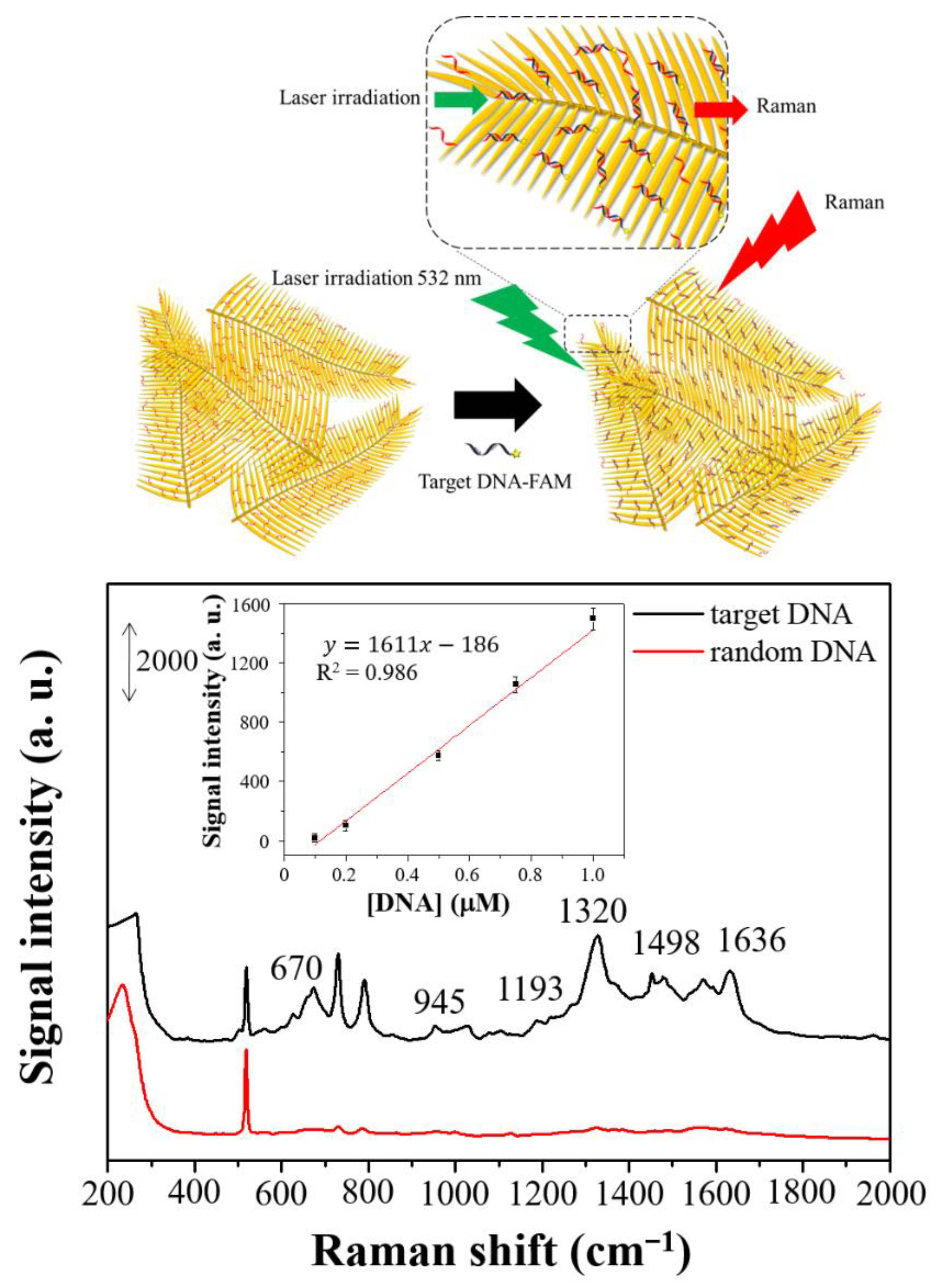
3.3. Precision and Application of Dendritic Forest-Like Ag Nanostructures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, H.S.; Li, G.K.; Xu, F.G.; Zhang, Z.M. Metal-organic frameworks: Opportunities and challenges for surface-enhanced Raman scattering—A review. J. Mater. Chem. C 2020, 8, 2952–2963. [Google Scholar] [CrossRef]
- Fan, M.K.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.B.; Ma, N.; Guo, S.; Yu, J.H.; Xiao, L.; Park, Y.; Park, E.; Jin, S.; Chen, L.; Jung, Y.M. Size-Dependent Surface-Enhanced Raman Scattering Activity of Ag@CuxOS Yolk-Shell Nanostructures: Surface Plasmon Resonance Induced Charge Transfer. J. Phys. Chem. C 2020, 124, 16616–16623. [Google Scholar] [CrossRef]
- Chang, T.H.; Chang, Y.C.; Wu, S.H. Ag nanoparticles decorated ZnO: Al nanoneedles as a high-performance surface-enhanced Raman scattering substrate. J. Alloy. Compd. 2020, 843, 156044. [Google Scholar] [CrossRef]
- Li, Y.E.; Tang, S.Q.; Xu, S.S.; Duan, Z.Z.; Wang, Z.; Zhang, Y. Ag Nanoframes Deposited on Au Films Generate Optical Cavities for Surface-Enhanced Raman Scattering. ACS Appl. Nano Mater. 2020, 3, 5116–5122. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, M.H.; Nguyen, T.H.T.; Dao, V.P.T.; Nguyen, Q.H.; Sai, C.D.; Pham, N.H.; Bach, T.C.; Ngac, A.B.; Nguyen, T.T.; et al. Facile fabrication of sensitive surface enhanced Raman scattering substrate based on CuO/Ag core/shell nanowires. Appl. Surf. Sci. 2020, 509, 145325. [Google Scholar] [CrossRef]
