Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PCL/PLA Nanofibers
2.3. Characterization
2.4. Antibacterial Evaluation
2.5. Drug Release Studies
3. Results and Discussion
3.1. Characterization of the Drug-Loaded PCL/PLA Nanofibers
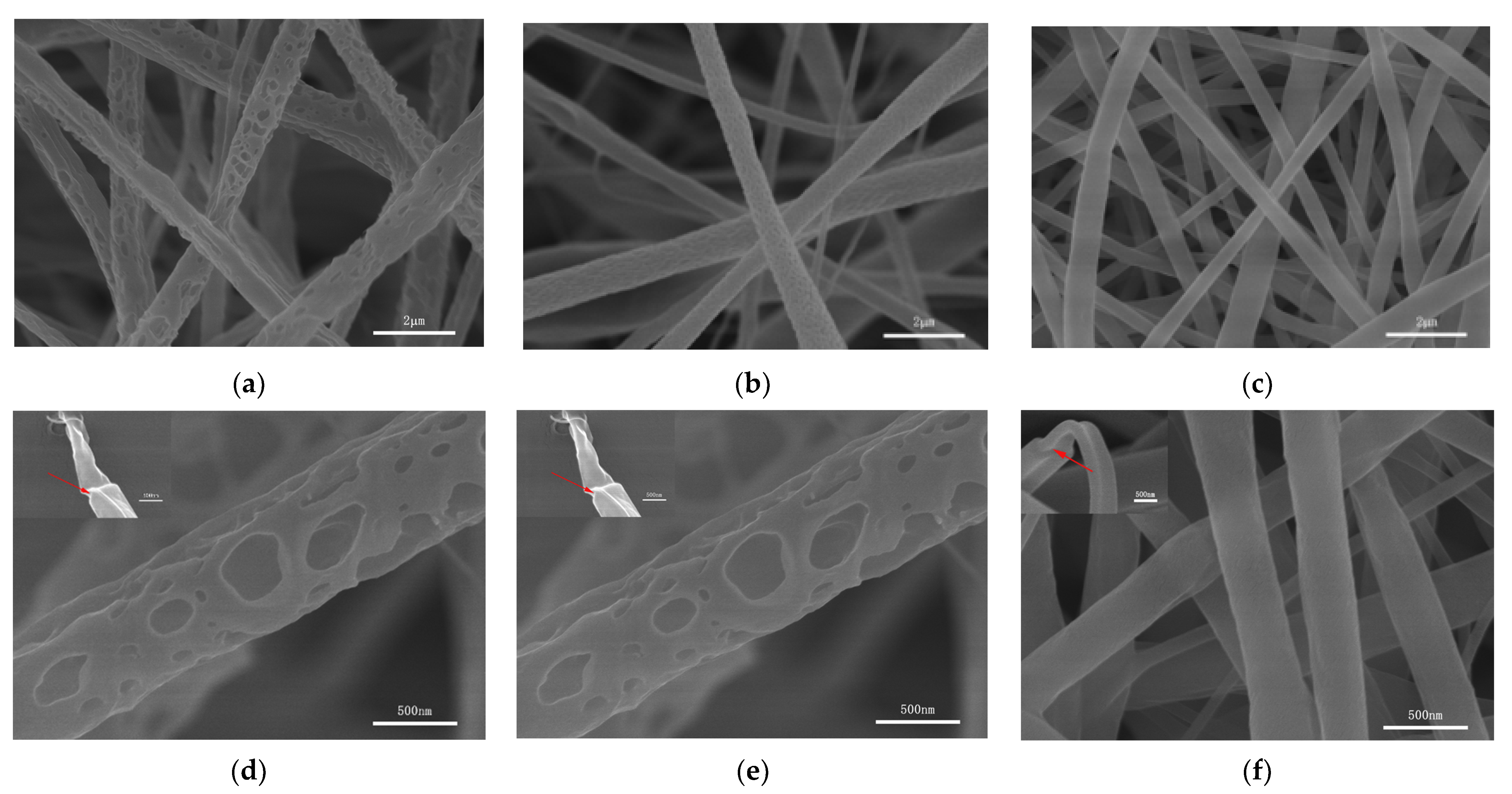
3.1.1. Morphological Properties
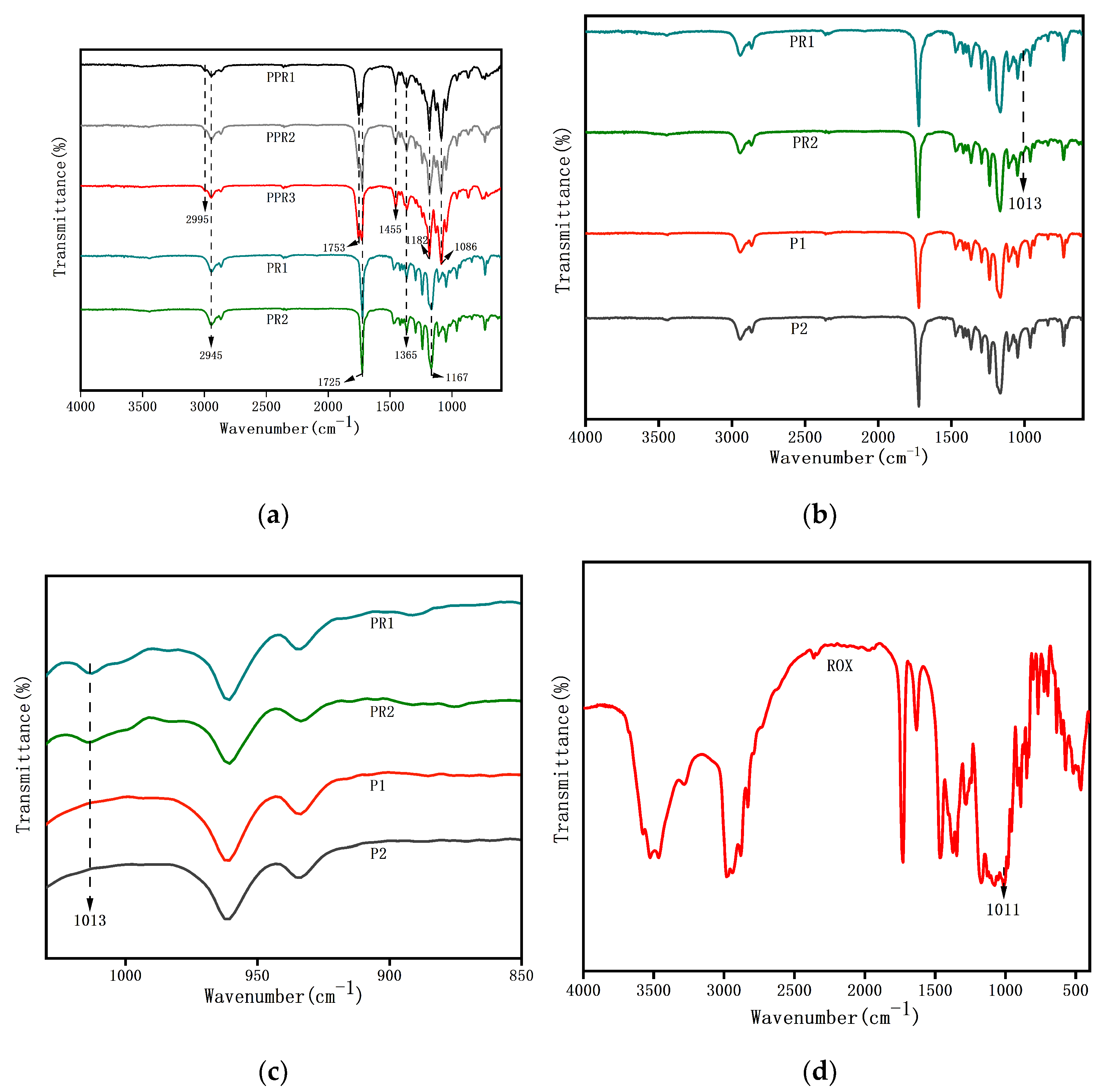
3.1.2. FTIR Analysis
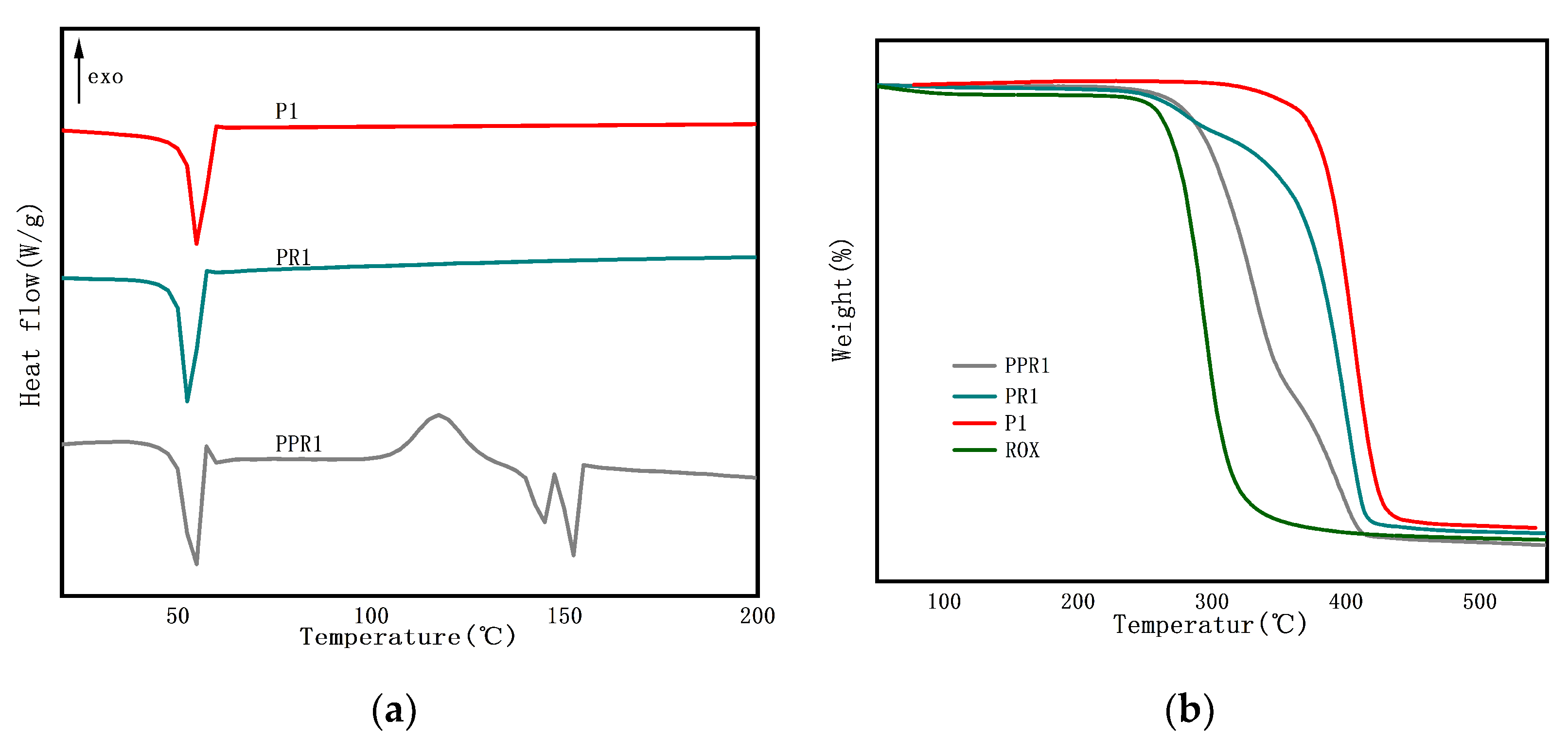
3.1.3. Thermal Characteristics
3.2. Antibacterial Activity
3.3. In Vitro Drug Release
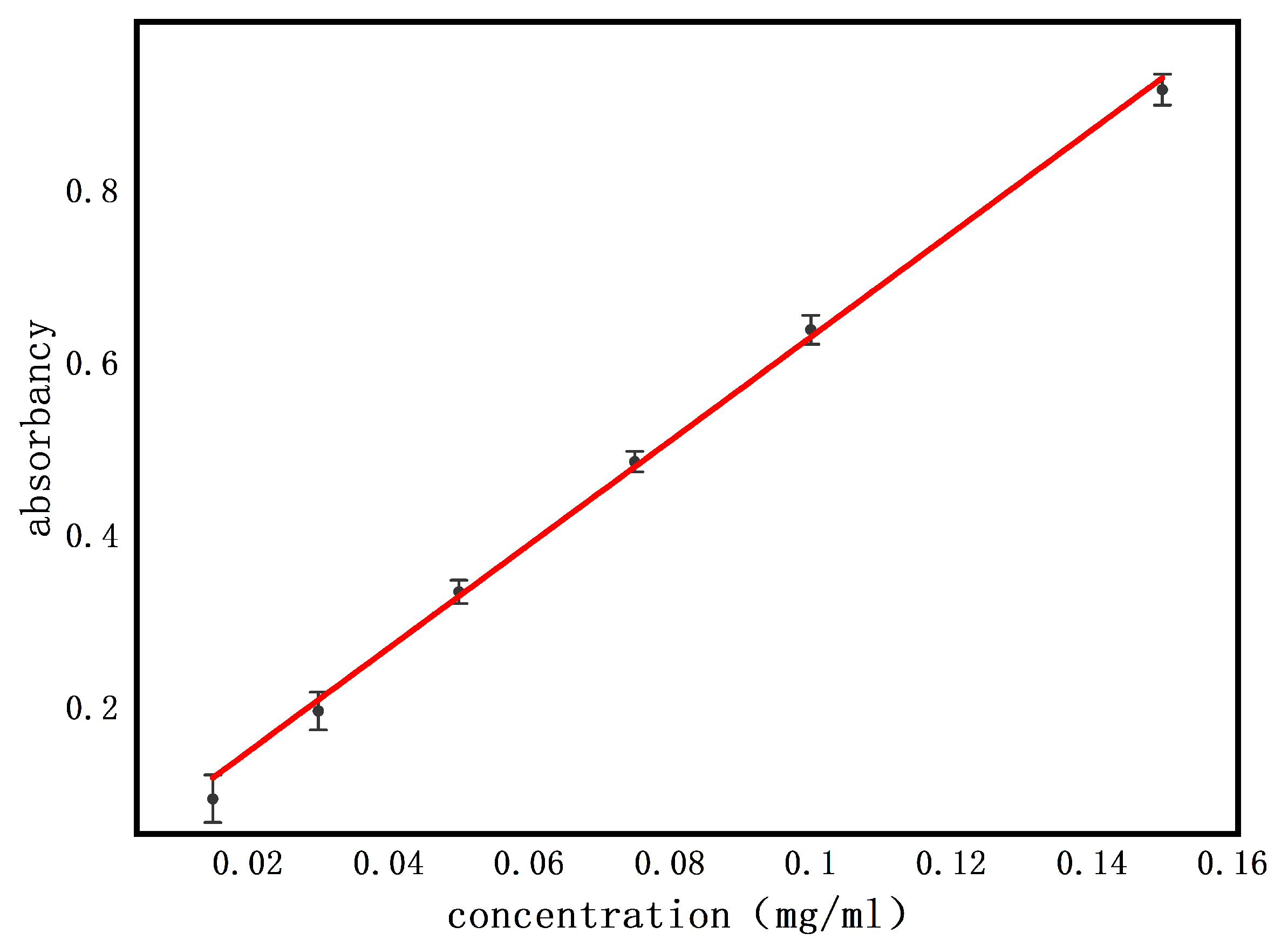
3.3.1. Standard Curve
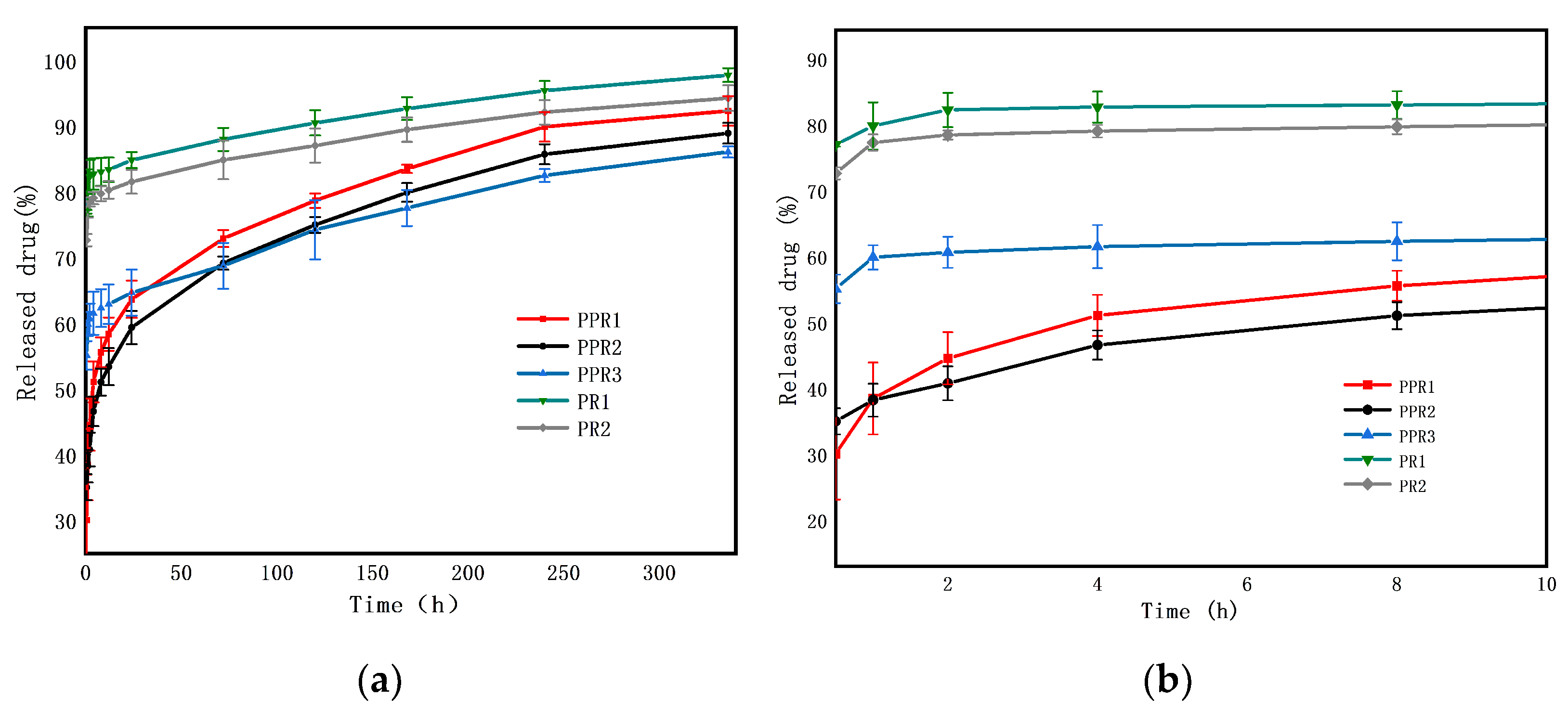
3.3.2. Drug Release Profile
3.3.3. Drug Release Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Monfared, M.; Taghizadeh, S.; Zare-Hoseinabadi, A.; Mousavi, S.M.; Hashemi, S.A.; Ranjbar, S.; Amani, A.M. Emerging frontiers in drug release control by core-shell nanofibers: A review. Drug. Metab. Rev. 2019, 51, 589–611. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Ghasemkhah, F. Design of Porous, Core-Shell, and Hollow Nanofibers. In Handbook of Nanofibers; Springer International Publishing: Berlin, Germany, 2018; pp. 1–58. [Google Scholar]
