Multifunctional Magneto-Plasmonic Fe3O4/Au Nanocomposites: Approaching Magnetophoretically-Enhanced Photothermal Therapy
Abstract
1. Introduction
2. Materials and Methods
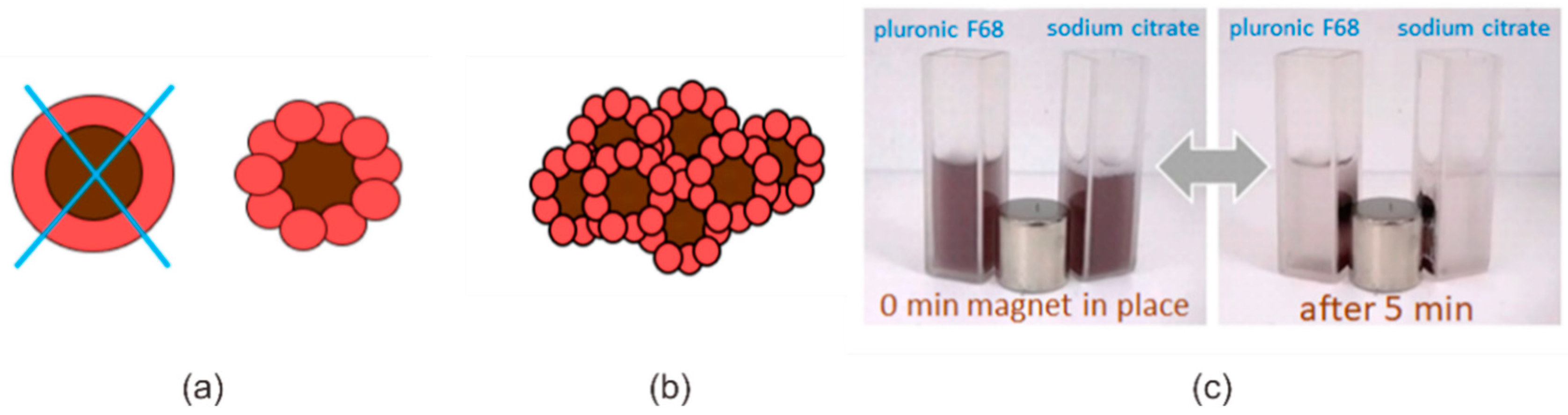
2.1. Synthesis of Fe3O4/Au Nanocomposites
2.2. Characterization of Fe3O4/Au Nanocomposites
3. Results and Discussion
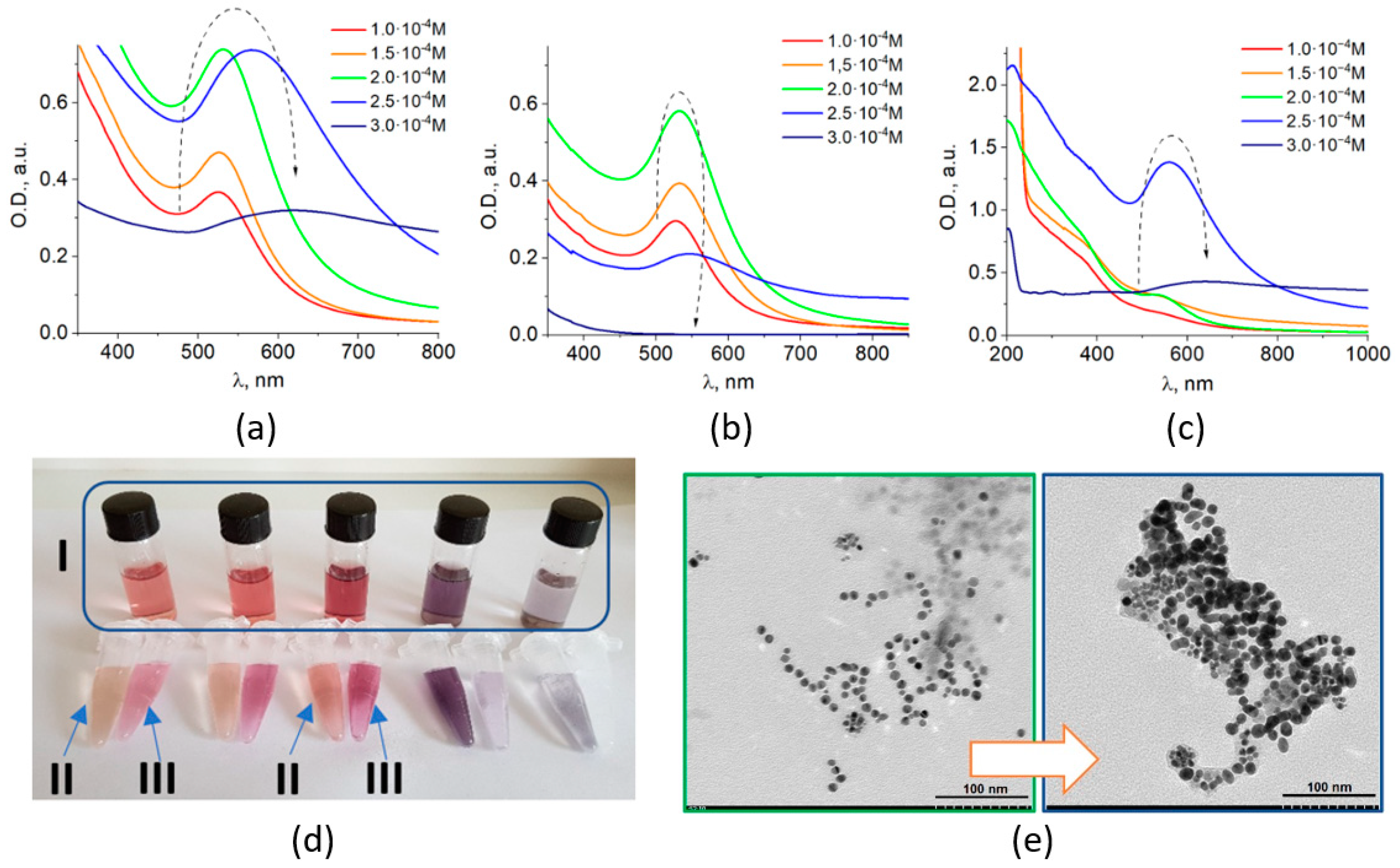
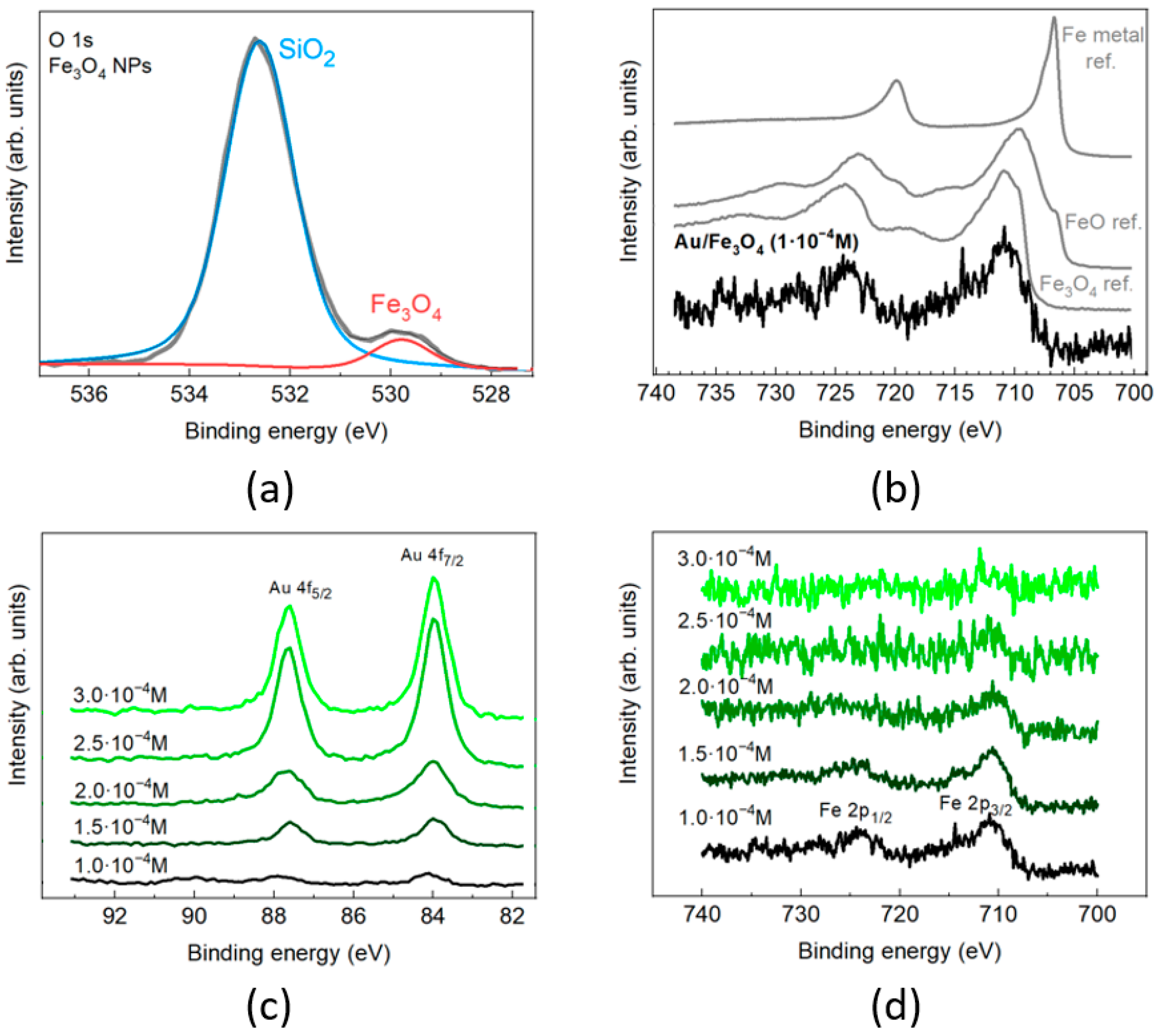
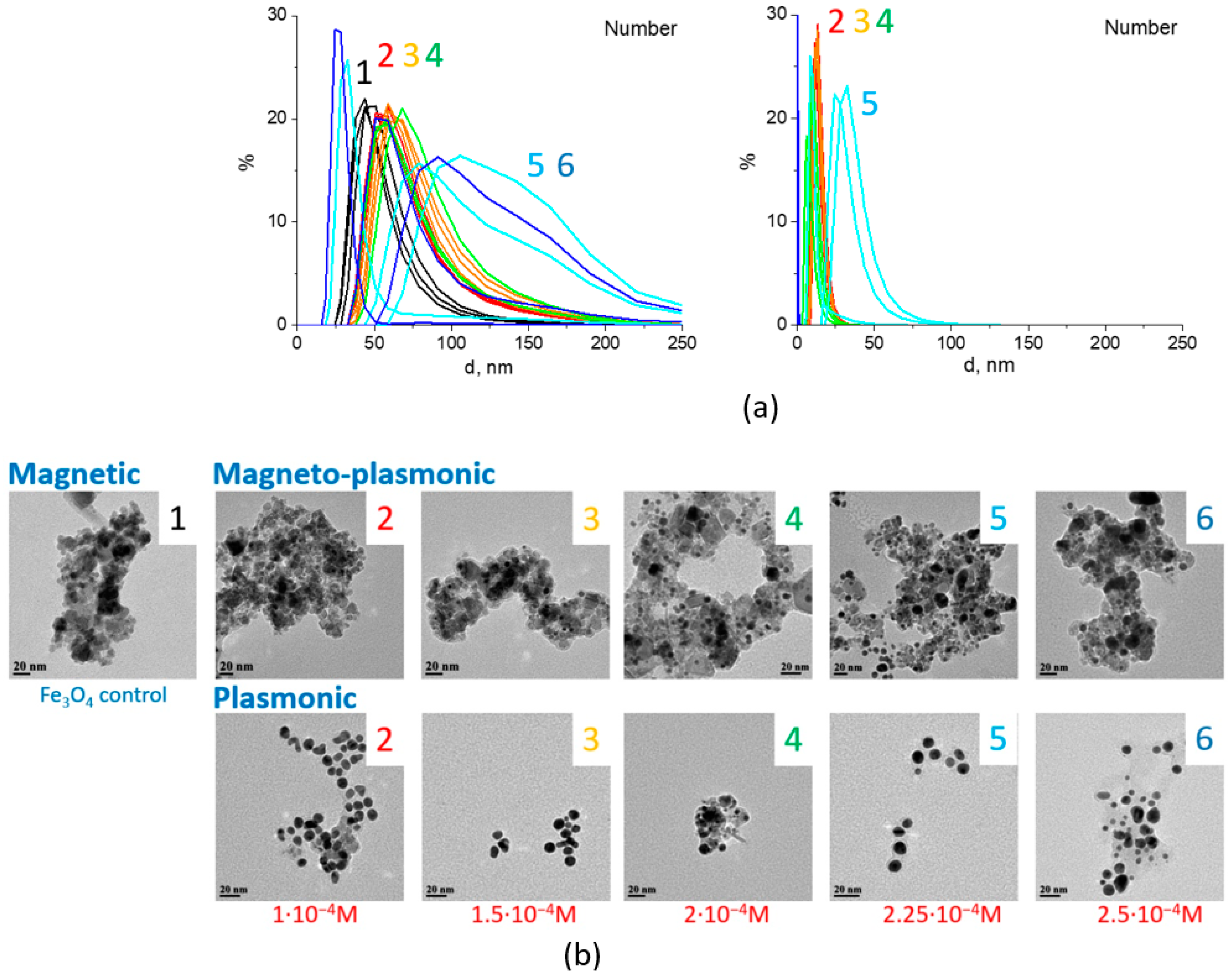
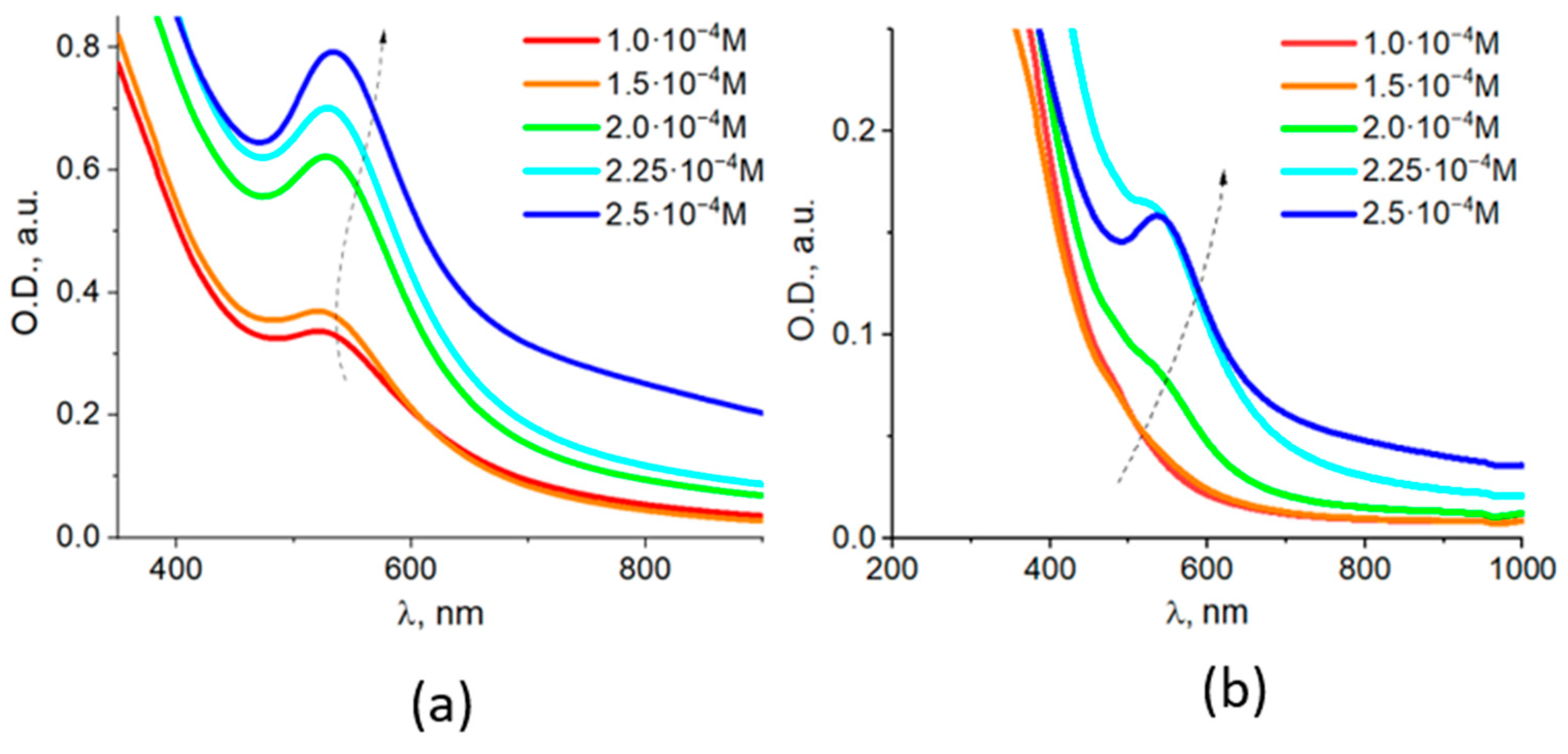
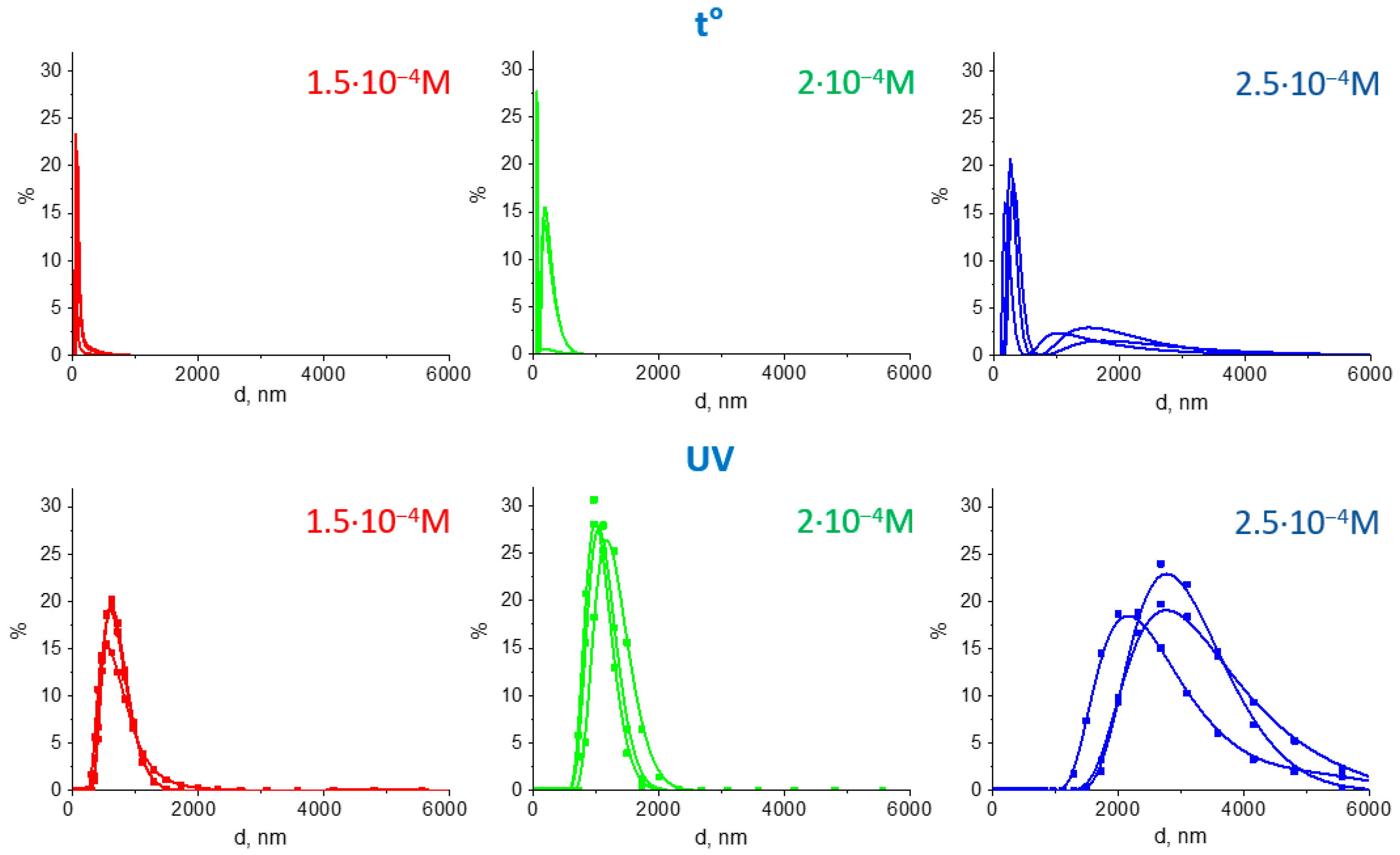
3.1. Characterization of Fe3O4/Au Nanocomposites and Their Optical Properties
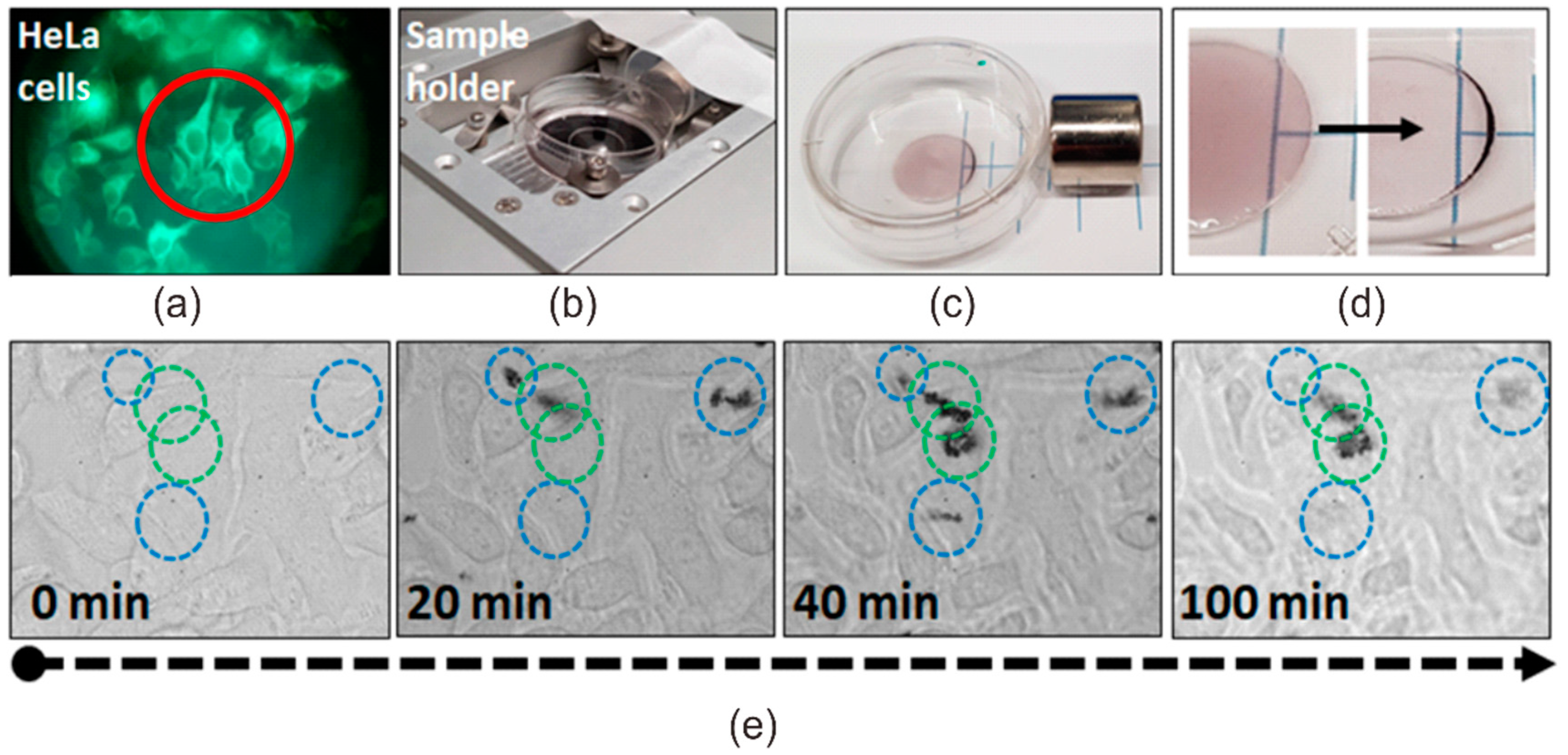
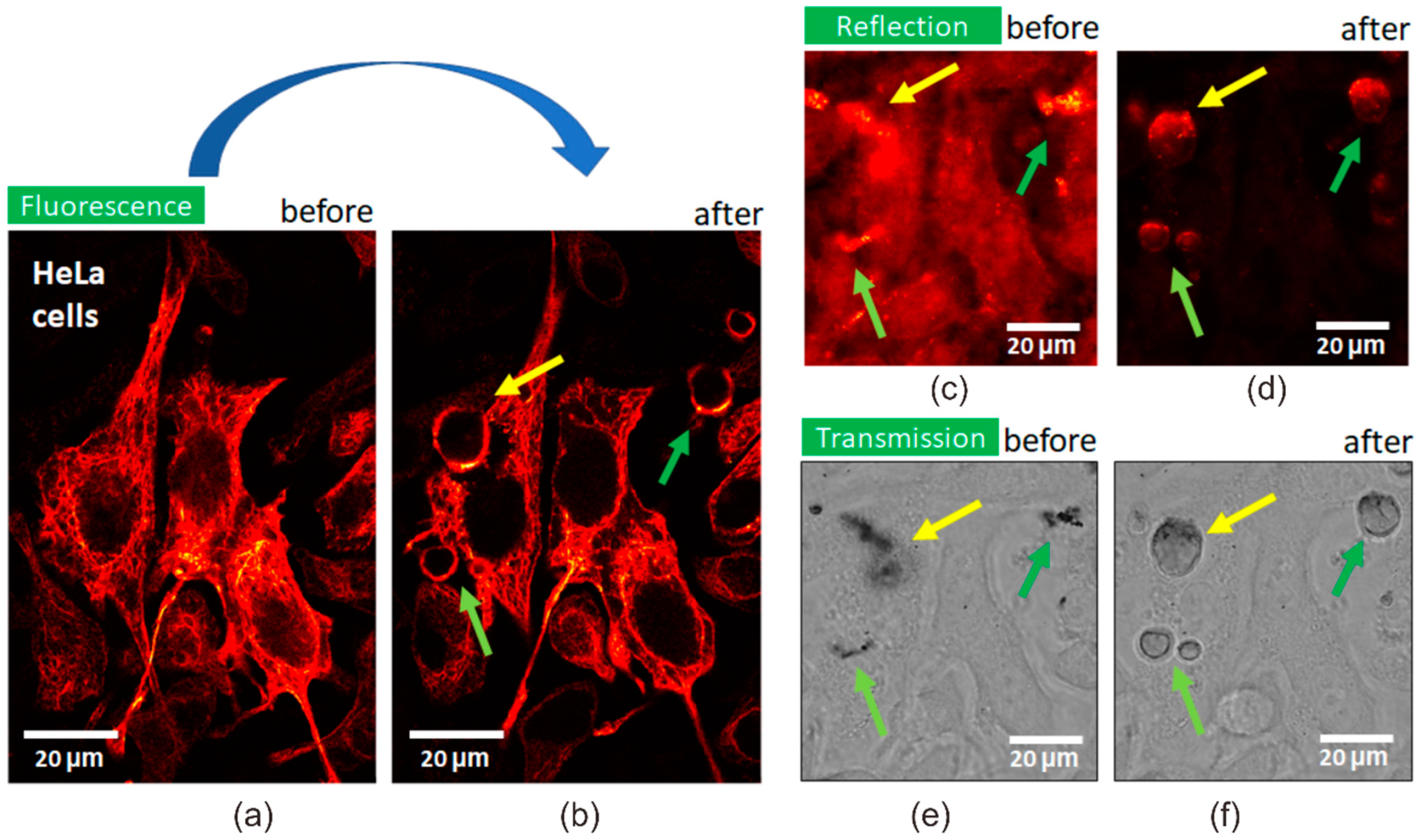
3.2. Photothermal Effect of Fe3O4/Au Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2007, 23, 217–228. [Google Scholar] [CrossRef]
- Pluchery, O. Chapter 3. Optical Properties of Gold Nanoparticles. In Gold Nanoparticles for Physics, Chemistry, and Biology; Louis, C., Pluchery, O., Eds.; Imperial College Press: London, UK, 2012. [Google Scholar]
- Taylor, A.B.; Siddiquee, A.M.; Chon, J.W.M. Below Melting Point Photothermal Reshaping of Single Gold Nanorods Driven by Surface Diffusion. ACS Nano 2014, 8, 12071–12079. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Plasmonic coupling in noble metal nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Westcott, S.L.; Oldenburg, S.J.; Lee, T.R.; Halas, N.J. Construction of simple gold nanoparticle aggregates with controlled plasmon–plasmon interactions. Chem. Phys. Lett. 1999, 300, 651–655. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Rosenzweig, Z. Development of an Aggregation-Based Immunoassay for Anti-Protein A Using Gold Nanoparticles. Anal. Chem. 2002, 74, 1624–1628. [Google Scholar] [CrossRef]
- Yang, Y.; Matsubara, S.; Nogami, M.; Shi, J. Controlling the aggregation behavior of gold nanoparticles. Mater. Sci. Eng. B 2007, 140, 172–176. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Jin, H.; Chung, H.; Kim, S. pH-Induced Aggregation of Gold Nanoparticles for Photothermal Cancer Therapy. J. Am. Chem. Soc. 2009, 131, 13639–13645. [Google Scholar] [CrossRef]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold Nanocages as Photothermal Transducers for Cancer Treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT Peptide-Functionalized Gold Nanostars: Enhanced Intracellular Delivery and Efficient NIR Photothermal Therapy Using Ultralow Irradiance. J. Am. Chem. Soc. 2012, 134, 11358–11361. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zeng, H.; Sahoo, Y.; Ohulchanskyy, T.Y.; Ding, Y.; Wang, Z.L.; Swihart, M.; Prasad, P.N. A General Approach to Binary and Ternary Hybrid Nanocrystals. Nano Lett. 2006, 6, 875–881. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Y.; Li, Z.; Li, L.; Saint-Cricq, P.; Li, C.; Lin, J.; Wang, C.; Su, Z.; Zink, J.I. Tailored Synthesis of Octopus-type Janus Nanoparticles for Synergistic Actively-Targeted and Chemo-Photothermal Therapy. Angew. Chem. Int. Ed. 2016, 55, 2118–2121. [Google Scholar] [CrossRef]
- Manohar, S.; Ungureanu, C.; Van Leeuwen, T.G. Gold nanorods as molecular contrast agents in photoacoustic imaging: The promises and the caveats. Contrast Media Mol. Imaging 2011, 6, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, S.; Wang, L.; Liu, G.; Cheng, Y.; Lin, M.; Sui, K.; Zhang, H. Alginate mediated functional aggregation of gold nanoclusters for systemic photothermal therapy and efficient renal clearance. Carbohydr. Polym. 2020, 241, 116344. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, J.; Huang, F.; Yang, L.; Ren, C.; Ma, L.; Zhang, W.; Dong, H.; Liu, J.; Liu, J. Acid-Triggered in Situ Aggregation of Gold Nanoparticles for Multimodal Tumor Imaging and Photothermal Therapy. ACS Biomater. Sci. Eng. 2019, 5, 1589–1601. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mammeri, F.; Ammar, S. Iron Oxide and Gold Based Magneto-Plasmonic Nanostructures for Medical Applications: A Review. Nanomaterials 2018, 8, 149. [Google Scholar] [CrossRef]