- Li, Z.B.; Zhang, L.J.; He, X.; Chen, B.S. Urchin-like ZnO-nanorod arrays templated growth of ordered hierarchical Ag/ZnO hybrid arrays for surface-enhanced Raman scattering. Nanotechnology 2020, 31, 165301. [Google Scholar] [CrossRef]
- Ren, X.H.; Li, X. Flower-like Ag coated with molecularly imprinted polymers as a surface-enhanced Raman scattering substrate for the sensitive and selective detection of glibenclamide. Anal. Methods 2020, 12, 2858–2864. [Google Scholar] [CrossRef]
- Wu, T.H.; Lin, Y.W. Surface-enhanced Raman scattering active gold nanoparticle/nanohole arrays fabricated through electron beam lithography. Appl. Surf. Sci. 2018, 435, 1143–1149. [Google Scholar] [CrossRef]
- Salinas, C.; Rodriguez-Sevilla, E.; Flores-Romero, E.; Cheang-Wong, J.C. Fabrication and characterization of surface- enhanced Raman scattering substrates with ordered arrays of gold nanopyramids by means of nanosphere lithography. Mater. Express 2019, 9, 141–149. [Google Scholar] [CrossRef]
- Lin, C.W.; Chang, S.H.; Huang, C.C.; Lin, C.H. Plasmonic nanocavities fabricated by directed self-assembly lithography and nanotransfer printing and used as surface-enhanced Raman scattering substrates. Microelectron. Eng. 2020, 227, 111309. [Google Scholar] [CrossRef]
- Wu, X.C.; Lv, L.L.; Han, X.S.; Li, C.X. Bovine serum albumin fibrous biofilm template synthesis of metallic nanomeshes for surface-enhanced Raman scattering and electrocatalytic detection. Mater. Des. 2020, 192, 108777. [Google Scholar] [CrossRef]