- Sharifi, F.; Sooriyarachchi, A.C.; Altural, H.; Montazami, R.; Rylander, M.N.; Hashemi, N. Fiber based approaches as medicine delivery systems. ACS Biomater. Sci. Eng. 2016, 2, 1411–1431. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core-shell fibers: Design, roles, and controllable release strategies in tissue engineering and drug delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef]
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun nanofibers for drug delivery and biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef]
- Rathore, P.; Schiffman, J.D. Beyond the single-nozzle: Coaxial electrospinning enables innovative nanofiber chemistries, geometries, and applications. ACS Appl. Mater. Interfaces 2021, 13, 48–66. [Google Scholar] [CrossRef]
- Valério, A.; Mancusi, E.; Ferreira, F.; Guelli Ulson de Souza, S.M.A.; de Souza, A.A.U.; González, S.Y.G. Biopolymer-hydrophobic drug fibers and the delivery mechanisms for sustained release applications. Eur. Polym. J. 2019, 112, 400–410. [Google Scholar] [CrossRef]
- Huang, H.H.; He, C.L.; Wang, H.S.; Mo, X.M. Preparation of core-shell biodegradable microfibers for long-term drug delivery. J. Biomed. Mater. Res. A 2009, 90, 1243–1251. [Google Scholar] [CrossRef]
