Enhanced Mechanical Properties and Microstructure of Accumulative Roll-Bonded Co/Pb Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
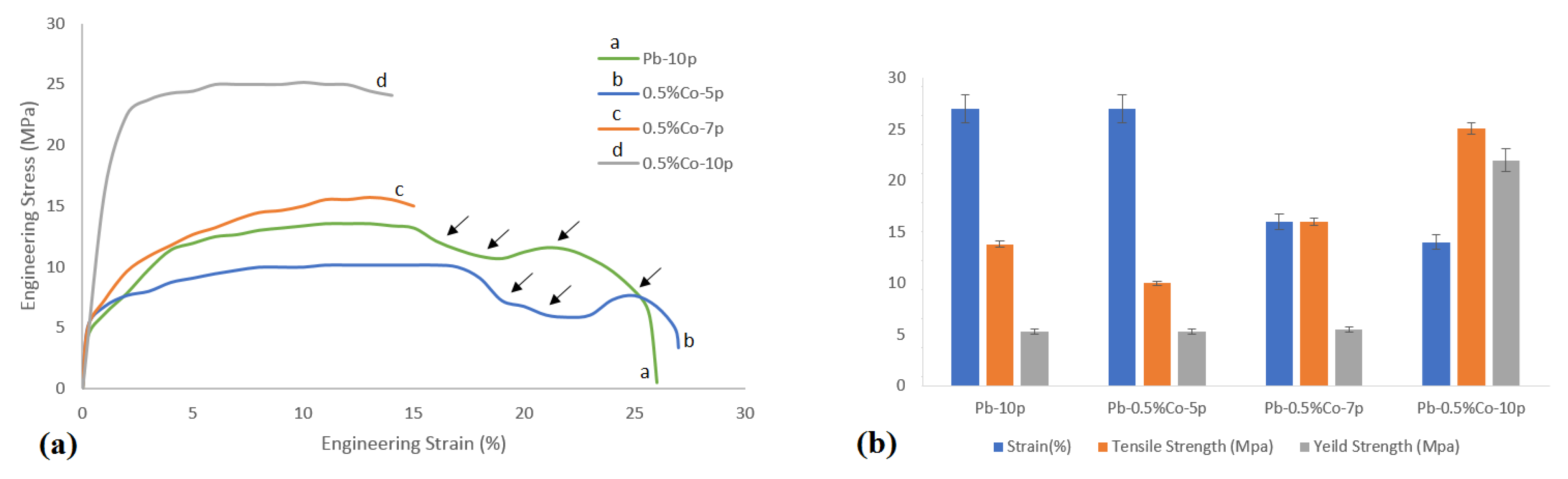
