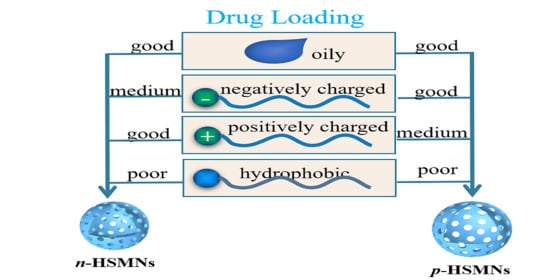
A Comprehensive Study of Drug Loading in Hollow Mesoporous Silica Nanoparticles: Impacting Factors and Loading Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
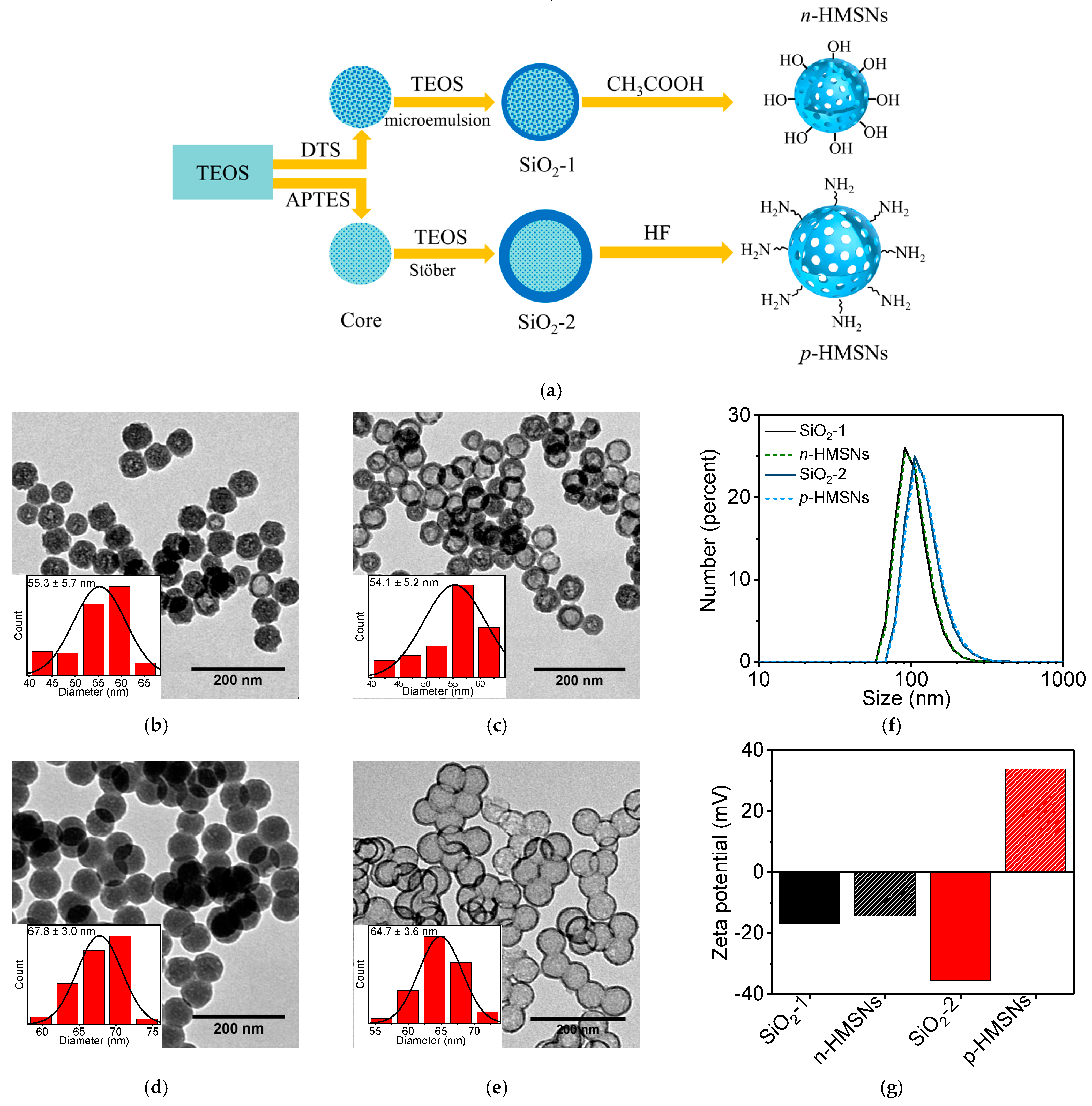
2.3. Synthesis of Negatively Charged HMSNs (n-HMSNs)
2.4. Synthesis of Positively Charged HMSNs (p-HMSNs)
2.5. Drug Loading and Calculation of Loading Efficiency
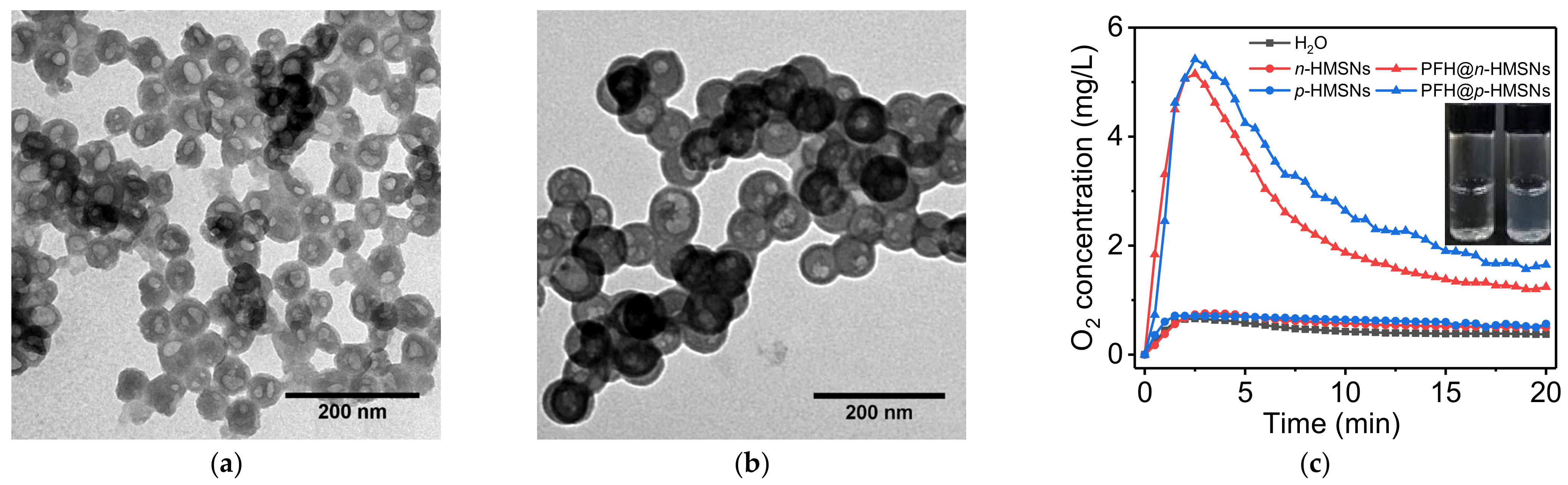
2.6. Measurement of Dissolved Oxygen in HMSNs and PFH@HMSNs
2.7. Cell Culture, NPs Incubation, and Intracellular Uptake Imaged by Confocal Microscopy
3. Results and Discussion
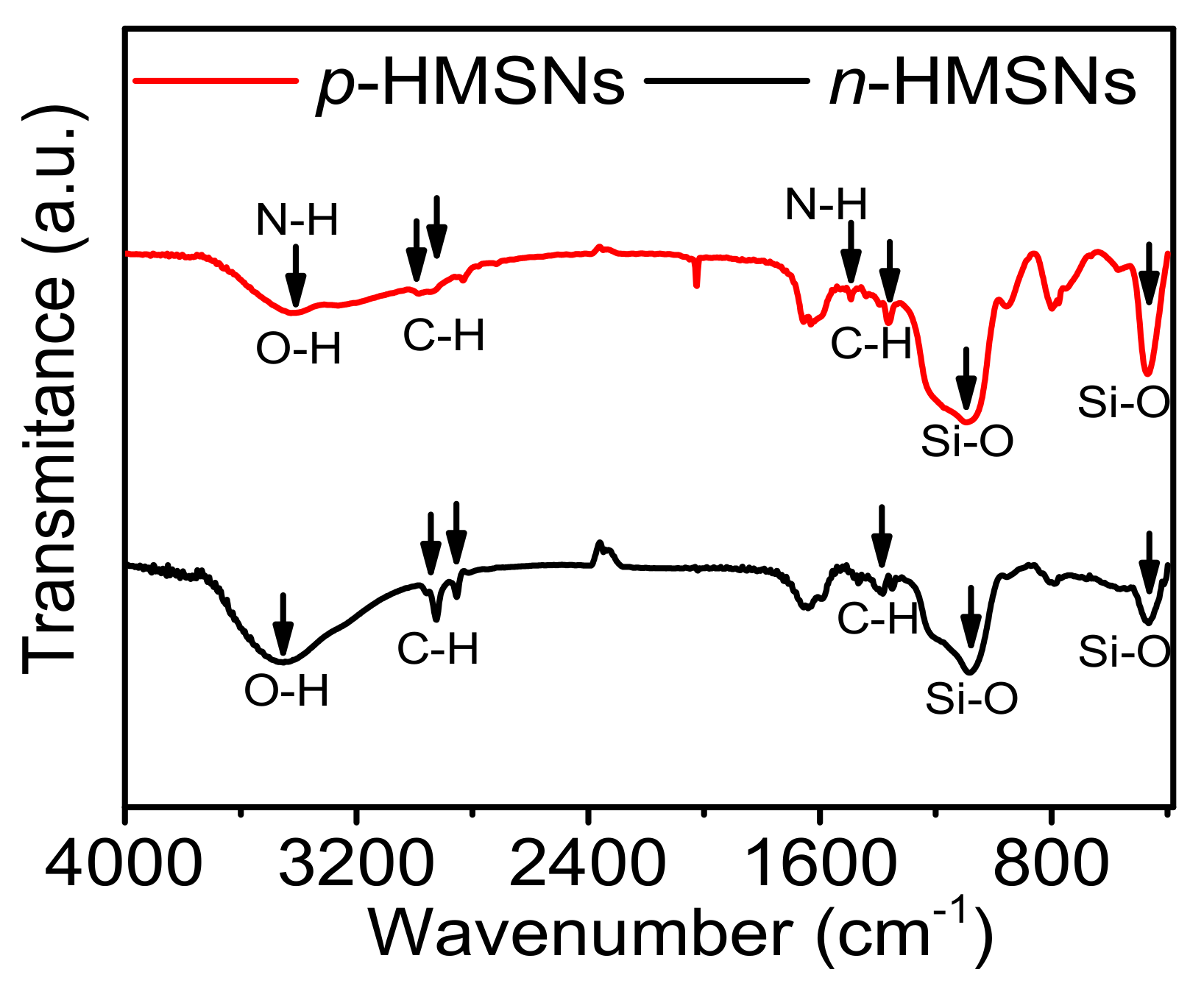
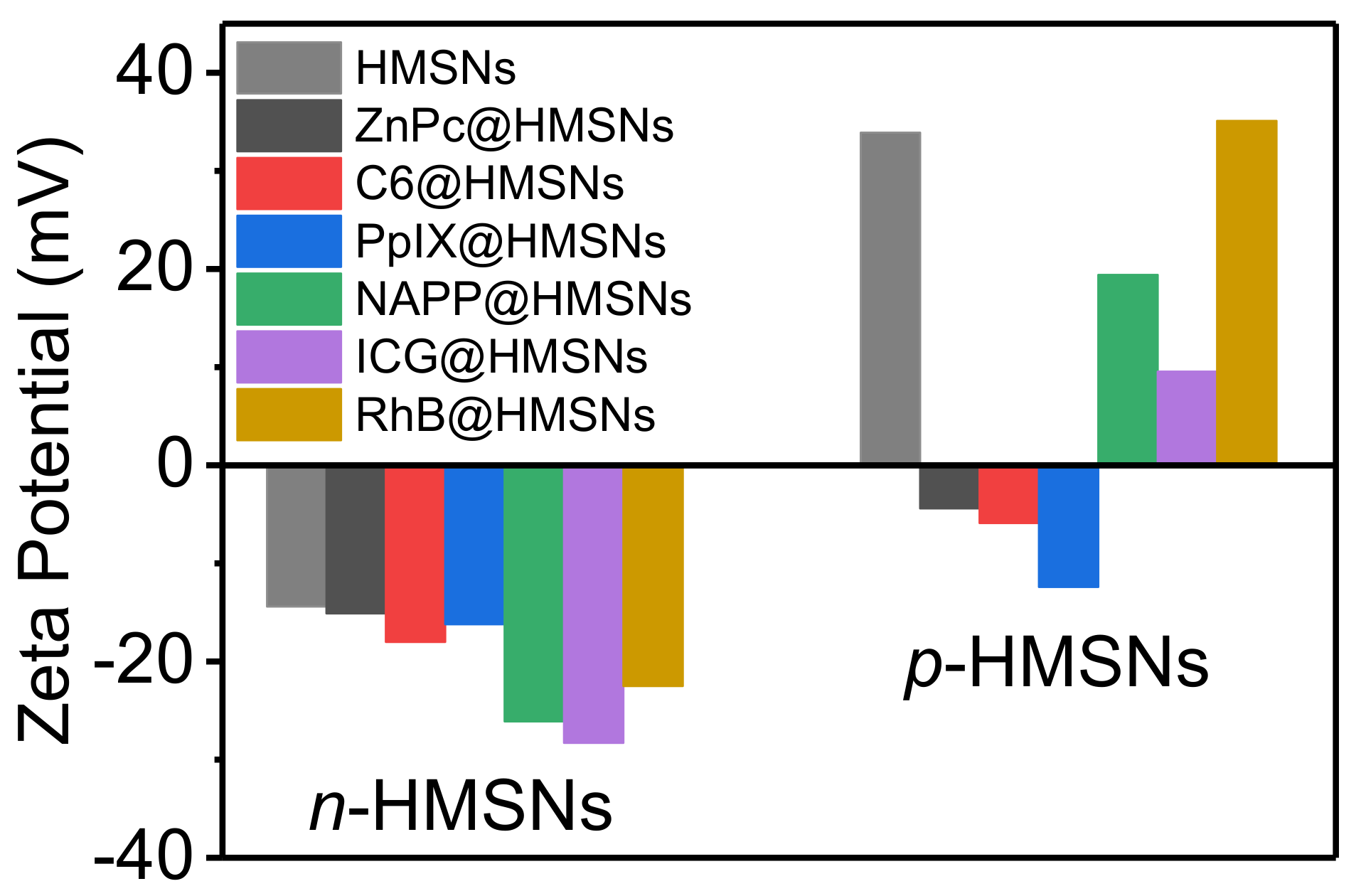
3.1. Synthesis and Characterization of HMSNs
3.2. Analysis of Pore Size and Porosity of HMSNs
3.3. Study of Loading Efficiency of HMSNs towards Different Drugs
3.3.1. Drug Loading of Highly Hydrophobic and Lipophobic Perflurohexane
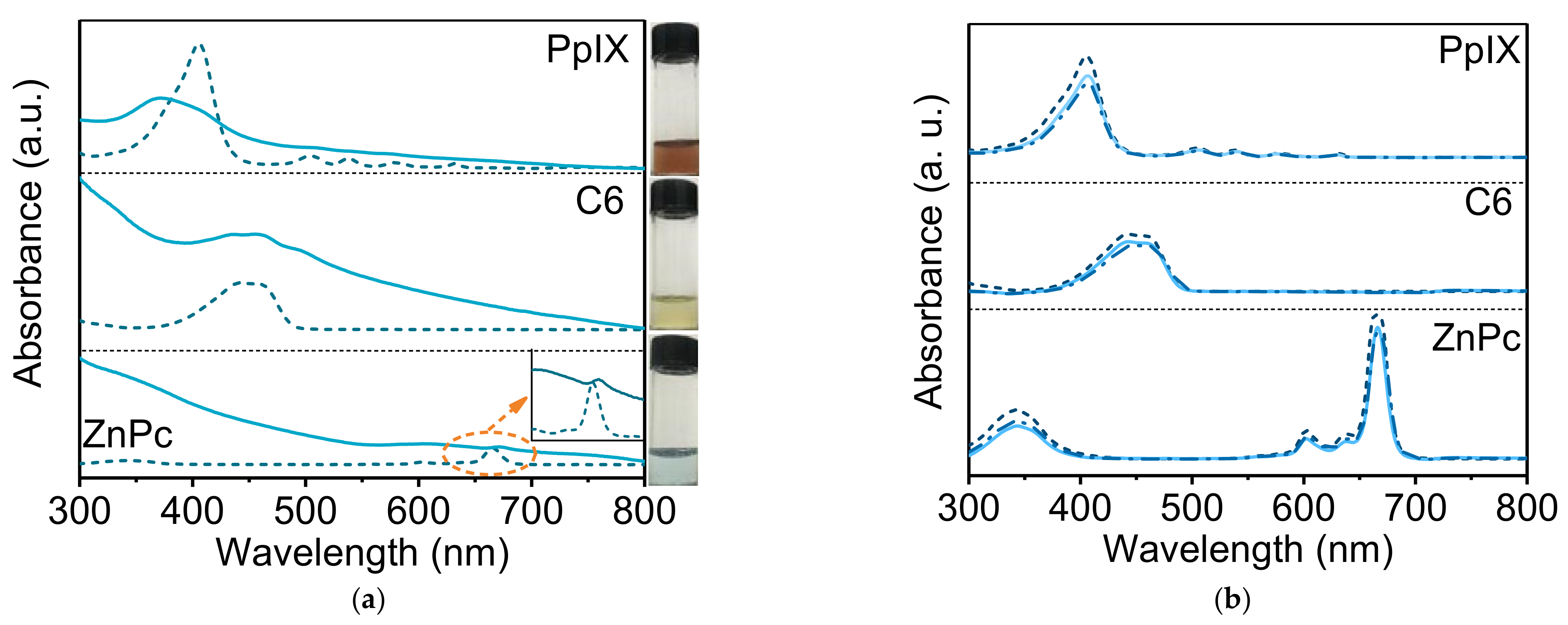
3.3.2. Drug Loading of Hydrophobic Molecules
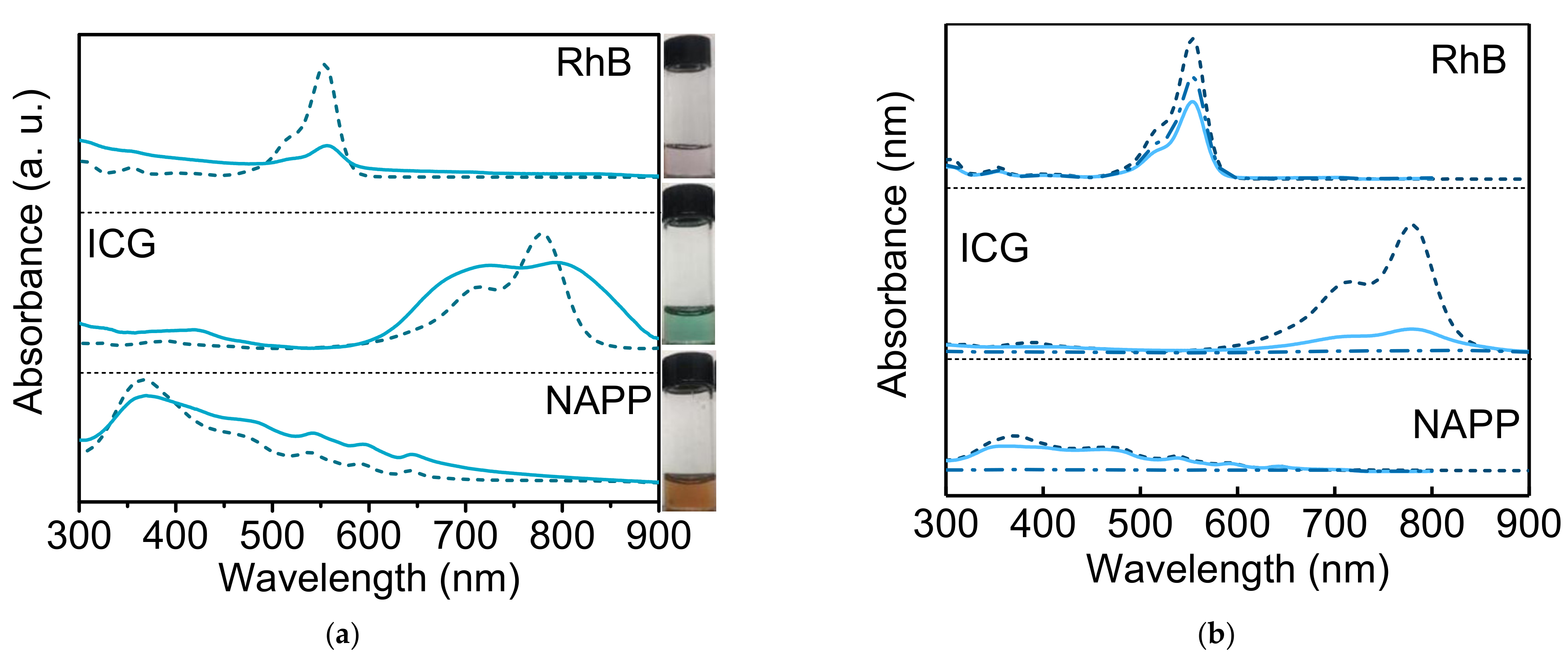
3.3.3. Drug Loading of Hydrophilic Molecules