- Tran, V.T.; Kim, J.; Tufa, L.T.; Oh, S.; Kwon, J.; Lee, J. Magnetoplasmonic Nanomaterials for Biosensing/Imaging and In Vitro/In Vivo Biousability. Anal. Chem. 2017, 90, 225–239. [Google Scholar] [CrossRef]
- Stafford, S.; Serrano Garcia, R.; Gun’ko, Y. Multimodal Magnetic-Plasmonic Nanoparticles for Biomedical Applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Espinosa, A.; Reguera, J.; Curcio, A.; Muñoz-Noval, Á.; Kuttner, C.; Van de Walle, A.; Liz-Marzán, L.M.; Wilhelm, C. Janus Magnetic-Plasmonic Nanoparticles for Magnetically Guided and Thermally Activated Cancer Therapy. Small 2020, 16, e1904960. [Google Scholar] [CrossRef]
- Multari, C.; Miola, M.; Laviano, F.; Gerbaldo, R.; Pezzotti, G.; Debellis, D.; Verné, E. Magnetoplasmonic nanoparticles for photothermal therapy. Nanotechnology 2019, 30, 255705. [Google Scholar] [CrossRef]
- Ohulchanskyy, T.Y.; Kopwitthaya, A.; Jeon, M.; Guo, M.; Law, W.-C.; Furlani, E.P.; Kim, C.; Prasad, P.N. Phospholipid micelle-based magneto-plasmonic nanoformulation for magnetic field-directed, imaging-guided photo-induced cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Cook, J.; Emelianov, S.; Sokolov, K. Multimodal Magneto-Plasmonic Nanoclusters for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 6862–6871. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Adams, S.A.; López-Luke, T.; Torres-Castro, A.; Zhang, J.Z. Magnetic Fe3O4-Au core-shell nanostructures for surface enhanced Raman scattering. Ann. Phys. 2012, 524, 670–679. [Google Scholar] [CrossRef]