- Raveendran, J.; Docoslis, A. Portable surface-enhanced Raman scattering analysis performed with microelectrode-templated silver nanodendrites. Analyst 2020, 145, 4467–4476. [Google Scholar] [CrossRef]
- Raveendran, J.; Stamplecoskie, K.G.; Docoslis, A. Tunable Fractal Nanostructures for Surface-Enhanced Raman Scattering via Templated Electrodeposition of Silver on Low-Energy Surfaces. ACS Appl. Nano Mater. 2020, 3, 2665–2679. [Google Scholar] [CrossRef]
- Ye, P.Y.; Xin, W.B.; De Rosa, I.M.; Wang, Y.K.; Goorsky, M.S.; Zheng, L.; Yin, X.Q.; Xie, Y.H. One-Pot Self-Templated Growth of Gold Nanoframes for Enhanced Surface-Enhanced Raman Scattering Performance. ACS Appl. Mater. Inter. 2020, 12, 22050–22057. [Google Scholar] [CrossRef]
- Yang, L.; Gao, M.X.; Zhan, L.; Gong, M.; Zhen, S.J.; Huang, C.Z. An enzyme-induced Au@Ag core-shell nanoStructure used for an ultrasensitive surface-enhanced Raman scattering immunoassay of cancer biomarkers. Nanoscale 2017, 9, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Lin, Y.W.; Chen, C.Y. Surface-enhanced Raman scattering-active desert-rose-like Ag mesoparticles prepared using cyclic voltammetric methods. RSC Adv. 2015, 5, 93293–93300. [Google Scholar] [CrossRef]
- Lin, Y.W.; Tang, C. Electrochemical Synthesis and Deposition of Surface-Enhanced Raman Scattering-Active Silver Microstructures on a Screen-Printed Carbon Electrode. J. Phys. Chem. C 2015, 119, 24865–24874. [Google Scholar] [CrossRef]
