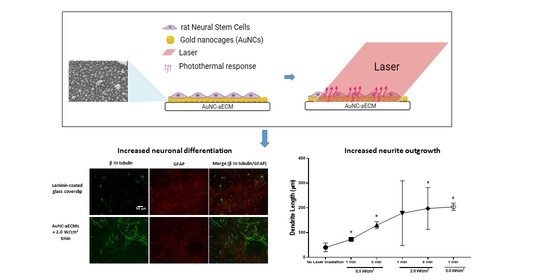
Photothermal Response Induced by Nanocage-Coated Artificial Extracellular Matrix Promotes Neural Stem Cell Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Gold Nanocage (AuNC) Preparation
2.3. Gold Nanocage-Artificial Extracellular Matrices (AuNC-aECMs) Preparation
2.4. AuNC-aECM Characterization
2.5. Laminin Coating
2.6. Cell Culture and rNSC Differentiation
2.7. Photothermal Treatment
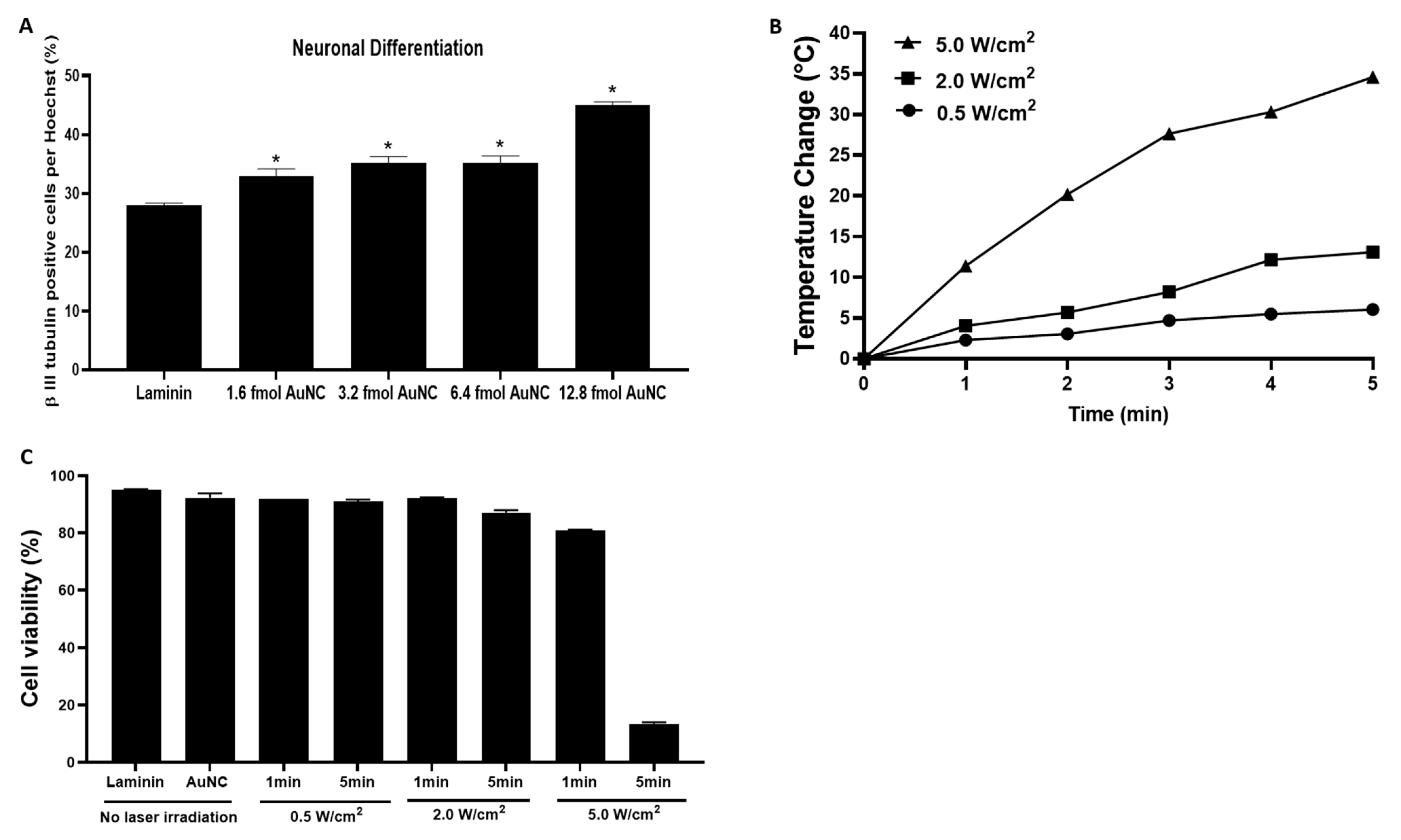
2.8. Measurement of Cell Viability
2.9. Immunocytochemistry
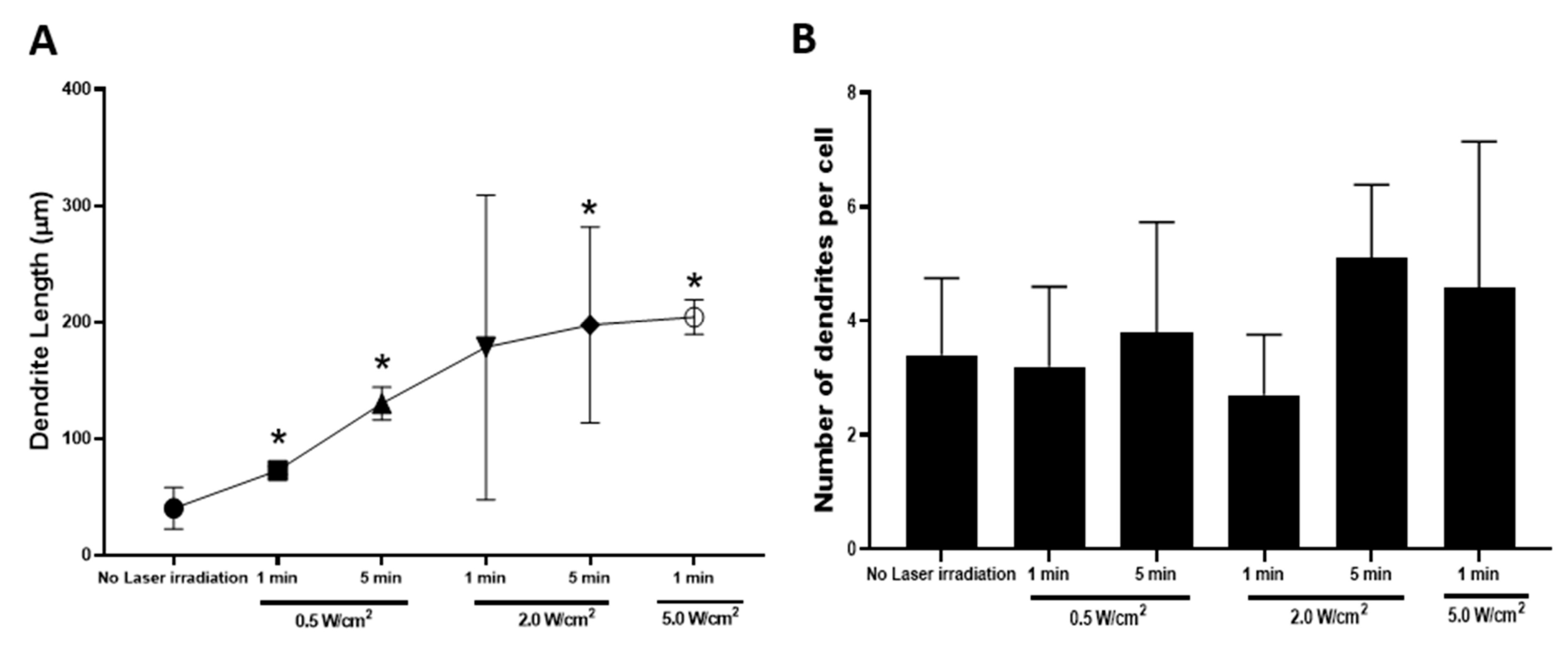
2.10. Assessment of Dendrite Length and Number Using the Neurite Tracing Program
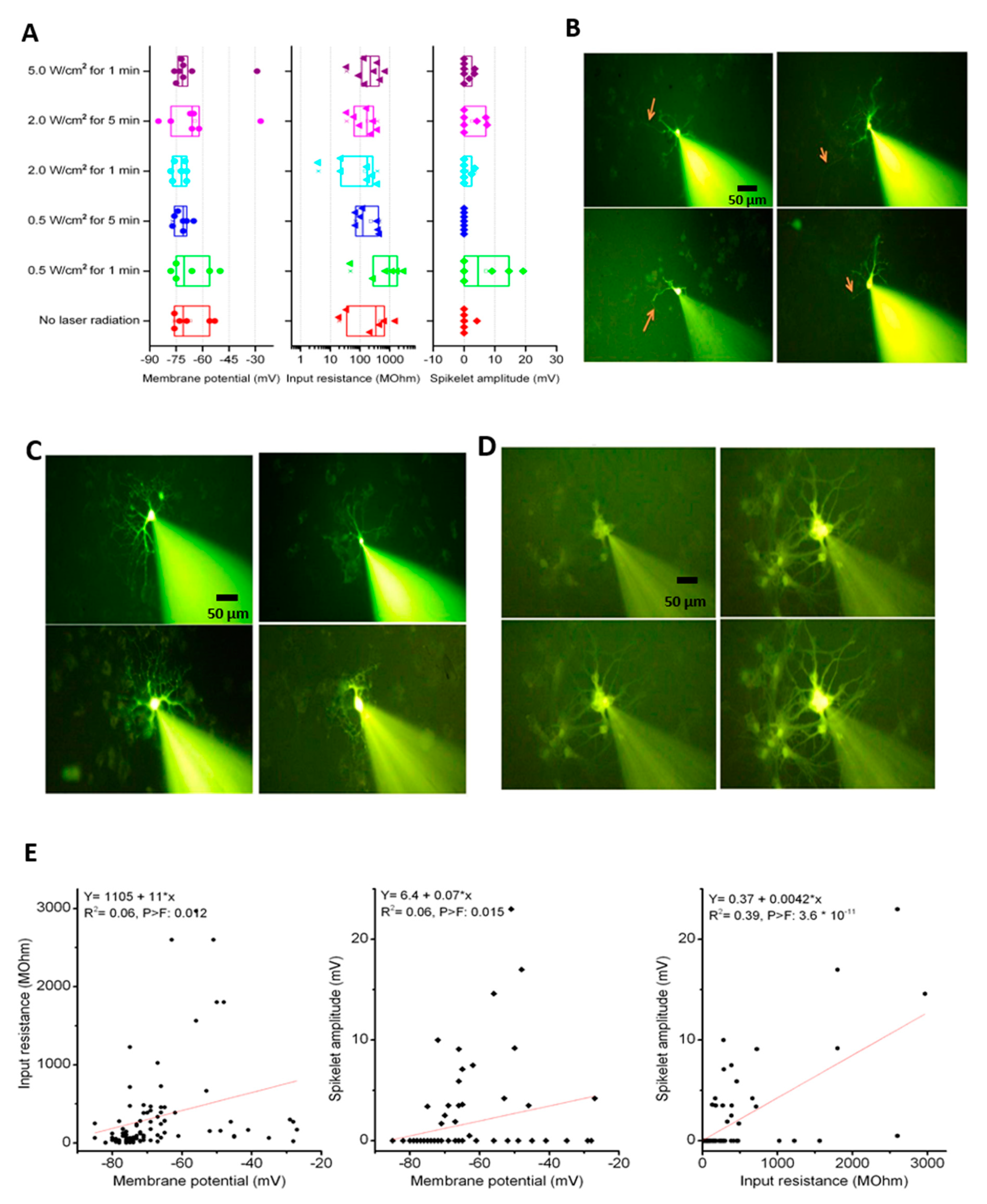
2.11. Patch-Clamp Recording
2.12. Measurement of Heat Shock Protein 27, 70, and 90α Expression
2.13. Statistical Analysis
3. Results
3.1. Characterization of the AuNC-aECMs and Laminin-Coated AuNC-aECMs
3.2. Effects of the Varying Coverage Densities of AuNCs onto Laminin-Coated AuNC-aECMs and Photothermal Response of Laminin-Coated AuNC-aECMs on Neuronal Differentiation Capacity in Rat Neural Stem Cells
3.3. Effects of Photothermal Response of Laminin-Coated AuNC-aECMs on Neuronal Differentiation Capacity in Rat Neural Stem Cells
3.4. Effects of Photothermal Response of Laminin-Coated AuNC-aECMs on Morphological Changes of Neuronal Maturation in Rat Neural Stem Cells
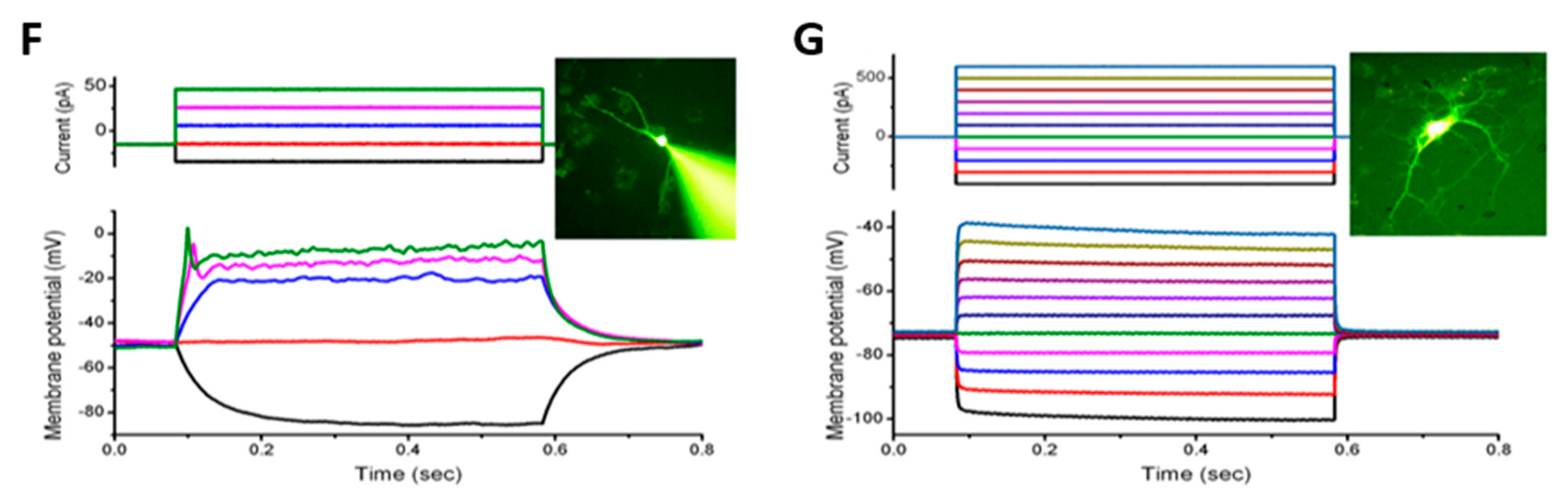
3.5. Effects of Photothermal Response of Laminin-Coated AuNC-aECMs on Neuronal Electrophysiological Activity in Differentiated Neurons
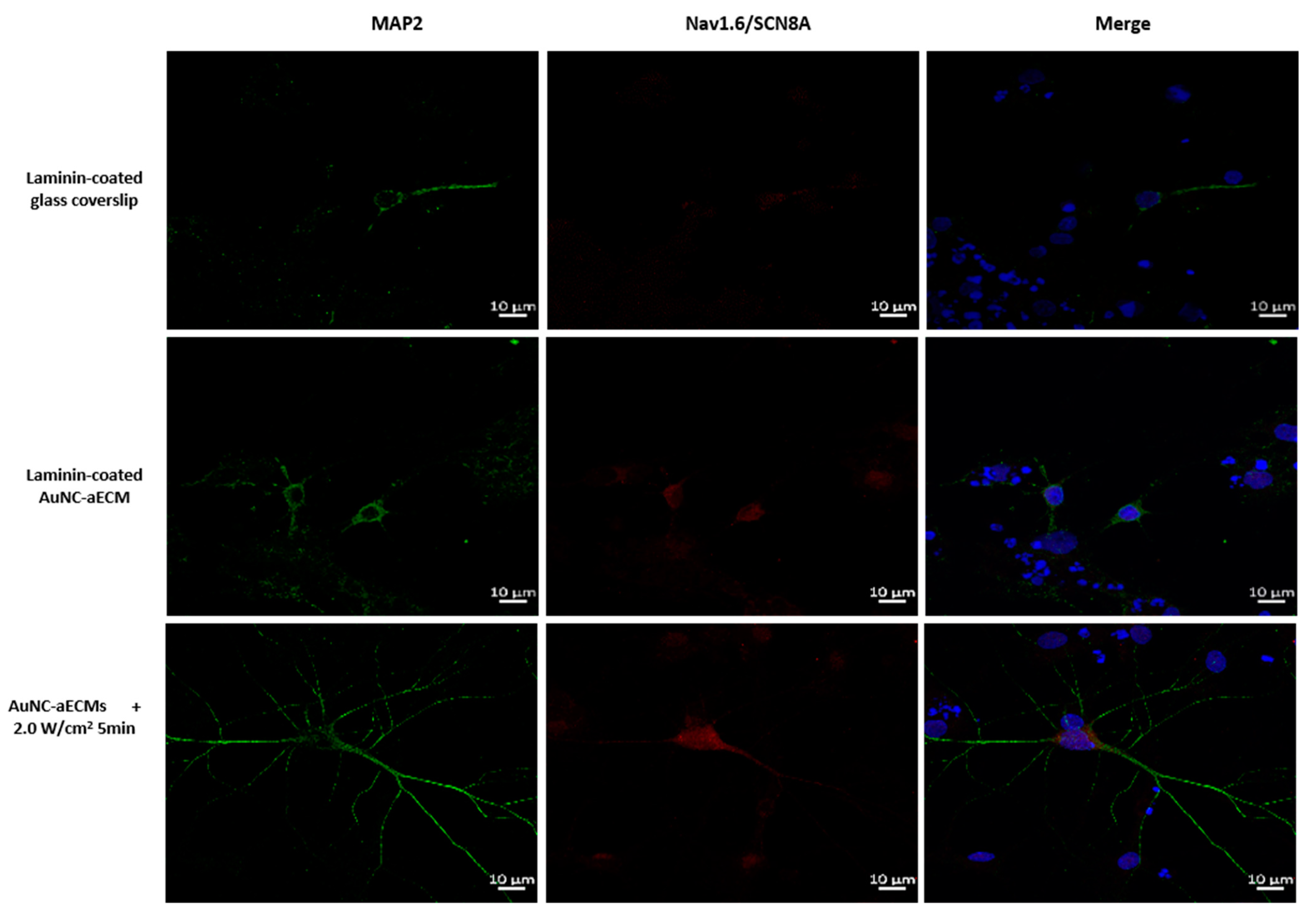
3.6. Effects of Photothermal Response of Laminin-Coated AuNC-aECMs on the Expression of Voltage-Gated Na+ Channels in Differentiated Mature Neurons