- Chang, S.; Yun, W.; Eichmann, S.L.; Poitzsch, M.E.; Wang, W. Magnetic SERS Composite Nanoparticles for Microfluidic Oil Reservoir Tracer Detection and Nanoprobe Applications. ACS Appl. Nano Mater. 2018, 2, 997–1004. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Giorgetti, E. Spectroscopic and Microscopic Analyses of Fe3O4/Au Nanoparticles Obtained by Laser Ablation in Water. Nanomaterials 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Jiménez de Aberasturi, D.; Winckelmans, N.; Langer, J.; Bals, S.; Liz-Marzán, L.M. Synthesis of Janus plasmonic–magnetic, star–sphere nanoparticles, and their application in SERS detection. Faraday Discuss. 2016, 191, 47–59. [Google Scholar] [CrossRef]
- Kuttner, C.; Höller, R.P.M.; Quintanilla, M.; Schnepf, M.J.; Dulle, M.; Fery, A.; Liz-Marzán, L.M. SERS and Plasmonic Heating Efficiency from Anisotropic Core/Satellite Superstructures. Nanoscale 2019, 11, 17655–17663. [Google Scholar] [CrossRef]
- Storozhuk, L.; Iukhymenko, N. Iron oxide nanoparticles modified with silanes for hyperthermia applications. App. Nanosci. 2018, 9, 889–898. [Google Scholar] [CrossRef]
- Briggs, M.P.; Seah, D. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley & Sons: Chichester, UK, 1983. [Google Scholar]
