Sequential Changes in Antioxidant Potential of Oakleaf Lettuce Seedlings Caused by Nano-TiO2 Treatment
Abstract
1. Introduction
2. Materials and Methods
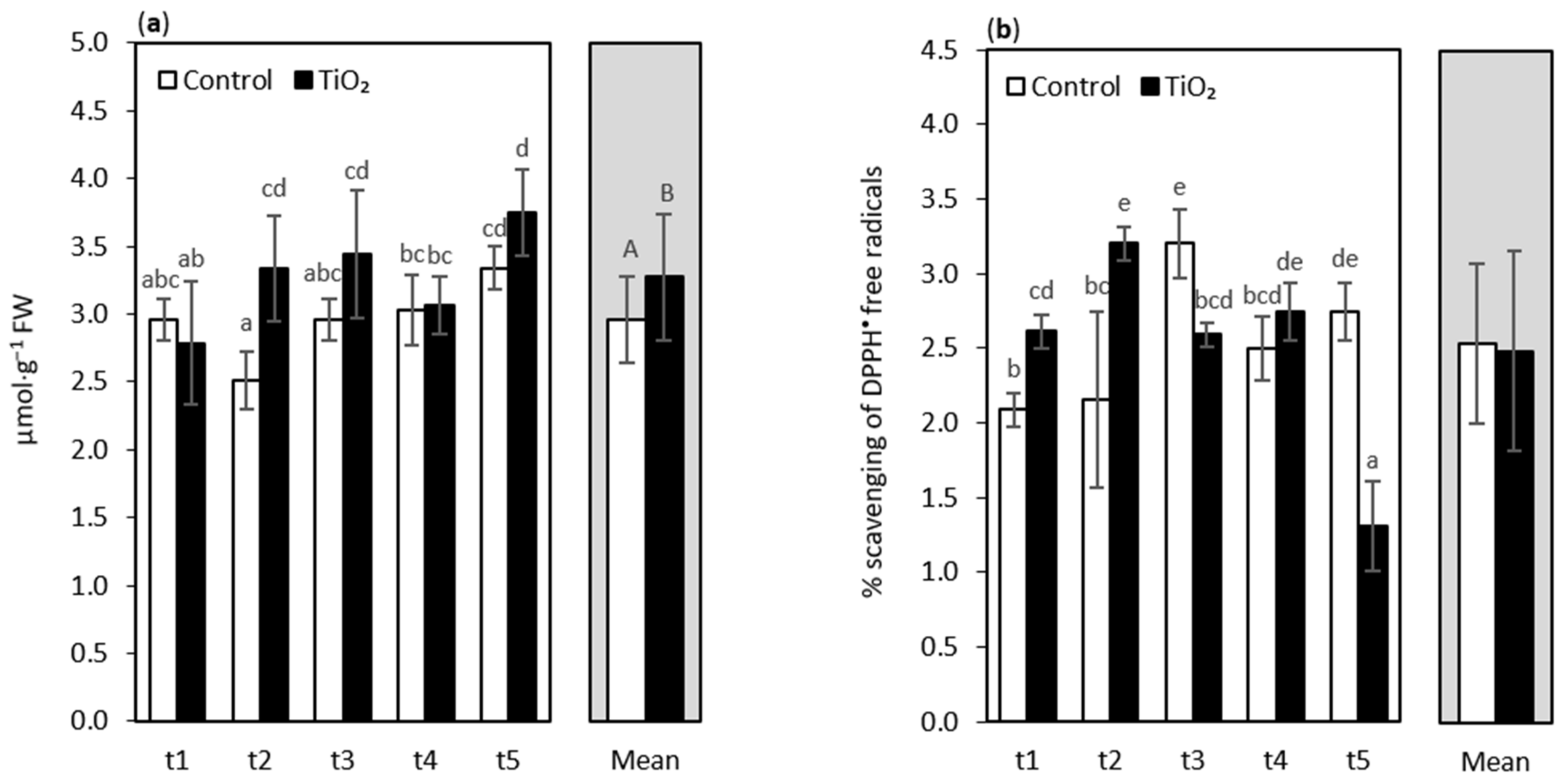
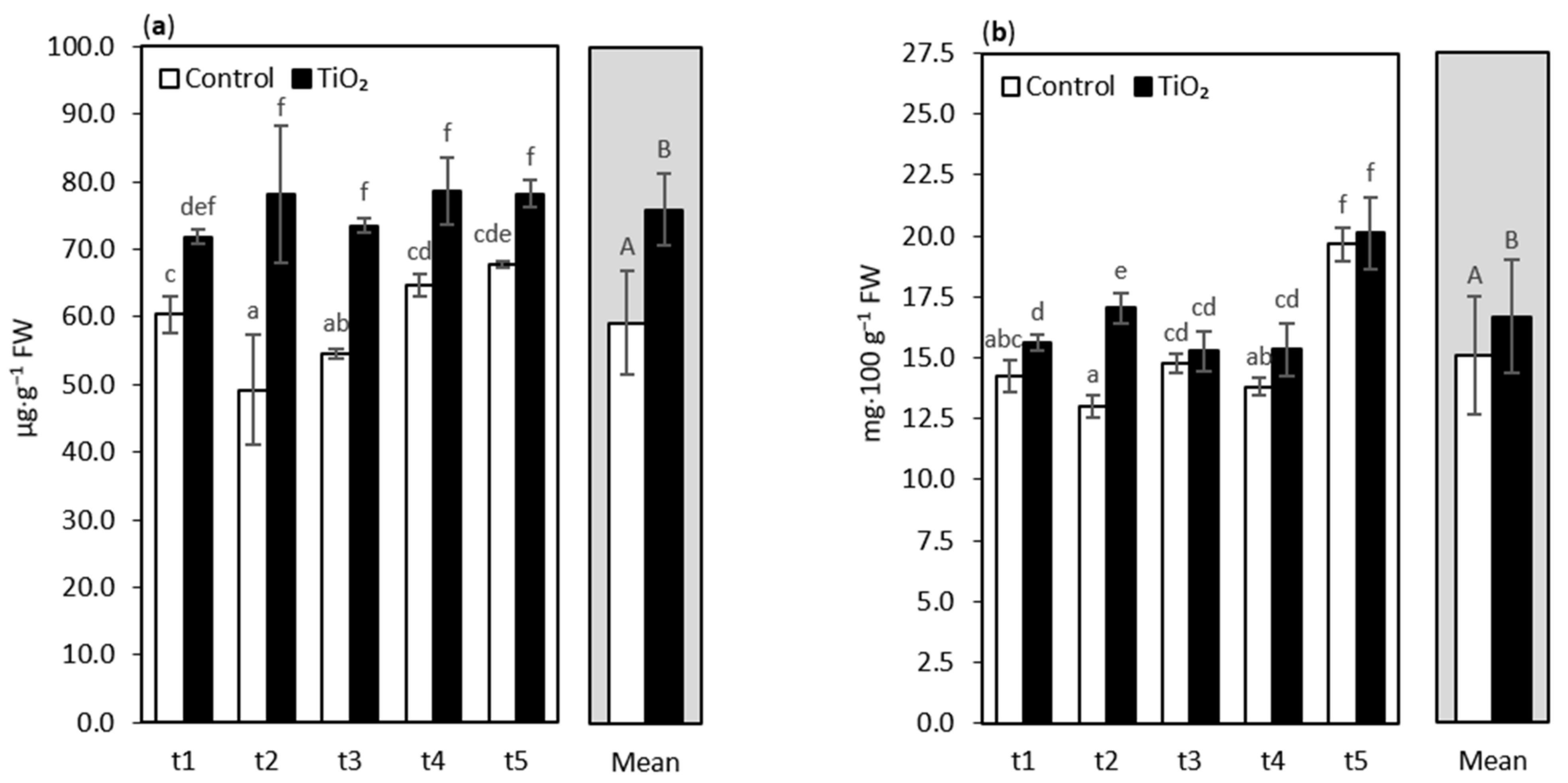
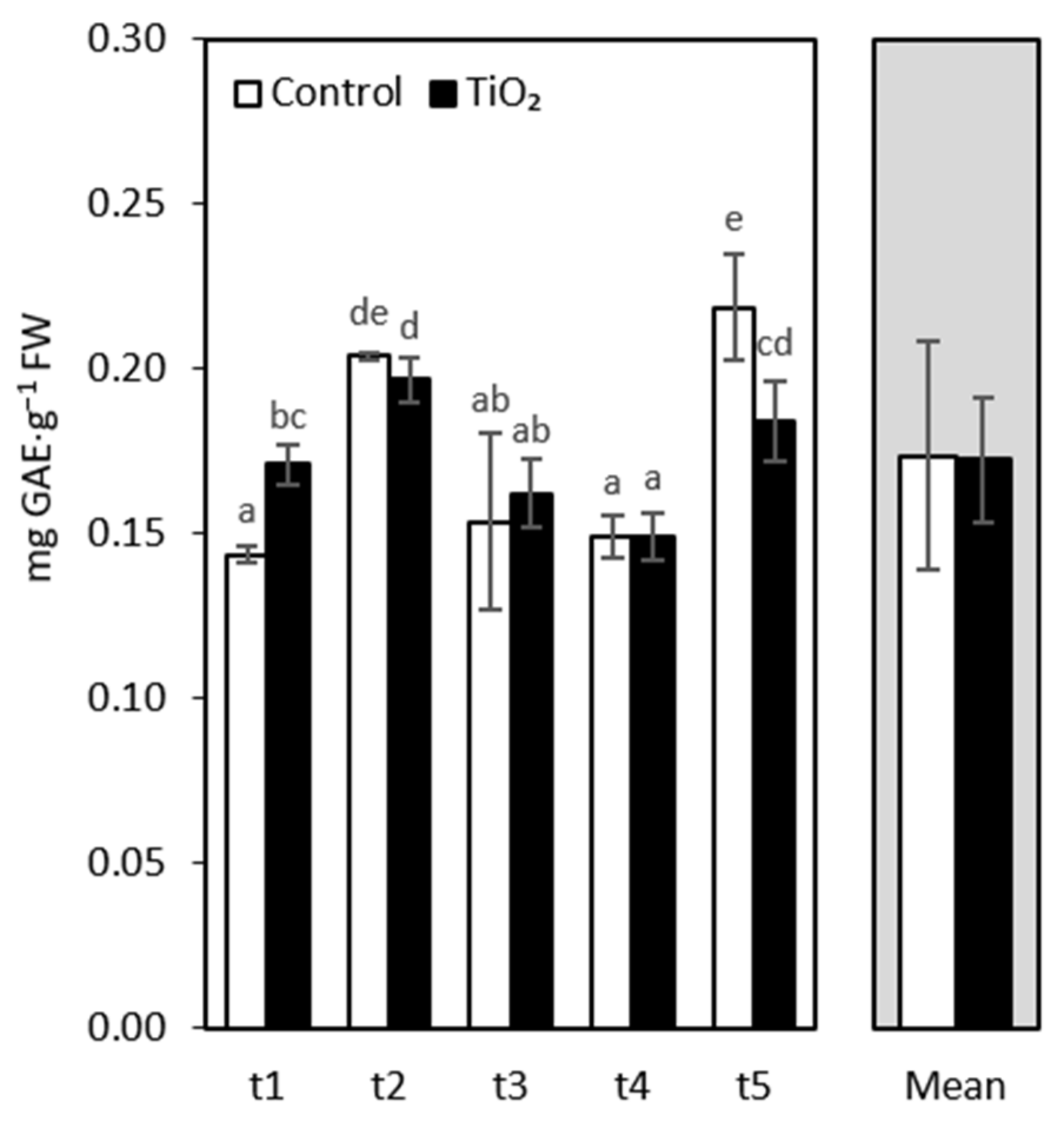
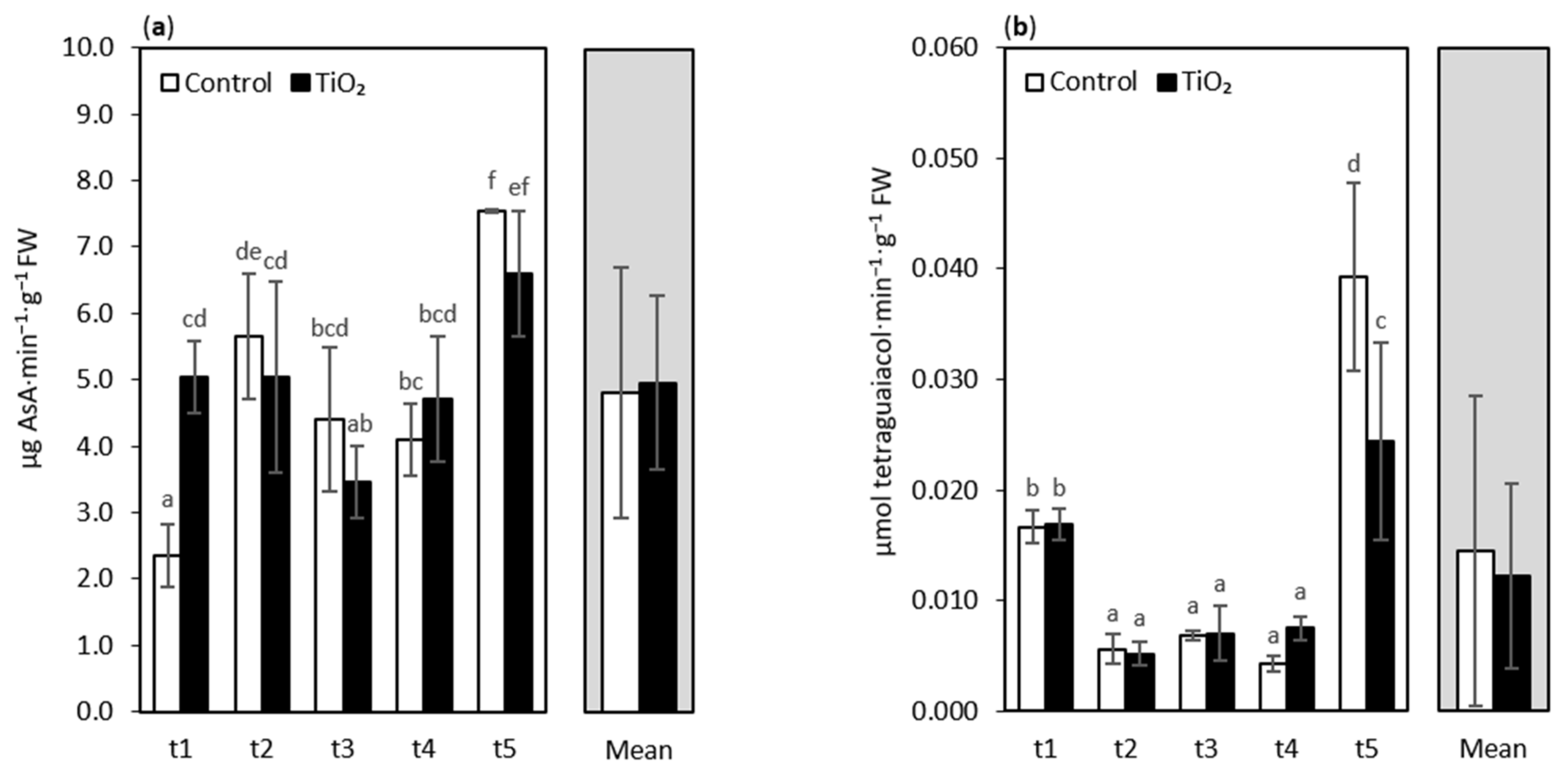
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Langauer-Lewowicka, H.; Pawlas, K. Nanoparticles, nanotechnology–potential environmental and occupational hazards. Med. Sr. Environ. Med. 2014, 17, 7–14. [Google Scholar]
- Radad, K.; Al-Shraim, M.; Moldzio, R.; Rausch, W.D. Recent advances in benefits and hazards of engineered nanoparticles. Environ. Toxicol. Pharmacol. 2012, 34, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J. Exposure of engineered nano-materials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Goswami, P.; Yadav, S.; Mathur, J. Positive and negative effects of nanoparticles on plants and their applications in agriculture. Plant Sci. Today 2019, 6, 233–242. [Google Scholar] [CrossRef]
- Arruda, S.C.; Silva, A.L.; Galazzi, R.M.; Azevedo, R.A.; Arruda, M.A. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Tan, W.; Peralta-Videa, J.P.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Costa, M.V.J.D.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Shallan, M.A.; Hassan, H.M.M.; Namich, A.A.M.; Ibrahim, A.A. Biochemical and physiological effects of TiO2 and SiO2 nanoparticles on cotton plant under drought stress. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1540–1551. [Google Scholar]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium dioxide nanoparticles in food and personal care products—What do we know about their safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef]