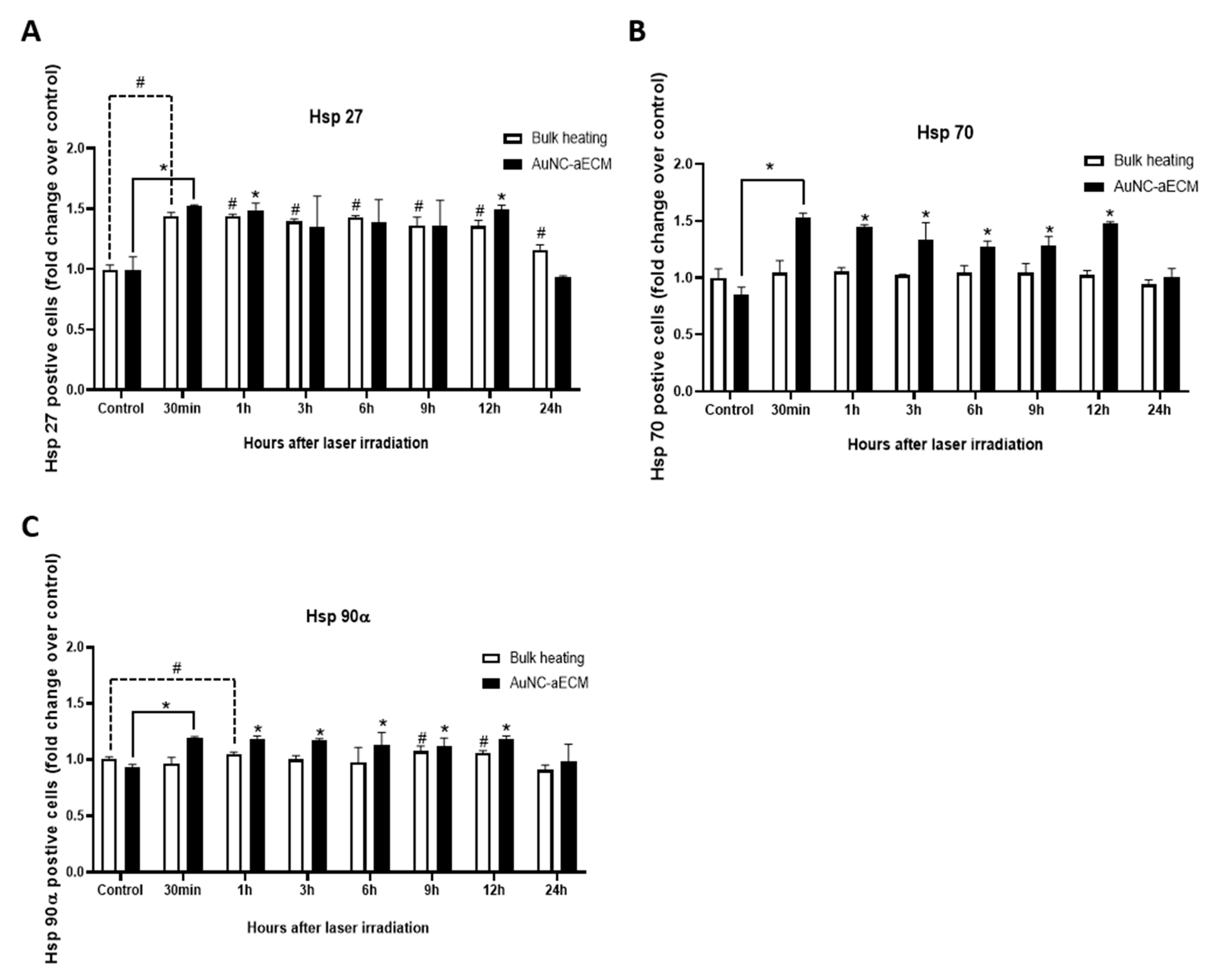
3.7. Effects of Photothermal Response of Laminin-Coated AuNC-aECMs on the Expression of Heat Shock Protein 27, 70, and 90α in Rat Neural Stem Cells
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.B.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Ransom, B.; Goldman, S.A. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003, 26, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Volpi, L.; Pasquali, L.; Petrozzi, L.; Siciliano, G. Astrocyte-neuron interactions in neurological disorders. J. Biol. Phys. 2009, 35, 317–336. [Google Scholar] [CrossRef]
- Stobart, J.L.; Anderson, C.M. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front. Cell. Neurosci. 2013, 7, 1–21. [Google Scholar] [CrossRef]
- Gomez-Arboledas, A.; Davila, J.C.; Sanchez-Mejias, E.; Navarro, V.; Nuñez-Diaz, C.; Sanchez-Varo, R.; Sanchez-Mico, M.V.; Trujillo-Estrada, L.; Fernandez-Valenzuela, J.J.; Vizuete, M.; et al. Phagocytic clearance of presynaptic dystrophies by reactive astrocytes in Alzheimer’s disease. Glia 2018, 66, 637–653. [Google Scholar] [CrossRef] [PubMed]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of astrocytes in Alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front. Mol. Neurosci. 2017, 10, 1–20. [Google Scholar] [CrossRef]
- Lee, H.J.; Suk, J.E.; Patrick, C.; Bae, E.J.; Cho, J.H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.J. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 2018, 12, 1–17. [Google Scholar] [CrossRef]
- Streit, W.J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 2002, 40, 133–139. [Google Scholar] [CrossRef]