- Grotjohann, T.; Testa, I.; Reuss, M.; Brakemann, T.; Eggeling, C.; Hell, S.W.; Jakobs, S. rsEGFP2 enables fast RESOLFT nanoscopy of living cells. eLife 2012, 1, e00248. [Google Scholar] [CrossRef]
- Mukha, I.; Vityuk, N.; Grodzyuk, G.; Shcherbakov, S.; Lyberopoulou, A.; Efstathopoulos, E.P.; Gazouli, M. Anticancer Effect of Ag, Au, and Ag/Au Bimetallic Nanoparticles Prepared in the Presence of Tryptophan. J. Nanosci. Nanotechnol. 2017, 17, 8987–8994. [Google Scholar] [CrossRef]
- Mukha, I.; Khodko, A.; Vityuk, N.; Severynovska, O.; Pivovarenko, V.; Kachalova, N.; Smirnova, N.; Eremenko, A. Light-driven formation of gold/tryptophan nanoparticles. Appl. Nanosci. 2020, 10, 2827–2833. [Google Scholar] [CrossRef]
- Mukha, I.; Vityuk, N.; Khodko, A.; Kachalova, O.N.; Fedyshyn, O.; Malysheva, M.; Eremenko, A. Photo- and temperature-dependent formation of tryptophan/silver nanoparticles. Res. Chem. Intermed. 2019, 45, 4053–4066. [Google Scholar] [CrossRef]
- Sahoo, Y.; Goodarzi, A.; Swihart, M.T.; Ohulchanskyy, T.Y.; Kaur, N.; Furlani, E.P.; Prasad, P.N. Aqueous Ferrofluid of Magnetite Nanoparticles: Fluorescence Labeling and Magnetophoretic Control. J. Phys. Chem. B 2005, 109, 3879–3885. [Google Scholar] [CrossRef]
- Hleb, E.Y.; Hafner, J.H.; Myers, J.N.; Hanna, E.Y.; Rostro, B.C.; Zhdanok, S.A.; Lapotko, D.O. LANTCET: Elimination of solid tumor cells with photothermal bubbles generated around clusters of gold nanoparticles. Nanomedicine 2008, 3, 647–667. [Google Scholar] [CrossRef]
