Impedance Spectroscopic Study of Nickel Sulfide Nanostructures Deposited by Aerosol Assisted Chemical Vapor Deposition Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bis(di-isobutyldithiophosphinato)nickel(II) Precursor
2.2. AACVD Technique
2.3. Dielectric and Impedance Studies
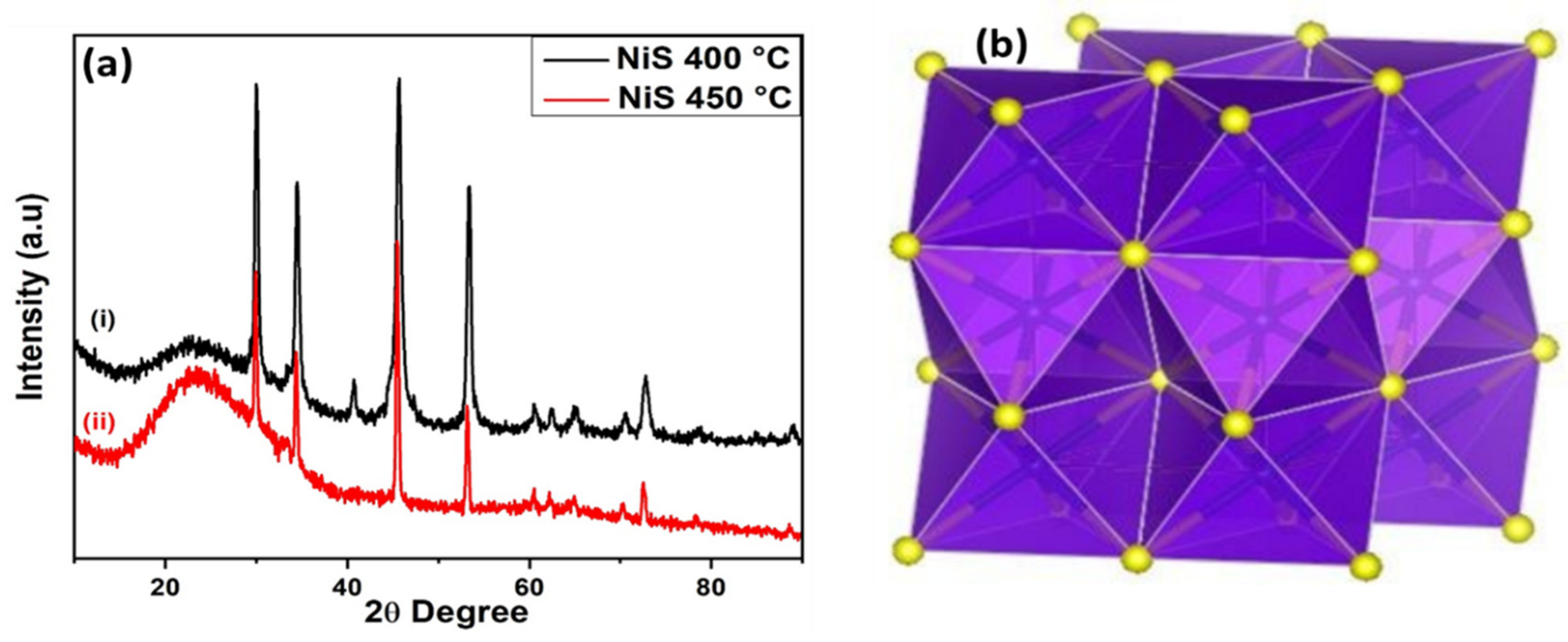
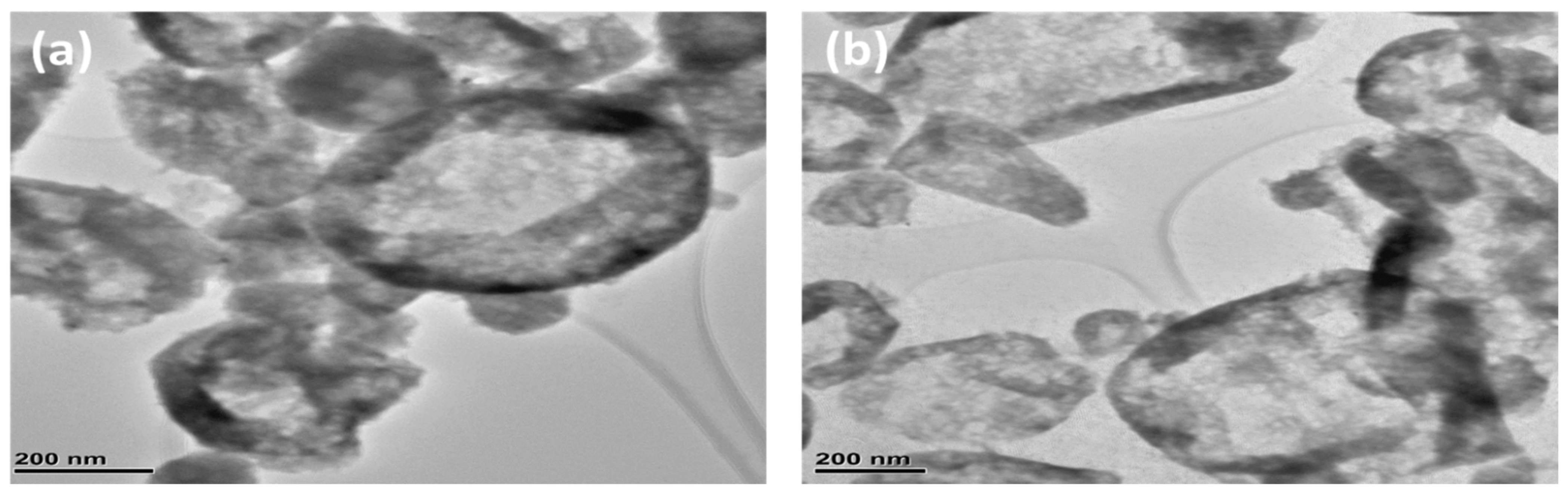
2.4. Structure and Morphology
3. Results
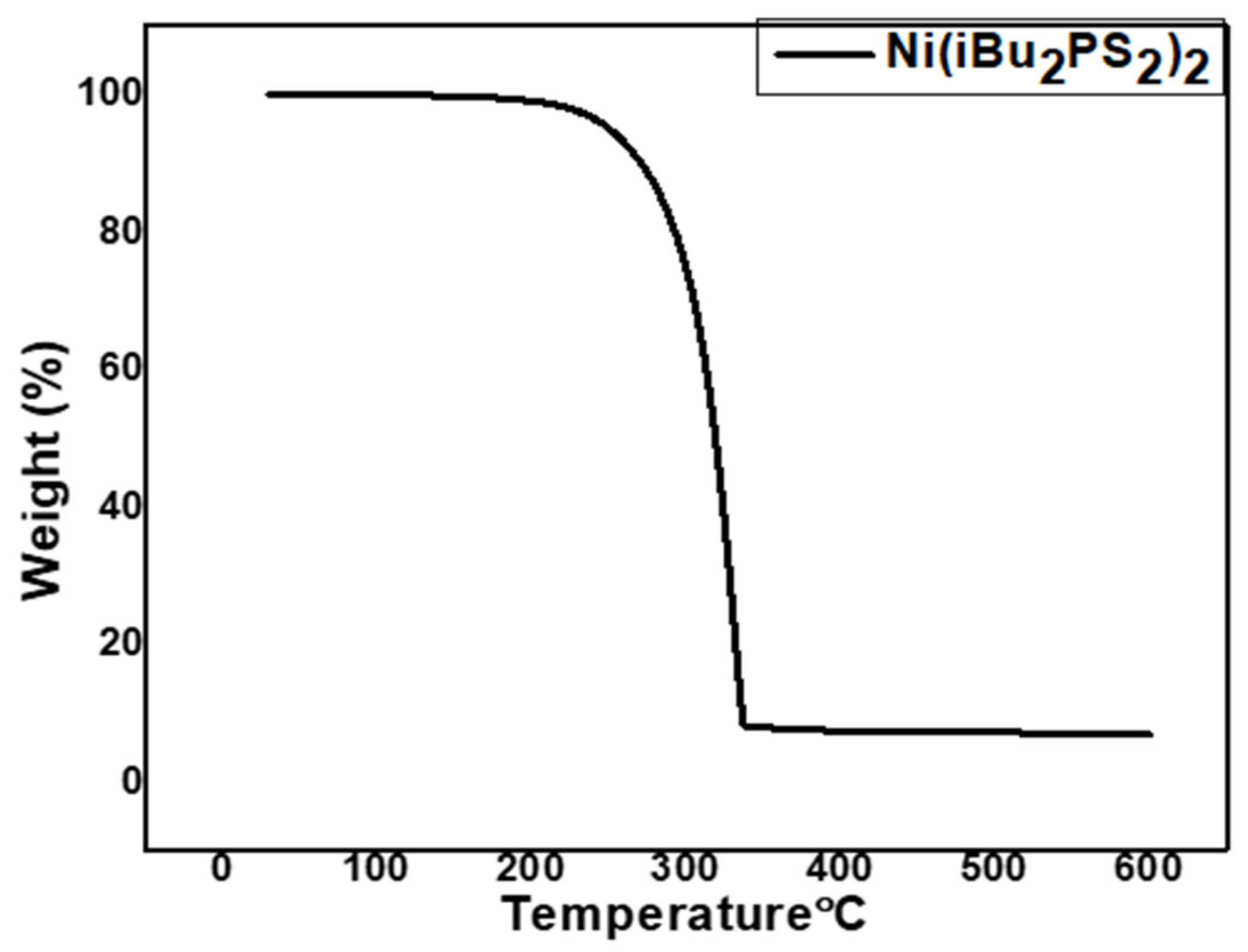
3.1. Gravimetric and Spectroscopic Analysis
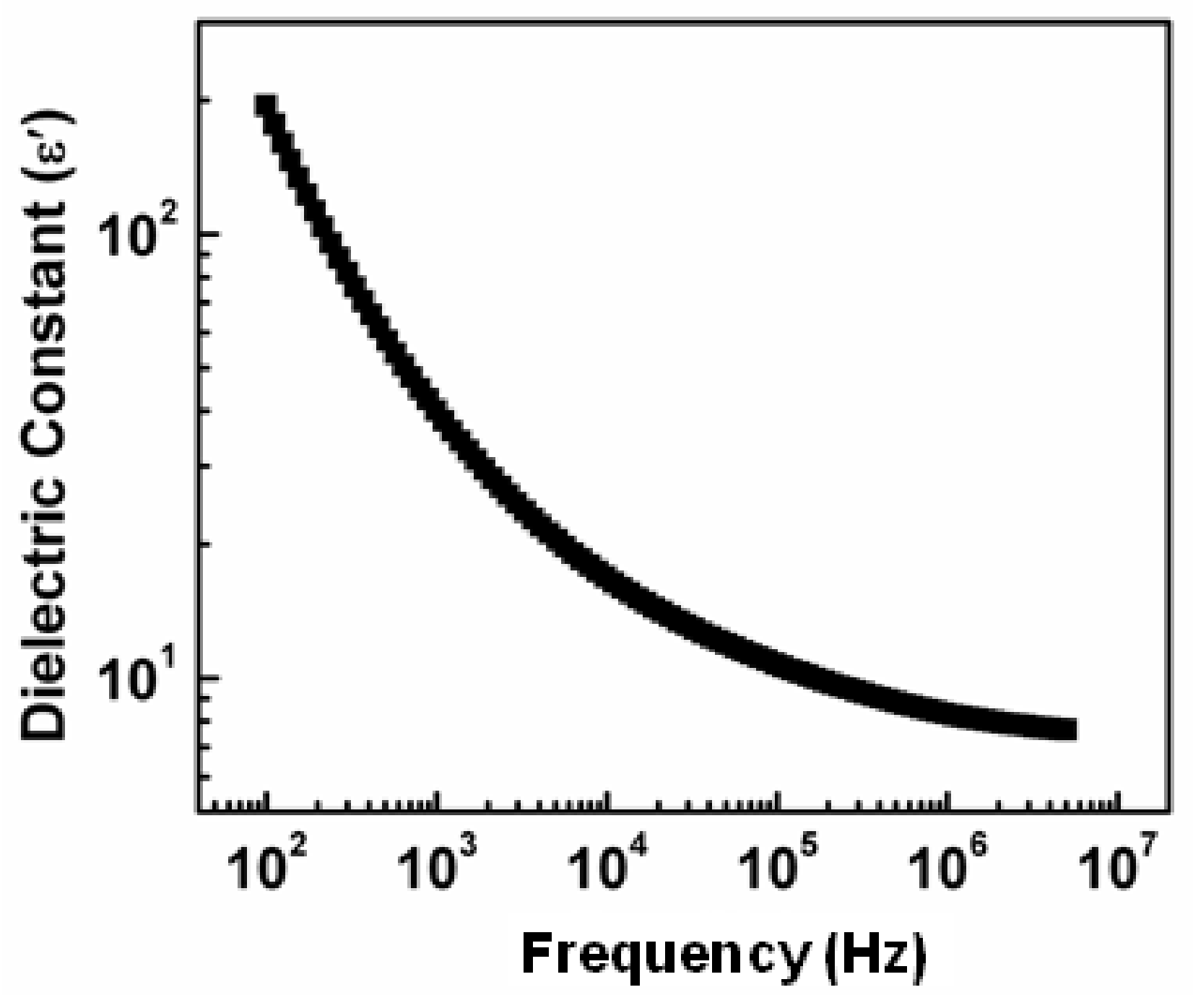
3.2. Dielectric Constant
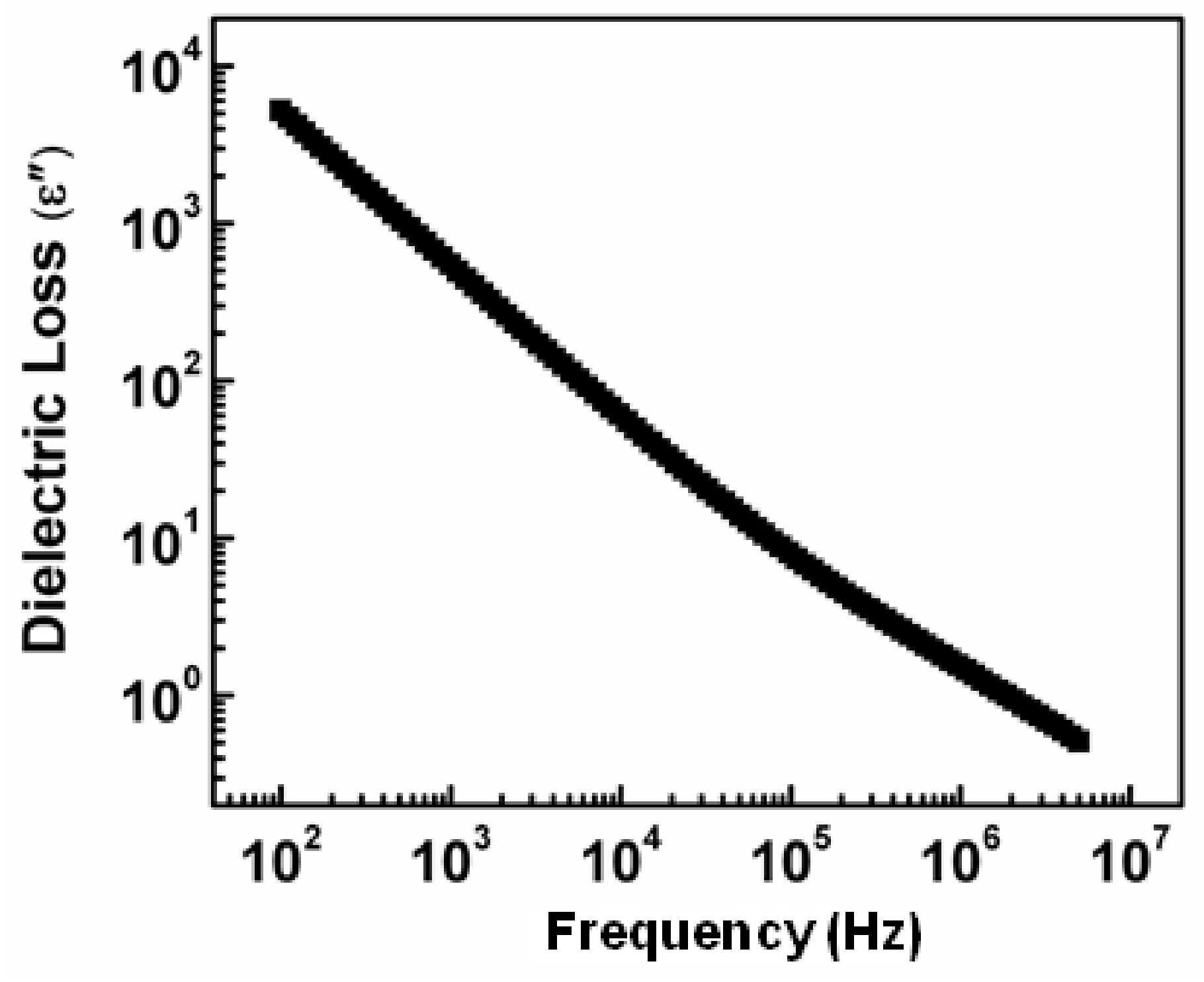
3.3. The Dielectric Loss Behavior
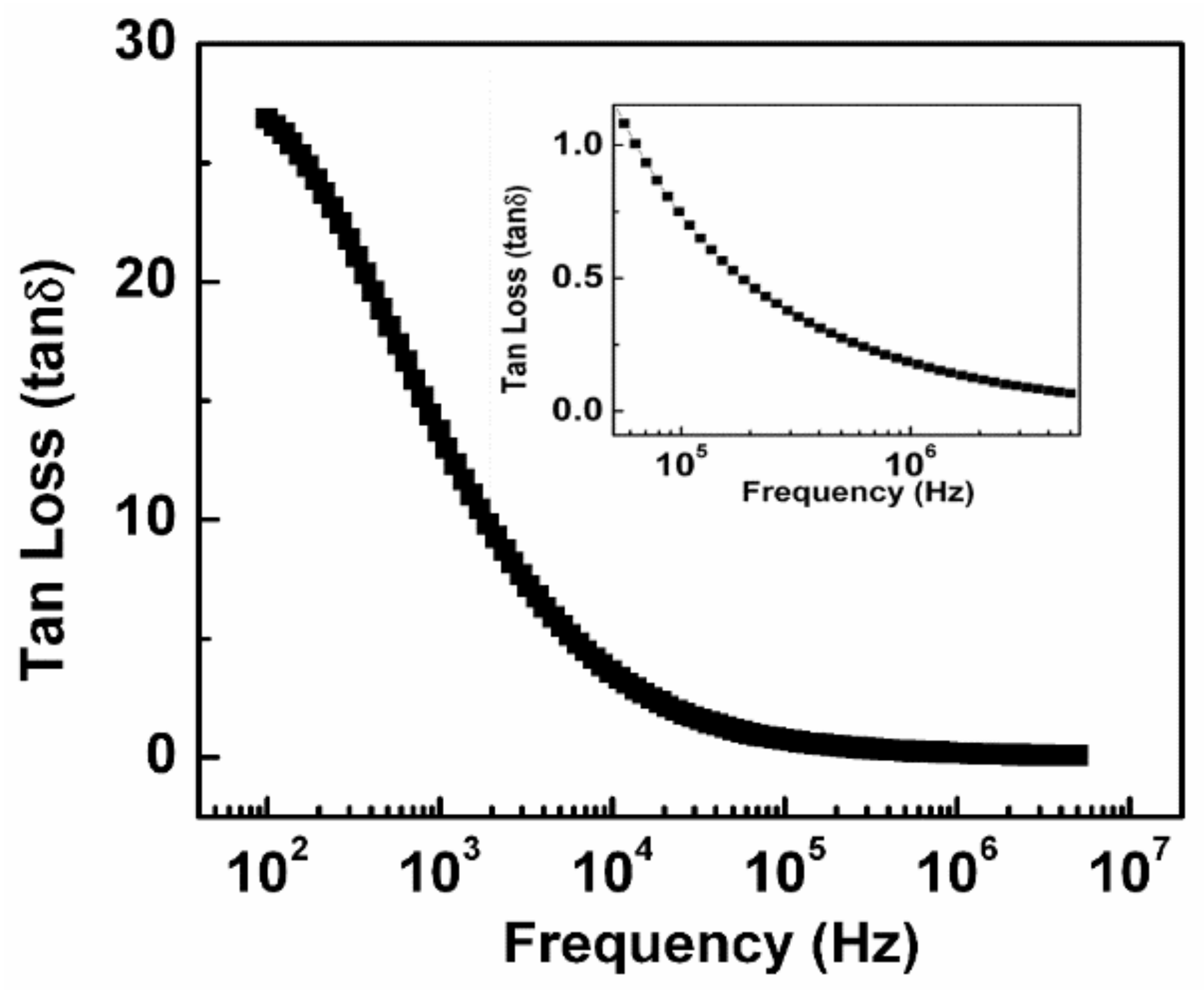
3.4. Tangent Loss Behavior
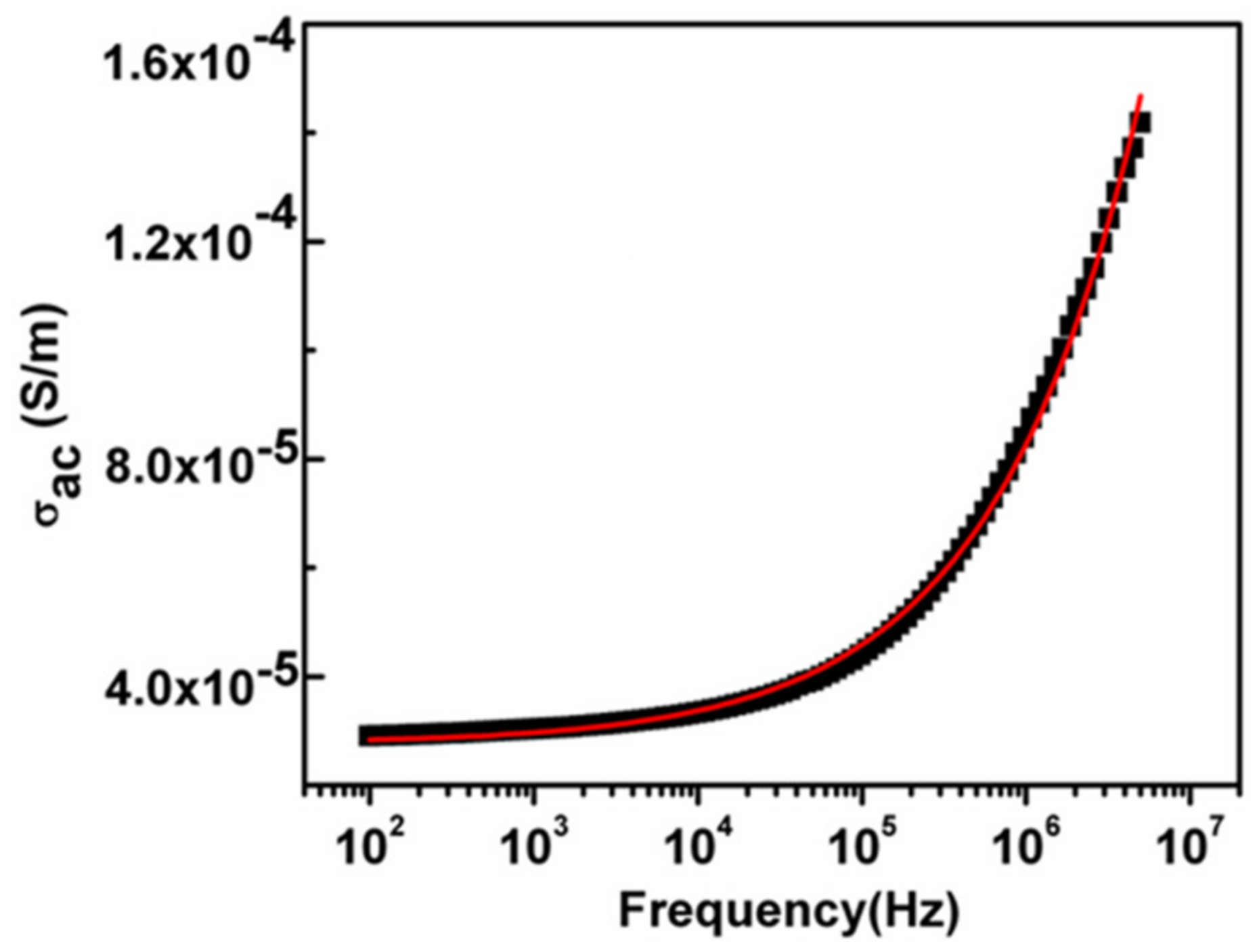
3.5. The Ac Conductivity (σac)
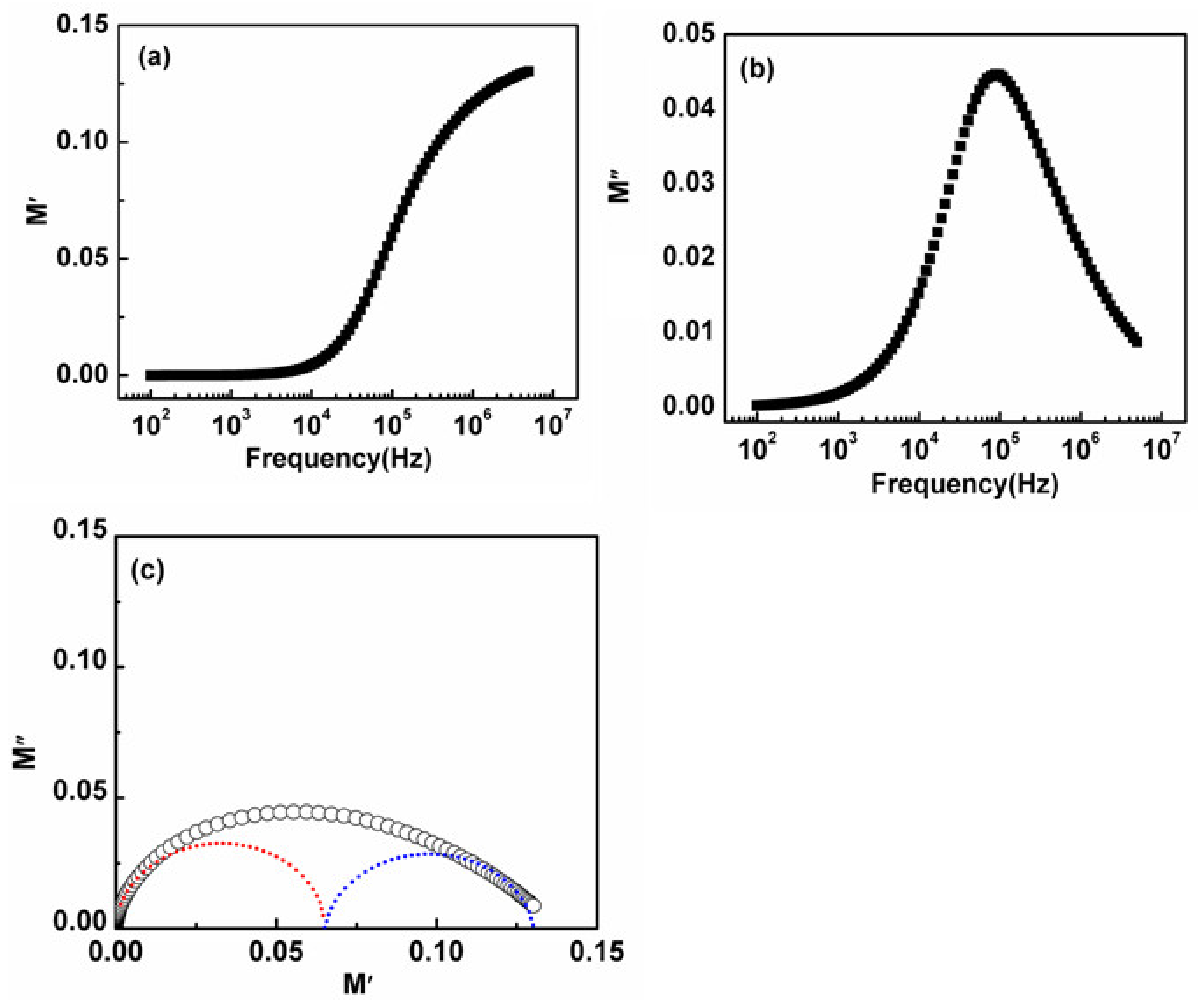
3.6. Electric Modulus
3.7. Impedance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.U.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.S. Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials 2018, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; Al-Safty, S.A. Haemoproteins nickel foam hybrid as effective supercapacitors. Chem. Commun. 2014, 50, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Kullerud, G.; Yund, R.A. The Ni-S System and Related Minerals. J. Petrol. 1962, 3, 126–175. [Google Scholar] [CrossRef]