- Cohen, C.C.H.; Popovic, M.A.; Klooster, J.; Weil, M.T.; Möbius, W.; Nave, K.A.; Kole, M.H.P. Saltatory Conduction along Myelinated Axons Involves a Periaxonal Nanocircuit. Cell 2020, 180, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A.; Werner, H.B. Myelination of the nervous system: Mechanisms and functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef]
- Trevisiol, A.; Saab, A.S.; Winkler, U.; Marx, G.; Imamura, H.; Möbius, W.; Kusch, K.; Nave, K.A.; Hirrlinger, J. Monitoring ATP dynamics in electrically active white matter tracts. Elife 2017, 6, 1–17. [Google Scholar] [CrossRef]
- Bath, K.; Lee, F. Neurotrophic factor control of adult SVZ neurogenesis. Dev. Neurobiol. 2010, 70, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.; Shah, S.; Memoli, K.A.; Park, S.Y.; Hong, S.; Lee, K.B. Controlling differentiation of neural stem cells using extracellular matrix protein patterns. Small 2010, 6, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Luo, Z.; Yan, S.; You, R. Biofunctionalized silk fibroin nanofibers for directional and long neurite outgrowth. Biointerphases 2019, 14, 061001. [Google Scholar] [CrossRef]
- Paviolo, C.; Haycock, J.W.; Yong, J.; Yu, A.; Stoddart, P.R.; Mcarthur, S.L. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol. Bioeng. 2013, 110, 2277–2291. [Google Scholar] [CrossRef]
- Pandanaboina, S.C.; Alghazali, K.M.; Nima, Z.A.; Alawajji, R.A.; Sharma, K.D.; Watanabe, F.; Saini, V.; Biris, A.S.; Srivatsan, M. Plasmonic nano surface for neuronal differentiation and manipulation. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102048. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Shi, P.; Laude, A.; Yeong, W.Y. Investigation of cell viability and morphology in 3D bio-printed alginate constructs with tunable stiffness. J. Biomed. Mater. Res. Part A 2017, 105, 1009–1018. [Google Scholar] [CrossRef]