- Kosmala, K.; Szymańska, R. Titanium dioxide (IV) nanoparticles. Production, properties and application. Kosmos 2016, 65, 235–245. (In Polish) [Google Scholar]
- Chaudhary, I.J.; Singh, V. Titanium dioxide nanoparticles and its impact on growth, biomass and yield of agricultural crops under environmental stress: A review. Res. J. Nanosci. Nanotechnol. 2020, 10, 1–8. [Google Scholar]
- Li, J.; Naeem, M.S.; Wang, X.; Liu, L.; Chen, C.; Ma, N.; Zhang, C. Nano-TiO2 is not phytotoxic as revealed by the oilseed rape growth and photosynthetic apparatus ultra-structural response. PLoS ONE 2015, 10, e0143885. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Song, G.; Gao, Y.; Wu, H.; Hou, W.; Zhang, C.; Ma, H. Physiological effect of anatase TiO2 nanoparticles on Lemna minor. Environ. Toxicol. Chem. 2012, 31, 2147–2152. [Google Scholar] [CrossRef]
- Frazier, T.P.; Burklew, C.E.; Zhang, B. Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum). Funct. Integr. Genom. 2014, 14, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Phytochemicals and overall quality of leafy lettuce (Lactuca sativa L.) varieties grown in closed hydroponic system. J. Food Qual. 2016, 39, 805–815. [Google Scholar] [CrossRef]
- Van Treuren, R.; van Eekelen, H.D.L.M.; Wehrens, R.; de Vos, R.C.H. Metabolite variation in the lettuce gene pool: Towards healthier crop varieties and food. Metabolomics 2018, 14, 146. [Google Scholar] [CrossRef]