- Ye, W.C.; Chen, Y.; Zhou, F.; Wang, C.M.; Li, Y.M. Fluoride-assisted galvanic replacement synthesis of Ag and Au dendrites on aluminum foil with enhanced SERS and catalytic activities. J. Mater. Chem. 2012, 22, 18327–18334. [Google Scholar] [CrossRef]
- Lin, G.H.; Dong, W.J.; Wang, C.; Lu, W.S. Mechanistic study on galvanic replacement reaction and synthesis of Ag-Au alloy nanoboxes with good surface-enhanced Raman scattering activity to detect melamine. Sens. Actuators B Chem. 2018, 263, 274–280. [Google Scholar] [CrossRef]
- Chen, J.L.; Yang, P.C.; Wu, T.; Lin, Y.W. Determination of mercury (II) ions based on silver-nanoparticles-assisted growth of gold nanostructures: UV-Vis and surface enhanced Raman scattering approaches. Spectrochim. Acta A 2018, 199, 301–307. [Google Scholar] [CrossRef]
- Chen, J.L.; Wu, T.; Lin, Y.W. Surface-enhanced Raman scattering enhancement due to localized surface plasmon resonance coupling between metallic nanoparticles and substrate. Microchem. J. 2018, 138, 340–347. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.B.; Kim, K.; Kim, M.S. Surface-enhanced Raman scattering of p-aminobenzoic acid at silver electrode. J. Phys. Chem. 1990, 94, 7576–7580. [Google Scholar] [CrossRef]
