In Situ Raman Microdroplet Spectroelectrochemical Investigation of CuSCN Electrodeposited on Different Substrates
Abstract
1. Introduction
2. Materials and Methods
3. Results
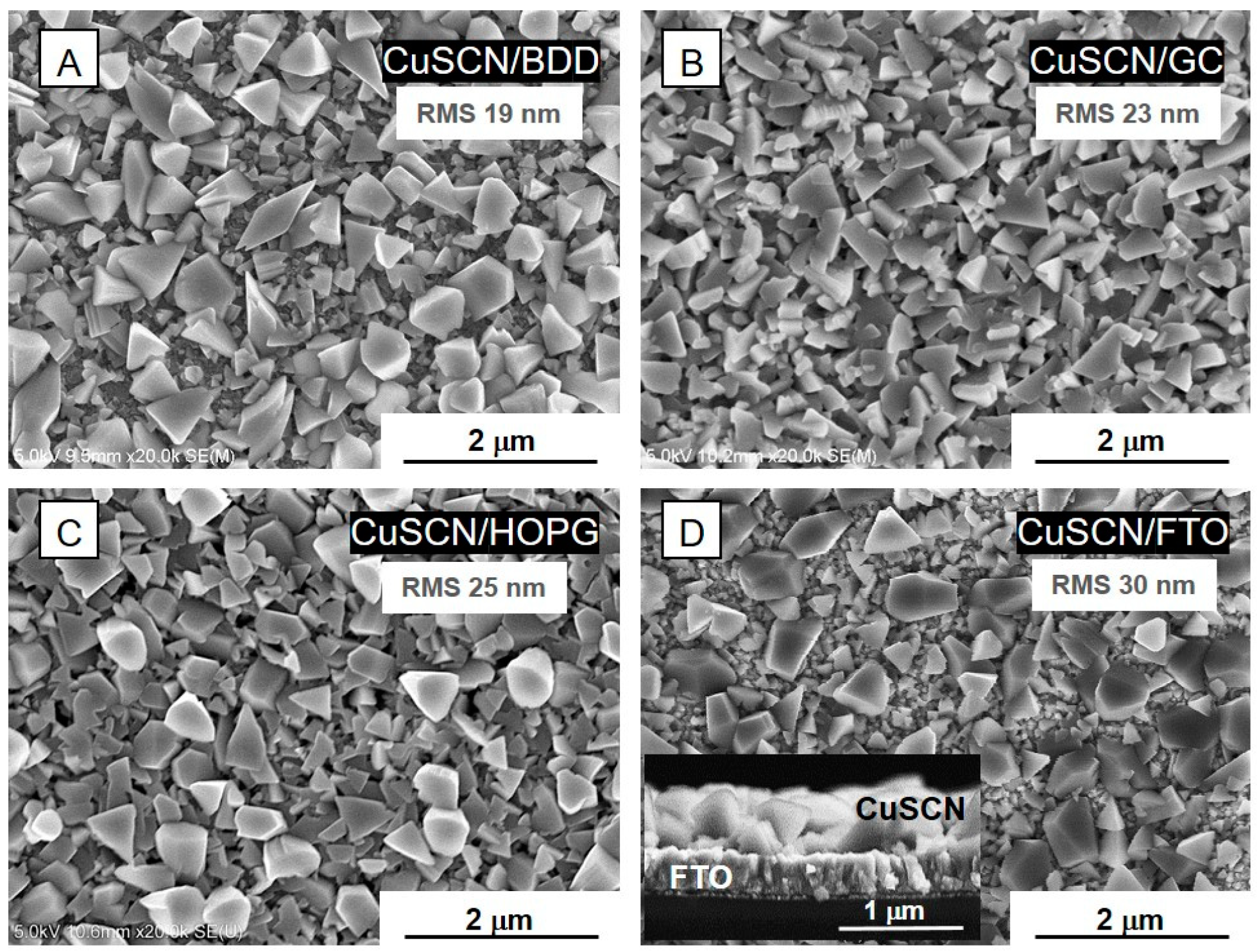
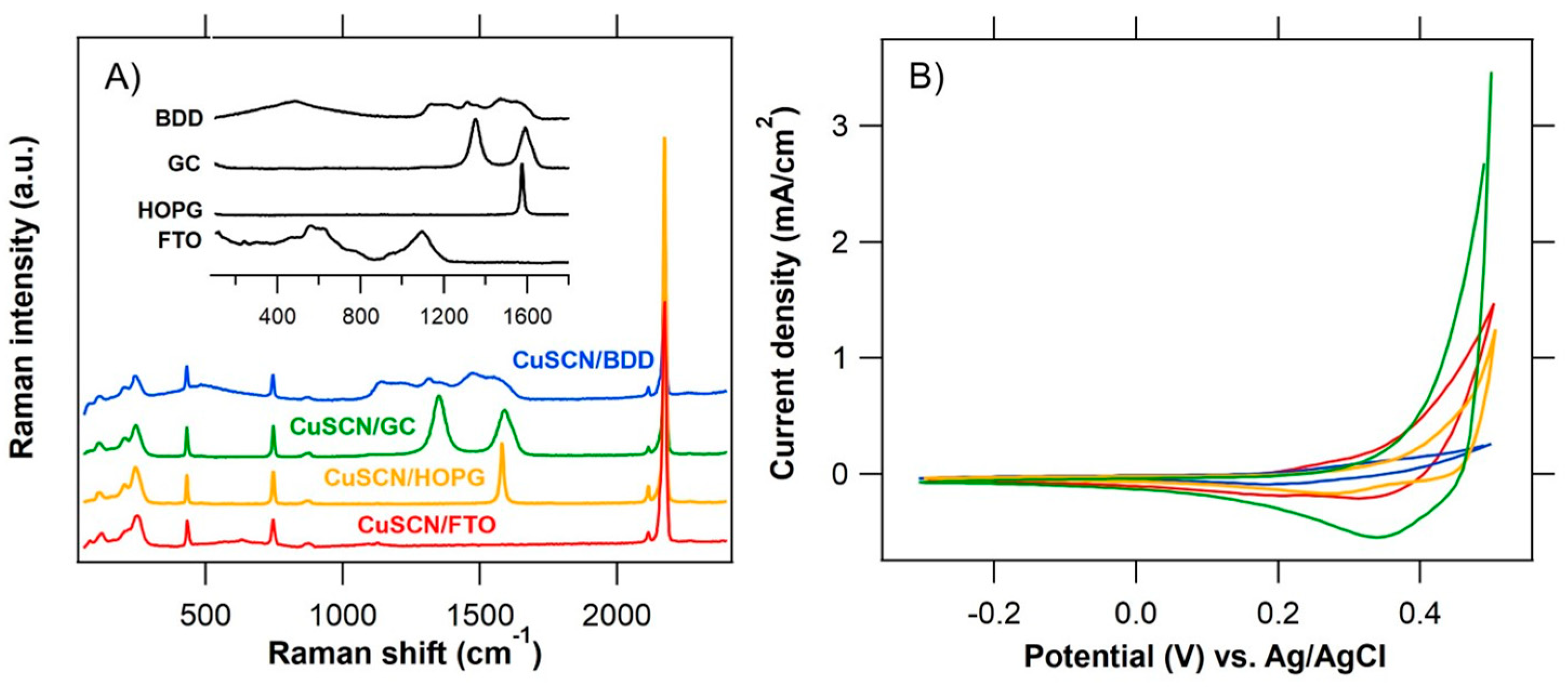
3.1. Structural and Electrochemical Characterization of CuSCN Layers
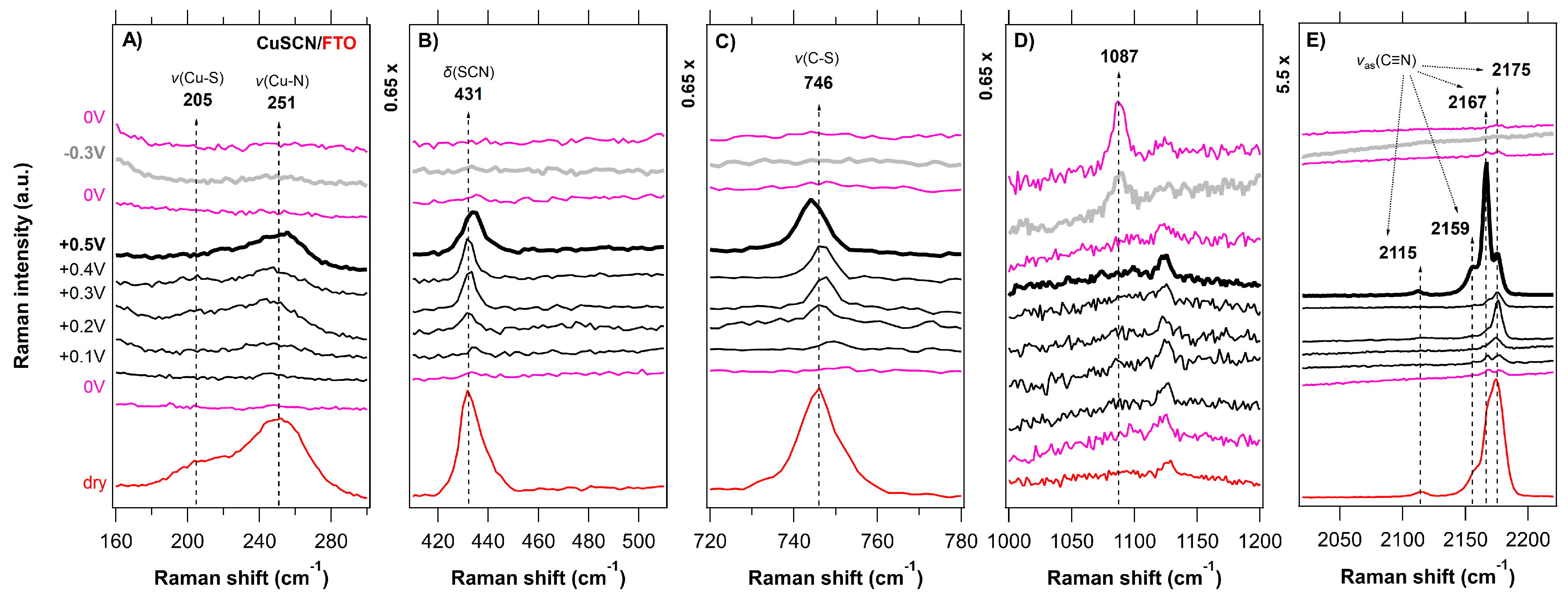
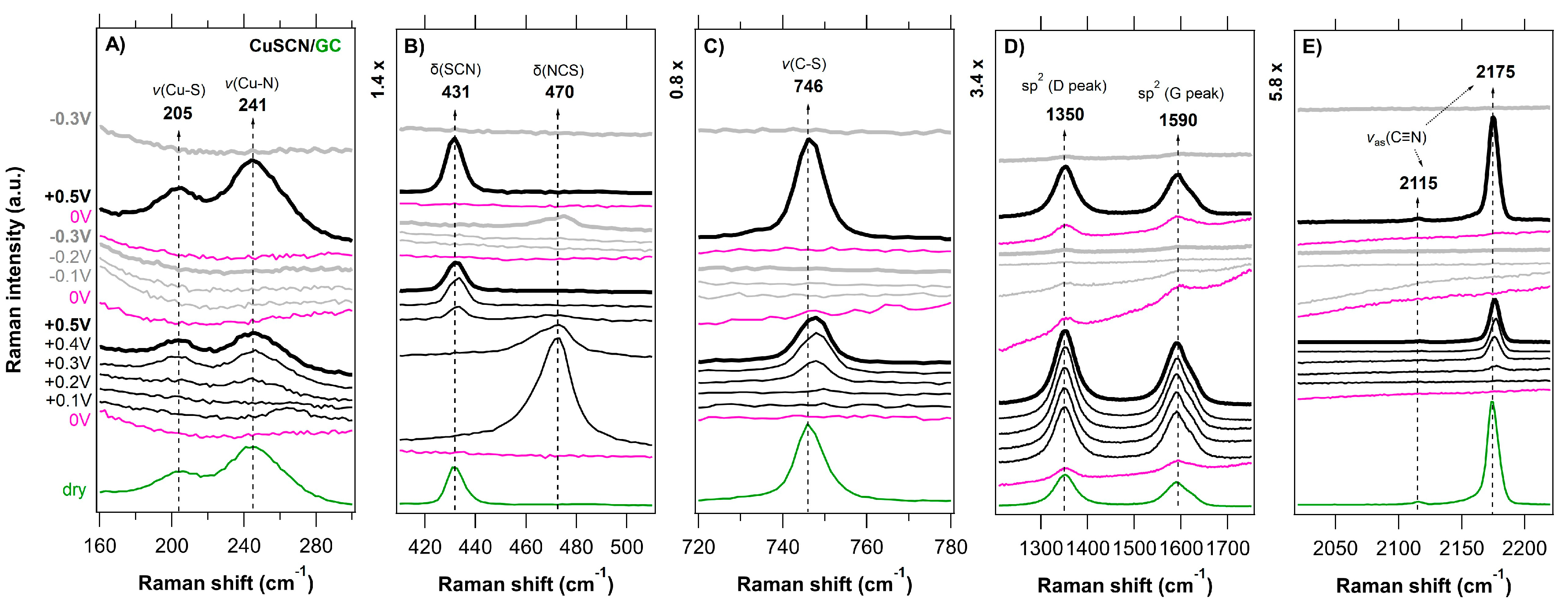
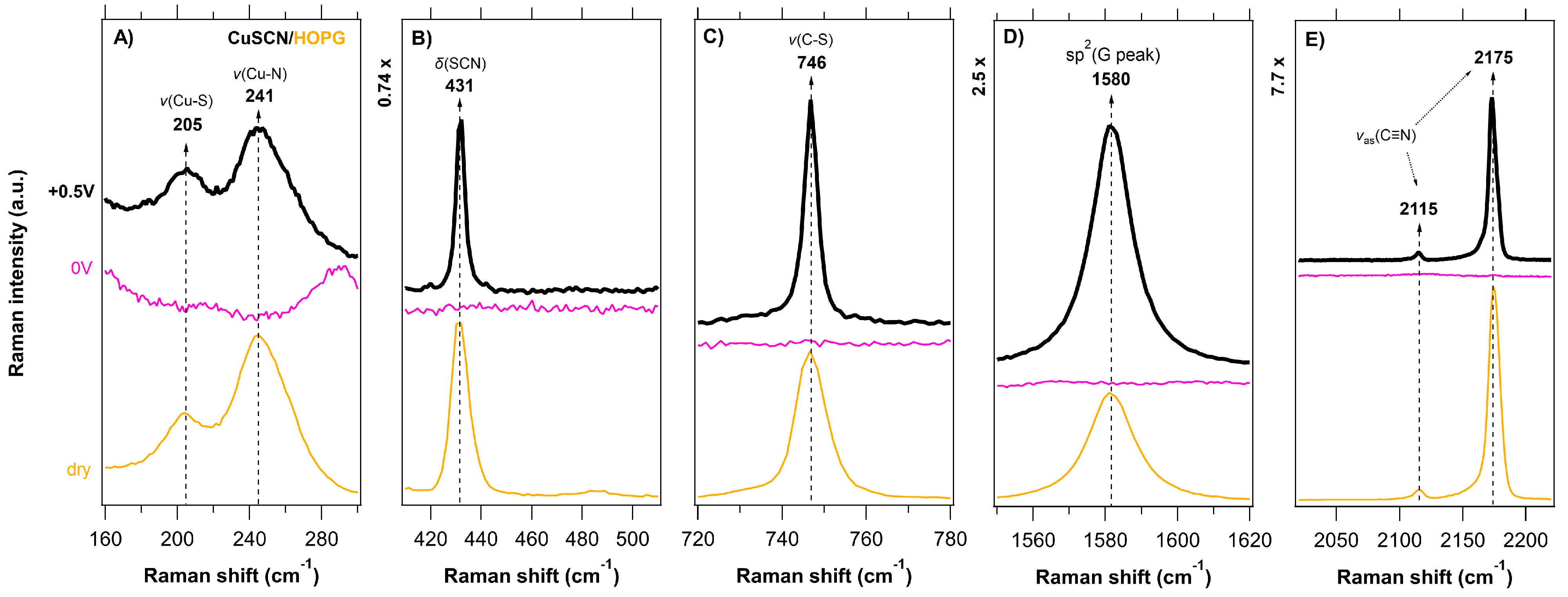
3.2. In Situ Raman Microdroplet Spectroelectrochemistry (Raman-μSEC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bach, U.; Lupo, D.; Comte, P.; Moser, J.E.; Weissörtel, F.; Salbeck, J.; Spreitzer, H.; Gratzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nat. Cell Biol. 1998, 395, 583–585. [Google Scholar] [CrossRef]
- Kavan, L. Electrochemistry and dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 88–96. [Google Scholar] [CrossRef]
- Zhang, J.; Freitag, M.; Hagfeldt, A.; Boschloo, G. Solid-State Dye-Sensitized Solar Cells; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; pp. 151–185. [Google Scholar]
- Kavan, L. Electrochemistry and perovskite photovoltaics. Curr. Opin. Electrochem. 2018, 11, 122–129. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Hagen, J.; Schaffrath, W.; Otschik, P.; Fink, R.; Bacher, A.; Schmidt, H.-W.; Haarer, D. Novel hybrid solar cells consisting of inorganic nanoparticles and an organic hole transport material. Synth. Met. 1997, 89, 215–220. [Google Scholar] [CrossRef]
- Sallenave, X.; Shasti, M.; Anaraki, E.H.; Volyniuk, D.; Grazulevicius, J.V.; Zakeeruddin, S.M.; Mortezaali, A.; Grätzel, M.; Hagfeldt, A.; Sini, G. Interfacial and bulk properties of hole transporting materials in perovskite solar cells: Spiro-MeTAD versus spiro-OMeTAD. J. Mater. Chem. A 2020, 8, 8527–8539. [Google Scholar] [CrossRef]
- O’Regan, B.; Schwartz, D.T. Efficient Photo-Hole Injection from Adsorbed Cyanine Dyes into Electrodeposited Copper(I) Thiocyanate Thin Films. Chem. Mater. 1995, 7, 1349–1354. [Google Scholar] [CrossRef]