- Babaie, E.; Bhaduri, S.B. Fabrication aspects of porous biomaterials in orthopedic applications: A review. ACS Biomater. Sci. Eng. 2017, 4, 1–39. [Google Scholar] [CrossRef]
- Qu, H.; Wei, S.; Guo, Z. Coaxial electrospun nanostructures and their applications. J. Mater. Chem. A 2013, 1, 11513–11528. [Google Scholar] [CrossRef]
- He, C.L.; Huang, Z.M.; Han, X.J. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J. Biomed. Mater. Res. A 2009, 89, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.M.; Correa, D.S.; Medeiros, E.S.; Oliveira, J.E.; Mattoso, L.H.C. Advances in functional polymer nanofibers: From spinning fabrication techniques to recent biomedical applications. ACS Appl. Mater. Interfaces 2020, 12, 45673–45701. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ghosh, C.; Hwang, S.G.; Chanunpanich, N.; Park, J.S. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int. J. Pharm. 2012, 439, 296–306. [Google Scholar] [CrossRef]
- Gong, M.; Huang, C.; Huang, Y.; Li, G.; Chi, C.; Ye, J.; Xie, W.; Shi, R.; Zhang, L. Core-sheath micro/nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 124–136. [Google Scholar] [CrossRef]
- Sattari, S.; Tehrani, A.D.; Adeli, M.; Soleimani, K.; Rashidipour, M. Fabrication of new generation of co-delivery systems based on graphene-g-cyclodextrin/chitosan nanofiber. Int. J. Biol. Macromol. 2020, 156, 1126–1134. [Google Scholar] [CrossRef]
- Yu, D.G.; Wang, X.; Li, X.Y.; Chian, W.; Li, Y.; Liao, Y.Z. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers. Acta Biomater. 2013, 9, 5665–5672. [Google Scholar] [CrossRef]
- Zamani, F.; Jahanmard, F.; Ghasemkhah, F.; Amjad-Iranagh, S.; Bagherzadeh, R.; Amani-Tehran, M.; Latifi, M. Nanofibrous and nanoparticle materials as drug-delivery systems. In Nanostructures for Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 239–270. [Google Scholar]
- Ramos, C.; Lanno, G.-M.; Laidmäe, I.; Meos, A.; Härmas, R.; Kogermann, K. High humidity electrospinning of porous fibers for tuning the release of drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–13. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, K.; Jiao, X.; Cheng, Y.; Zhang, Y.; Zhang, P.; Zhang, X.; Wen, Y. A controllable local drug delivery system based on porous fibers for synergistic treatment of melanoma and promoting wound healing. Biomater. Sci. 2019, 7, 5084–5096. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Xiang, C.; Li, L. Electrospun porous PLLA and poly(LLA-co-CL) fibers by phase separation. New J. Chem. 2018, 42, 5102–5108. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel antimicrobial packaging film based on porous poly(lactic acid) nanofiber and polymeric coating for humidity-controlled release of thyme essential oil. Lwt 2021, 135, 110034. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Li, Y.; Wang, M.; Fan, T.; Liu, M.; Ke, Q.; Xu, H.; Yi, Z. An aligned porous electrospun fibrous scaffold with embedded asiatic acid for accelerating diabetic wound healing. J. Mater. Chem. B 2019, 7, 6125–6138. [Google Scholar] [CrossRef]
- Lanno, G.M.; Ramos, C.; Preem, L.; Putrins, M.; Laidmae, I.; Tenson, T.; Kogermann, K. Antibacterial porous electrospun fibers as skin scaffolds for wound healing applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Choi, K.; Choi, S.-O.; Kim, J. Early stage release control of an anticancer drug by drug-polymer miscibility in a hydrophobic fiber-based drug delivery system. RSC Adv. 2018, 8, 19791–19803. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, L.; Huang, C.; Jin, X. Direct electrospinning of ultrafine fibers with interconnected macropores enabled by in situ mixing microfluidics. ACS Appl. Mater. Interfaces 2016, 8, 34870–34878. [Google Scholar] [CrossRef] [PubMed]
- Rade, P.P.; Garnaik, B. Ofloxacin-loaded PLLA nanofibrous mats for wound dressing applications. ACS Appl. Bio. Mater. 2020, 3, 6648–6660. [Google Scholar] [CrossRef]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y. Electrospun microfiber membranes embedded with drug-loaded clay nanotubes for sustained antimicrobial protection. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Srikar, R.; Yarin, A.L.; Megaridis, C.M.; Bazilevsky, A.V. Desorption-limited mechanism of release from polymer nanofibers. Langmuir 2008, 24, 965–974. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. fickian and non-fickian release from non-sweliable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. fickian and anomalous release from sweliable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
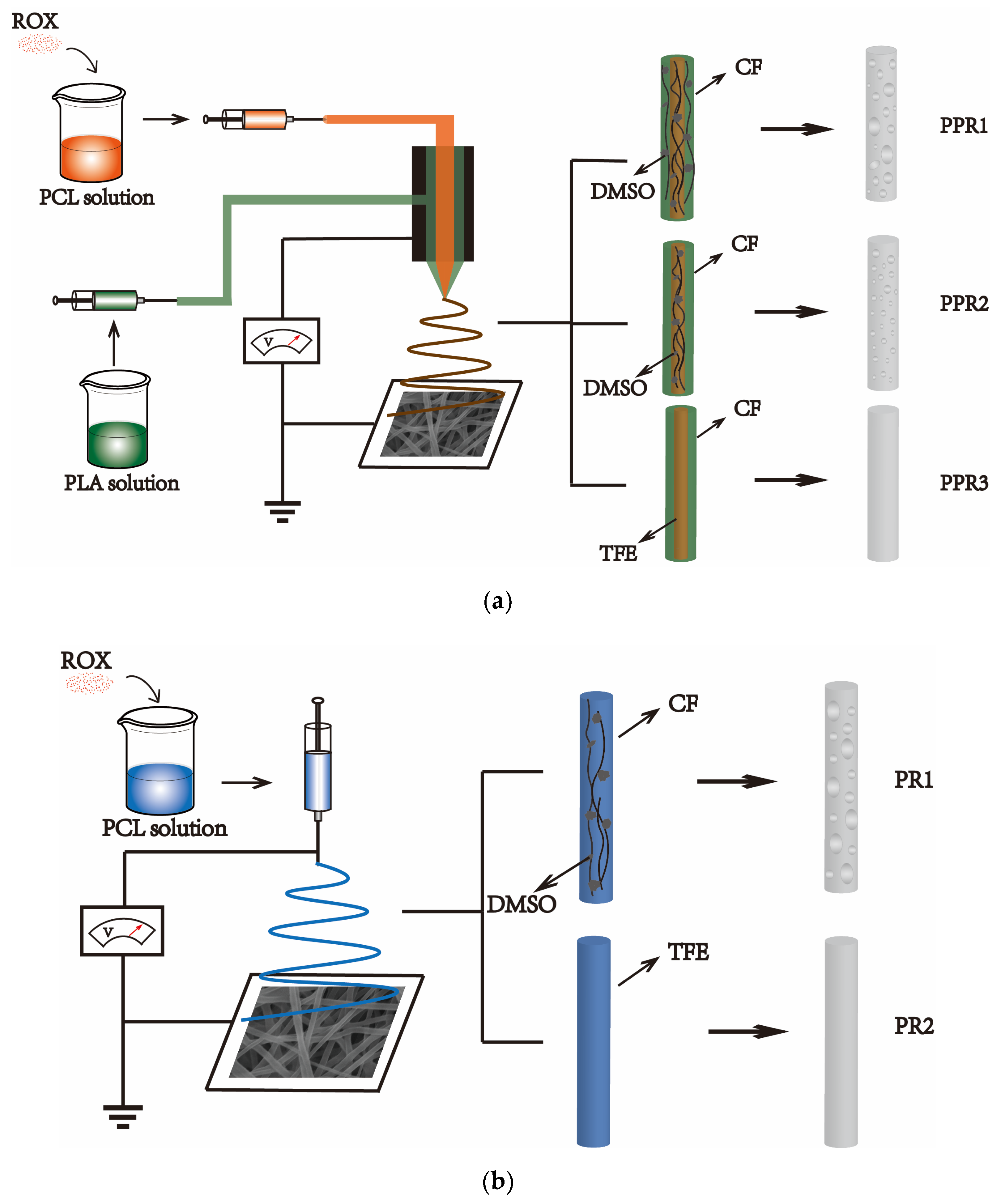


| No. | Sample | Process | Solvent Type | Flow Rate (mL/h) | ROX Content 3 (%) | ||
|---|---|---|---|---|---|---|---|
| Sheath 1 | Core 2 | Sheath | Core | ||||
| PPR1 | PCL/PLA | Coaxial | CF/DMSO | CF/DMSO | 0.2 | 0.1 | 15 |
| PPR2 | PCL/PLA | Coaxial | CF | CF/DMSO | 0.2 | 0.1 | 15 |