- Li, R.; Lv, H.M.; Zhang, X.L.; Liu, P.P.; Chen, L.; Cheng, J.B.; Zhao, B. Vibrational spectroscopy and density functional theory study of 4-mercaptobenzoic acid. Spectrochim. Acta A 2015, 148, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yu, M.; Jiang, T.; Wang, E.Y.; Ge, C.L.; Chen, Z.R. A green approach for the synthesis of silver dendrites and their superior SERS performance. Optik 2017, 136, 244–248. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, L.; Zhou, J. Silver nanocube-mediated sensitive immunoassay based on surface-enhanced Raman scattering assisted by etched silicon nanowire arrays. Analyst 2014, 139, 5893–5900. [Google Scholar] [CrossRef]
- Xia, Y.Y.; Li, T.J.; Gao, C.; Ma, C.; Chen, J. Facile fabrication of hierarchically flowerlike Ag microstructure for SERS application. J. Mater. Sci. 2014, 49, 2781–2786. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.L.; Xue, B.; Peng, Z.H.; Cao, Z.L.; Mu, Q.Q.; Xuan, L. Highly effective and chemically stable surface enhanced Raman scattering substrates with flower-like 3D Ag-Au hetero-nanostructures. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cardinal, M.F.; Vander Ende, E.; Hackler, R.A.; McAnally, M.O.; Stair, P.C.; Schatz, G.C.; Van Duyne, R.P. Expanding applications of SERS through versatile nanomaterials engineering. Chem. Soc. Rev. 2017, 46, 3886–3903. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.J.; Chang, H.W.; Lin, Y.W.; Chuang, S.Y.; Lin, Y.S.; Shiao, M.H. Silicon-Based Ag Dendritic Nanoforests for Light-Assisted Bacterial Inhibition. Nanomaterials 2020, 10, 2244. [Google Scholar] [CrossRef] [PubMed]