3.4. Intracellular Study of Drug-Loaded HMSNs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Y.; Cui, J.; Hosta-Rigau, L.; Heath, J.K.; Nice, E.C.; Caruso, F. Encapsulation of water-insoluble drugs in polymer capsules prepared using mesoporous silica templates for intracellular drug delivery. Adv. Mater. 2010, 22, 4293–4297. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, e1705328. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Patel, R.J.; Ajazuddin; Ravichandiran, V.; Murty, U.S.; Alexander, A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. J. Control. Release 2020, 321, 372–415. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for cancer theranostic applications. Adv. Mater. 2020, 32, e1902333. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J. Hollow-structured mesoporous materials: Chemical synthesis, functionalization and applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef]
- Lin, L.-S.; Song, J.; Yang, H.-H.; Chen, X. Yolk-shell nanostructures: Design, synthesis, and biomedical applications. Adv. Mater. 2017, 30, 30. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Shi, J.-L.; Li, Y.-S.; Chen, H.-R.; Shen, W.-H.; Dong, X.-P. Hollow mesoporous spheres with cubic pore network as a potential carrier for drug storage and its in vitro release kinetics. J. Mater. Res. 2005, 20, 54–61. [Google Scholar] [CrossRef]
- Tan, L.; Liu, T.; Li, L.; Liu, H.; Wu, X.; Gao, F.; He, X.; Meng, X.; Chen, D.; Tang, F. Uniform double-shelled silica hollow spheres: Acid/base selective-etching synthesis and their drug delivery application. RSC Adv. 2013, 3, 5649–5655. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Chen, Y.; Ma, M.; Zhang, K.; Li, F.; Zheng, Y.; Zeng, D.; Wang, Q.; Shi, J. Perfluorohexane-encapsulated mesoporous silica nanocapsules as enhancement agents for highly efficient high intensity focused ultrasound (HIFU). Adv. Mater. 2012, 24, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Hollow mesoporous silica nanoparticles as nanocarriers employed in cancer therapy: A review. Front. Mater. Sci. 2020, 14, 373–386. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.; Na, J.; Lee, C.; Sun, Z.; Wang, S.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.; et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, J.H.; Yeh, Y.Q.; Wu, S.H.; Liu, Y.H.; Mou, C.Y. Formation of hollow silica nanospheres by reverse micro-emulsion. Nanoscale 2015, 7, 9614–9626. [Google Scholar] [CrossRef]