- Huang, H.; Deng, X.; Yan, L.; Wei, G.; Zhou, W.; Liang, X.; Guo, J. One-Step Synthesis of Self-Supported Ni3S2/NiS Composite Film on Ni Foam by Electrodeposition for High-Performance Supercapacitors. Nanomaterials 2019, 9, 1718. [Google Scholar] [CrossRef]
- Anzai, S.; Ozawa, K. Pressure effect on metal-nonmetal transition in NiS. Phys. B + C 1977, 86, 1001–1002. [Google Scholar] [CrossRef]
- Barthelemy, E.; Gorochov, O.; McKinzie, H. The electrical and magnetic properties of the transition in nickel sulfide. Mater. Res. Bull. 1973, 8, 1401–1412. [Google Scholar] [CrossRef]
- Olivas, A.; Cruz-Reyes, J.; Petranovskii, V.; Avalos, M.; Fuentes, S. Synthesis and characterization of nickel sulfide catalysts. J. Vac. Sci. Technol. A 1998, 16, 3515–3520. [Google Scholar] [CrossRef]
- Li, X.; Fang, Z.P.; Ng, F.L.; Zhao, L.P.; Zhao, L. Inspection and Image Analysis of Nickel Sulphide Inclusions in Toughened Glass Panels. In Proceedings of the 2006 9th International Conference on Control, Automation, Robotics and Vision, Singapore, 5–8 December 2006; pp. 1–6. [Google Scholar]
- Huang, X.-Y.; Chi, Z.T.; Liu, J.; Li, D.H.; Sun, X.J.; Yan, C.; Wang, Y.C.; Li, H.; Wang, X.D.; Xie, W.F. Enhanced gas sensing performance based on p-NiS/n-In2O3 heterojunction nanocomposites. Sens. Actuators B Chem. 2019, 304, 127305. [Google Scholar] [CrossRef]