- Gutes, A.; Carraro, C.; Maboudian, R. Silver Nanodesert Rose as a Substrate for Surface-Enhanced Raman Spectroscopy. Acs Appl. Mater. Inter. 2009, 1, 2551–2555. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.Y.; Li, Z.P.; Wang, W.Z.; Wu, Y.S.; Xu, H.X. Highly Surface-roughened “Flower-like” Silver Nanoparticles for Extremely Sensitive Substrates of Surface-enhanced Raman Scattering. Adv. Mater. 2009, 21, 4614–4618. [Google Scholar] [CrossRef]
- Bian, J.C.; Shu, S.W.; Li, J.F.; Huang, C.; Li, Y.Y.; Zhang, R.Q. Reproducible and recyclable SERS substrates: Flower-like Ag structures with concave surfaces formed by electrodeposition. Appl. Surf. Sci. 2015, 333, 126–133. [Google Scholar] [CrossRef]
- Nistico, R.; Rivolo, P.; Novara, C.; Giorgis, F. New branched flower-like Ag nanostructures for SERS analysis. Colloids Surf. A 2019, 578, 123600. [Google Scholar] [CrossRef]
- Xia, Y.; Gao, Z.N.; Liao, X.M.; Pan, C.C.; Zhang, Y.F.; Feng, X.S. Rapid synthesis of hierarchical, flower-like Ag microstructures with a gemini surfactant as a directing agent for SERS applications. Crystengcomm 2017, 19, 6547–6555. [Google Scholar] [CrossRef]
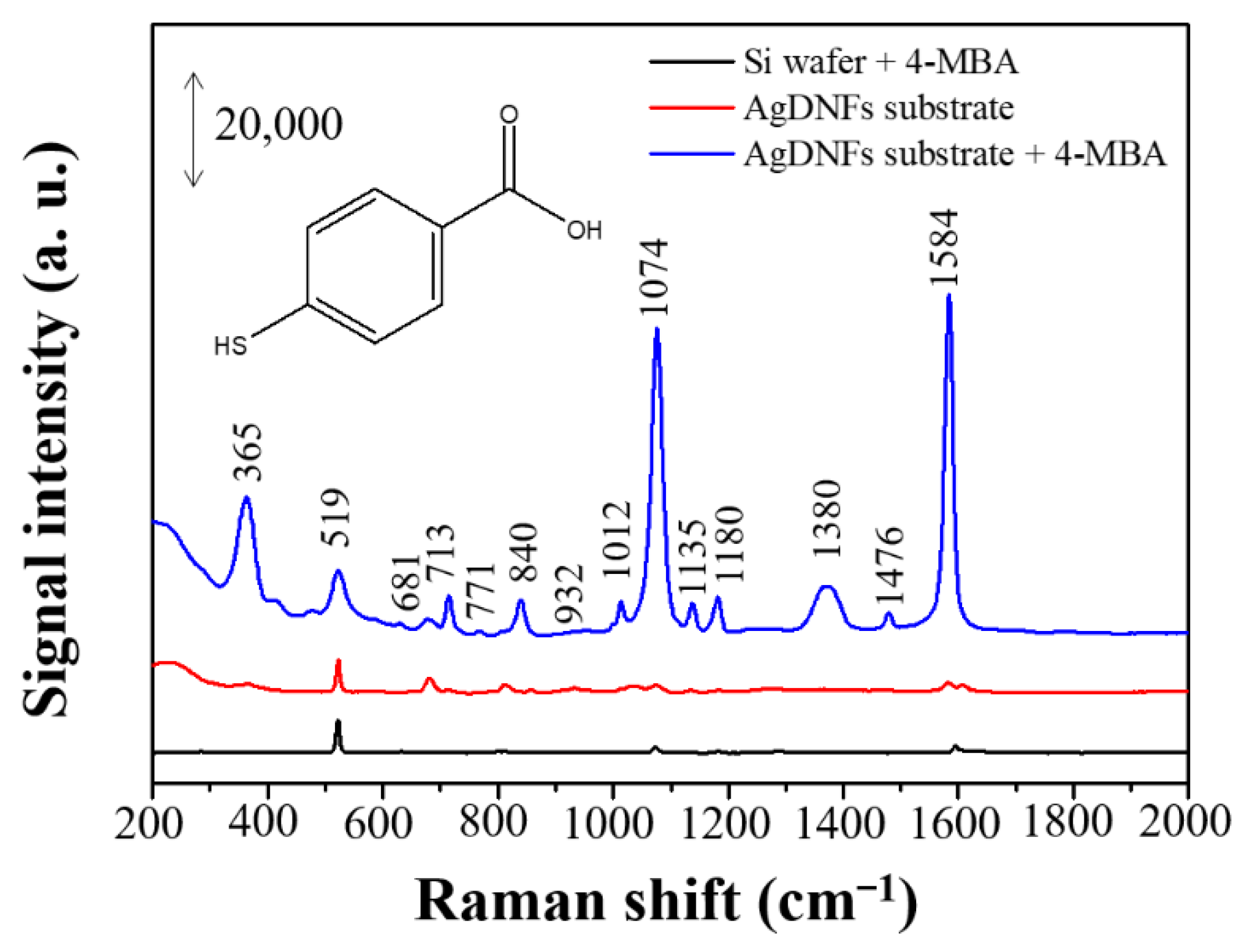
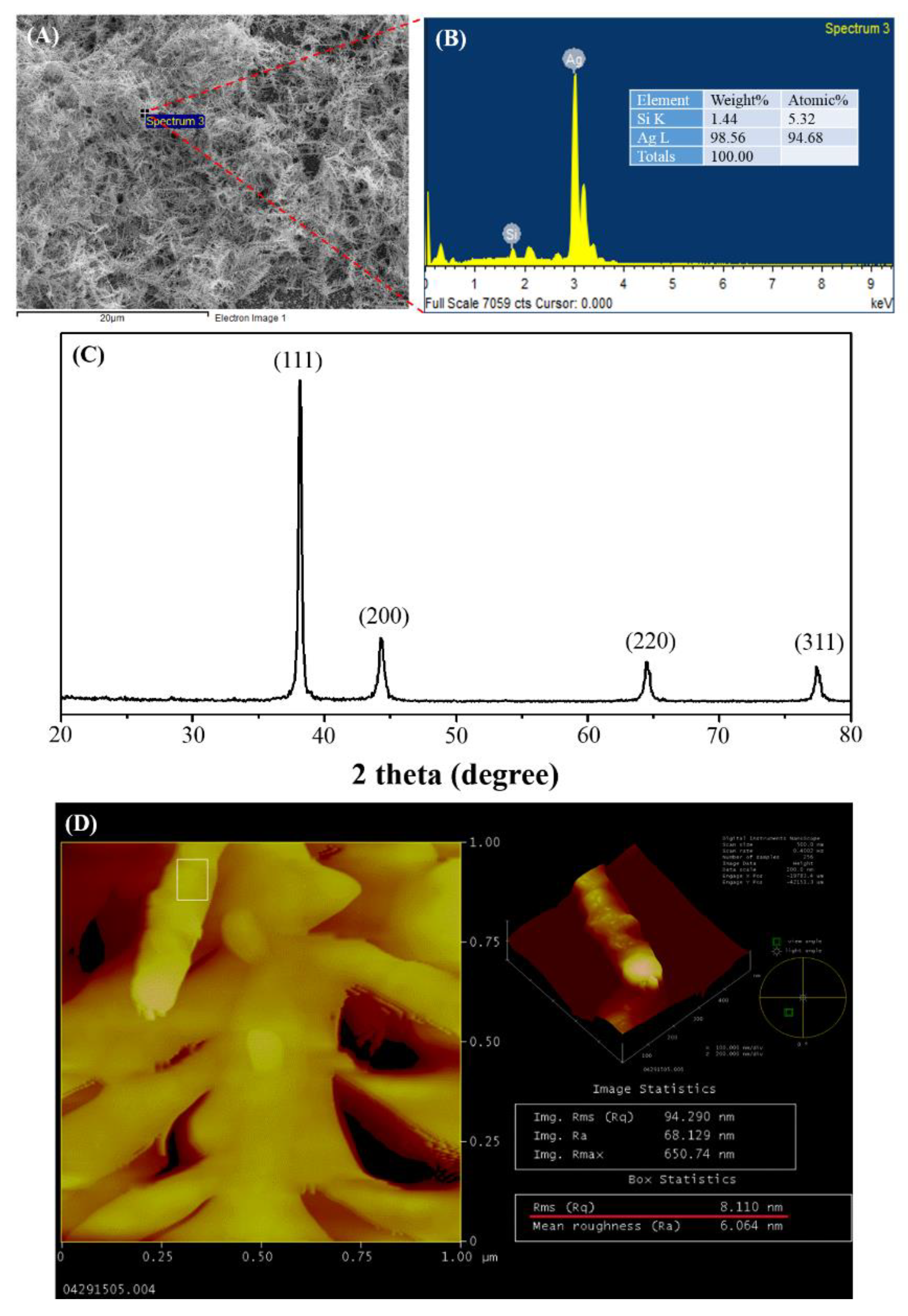
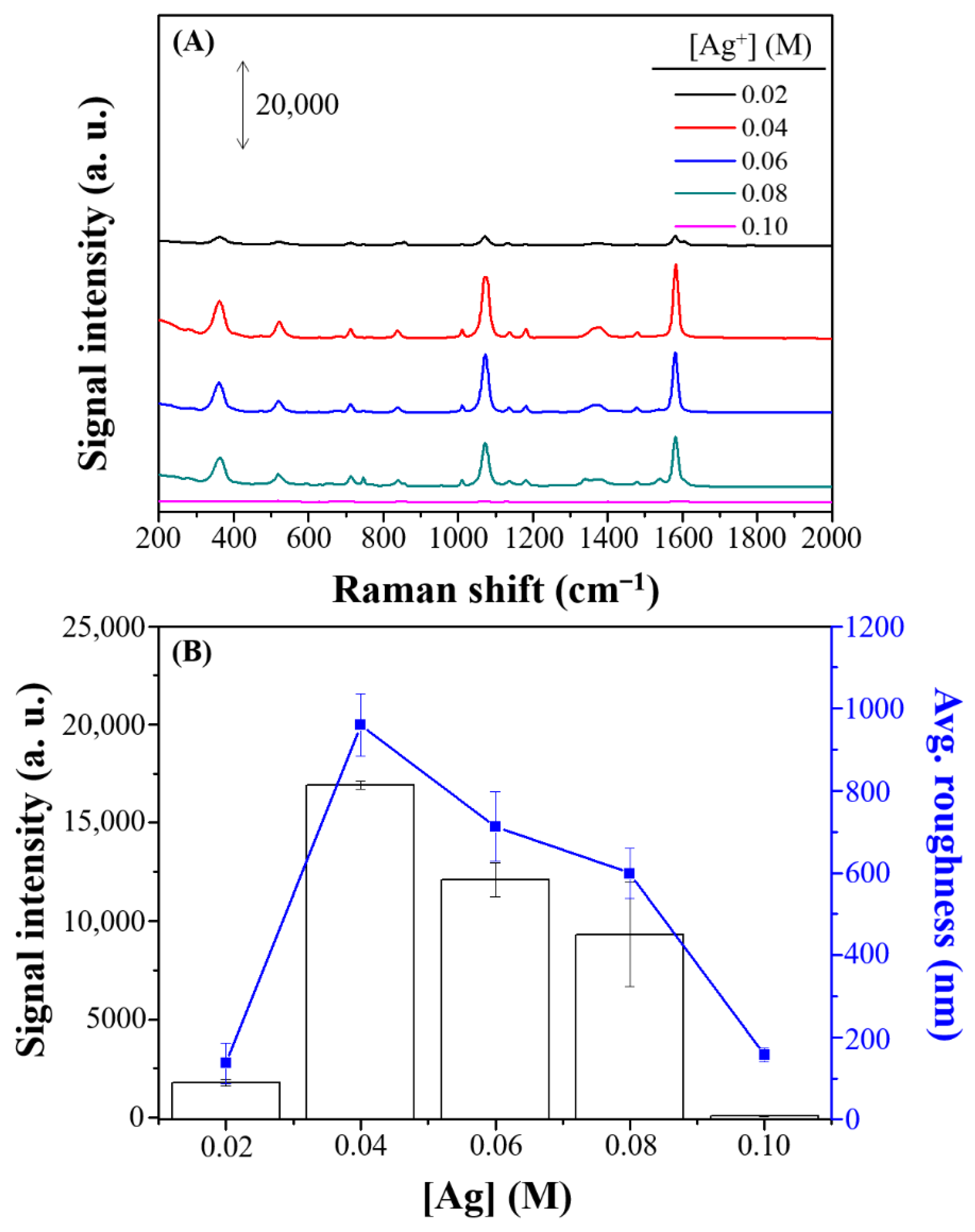
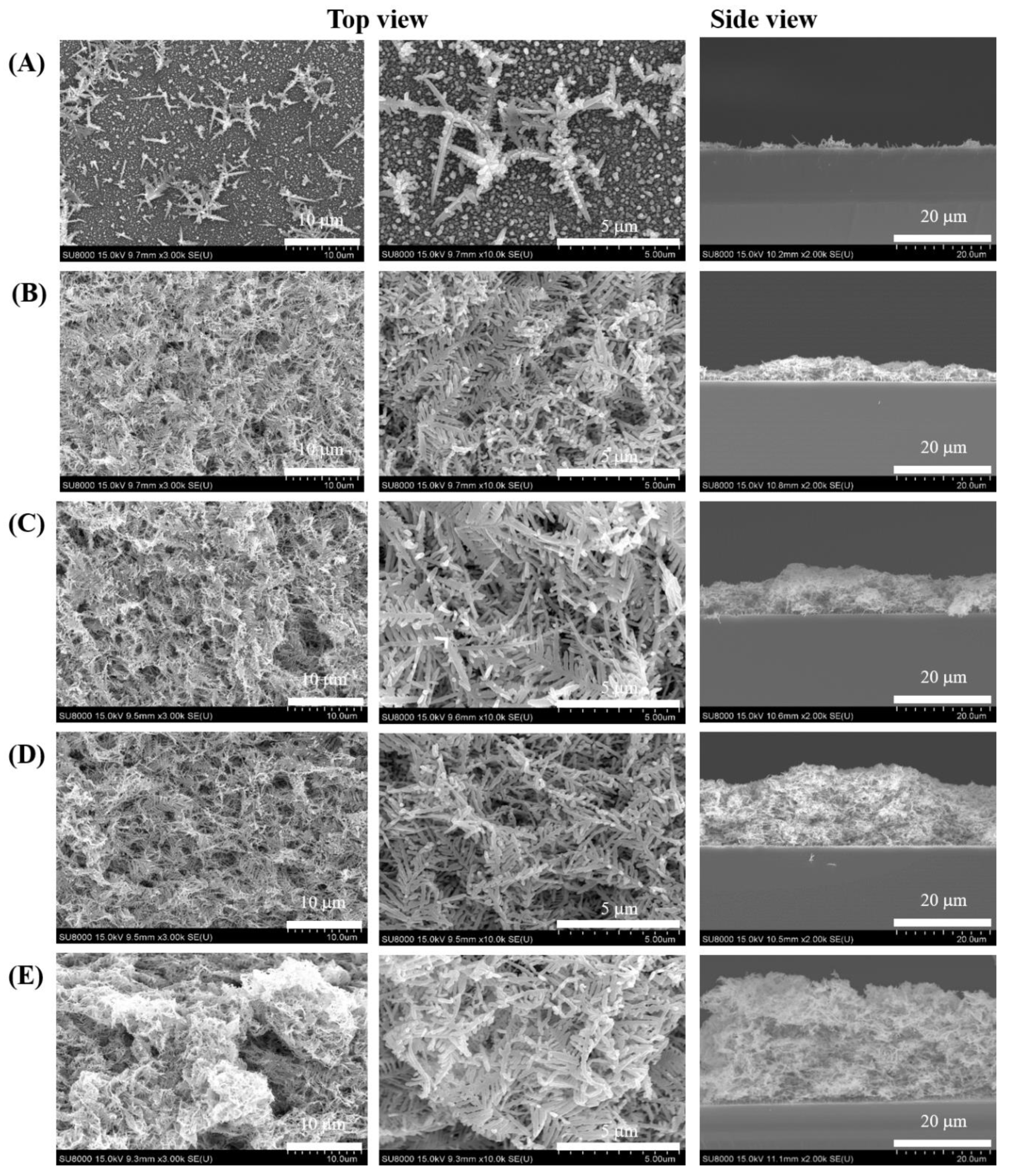


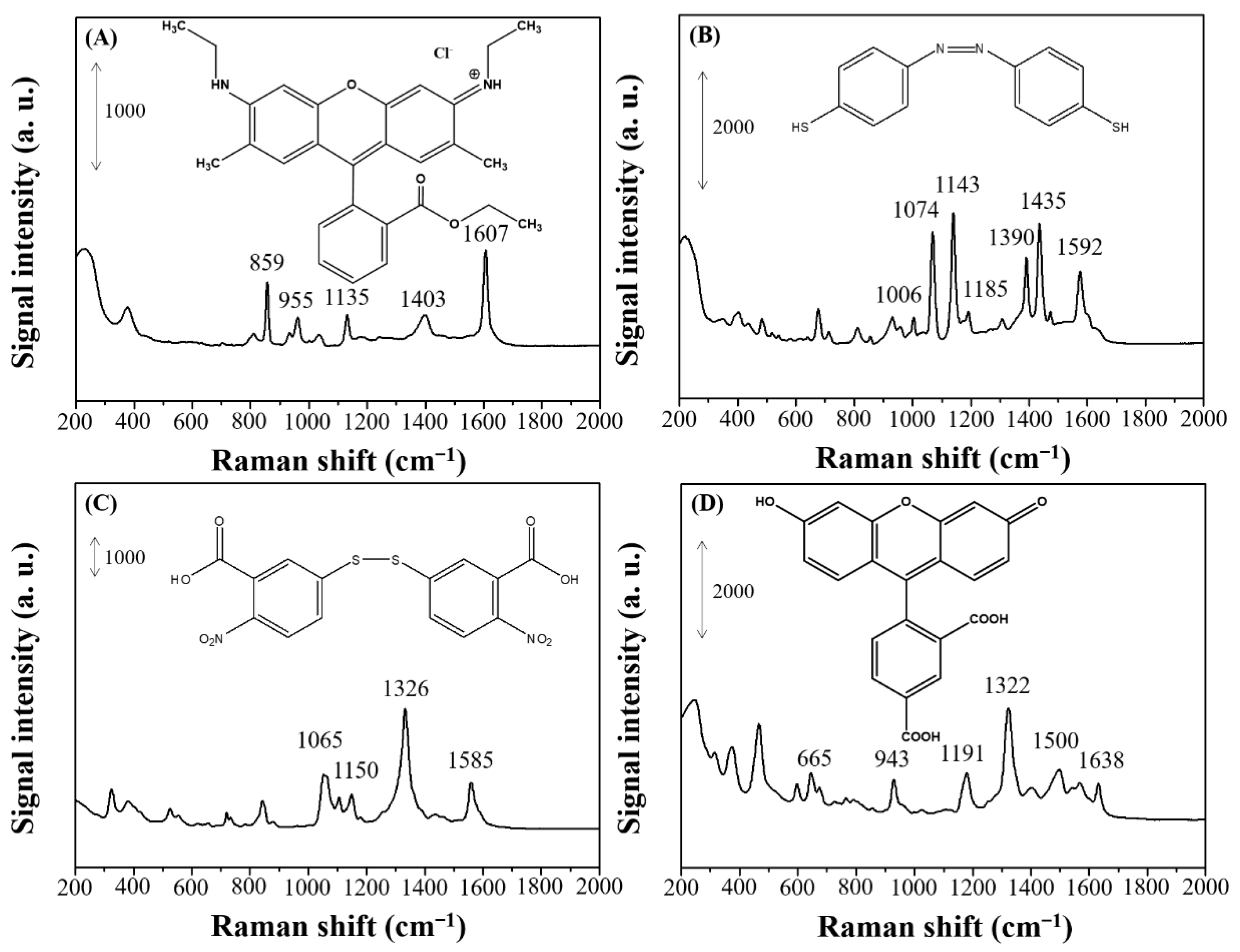
| Raman Shift (cm−1) | Assignment a |
|---|---|
| 1584 | 9a |
| 1476 | 15 |
| 1380 | βO-H & υC-ph & 19a & as.υCO2 |
| 1180 | βO–H & 9a |