- O’Regan, B.; Schwartz, D.T. Large Enhancement in Photocurrent Efficiency Caused by UV Illumination of the Dye-Sensitized Heterojunction TiO2/RuLL‘NCS/CuSCN: Initiation and Potential Mechanisms. Chem. Mater. 1998, 10, 1501–1509. [Google Scholar] [CrossRef]
- Perera, V.P.S.; Pitigala, P.K.D.D.P.; Jayaweera, P.V.V.; Bandaranayake, K.M.P.; Tennakone, K. Dye-Sensitized Solid-State Photovoltaic Cells Based on Dye Multilayer−Semiconductor Nanostructures. J. Phys. Chem. B 2003, 107, 13758–13761. [Google Scholar] [CrossRef]
- Perera, V.; Senevirathna, M.; Pitigala, P.; Tennakone, K. Doping CuSCN films for enhancement of conductivity: Application in dye-sensitized solid-state solar cells. Sol. Energy Mater. Sol. Cells 2005, 86, 443–450. [Google Scholar] [CrossRef]
- Sun, L.; Ichinose, K.; Sekiya, T.; Sugiura, T.; Yoshida, T. Cathodic electrodeposition of p-CuSCN nanorod and its dye-sensitized photocathodic property. Phys. Procedia 2011, 14, 12–24. [Google Scholar] [CrossRef][Green Version]
- Odobel, F.; Pellegrin, Y.; Gibson, E.A.; Hagfeldt, A.; Smeigh, A.L.; Hammarström, L. Recent advances and future directions to optimize the performances of p-type dye-sensitized solar cells. Co-Ord. Chem. Rev. 2012, 256, 2414–2423. [Google Scholar] [CrossRef]
- Premalal, E.; Dematage, N.; Kumara, G.; Rajapakse, R.; Shimomura, M.; Murakami, K.; Konno, A. Preparation of structurally modified, conductivity enhanced-p-CuSCN and its application in dye-sensitized solid-state solar cells. J. Power Sources 2012, 203, 288–296. [Google Scholar] [CrossRef]
- Iwamoto, T.; Ogawa, Y.; Sun, L.; White, M.S.; Glowacki, E.D.; Scharber, M.C.; Sariciftci, N.S.; Manseki, K.; Sugiura, T.; Yoshida, T. Electrochemical Self-Assembly of Nanostructured CuSCN/Rhodamine B Hybrid Thin Film and Its Dye-Sensitized Photocathodic Properties. J. Phys. Chem. C 2014, 118, 16581–16590. [Google Scholar] [CrossRef]