- Tao, G.; He, W.; Wang, Y.; Yu, F.; Ge, J.; Yang, W. Dispersity, mesoporous structure and particle size modulation of hollow mesoporous silica nanoparticles with excellent adsorption performance. Dalton Trans. 2018, 47, 13345–13352. [Google Scholar] [CrossRef]
- Xie, J.; Yang, C.; Liu, Q.; Liping, L.; Liang, R.; Shen, C.; Zhang, Y.; Wang, K.; Liu, L.; Shezad, K.; et al. Encapsulation of hydrophilic and hydrophobic peptides into hollow mesoporous silica nanoparticles for enhancement of antitumor immune response. Small 2017, 13, 13. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Y.; Xu, S.; Li, G. A wrinkle to sub-100 nm yolk/shell Fe3O4@SiO2 nanoparticles. Nano Res. 2016, 9, 3632–3643. [Google Scholar] [CrossRef]
- Cai, X.; Jia, X.; Gao, W.; Zhang, K.; Ma, M.; Wang, S.; Zheng, Y.; Shi, J.; Chen, H. A versatile nanotheranostic agent for efficient dual-mode imaging guided synergistic chemo-thermal tumor therapy. Adv. Funct. Mater. 2015, 25, 2520–2529. [Google Scholar] [CrossRef]