| PPR3 | PCL/PLA | Coaxial | CF | TFE | 0.2 | 0.1 | 15 |
| PR1 | PCL | Single | -- | CF/DMSO | -- | 0.3 | 15 |
| PR2 | PCL | Single | -- | TFE | -- | 0.3 | 15 |
| No. | Model | Fitted Equation | R2 | n |
|---|---|---|---|---|
| PPR1 | zero-order model | Mt/M∞ = 0.00502 + 0.00002 t | 0.73113 | 0.1484 |
| the first-order model | ln (1 − Mt/M∞) = -0.00860 t − 0.59935 | 0.87218 | ||
| Higuchi model | Mt/M∞ = 0.00416 + 0.00032 t0.5 | 0.89865 | ||
| Ritger-Peppas model | Mt/M∞ = 0.00393 t0.1484 | 0.9876 | ||
| PPR2 | zero-order model | Mt/M∞ = 0.00477 + 0.00002 t | 0.79783 | 0.14748 |
| the first-order model | ln (1 − Mt/M∞) = −0.00671 t − 0.57268 | 0.92144 | ||
| Higuchi model | Mt/M∞ = 0.00396 + 0.00030 t0.5 | 0.95075 | ||
| Ritger-Peppas model | Mt/M∞ = 0.00375 t0.14748 | 0.99752 | ||
| PPR3 | zero-order model | Mt/M∞ = 0.00611 + 0.00001 t | 0.92653 | 0.06311 |
| the first-order model | ln (1 − Mt/M∞) = −0.00342 t − 0.91761 | 0.9654 | ||
| Higuchi model | Mt/M∞ = 0.00572 + 0.00016 t0.5 | 0.98022 | ||
| Ritger-Peppas model | Mt/M∞ = 0.00562 t0.06311 | 0.88921 | ||
| PR1 | zero-order model | Mt/M∞ = 0.00821+ 0.00001 t | 0.87736 | 0.03243 |
| the first-order model | ln (1 − Mt/M∞) = -0.00616 t − 1.65887 | 0.93903 | ||