- Yoo, J.; Kwak, I.H.; Kwon, I.S.; Park, K.; Kim, D.; Lee, J.H.; Lim, S.A.; Cha, E.H.; Park, J. Nickel Sulfide Nanocrystals on Silicon Nanowire Array for Solar-Driven Photoelectrochemical Hydrogen Generation. J. Mater. Chem. C 2020, 8, 3240–3247. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, Q.; Chen, Y.; Yu, H.; Liu, H.; Cui, G.; Sun, X.; Li, L. NiS2@ rGO Nanosheet Wrapped with PPy Aerogel: A Sandwich-Like Structured Composite for Excellent Microwave Absorption. Nanomaterials 2019, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qin, D.; Huang, S.; Guo, X.; Li, D.; Luo, Y.; Meng, Q. Dye-sensitized solar cells with NiS counter electrodes electrodeposited by a potential reversal technique. Energy Environ. Sci. 2011, 4, 2630–2637. [Google Scholar] [CrossRef]
- Sonia, T.S.; Anjali, P.; Roshny, S.; Lakshmi, V.; Ranjusha, R.; Subramanian, K.R.; Nair, S.V.; Balakrishnan, A. Nano/micro-hybrid NiS cathodes for lithium ion batteries. Ceram. Int. 2014, 40, 8351–8356. [Google Scholar] [CrossRef]