| 1135 | 9a & γO–H |
| 1074 | 1 & υC–S |
| 1012 | υC–O & 1 |
| 932 | 17a |
| 840 | 10b |
| 771 | 6a & υC-ph & υC–O |
| 713 | 11 & ωCO2 |
| 681 | βCO2 & 19a & υC–S |
| Substrate | Preparation Process | EF | Applications | Ref. |
|---|---|---|---|---|
| Desert-rose-like Ag mesoparticles | One-step electrochemical reduction | 1.3 × 105 for 4-MBA | FAH gene | [18] |
| Silver dendrites | A facile in situ reduction method | 7.0 × 105 for 4-MBA | a | [26] |
| Ag nanodesert rose | Galvanic displacement process (one-step method) | 2.0 × 1010 for R6G | a | [32] |
| Flower-like Ag microstructures | Chemical reduction on polyaniline/polyvinyl alcohol composite film (two-step method) | 1.9 × 105 for 4-MBA | a | [28] |
| Flower-like Ag-Au hetero-nanoparticles | Electrodeposition and galvanic replacement reaction | 8.6 × 107 for 4-MBA | a | [29] |
| Flower-like Silver Nanoparticles | Chemical reduction in the presence of ascorbic acid | 107 ~ 108 for malachite green isothiocyanate | a | [33] |
| Flower-like Ag structures with concave surfaces | Electrodeposition | 2.1 × 109 for 4-ATP | a | [34] |
| Flower-like Ag nanostructures | Chemical reduction in the presence of L-cysteine | a | a | [35] |
| Flower-like Ag microstructures | Chemical reduction in the presence of surfactant | 1.84 × 1014 for crystal violet | a | [36] |
| Dendritic forest-like Ag nanostructures | FAGRR process | 9.18 × 108 for 4-MBA | FAH gene | This study |
| Raman Shift | 1074 (cm−1) | 1584 (cm−1) | ||