- Park, S.; Park, H.H.; Sun, K.; Gwon, Y.; Seong, M.; Kim, S.; Park, T.E.; Hyun, H.; Choung, Y.H.; Kim, J.; et al. Hydrogel Nanospike Patch as a Flexible Anti-Pathogenic Scaffold for Regulating Stem Cell Behavior. ACS Nano 2019, 13, 11181–11193. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Srivatsan, A.; Jenkins, S.V.; Jeon, M.; Wu, Z.; Kim, C.; Chen, J.; Pandey, R.K. Gold nanocage-photosensitizer conjugates for dual-modal image-guided enhanced photodynamic therapy. Theranostics 2014, 4, 163–174. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Wang, Y.; Black, K.C.L.; Luehmann, H.; Li, W.; Zhang, Y.; Cai, X.; Wan, D.; Liu, S.Y.; Li, M.; Kim, P.; et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Kuttner, C.; Höller, R.P.M.; Quintanilla, M.; Schnepf, M.J.; Dulle, M.; Fery, A.; Liz-Marzán, L.M. SERS and plasmonic heating efficiency from anisotropic core/satellite superstructures. Nanoscale 2019, 11, 17655–17663. [Google Scholar] [CrossRef]
- Cho, E.C.; Kim, C.; Zhou, F.; Cobley, C.M.; Song, K.H.; Chen, J.; Li, Z.Y.; Wang, L.V.; Xia, Y. Measuring the optical absorption cross sections of Au-Ag nanocages and au nanorods by photoacoustic imaging. J. Phys. Chem. C 2009, 113, 9023–9028. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Johannsmeier, S.; Heeger, P.; Terakawa, M.; Kalies, S.; Heisterkamp, A.; Ripken, T.; Heinemann, D. Gold nanoparticle-mediated laser stimulation induces a complex stress response in neuronal cells. Sci. Rep. 2018, 8, 3–14. [Google Scholar] [CrossRef]