- Wijeyasinghe, N.; Regoutz, A.; Eisner, F.; Du, T.; Tsetseris, L.; Lin, Y.-H.; Faber, H.; Pattanasattayavong, P.; Li, J.; Yan, F.; et al. Copper(I) Thiocyanate (CuSCN) Hole-Transport Layers Processed from Aqueous Precursor Solutions and Their Application in Thin-Film Transistors and Highly Efficient Organic and Organometal Halide Perovskite Solar Cells. Adv. Funct. Mater. 2017, 27, 1701818. [Google Scholar] [CrossRef]
- Matebese, F.; Taziwa, R.; Mutukwa, D. Progress on the Synthesis and Application of CuSCN Inorganic Hole Transport Material in Perovskite Solar Cells. Materials 2018, 11, 2592. [Google Scholar] [CrossRef]
- Yang, I.S.; Lee, S.; Choi, J.; Jung, M.T.; Kim, J.; Lee, W.I. Enhancement of open circuit voltage for CuSCN-based perovskite solar cells by controlling the perovskite/CuSCN interface with functional molecules. J. Mater. Chem. A 2019, 7, 6028–6037. [Google Scholar] [CrossRef]
- Kavan, L.; Zivcova, Z.V.; Hubik, P.; Arora, N.; Dar, M.I.; Zakeeruddin, S.M.; Grätzel, M. Electrochemical Characterization of CuSCN Hole-Extracting Thin Films for Perovskite Photovoltaics. ACS Appl. Energy Mater. 2019, 2, 4264–4273. [Google Scholar] [CrossRef]
- Wijeyasinghe, N.; Eisner, F.; Tsetseris, L.; Lin, Y.-H.; Seitkhan, A.; Li, J.; Yan, F.; Solomeshch, O.; Tessler, N.; Patsalas, P.; et al. p-Doping of Copper(I) Thiocyanate (CuSCN) Hole-Transport Layers for High-Performance Transistors and Organic Solar Cells. Adv. Funct. Mater. 2018, 28, 1802055. [Google Scholar] [CrossRef]
- Patel, M.J.; Gupta, S.K.; Gajjar, P. Electronic structure and optical properties of β-CuSCN: A DFT study. Mater. Today Proc. 2020, 28, 164–167. [Google Scholar] [CrossRef]
- Pattanasattayavong, P.; Packwood, D.M.; Harding, D.J. Structural versatility and electronic structures of copper(i) thiocyanate (CuSCN)–ligand complexes. J. Mater. Chem. C 2019, 7, 12907–12917. [Google Scholar] [CrossRef]
- Ni, Y.; Jin, Z.; Fu, Y. Electrodeposition of p-Type CuSCN Thin Films by a New Aqueous Electrolyte With Triethanolamine Chelation. J. Am. Ceram. Soc. 2007, 90, 2966–2973. [Google Scholar] [CrossRef]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 5th ed.; Wiley Interscience: New York, NY, USA, 1997; pp. 1–273. [Google Scholar]