- Cui, L.; Liu, W.; Liu, H.; Qin, Q.; Wu, S.; He, S.; Pang, X.; Zhu, C.; Shen, P. pH-triggered charge-reversal mesoporous silica nanoparticles stabilized by chitosan oligosaccharide/carboxymethyl chitosan hybrids for effective intracellular delivery of doxorubicin. ACS Appl. Bio. Mater. 2019, 2, 1907–1919. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Ma, M.; Chen, F.; Guo, L.; Zhang, L.; Shi, J. Double mesoporous silica shelled spherical/ellipsoidal nanostructures: Synthesis and hydrophilic/hydrophobic anticancer drug delivery. J. Mater. Chem. 2011, 21, 5290–5298. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, P.; Pan, W.; Li, N.; Tang, B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat. Commun. 2018, 9, 5044. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, B.; Wang, H.; Yuan, A.; Hu, Y.; Wu, J. Two-stage oxygen delivery for enhanced radiotherapy by perfluoro-carbon nanoparticles. Theranostics 2018, 8, 4898–4911. [Google Scholar] [CrossRef]
- Pochert, A.; Vernikouskaya, I.; Pascher, F.; Rasche, V.; Linden, M. Cargo-influences on the biodistribution of hollow mesopo-rous silica nanoparticles as studied by quantitative (19)F-magnetic resonance imaging. J. Colloid. Interf. Sci. 2017, 488, 1–9. [Google Scholar] [CrossRef]