- Jenkins, S.V.; Jung, S.; Shah, S.; Millett, P.C.; Dings, R.P.; Borrelli, M.J.; Griffin, R.J. Nanoscale Investigation and Control of Photothermal Action of Gold Nanostructure-coated Surfaces. J. Mater. Sci. 2021, 56, 10249–10263. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Au, L.; Li, X.; Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.V.; Nedosekin, D.A.; Shaulis, B.J.; Wang, T.; Jamshidi-Parsian, A.; Pollock, E.D.; Chen, J.; Dings, R.P.M.; Griffin, R.J. Enhanced photothermal treatment efficacy and normal tissue protection via vascular targeted gold nanocages. Nanotheranostics 2019, 3, 145–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hayar, A.; Gu, C.; Al-Chaer, E.D. An improved method for patch clamp recording and calcium imaging of neurons in the intact dorsal root ganglion in rats. J. Neurosci. Methods 2008, 173, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Hayar, A.; Shipley, M.T.; Ennis, M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J. Neurosci. 2005, 25, 8197–8208. [Google Scholar] [CrossRef]
- Quintanilla, M.; Kuttner, C.; Smith, J.D.; Seifert, A.; Skrabalak, S.E.; Liz-Marzán, L.M. Heat generation by branched Au/Pd nanocrystals: Influence of morphology and composition. Nanoscale 2019, 11, 19561–19570. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Lim, S.T.; Antonucci, D.E.; Scannevin, R.H.; Trimmer, J.S. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron 2000, 25, 385–397. [Google Scholar] [CrossRef]
- Trimmer, J.S.; Rhodes, K.J. Localization of voltage-gated ion channels in mammalian brain. Annu. Rev. Physiol. 2004, 66, 477–519. [Google Scholar] [CrossRef]
- Katz, E.; Stoler, O.; Scheller, A.; Khrapunsky, Y.; Goebbels, S.; Kirchhoff, F.; Gutnick, M.J.; Wolf, F.; Fleidervish, I.A. Role of sodium channel subtype in action potential generation by neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA 2018, 115, E7184–E7192. [Google Scholar] [CrossRef]