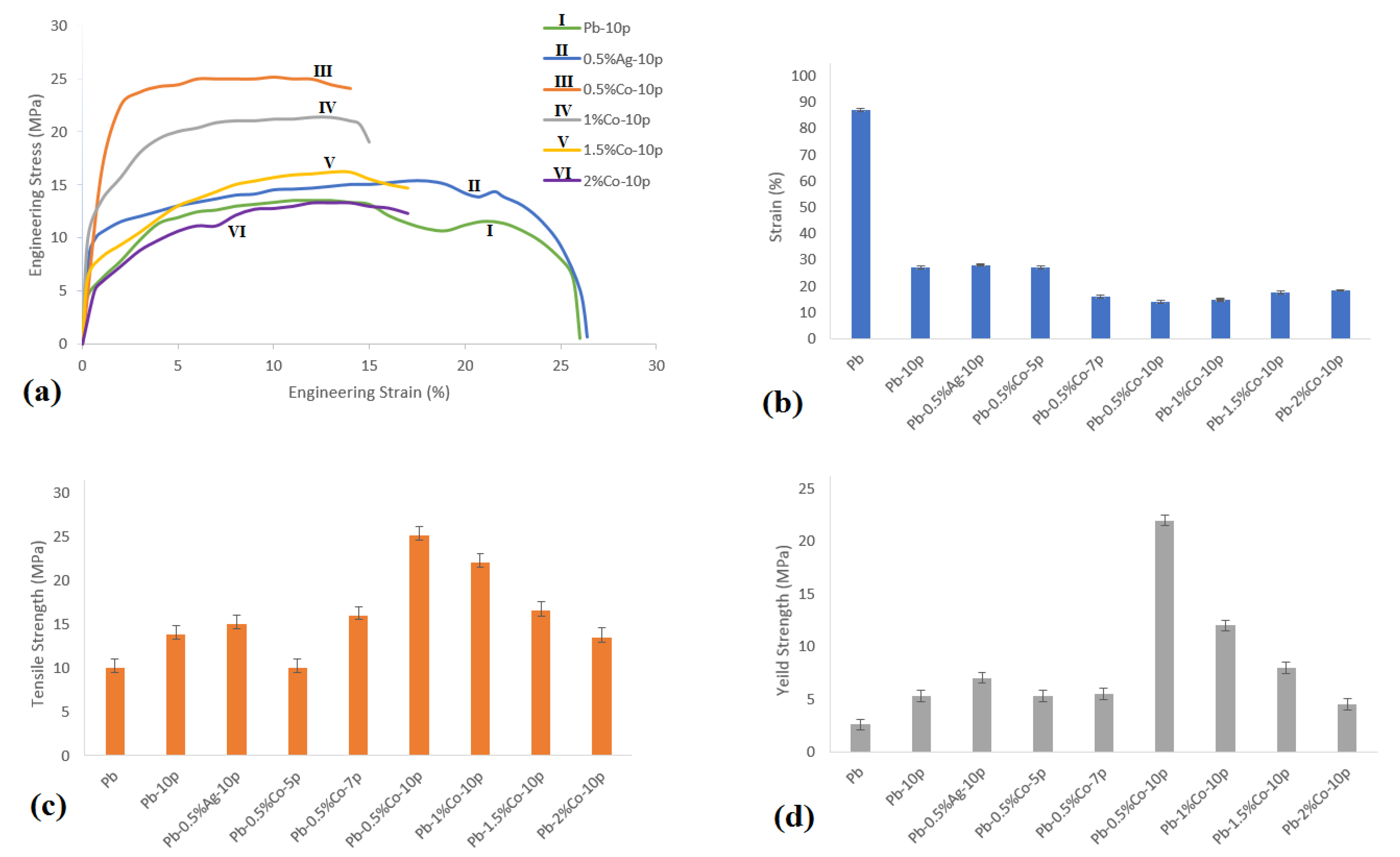
3.1. Mechanical Properties
3.1.1. Tensile Test
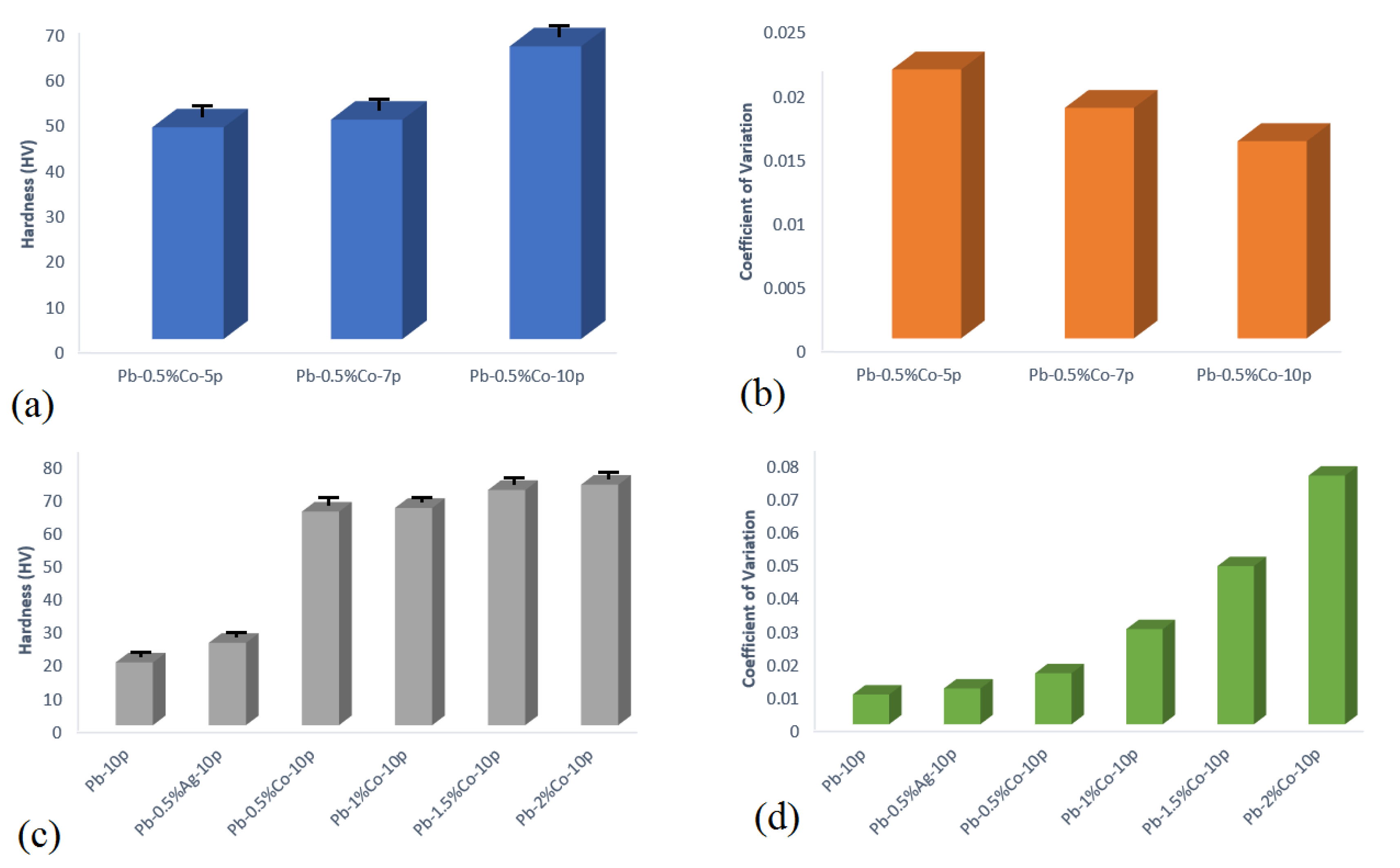
3.1.2. Hardness Test
3.1.3. Shear Punch Test