| Higuchi model | Mt/M∞ = 0.00794 + 0.00010 t0.5 | 0.96394 | ||
| Ritger-Peppas model | Mt/M∞ = 0.00785 t0.03243 | 0.92104 | ||
| PR2 | zero-order model | Mt/M∞ = 0.00786 + 0.00001 t | 0.8569 | 0.03431 |
| the first-order model | ln (1 − Mt/M∞) = −0.00454 t − 1.5017 | 0.91754 | ||
| Higuchi model | Mt/M∞ = 0.00760 + 0.00010 t0.5 | 0.95155 | ||
| Ritger-Peppas model | Mt/M∞ = 0.00750 t0.03431 | 0.92593 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, H.; Lu, W.; Guo, Y. Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications. Nanomaterials 2021, 11, 1316. https://doi.org/10.3390/nano11051316
Chen X, Li H, Lu W, Guo Y. Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications. Nanomaterials. 2021; 11(5):1316. https://doi.org/10.3390/nano11051316
Chicago/Turabian StyleChen, Xin, Honghai Li, Weipeng Lu, and Yanchuan Guo. 2021. "Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications" Nanomaterials 11, no. 5: 1316. https://doi.org/10.3390/nano11051316
APA StyleChen, X., Li, H., Lu, W., & Guo, Y. (2021). Antibacterial Porous Coaxial Drug-Carrying Nanofibers for Sustained Drug-Releasing Applications. Nanomaterials, 11(5), 1316. https://doi.org/10.3390/nano11051316