- Guan, X.; Liu, X.; Xu, B.; Liu, X.; Kong, Z.; Song, M.; Fu, A.; Li, Y.; Guo, P.; Li, H. Carbon wrapped Ni3S2 nanocrystals anchored on graphene sheets as anode materials for lithium-ion battery and the study on their capacity evolution. Nanomaterials 2018, 8, 760. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Li, H.; Li, Q.; Qian, Y. Hydrothermal synthesis of NiS nanobelts and NiS2 microspheres constructed of cuboids architectures. J. Solid State Chem. 2010, 183, 223–227. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakraborty, A.; Bera, S. NiS/rGO nanohybrid: An excellent counter electrode for dye sensitized solar cell. Solar Energy Mater. Solar Cells 2018, 182, 314–320. [Google Scholar] [CrossRef]
- Artal, R.; Serrà, A.; Michler, J.; Philippe, L.; Gómez, E. Electrodeposition of Mesoporous Ni-Rich Ni-Pt Films for Highly Efficient Methanol Oxidation. Nanomaterials 2020, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Sartale, S.D.; Lokhande, C. Preparation and characterization of nickel sulphide thin films using successive ionic layer adsorption and reaction (SILAR) method. Mater. Chem. Phys. 2001, 72, 101–104. [Google Scholar] [CrossRef]
- Jarvis, K.E.; Williams, J.G.; Parry, S.J.; Bertalan, E. Quantitative determination of the platinum-group elements and gold using NiS fire assay with laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS ). Chem. Geol. 1995, 124, 37–46. [Google Scholar] [CrossRef]