- Viacava, G.E.; Roura, S.I.; Berrueta, L.A.; Iriondo, C.; Gallo, B.; Alonso-Salces, R.M. Characterization of phenolic compounds in green and red oak-leaf lettuce cultivars by UHPLC-DAD-ESI-QToF/MS using MSE scan mode. J. Mass Spectrom. 2017, 52, 873–902. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Trcera, N.; Sorieul, S.; Cécillon, L.; Ouerdane, L.; Legros, S.; Sarret, G. Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J. Hazard. Mater. 2014, 273, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, X.; Wang, Y.; Feng, Y. Evaluation of zinc oxide nanoparticles on lettuce (Lactuca sativa L.) growth and soil bacterial community. Environ. Sci. Pollut. Res. 2018, 25, 6026–6035. [Google Scholar] [CrossRef]
- Jurkow, R.; Pokluda, R.; Sękara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 290. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; Kohatsu, M.Y.; Seabra, A.B.; Monteiro, L.R.; Gomes, D.G.; Oliveira, H.C.; Rolim, W.R.; Jesus, T.A.; Batista, B.L.; Lange, C.N. Effects of copper oxide nanoparticles on growth of lettuce (Lactuca sativa L.) seedlings and possible implications of nitric oxide in their antioxidative defense. Environ. Monit. Assess. 2020, 192, 232. [Google Scholar] [CrossRef]
- Jurkow, R.; Sękara, A.; Pokluda, R.; Smoleń, S.; Kalisz, A. Biochemical response of oakleaf lettuce seedlings to different concentrations of some metal(oid) oxide nanoparticles. Agronomy 2020, 10, 997. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defense against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Guri, A. Variation in glutathione and ascorbic acid content among selected cultivars of Phaseolus vulgaris prior to and after exposure to ozone. Can. J. Plant Sci. 1983, 63, 733–737. [Google Scholar] [CrossRef]