- Aldakov, D.; Chappaz-Gillot, C.; Salazar, R.; Delaye, V.; Welsby, K.A.; Ivanova, V.; Dunstan, P.R. Properties of Electrodeposited CuSCN 2D Layers and Nanowires Influenced by Their Mixed Domain Structure. J. Phys. Chem. C 2014, 118, 16095–16103. [Google Scholar] [CrossRef]
- Yoshida, T.; Zhang, J.; Komatsu, D.; Sawatani, S.; Minoura, H.; Pauporté, T.; Lincot, D.; Oekermann, T.; Schlettwein, D.; Tada, H.; et al. Electrodeposition of Inorganic/Organic Hybrid Thin Films. Adv. Funct. Mater. 2008, 19, 17–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, H.; Feng, Z.; Lin, L.; Zhou, J.; Lin, Z. n-ZnO nanorods/p-CuSCN heterojunction light-emitting diodes fabricated by electrochemical method. Electrochim. Acta 2010, 55, 4889–4894. [Google Scholar] [CrossRef]
- Chappaz-Gillot, C.; Salazar, R.; Berson, S.; Ivanova, V. Room temperature template-free electrodeposition of CuSCN nanowires. Electrochem. Commun. 2012, 24, 1–4. [Google Scholar] [CrossRef]
- Sanchez, S.; Chappaz-Gillot, C.; Salazar, R.; Muguerra, H.; Arbaoui, E.; Berson, S.; Lévy-Clément, C.; Ivanova, V. Comparative study of ZnO and CuSCN semiconducting nanowire electrodeposition on different substrates. J. Solid State Electrochem. 2012, 17, 391–398. [Google Scholar] [CrossRef]
- Sun, L.; Huang, Y.; Hossain, A.; Li, K.; Adams, S.; Wang, Q. Fabrication of TiO2/CuSCN Bulk Heterojunctions by Profile-Controlled Electrodeposition. J. Electrochem. Soc. 2012, 159, D323–D327. [Google Scholar] [CrossRef]
- Chappaz-Gillot, C.; Salazar, R.; Berson, S.; Ivanova, V. Insights into CuSCN nanowire electrodeposition on flexible substrates. Electrochim. Acta 2013, 110, 375–381. [Google Scholar] [CrossRef]
- Ramírez, D.; Álvarez, K.; Riveros, G.; González, B.; Dalchiele, E.A. Electrodeposition of CuSCN seed layers and nanowires: A microelectrogravimetric approach. Electrochim. Acta 2017, 228, 308–318. [Google Scholar] [CrossRef]
- Shlenskaya, N.N.; Tutantsev, A.S.; Belich, N.A.; Goodilin, E.A.; Grätzel, M.; Tarasov, A.B. Electrodeposition of porous CuSCN layers as hole-conducting material for perovskite solar cells. Mendeleev Commun. 2018, 28, 378–380. [Google Scholar] [CrossRef]