- Lee, A.L.; Gee, C.T.; Weegman, B.P.; Einstein, S.A.; Juelfs, A.R.; Ring, H.L.; Hurley, K.R.; Egger, S.M.; Swindlehurst, G.; Garwood, M.; et al. Oxygen sensing with perfluorocarbon-loaded ultraporous mesostructured silica nanoparticles. ACS Nano 2017, 11, 5623–5632. [Google Scholar] [CrossRef]
- Tu, J.; Wang, T.; Shi, W.; Wu, G.; Tian, X.; Wang, Y.; Ge, D.; Ren, L. Multifunctional ZnPc-loaded mesoporous silica nanopar-ticles for enhancement of photodynamic therapy efficacy by endolysosomal escape. Biomaterials 2012, 33, 7903–7914. [Google Scholar] [CrossRef]
- Qian, H.S.; Guo, H.C.; Ho, P.C.-L.; Mahendran, R.; Zhang, Y. Mesoporous-silica-coated up-conversion fluorescent nanoparticles for photodynamic therapy. Small 2009, 5, 2285–2290. [Google Scholar] [CrossRef]
- Sixiang, S.; Chen, F.; Cai, W. Biomedical applications of functionalized hollow mesoporous silica nanoparticles focusing on molecular imaging. Nanomedicine 2013, 8, 2027–2039. [Google Scholar]


| Cavity Diameter (nm) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | |
|---|---|---|---|---|