- Krełowska-Kułas, M. Badanie Jakości Produktów Spożywczych; PWE: Warsaw, Poland, 1993. (In Polish) [Google Scholar]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Zhang, Z.; Pang, X.; Duan, X.; Ji, Z.L.; Jiang, Y. Role of peroxidase in anthocyanine degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]
- Pijanowski, E.; Mrożewski, S.; Horubała, A. Technologia Produktów Owocowych i Warzywnych; PWRiL: Warsaw, Poland, 1964. (In Polish) [Google Scholar]
- Kalisz, A.; Sękara, A.; Smoleń, S.; Grabowska, A.; Gil, J.; Komorowska, M.; Kunicki, E. Survey of 17 elements, including rare earth elements, in chilled and non-chilled cauliflower cultivars. Sci. Rep. 2019, 9, 5416. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Mohammadi, H.; Esmailpour, M.; Gheranpaye, A. Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L.) plants. Acta Agric. Slov. 2016, 107, 385–396. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Srivastava, A.K.; El-Sadek, M.S.A.; Kordrostami, M.; Tran, L.-S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Kőrösi, L.; Bouderias, S.; Csepregi, K.; Bognár, B.; Teszlák, P.; Scarpellini, A.; Castelli, A.; Hideg, É.; Jakab, G. Nanostructured TiO2-induced photocatalytic stress enhances the antioxidant capacity and phenolic content in the leaves of Vitis vinifera on a genotype-dependent manner. J. Photochem. Photobiol. B Biol. 2019, 190, 137–145. [Google Scholar]
- Ghorbanpour, M. Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Indian J. Plant Physiol. 2015, 20, 249–256. [Google Scholar] [CrossRef]
- Kang, H.-M.; Saltveit, M.E. Effect of chilling on antioxidant enzymes and DPPH-radical scavenging activity of high- and low-vigour cucumber seedling radicles. Plant Cell Environ. 2002, 25, 1233–1238. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Hicks, L.M.; Cahoon, R.E.; Bonner, E.R.; Rivard, R.S.; Sheffield, J.; Jez, J.M. Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana. Plant Cell 2007, 19, 2653–2661. [Google Scholar] [PubMed]