- Diao, C.-C.; Huang, C.Y.; Yang, C.F.; Wu, C.C. Morphological, Optical, and Electrical Properties of p-Type Nickel Oxide Thin Films by Nonvacuum Deposition. Nanomaterials 2020, 10, 636. [Google Scholar] [CrossRef]
- Yang, J.; Duan, X.; Qin, Q.; Zheng, W. Solvothermal synthesis of hierarchical flower-like β-NiS with excellent electrochemical performance for supercapacitors. J. Mater. Chem. A 2013, 1, 7880–7884. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Tang, K.; Li, F.; Qian, Y. Solvothermal synthesis of NiS 3D nanostructures. Eur. J. Inorg. Chem. 2005, 2005, 653–656. [Google Scholar] [CrossRef]
- Reddy, L.; Deshmukh, K.; Kovářík, T.; Reiger, D.; Nambiraj, N.A.; Lakshmipathy, R. Enhanced dielectric properties of green synthesized Nickel Sulphide (NiS) nanoparticles integrated polyvinylalcohol nanocomposites. Mater. Res. Express 2020, 7, 64007. [Google Scholar] [CrossRef]
- Bose, A.; Mandal, A.; Mitra, S.; De, S.K.; Banerjee, S.; Chakravorty, D. Dielectric relaxation studies on two-dimensional nanocomposites of NiS and Na-4 mica. Indian J. Phys. 2013, 87, 977–981. [Google Scholar] [CrossRef]
- Jamil, A.; Batool, S.S.; Sher, F.; Rafiq, M.A. Determination of density of states, conduction mechanisms and dielectric properties of nickel disulfide nanoparticles. AIP Adv. 2016, 6, 55120. [Google Scholar] [CrossRef]