- Chavhan, S.; Miguel, O.; Grande, H.-J.; Gonzalez-Pedro, V.; Sánchez, R.S.; Barea, E.M.; Mora-Seró, I.; Tena-Zaera, R. Organo-metal halide perovskite-based solar cells with CuSCN as the inorganic hole selective contact. J. Mater. Chem. A 2014, 2, 12754–12760. [Google Scholar] [CrossRef]
- Yang, I.S.; Sohn, M.R.; Sung, S.D.; Kim, Y.J.; Yoo, Y.J.; Kim, J.; Lee, W.I. Formation of pristine CuSCN layer by spray deposition method for efficient perovskite solar cell with extended stability. Nano Energy 2017, 32, 414–421. [Google Scholar] [CrossRef]
- Kavan, L.; Dunsch, L. Spectroelectrochemistry of Carbon Nanostructures. ChemPhysChem 2007, 8, 974–998. [Google Scholar] [CrossRef]
- Velický, M.; Bradley, D.F.; Cooper, A.J.; Hill, E.W.; Kinloch, I.A.; Mishchenko, A.; Novoselov, K.S.; Patten, H.V.; Toth, P.S.; Valota, A.T.; et al. Electron Transfer Kinetics on Mono- and Multilayer Graphene. ACS Nano 2014, 8, 10089–10100. [Google Scholar] [CrossRef]
- Velický, M.; Bissett, M.A.; Woods, C.R.; Toth, P.S.; Georgiou, T.; Kinloch, I.A.; Novoselov, K.S.; Dryfe, R.A.W. Photoelectrochemistry of Pristine Mono- and Few-Layer MoS2. Nano Lett. 2016, 16, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Velický, M.; Toth, P.S.; Woods, C.R.; Novoselov, K.S.; Dryfe, R.A.W. Electrochemistry of the Basal Plane versus Edge Plane of Graphite Revisited. J. Phys. Chem. C 2019, 123, 11677–11685. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Swain, G.M.; Ramesham, R. The electrochemical activity of boron-doped polycrystalline diamond thin film electrodes. Anal. Chem. 1993, 65, 345–351. [Google Scholar] [CrossRef]
- Pleskov, Y.; Mishuk, V.; Abaturov, M.; Elkin, V.; Krotova, M.; Varnin, V.; Teremetskaya, I. Synthetic semiconductor diamond electrodes: Determination of acceptor concentration by linear and non-linear impedance measurements. J. Electroanal. Chem. 1995, 396, 227–232. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]