- Li, W.; Hou, W.; Guo, X.; Luo, L.; Li, Q.; Zhu, C.; Yang, J.; Zhu, J.; Du, Y.; You, J. Temperature-controlled, phase-transition ultrasound imaging-guided photothermal-chemotherapy triggered by NIR light. Theranostics 2018, 8, 3059–3073. [Google Scholar] [CrossRef]

| CAu, M in Colloid (Initial) | Au:Fe in Colloid (Initial) | Au:Fe 2p in Magnetic Component (XPS) |
|---|---|---|
| 1.0 × 10−4 | 0.667 | 0.053 (5:95) |
| 1.5 × 10−4 | 1.000 | 0.111 (10:90) |
| 2.0 × 10−4 | 1.333 | 0.333 (25:75) |
| 2.5 × 10−4 | 1.667 | 2.333 (70:30) |
| 3.0 × 10−4 | 2.000 | 2.333 (70:30) |
| Sample | λmax 0, nm | λmax 1, nm | ΔλH+, nm | λmax 2, nm | r 800 | r 900 | Fe3O4/Au Size, nm (DLS) | Gold NPs Size, nm (TEM) |
|---|---|---|---|---|---|---|---|---|
| t-1.5 | 527 | 549 | 22 | 549 | 1.30 | 1.35 | <200 | 11.8 ± 2.5 |
| t-2.0 | 532 | 552 | 20 | 549 | 1.22 | 1.28 | 100–400 | 8.9 ± 1,4 |
| t-2.5 | 567 | 602 | 35 | 600 | 1.07 | 1.20 | 200–500 (1000–3000) | 10.6 ± 2.7 |
| UV-1.5 | 523 | 540 | 17 | 536 | 1.45 | 1.50 | 400–1000 | 8.7 ± 1.4 |
| UV-2.0 | 527 | 545 | 18 | 543 | 1.55 | 1.66 | 800–1500 | 10.6 ± 2.8 |