- King, A.N.; Manning, C.F.; Trimmer, J.S. A unique ion channel clustering domain on the axon initial segment of mammalian neurons. J. Comp. Neurol. 2014, 522, 2594–2608. [Google Scholar] [CrossRef]
- Afzal, E.; Ebrahimi, M.; Arab Najafi, S.M.; Daryadel, A.; Baharvand, H. Potential role of heat shock proteins in neural differentiation of murine embryonal carcinoma stem cells (P19). Cell Biol. Int. 2011, 35, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.E.; Matsuzaki, K.; Katakura, M.; Sugimoto, N.; Mamun, A.A.; Islam, R.; Hashimoto, M.; Shido, O. Direct exposure to mild heat promotes proliferation and neuronal differentiation of neural stem/progenitor cells in vitro. PLoS ONE 2017, 12, e0190356. [Google Scholar] [CrossRef]
- Wattanapanitch, M.; Klincumhom, N.; Potirat, P.; Amornpisutt, R.; Lorthongpanich, C.; U-Pratya, Y.; Laowtammathron, C.; Kheolamai, P.; Poungvarin, N.; Iaragrisil, S. Dual small-molecule targeting of SMAD signaling stimulates human induced pluripotent stem cells toward neural lineages. PLoS ONE 2014, 9, e106952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, C.; Zheng, J.C. FoxO3a contributes to the reprogramming process and the differentiation of induced pluripotent stem cells. Stem Cells Dev. 2013, 22, 2954–2963. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kageyama, R. Hes1 regulates embryonic stem cell differentiation by suppressing Notch signaling. Genes Cells 2010, 15, 689–698. [Google Scholar] [CrossRef]
- Chen, D.; Hu, S.; Wu, Z.; Liu, J.; Li, S. The role of MiR-132 in regulating neural stem cell proliferation, differentiation and neuronal maturation. Cell. Physiol. Biochem. 2018, 47, 2319–2330. [Google Scholar] [CrossRef]