|---|---|---|---|---|
| Day | Raman Intensity | RSD (%) a | Raman Intensity | RSD (%) |
| Day 1 | 11,893 ± 25 | 0.210 | 19,909 ± 26 | 0.130 |
| Day 2 | 11,747 ± 53 | 0.451 | 19,509 ± 95 | 0.487 |
| Day 3 | 11,828 ± 73 | 0.617 | 19,588 ± 97 | 0.495 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiao, M.-H.; Wu, T.; Huang, H.J.; Peng, C.-Y.; Lin, Y.-S.; Lai, T.-Y.; Lin, Y.-W. Dendritic Forest-Like Ag Nanostructures Prepared Using Fluoride-Assisted Galvanic Replacement Reaction for SERS Applications. Nanomaterials 2021, 11, 1359. https://doi.org/10.3390/nano11061359
Shiao M-H, Wu T, Huang HJ, Peng C-Y, Lin Y-S, Lai T-Y, Lin Y-W. Dendritic Forest-Like Ag Nanostructures Prepared Using Fluoride-Assisted Galvanic Replacement Reaction for SERS Applications. Nanomaterials. 2021; 11(6):1359. https://doi.org/10.3390/nano11061359
Chicago/Turabian StyleShiao, Ming-Hua, Tsunghsueh Wu, Hung Ji Huang, Ching-Yi Peng, Yung-Sheng Lin, Ting-Yu Lai, and Yang-Wei Lin. 2021. "Dendritic Forest-Like Ag Nanostructures Prepared Using Fluoride-Assisted Galvanic Replacement Reaction for SERS Applications" Nanomaterials 11, no. 6: 1359. https://doi.org/10.3390/nano11061359
APA StyleShiao, M.-H., Wu, T., Huang, H. J., Peng, C.-Y., Lin, Y.-S., Lai, T.-Y., & Lin, Y.-W. (2021). Dendritic Forest-Like Ag Nanostructures Prepared Using Fluoride-Assisted Galvanic Replacement Reaction for SERS Applications. Nanomaterials, 11(6), 1359. https://doi.org/10.3390/nano11061359