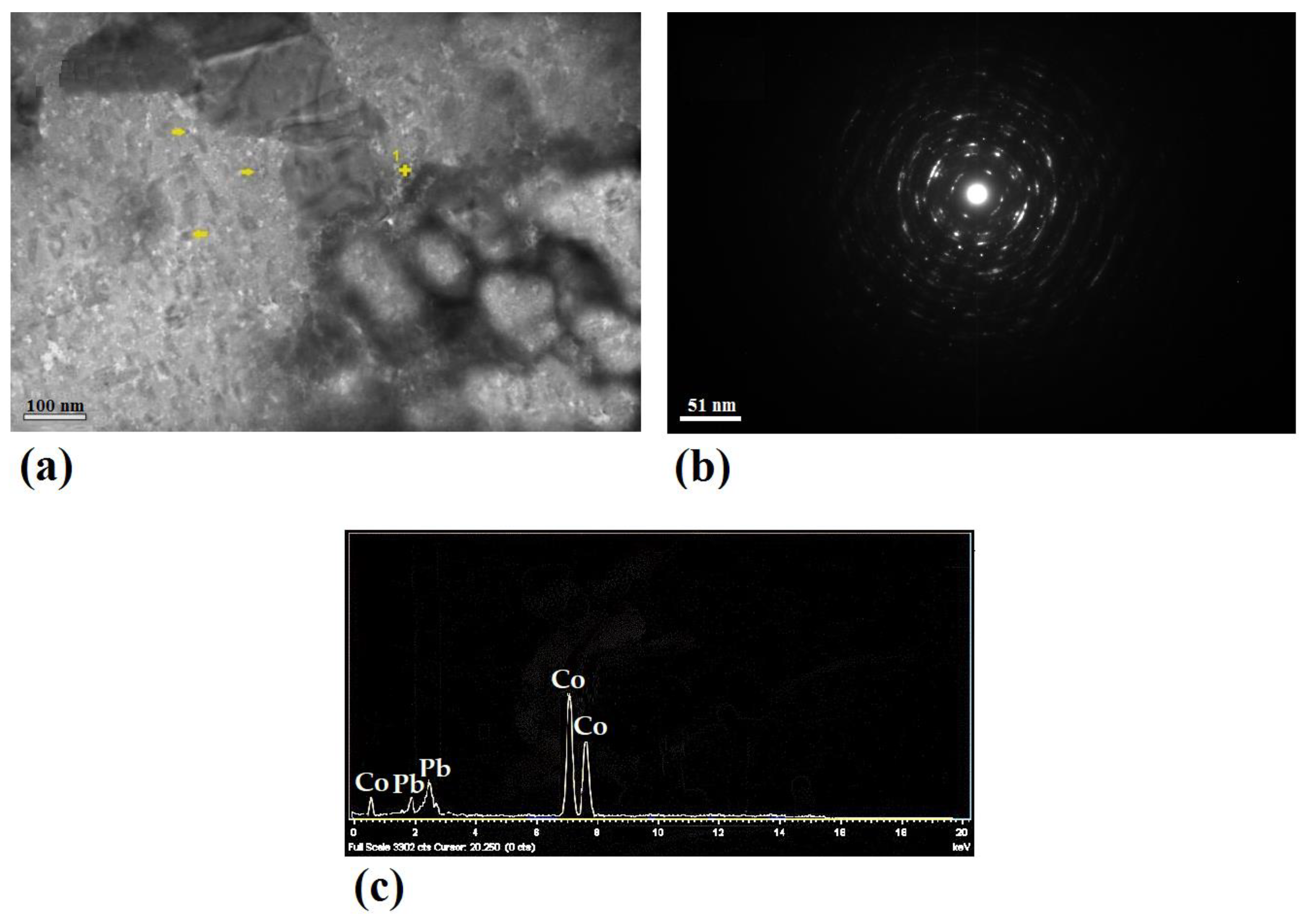
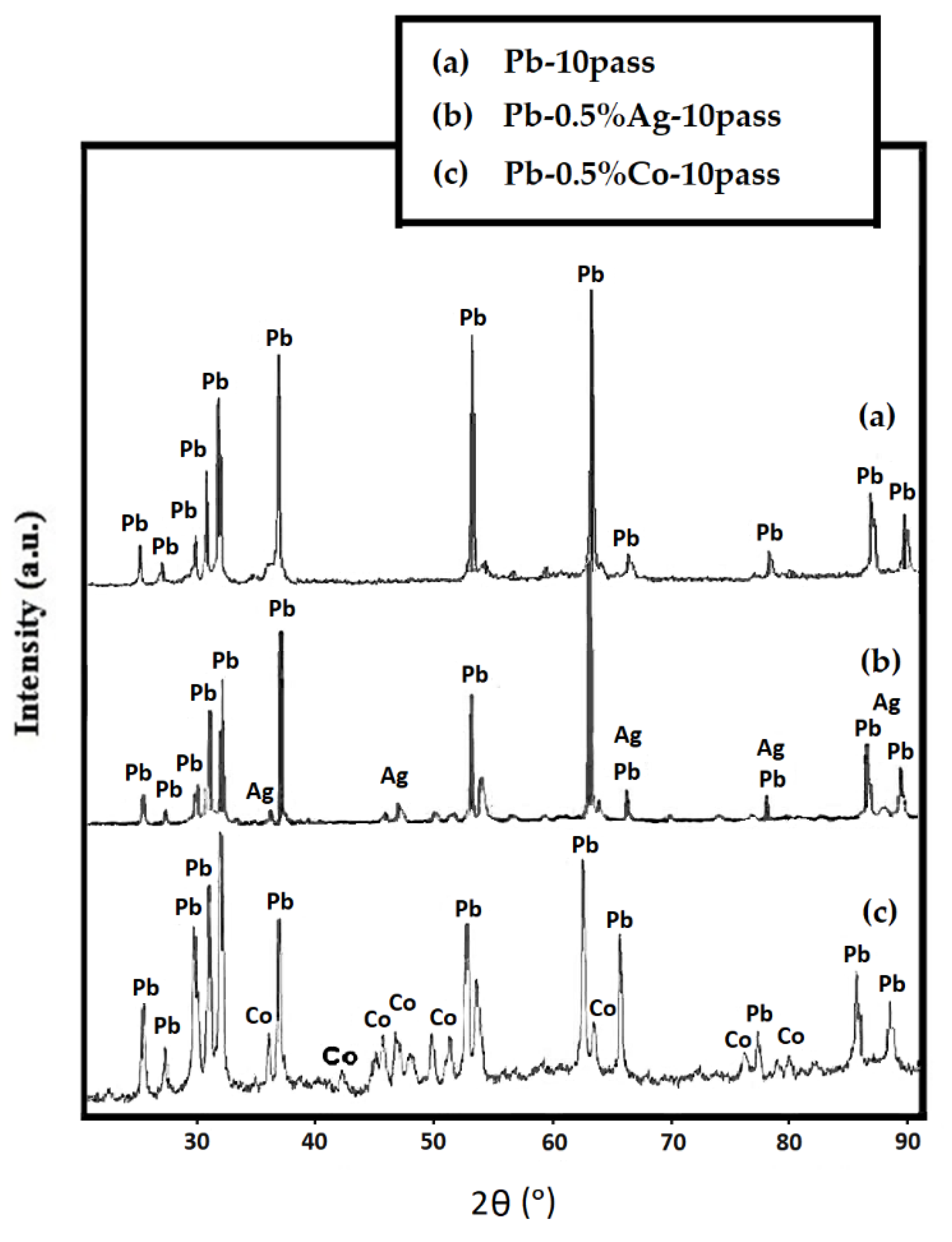
3.2. Microstructure Analysis
4. Conclusions
- (1)
- ARB was used to fabricate nano-structured Co/Pb composite anodes.
- (2)
- The maximum tensile strength was gained in the Pb–0.5%Co–10pass sample (1.5 times compared to Pb–0pass).
- (3)
- Creep resistance increased for the Pb–0.5%Co–10pass up to 1.8 times compared to Pb–0pass.
- (4)
- Up to 2.5 times increased hardness was achieved in Pb–0.5%Co–10pass compared to Pb–0pass.