- Zivcova, Z.V.; Frank, O.; Petrák, V.; Tarábková, H.; Vacik, J.; Nesládek, M.; Kavan, L. Electrochemistry and in situ Raman spectroelectrochemistry of low and high quality boron doped diamond layers in aqueous electrolyte solution. Electrochim. Acta 2013, 87, 518–525. [Google Scholar] [CrossRef]
- MacPherson, J.V. A practical guide to using boron doped diamond in electrochemical research. Phys. Chem. Chem. Phys. 2015, 17, 2935–2949. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L.; Zivcova, Z.V.; Petrak, V.; Frank, O.; Janda, P.; Tarabkova, H.; Nesladek, M.; Mortet, V. Boron-doped Diamond Electrodes: Electrochemical, Atomic Force Microscopy and Raman Study towards Corrosion-modifications at Nanoscale. Electrochim. Acta 2015, 179, 626–636. [Google Scholar] [CrossRef]
- Živcová, Z.V.; Frank, O.; Drijkoningen, S.; Haenen, K.; Mortet, V.; Kavan, L. n-Type phosphorus-doped nanocrystalline diamond: Electrochemical and in situ Raman spectroelectrochemical study. Rsc Adv. 2016, 6, 51387–51393. [Google Scholar] [CrossRef]
- Zivcova, Z.V.; Petrák, V.; Frank, O.; Kavan, L. Electrochemical impedance spectroscopy of polycrystalline boron doped diamond layers with hydrogen and oxygen terminated surface. Diam. Relat. Mater. 2015, 55, 70–76. [Google Scholar] [CrossRef]
- Itoh, T.; McCreery, R.L. In Situ Raman Spectroelectrochemistry of Electron Transfer between Glassy Carbon and a Chemisorbed Nitroazobenzene Monolayer. J. Am. Chem. Soc. 2002, 124, 10894–10902. [Google Scholar] [CrossRef]
- Kalbac, M.; Kavan, L.; Dunsch, L. An in situ Raman spectroelectrochemical study of the controlled doping of semiconducting single walled carbon nanotubes in a conducting polymer matrix. Synth. Met. 2009, 159, 2245–2248. [Google Scholar] [CrossRef]
- Frank, O.; Dresselhaus, M.S.; Kalbac, M. Raman Spectroscopy and in Situ Raman Spectroelectrochemistry of Isotopically Engineered Graphene Systems. Acc. Chem. Res. 2015, 48, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, R.; Choy, K. Electrostatic spray assisted vapour deposition of fluorine doped tin oxide. J. Cryst. Growth 2001, 231, 215–221. [Google Scholar] [CrossRef]