- Frisca, F.; Crombie, D.E.; Dottori, M.; Goldshmit, Y.; Pébay, A. Rho/ROCK pathway is essential to the expansion, differentiation, and morphological rearrangements of human neural stem/progenitor cells induced by lysophosphatidic acid. J. Lipid Res. 2013, 54, 1192–1206. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Varela-Nallar, L. Wnt signalling in neuronal differentiation and development. Cell Tissue Res. 2015, 359, 215–223. [Google Scholar] [CrossRef]
- Hur, E.M.; Zhou, F.Q. GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010, 11, 539–551. [Google Scholar] [CrossRef]
- Alghazali, K.M.; Newby, S.D.; Nima, Z.A.; Hamzah, R.N.; Watanabe, F.; Bourdo, S.E.; Masi, T.J.; Stephenson, S.M.; Anderson, D.E.; Dhar, M.S.; et al. Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitors. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Tebbe, M.; Kuttner, C.; Männel, M.; Fery, A.; Chanana, M. Colloidally Stable and Surfactant-Free Protein-Coated Gold Nanorods in Biological Media. ACS Appl. Mater. Interfaces 2015, 7, 5984–5991. [Google Scholar] [CrossRef] [PubMed]
- Luarte, A.; Cisternas, P.; Caviedes, A.; Batiz, L.F.; Lafourcade, C.; Wyneken, U.; Henzi, R. Astrocytes at the Hub of the Stress Response: Potential Modulation of Neurogenesis by miRNAs in Astrocyte-Derived Exosomes. Stem Cells Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Faiz, M.; De Pablo, Y.; Sjöqvist, M.; Andersson, D.; Widestrand, Å.; Potokar, M.; Stenovec, M.; Smith, P.L.P.; Shinjyo, N.; et al. Astrocytes negatively regulate neurogenesis through the Jagged1-mediated notch pathway. Stem Cells 2012, 30, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Sourial, M.; Doering, L.C. Astrocyte-secreted factors selectively alter neural stem and progenitor cell proliferation in the fragile X mouse. Front. Cell. Neurosci. 2016, 10, 126. [Google Scholar] [CrossRef]