- Ma, C.; Chhikara, S.; Xing, B.; Musante, C.; White, J.C.; Dhankher, O.P. Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustain. Chem. Eng. 2013, 1, 768–778. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Munné-Bosch, S.; Burritt, D.J.; Diaz-Vivancos, P.; Fujita, M.; Lorence, A. Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Silva, S.; de Oliveira, J.M.P.F.; Dias, M.C.; Silva, A.M.S.; Santos, C. Antioxidant mechanisms to counteract TiO2-nanoparticles toxicity in wheat leaves and roots are organ dependent. J. Hazard. Mater. 2019, 380, 120889. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules, Role and Regulation under Stressful Environments; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Comotto, M.; Casazza, A.A.; Aliakbarian, B.; Caratto, V.; Ferretti, M.; Perego, P. Influence of TiO2 nanoparticles on growth and phenolic compounds production in photosynthetic microorganisms. Sci. World J. 2014, 2014, 961437. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35 (Suppl. 4), 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Shekhawat, G.S. Phytotoxicity and oxidative stress perspective of two selected nanoparticles in Brassica juncea. Biotech 2016, 6, 244. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Mingyu, S.; Xiao, W.; Chao, L.; Chunxiang, Q.; Liang, C.; Hao, H.; Xiaoqing, L.; Fashui, H. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem. Res. 2008, 121, 69–79. [Google Scholar] [CrossRef]