- Shiell, T.B.; Wong, S.; Yang, W.; Tanner, C.A.; Haberl, B.; Elliman, R.G.; McKenzie, D.R.; McCulloch, D.G.; Bradby, J.E. The composition, structure and properties of four different glassy carbons. J. Non-Cryst. Solids 2019, 522, 119561. [Google Scholar] [CrossRef]
- Kawashima, Y.; Katagiri, G. Fundamentals, overtones, and combinations in the Raman spectrum of graphite. Phys. Rev. B 1995, 52, 10053–10059. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Prawer, S.; Nemanich, R.J. Raman spectroscopy of diamond and doped diamond. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2537–2565. [Google Scholar] [CrossRef] [PubMed]
- Dennison, J.R.; Holtz, M.; Swain, G. Raman Spectroscopy of Carbon Materials. Spectroscopy 1996, 11, 38–45. [Google Scholar]
- Williams, A.W.S.; Lightowlers, E.C.; Collins, A.T. Impurity conduction in synthetic semiconducting diamond. J. Phys. C: Solid State Phys. 1970, 3, 1727–1735. [Google Scholar] [CrossRef]
- Mortet, V.; Taylor, A.; Živcová, Z.V.; Machon, D.; Frank, O.; Hubík, P.; Tremouilles, D.; Kavan, L. Analysis of heavily boron-doped diamond Raman spectrum. Diam. Relat. Mater. 2018, 88, 163–166. [Google Scholar] [CrossRef]
- Bian, H.; Chen, H.; Zhang, Q.; Li, J.; Wen, X.; Zhuang, W.; Zheng, J. Cation Effects on Rotational Dynamics of Anions and Water Molecules in Alkali (Li+, Na+, K+, Cs+) Thiocyanate (SCN–) Aqueous Solutions. J. Phys. Chem. B 2013, 117, 7972–7984. [Google Scholar] [CrossRef]
- Son, Y.; de Tacconi, N.R.; Rajeshwar, K. Photoelectrochemistry and Raman spectroelectrochemistry of cuprous thiocyanate films on copper electrodes in acidic media. J. Electroanal. Chem. 1993, 345, 135–146. [Google Scholar] [CrossRef]
- Laser, D.; Bard, A.J. Semiconductor electrodes. IV. Electrochemical behavior of n- and p-type silicon electrodes in acetonitrile solutions. J. Phys. Chem. 1976, 80, 459–466. [Google Scholar] [CrossRef]