- (5)
- The ARB process led to an appropriate distribution of Co and Ag secondary phase particles, with particle sizes of 353 ± 259 and 553 ± 286 nm, respectively.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Staff ASMI. ASM Handbook: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Novelty, OH, USA, 1991; Volume 2. [Google Scholar]
- Fushoku Bōshoku, K. Corrosion Engineering; Allerton Press: New York, NY, USA, 1987. [Google Scholar]
- Morgan, S.W.K. Zinc and Its Alloys and Compounds; E. Horwood: New York, NY, USA; Chichester, UK; West Sussex, UK, 1985. [Google Scholar]
- Karbasi, M.; Alamdari, E.K.; Dehkordi, E.A.; Tavangarian, F. Electrochemical and anodic behaviors of MnO2/Pb nanocomposite in zinc electrowinning. J. App. Elec. 2018, 48, 379–390. [Google Scholar] [CrossRef]
- Andersen, T.N.; Adamson, D.L.; Richards, K.J. The corrosion of lead anodes in copper electrowinning. Met. Mater. Trans. A 1974, 5, 1345–1349. [Google Scholar] [CrossRef]
- Nikoloski, A.; Nicol, M. The electrochemistry of the leaching reactions in the Caron process II. Cathodic processes. Hydrometallurgy 2010, 105, 54–59. [Google Scholar] [CrossRef]
- Clancy, M.; Bettles, C.J.; Stuart, A.; Birbilis, N. The influence of alloying elements on the electrochemistry of lead anodes for electrowinning of metals: A review. Hydrometallurgy 2013, 131, 144–157. [Google Scholar] [CrossRef]
- Lakshminarayanan, G.R.; Chen, E.S.; Sadak, J.C.; Sautter, F.K. Electrodeposition of Cobalt Using an Insoluble Anode. J. Electrochem. Soc. 1976, 123, 1612–1616. [Google Scholar] [CrossRef]
- Rashkov, S.; Dobrev, T.; Noncheva, Z.; Stefanov, Y.; Rashkova, B.; Petrova, M. Lead–cobalt anodes for electrowinning of zinc from sulphate electrolytes. Hydrometallurgy 1999, 52, 223–230. [Google Scholar] [CrossRef]
- Jamaati, R.; Toroghinejad, M.R. Cold roll bonding bond strengths: Review. Mater. Sci. Technol. 2011, 27, 1101–1108. [Google Scholar] [CrossRef]
- Jamaati, R.; Toroghinejad, M.R. Manufacturing of high-strength aluminum/alumina composite by accumulative roll bonding. Mater. Sci. Eng. A 2010, 527, 4146–4151. [Google Scholar] [CrossRef]
- Park, K.-T.; Kwon, H.-J.; Kim, W.-J.; Kim, Y.-S. Microstructural characteristics and thermal stability of ultrafine grained 6061 Al alloy fabricated by accumulative roll bonding process. Mater. Sci. Eng. A 2001, 316, 145–152. [Google Scholar] [CrossRef]
- Rezayat, M.; Akbarzadeh, A. Bonding behavior of Al–Al2O3 laminations during roll bonding process. Mater. Des. 2012, 36, 874–879. [Google Scholar] [CrossRef]
- Jamaati, R. Annealing Texture of Nanostructured Steel-Based Nanocomposite. J. Mater. Eng. Perform. 2015, 24, 3201–3208. [Google Scholar] [CrossRef]
- Karbasi, M.; Alamdari, E.K. Improving the Mechanical Properties and the Microstructure of Pb Electrowinning Anodes Using Accumulative Roll Bonding. Trans. Ind. Inst. Met. 2016, 69, 1097–1105. [Google Scholar] [CrossRef]