- Suresh, S.; Anand, S.S.; Arul, R.; Isha, D. Structural, morphological, optical and electrical properties of nickel sulphide thin films. Chalcogenide Lett. 2016, 13, 291–299. [Google Scholar]
- Atay, F.; Köse, S.; Bilgin, V.; Akyüz, İ. Electrical, Optical, Structural and Morphological Properties of NiS Films. Turk. J. Phys. 2003, 27, 285–291. [Google Scholar]
- Surendran, S.; Sankar, K.V.; Berchmans, L.J.; Selvan, R.K. Polyol synthesis of α-NiS particles and its physico-chemical properties. Mater. Sci. Semicond. Process. 2015, 33, 16–23. [Google Scholar] [CrossRef]
- Balayeva, O.O.; Azizov, A.A.; Muradov, M.B.; Eyvazova, G.M. Dielectric characterization of CuxS-NiySz/FNBR and CuS-NiySz/FNBR nanocomposites. Solid State Electron. 2017, 132, 31–38. [Google Scholar] [CrossRef]
- Iram, S.; Mahmood, A.; Sitara, E.; Batool Bukhari, S.A.; Fatima, S.A.; Shaheen, R.; Azad Malik, M. Nanostructured Lead Sulphide Depositions by AACVD Technique Using Bis(Isobutyldithiophosphinato)Lead(II) Complex as Single Source Precursor and Its Impedance Study. Nanomaterials 2020, 10, 1438. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Drew, M.G.B.; Singh, N. Self assembly of homoleptic Ni(ii) dithiocarbamates and dithiocarbimates via Ni⋯H–C anagostic and C–H⋯π (chelate) interactions. CrystEngComm 2013, 15, 10255–10265. [Google Scholar] [CrossRef]