- Wang, F.; Hao, H.; Zhao, S.; Zhang, Y.; Liu, Q.; Liu, H.; Liu, S.; Yuan, Q.; Bing, L.; Ling, E.A.; et al. Roles of activated astrocyte in neural stem cell proliferation and differentiation. Stem Cell Res. 2011, 7, 41–53. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Harris, N.; Niyonshuti, I.I.; Jenkins, S.V.; Hayar, A.M.; Watanabe, F.; Jamshidi-Parsian, A.; Chen, J.; Borrelli, M.J.; Griffin, R.J. Photothermal Response Induced by Nanocage-Coated Artificial Extracellular Matrix Promotes Neural Stem Cell Differentiation. Nanomaterials 2021, 11, 1216. https://doi.org/10.3390/nano11051216
Jung S, Harris N, Niyonshuti II, Jenkins SV, Hayar AM, Watanabe F, Jamshidi-Parsian A, Chen J, Borrelli MJ, Griffin RJ. Photothermal Response Induced by Nanocage-Coated Artificial Extracellular Matrix Promotes Neural Stem Cell Differentiation. Nanomaterials. 2021; 11(5):1216. https://doi.org/10.3390/nano11051216
Chicago/Turabian StyleJung, Seunghyun, Nathaniel Harris, Isabelle I. Niyonshuti, Samir V. Jenkins, Abdallah M. Hayar, Fumiya Watanabe, Azemat Jamshidi-Parsian, Jingyi Chen, Michael J. Borrelli, and Robert J. Griffin. 2021. "Photothermal Response Induced by Nanocage-Coated Artificial Extracellular Matrix Promotes Neural Stem Cell Differentiation" Nanomaterials 11, no. 5: 1216. https://doi.org/10.3390/nano11051216
APA StyleJung, S., Harris, N., Niyonshuti, I. I., Jenkins, S. V., Hayar, A. M., Watanabe, F., Jamshidi-Parsian, A., Chen, J., Borrelli, M. J., & Griffin, R. J. (2021). Photothermal Response Induced by Nanocage-Coated Artificial Extracellular Matrix Promotes Neural Stem Cell Differentiation. Nanomaterials, 11(5), 1216. https://doi.org/10.3390/nano11051216