| UV-2.5 | 533 | 551 | 18 | 548 | 1.36 | 1.52 | 2000–4000 | 9.5 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukha, I.; Chepurna, O.; Vityuk, N.; Khodko, A.; Storozhuk, L.; Dzhagan, V.; Zahn, D.R.T.; Ntziachristos, V.; Chmyrov, A.; Ohulchanskyy, T.Y. Multifunctional Magneto-Plasmonic Fe3O4/Au Nanocomposites: Approaching Magnetophoretically-Enhanced Photothermal Therapy. Nanomaterials 2021, 11, 1113. https://doi.org/10.3390/nano11051113
Mukha I, Chepurna O, Vityuk N, Khodko A, Storozhuk L, Dzhagan V, Zahn DRT, Ntziachristos V, Chmyrov A, Ohulchanskyy TY. Multifunctional Magneto-Plasmonic Fe3O4/Au Nanocomposites: Approaching Magnetophoretically-Enhanced Photothermal Therapy. Nanomaterials. 2021; 11(5):1113. https://doi.org/10.3390/nano11051113
Chicago/Turabian StyleMukha, Iuliia, Oksana Chepurna, Nadiia Vityuk, Alina Khodko, Liudmyla Storozhuk, Volodymyr Dzhagan, Dietrich R.T. Zahn, Vasilis Ntziachristos, Andriy Chmyrov, and Tymish Y. Ohulchanskyy. 2021. "Multifunctional Magneto-Plasmonic Fe3O4/Au Nanocomposites: Approaching Magnetophoretically-Enhanced Photothermal Therapy" Nanomaterials 11, no. 5: 1113. https://doi.org/10.3390/nano11051113
APA StyleMukha, I., Chepurna, O., Vityuk, N., Khodko, A., Storozhuk, L., Dzhagan, V., Zahn, D. R. T., Ntziachristos, V., Chmyrov, A., & Ohulchanskyy, T. Y. (2021). Multifunctional Magneto-Plasmonic Fe3O4/Au Nanocomposites: Approaching Magnetophoretically-Enhanced Photothermal Therapy. Nanomaterials, 11(5), 1113. https://doi.org/10.3390/nano11051113