- Jamaati, R.; Toroghinejad, M.R.; Amirkhanlou, S.; Edris, H. Strengthening mechanisms in nanostructured interstitial free steel deformed to high strain. Mater. Sci. Eng. A 2015, 639, 656–662. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy, 3rd ed.; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Rollett, A.D.; Rohrer, G.S.; Humphreys, F.J. Recrystallization and Related Annealing Phenomena, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Alizadeh, R.; Mahmudi, R.; Esfandyarpour, M. Shear punch creep test: A novel localized method for evaluating creep properties. Scr. Mater. 2011, 64, 442–445. [Google Scholar] [CrossRef]
- Roy, S.; Singh, S.D.; Suwas, S.; Kumar, S.; Chattopadhyay, K. Evolution of Texture and Microstructure during Accumulative Roll Bonding of Aluminum AA5086 Alloy. Mater. Sci. Eng. A 2011, 528, 8469. [Google Scholar] [CrossRef]



| Content | Pb | Sn | Zn | Ni | Sb | Bi | Ag |
|---|---|---|---|---|---|---|---|
| wt.% | 99.987% | 0.0011% | 0.0041% | 0.0003% | 0.0059% | 0.0012% | 0.0006% |
| Sample Code | Added Powder (wt.%) | Number of ARB Cycles |
|---|---|---|
| Pb–0 pass | 0 | 0 |
| Pb–5 pass | 0 | 5 |
| Pb–7 pass | 0 | 7 |
| Pb–10 pass | 0 | 10 |
| Pb–0.5%Ag–10 pass | 0.5 | 10 |
| Pb–0.5%Co–5 pass | 0.5 | 5 |
| Pb–0.5%Co–7 pass | 0.5 | 7 |
| Pb–0.5%Co–10 pass | 0.5 | 10 |
| Pb–1%Co–10 pass | 1 | 10 |
| Pb–1.5%Co–7 pass | 1.5 | 7 |
| Pb–2%Co–7 pass | 2 | 7 |
| Increase Yield Strength (Time) | Increase Tensile Strength (Time) | Decrease Strain% | |
|---|---|---|---|
| Pb–0pass | --- | --- | --- |
| Pb–5pass | 0.1 | 0.1 | 14 |
| Pb–7pass | 0.6 | 0.3 | 33 |
| Pb–10pass | 1.0 | 0.3 | 69 |
| Pb–0.5%Ag–5pass | 1.2 | 0.0 | 34 |
| Pb–0.5%Ag–7pass | 1.3 | 0.3 | 50 |
| Pb–0.5%Ag–10pass | 1.6 | 0.5 | 68 |
| Pb–0.5%Co–5pass | 1.0 | 0.0 | 69 |
| Pb–0.5%Co–7pass | 1.1 | 0.6 | 82 |
| Pb–0.5%Co–10pass | 7.4 | 1.1 | 84 |
| Sample | Sub Grain Size (nm) | Dislocation Density (nm/nm3) * 10−5 |
|---|---|---|
| Pb–10pass | 1160 | 0.926 |
| Pb–0.5%Ag–10pass | 815 | 1.230 |
| Pb–0.5%Co–10pass | 174 | 5.673 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbasi, M.; Keshavarz Alamdari, E.; Amirkhani Dehkordi, E.; Khan, Z.A.; Tavangarian, F. Enhanced Mechanical Properties and Microstructure of Accumulative Roll-Bonded Co/Pb Nanocomposite. Nanomaterials 2021, 11, 1190. https://doi.org/10.3390/nano11051190
Karbasi M, Keshavarz Alamdari E, Amirkhani Dehkordi E, Khan ZA, Tavangarian F. Enhanced Mechanical Properties and Microstructure of Accumulative Roll-Bonded Co/Pb Nanocomposite. Nanomaterials. 2021; 11(5):1190. https://doi.org/10.3390/nano11051190
Chicago/Turabian StyleKarbasi, Maryam, Eskandar Keshavarz Alamdari, Elahe Amirkhani Dehkordi, Zulfiqar A. Khan, and Fariborz Tavangarian. 2021. "Enhanced Mechanical Properties and Microstructure of Accumulative Roll-Bonded Co/Pb Nanocomposite" Nanomaterials 11, no. 5: 1190. https://doi.org/10.3390/nano11051190
APA StyleKarbasi, M., Keshavarz Alamdari, E., Amirkhani Dehkordi, E., Khan, Z. A., & Tavangarian, F. (2021). Enhanced Mechanical Properties and Microstructure of Accumulative Roll-Bonded Co/Pb Nanocomposite. Nanomaterials, 11(5), 1190. https://doi.org/10.3390/nano11051190