| n-HMSNs | 36.0 (4.7) | 450.77 (2.41) | 1.40 (0.06) | 3.99 (0.10) |
| p-HMSNs | 56.8 (2.2) | 562.03 (3.89) | 2.24 (0.08) | 4.00 (0.09) |
| Drug Name | PFH | ZnPc | C6 | PpIX | NAPP | ICG | RhB | |
|---|---|---|---|---|---|---|---|---|
| Chemical structure |  | 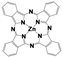 | 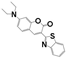 | 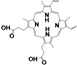 | 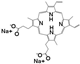 | 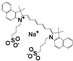 | 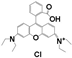 | |
| Configuration | linear | planar | linear | planar | planar | linear | linear | |
| Charge | NA b | NA b | NA b | NA b | negative | negative | positive | |
| Solubility a | insoluble | insoluble | insoluble | insoluble | good | good | good | |
| Loading method | vacuum pumping | capillary action | capillary action | capillary action | electrostatic adsorption | electrostatic adsorption | electrostatic adsorption | |
| Loading efficiency (%) | n-HMSNs | NA b | 9.63 (0.36) | 13.18 (0.78) | 19.49 (0.77) | 33.69 (1.33) | 81.84 (2.82) | 44.91 (1.21) |
| p-HMSNs | NA b | 12.99 (1.68) | 20.91 (1.59) | 26.96 (2.43) | 98.39 (1.58) | 98.40 (1.62) | 27.79 (1.21) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Ping, J.; Qin, J.; Yang, M.; Wu, X.; You, M.; You, F.; Peng, H. A Comprehensive Study of Drug Loading in Hollow Mesoporous Silica Nanoparticles: Impacting Factors and Loading Efficiency. Nanomaterials 2021, 11, 1293. https://doi.org/10.3390/nano11051293
Guo L, Ping J, Qin J, Yang M, Wu X, You M, You F, Peng H. A Comprehensive Study of Drug Loading in Hollow Mesoporous Silica Nanoparticles: Impacting Factors and Loading Efficiency. Nanomaterials. 2021; 11(5):1293. https://doi.org/10.3390/nano11051293
Chicago/Turabian StyleGuo, Lanying, Jiantao Ping, Jinglei Qin, Mu Yang, Xi Wu, Mei You, Fangtian You, and Hongshang Peng. 2021. "A Comprehensive Study of Drug Loading in Hollow Mesoporous Silica Nanoparticles: Impacting Factors and Loading Efficiency" Nanomaterials 11, no. 5: 1293. https://doi.org/10.3390/nano11051293
APA StyleGuo, L., Ping, J., Qin, J., Yang, M., Wu, X., You, M., You, F., & Peng, H. (2021). A Comprehensive Study of Drug Loading in Hollow Mesoporous Silica Nanoparticles: Impacting Factors and Loading Efficiency. Nanomaterials, 11(5), 1293. https://doi.org/10.3390/nano11051293