- Ramasamy, K.; Malik, M.A.; O’Brien, P.; Raftery, J.; Helliwell, M. Nickel Sulfide Thin Films from Thio- and Dithiobiuret Precursors. Chem. Mater. 2010, 22, 6328–6340. [Google Scholar] [CrossRef]
- Buchmaier, C.; Glänzer, M.; Torvisco, A.; Poelt, P.; Wewerka, K.; Kunert, B.; Gatterer, K.; Trimmel, G.; Rath, T. Nickel sulfide thin films and nanocrystals synthesized from nickel xanthate precursors. J. Mater. Sci. 2017, 52, 10898–10914. [Google Scholar] [CrossRef]
- Mgabi, L.P.; Dladla, B.S.; Malik, M.A.; Garje, S.S.; Akhtar, J.; Revaprasadu, N. Deposition of Cobalt and Nickel Sulfide Thin Films from Thio- and Alkylthio-urea Complexes as Precursors via the Aerosol Assisted Chemical Vapour Deposition Technique. Thin Solid Films 2014, 564, 51–57. [Google Scholar] [CrossRef]
- Kuchen, W.; Judat, A.; Metten, J.J.C.B. Metallkomplexe der Dialkyldithiophosphinsäuren, II. Synthese und Dipolmomente einiger Diäthyldithiophosphinato-Komplexe. Chem. Ber. 1965, 98, 3981–3987. [Google Scholar]
- Zheng, X.; Han, X.; Zhang, Y.; Wang, J.; Zhong, C.; Deng, Y.; Hu, W. Controllable synthesis of nickel sulfide nanocatalysts and their phase-dependent performance for overall water splitting. Nanoscale 2019, 11, 5646–5654. [Google Scholar] [CrossRef] [PubMed]