- Mahmoodzadeh, H.; Nabavi, M.; Kashefi, H. Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J. Ornam. Hortic. Plants 2013, 3, 25–32. [Google Scholar]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Nanoparticles (NPs) | Sampling Time (tn) | NPs × tn |
|---|---|---|---|
| Malondialdehyde | ** | ** | *** |
| Total antioxidant capacity | ns | *** | *** |
| Glutathione | *** | ** | *** |
| L-ascorbic acid | *** | *** | *** |
| Total phenolics | ns | *** | *** |
| Ascorbate peroxidase | ns | *** | *** |
| Guaiacol peroxidase | ns | *** | *** |
| Fresh weight | ns | *** | *** |
| Dry weight | ns | *** | *** |
| Titanium | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkow, R.; Kalisz, A.; Húska, D.; Sękara, A.; Dastborhan, S. Sequential Changes in Antioxidant Potential of Oakleaf Lettuce Seedlings Caused by Nano-TiO2 Treatment. Nanomaterials 2021, 11, 1171. https://doi.org/10.3390/nano11051171
Jurkow R, Kalisz A, Húska D, Sękara A, Dastborhan S. Sequential Changes in Antioxidant Potential of Oakleaf Lettuce Seedlings Caused by Nano-TiO2 Treatment. Nanomaterials. 2021; 11(5):1171. https://doi.org/10.3390/nano11051171
Chicago/Turabian StyleJurkow, Rita, Andrzej Kalisz, Dalibor Húska, Agnieszka Sękara, and Soheila Dastborhan. 2021. "Sequential Changes in Antioxidant Potential of Oakleaf Lettuce Seedlings Caused by Nano-TiO2 Treatment" Nanomaterials 11, no. 5: 1171. https://doi.org/10.3390/nano11051171
APA StyleJurkow, R., Kalisz, A., Húska, D., Sękara, A., & Dastborhan, S. (2021). Sequential Changes in Antioxidant Potential of Oakleaf Lettuce Seedlings Caused by Nano-TiO2 Treatment. Nanomaterials, 11(5), 1171. https://doi.org/10.3390/nano11051171