- Zou, Y.; Walton, A.S.; Kinloch, I.A.; Dryfe, R.A.W. Investigation of the Differential Capacitance of Highly Ordered Pyrolytic Graphite as a Model Material of Graphene. Langmuir 2016, 32, 11448–11455. [Google Scholar] [CrossRef] [PubMed]
- Van Duyne, R.P.; Haushalter, J.P. Resonance Raman spectroelectrochemistry of semiconductor electrodes: The photooxidation of tetrathiafulvalene at n-gallium arsenide(100) in acetonitrile. J. Phys. Chem. 1984, 88, 2446–2451. [Google Scholar] [CrossRef]
- Hao, H.; Xie, Q.; Ai, J.; Wang, Y.; Bian, H. Specific counter-cation effect on the molecular orientation of thiocyanate anions at the aqueous solution interface. Phys. Chem. Chem. Phys. 2020, 22, 10106–10115. [Google Scholar] [CrossRef]
- Gans, P.; Gill, J.B.; Griffin, M. Spectrochemistry of solutions. Part 5.—Raman spectroscopic study of the coordination of silver(I) ions in liquid ammonia by thiocyanate ions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1978, 74, 432–439. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlčková Živcová, Z.; Bouša, M.; Velický, M.; Frank, O.; Kavan, L. In Situ Raman Microdroplet Spectroelectrochemical Investigation of CuSCN Electrodeposited on Different Substrates. Nanomaterials 2021, 11, 1256. https://doi.org/10.3390/nano11051256
Vlčková Živcová Z, Bouša M, Velický M, Frank O, Kavan L. In Situ Raman Microdroplet Spectroelectrochemical Investigation of CuSCN Electrodeposited on Different Substrates. Nanomaterials. 2021; 11(5):1256. https://doi.org/10.3390/nano11051256
Chicago/Turabian StyleVlčková Živcová, Zuzana, Milan Bouša, Matěj Velický, Otakar Frank, and Ladislav Kavan. 2021. "In Situ Raman Microdroplet Spectroelectrochemical Investigation of CuSCN Electrodeposited on Different Substrates" Nanomaterials 11, no. 5: 1256. https://doi.org/10.3390/nano11051256
APA StyleVlčková Živcová, Z., Bouša, M., Velický, M., Frank, O., & Kavan, L. (2021). In Situ Raman Microdroplet Spectroelectrochemical Investigation of CuSCN Electrodeposited on Different Substrates. Nanomaterials, 11(5), 1256. https://doi.org/10.3390/nano11051256