- Lawton, S.L.; Kokotailo, G.T. Crystal and molecular structures of zinc and cadmium O,O-diisopropyl phosphorodithioates. Inorg. Chem. 1969, 8, 2410–2421. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Younas, M. Structural and transport properties of nanocrystalline MnFe2O4 synthesized by co-precipitation method. Solid State Sci. 2012, 14, 1536–1542. [Google Scholar] [CrossRef]
- Sagadevan, S.; Rajesh, S.; Das, I. Studies on Nanocrystalline Nickel Oxide Thin Films for Potential Applications. Mater. Today Proc. 2017, 4, 4123–4129. [Google Scholar] [CrossRef]
- Khan, M.Z.; Gul, I.H.; Baig, M.M.; Khan, A.N. Comprehensive study on structural, electrical, magnetic and photocatalytic degradation properties of Al3+ ions substituted nickel ferrites nanoparticles. J. Alloy. Compd. 2020, 848, 155795. [Google Scholar] [CrossRef]
- Halder, S.; Dey, A.; Sil, S.; Ray, P.P. Study of A.C. conductivity and dielectric behaviour of hydrothermally synthesised molybdenum disulphide. J. Mater. Sci. Mater. Electron. 2021, 32, 1–14. [Google Scholar] [CrossRef]
- Sengupta, P.; Sadhukhan, P.; Ray, A.; Ray, R.; Bhattacharyya, S.; Das, S. Temperature and frequency dependent dielectric response of C3H7NH3PbI3: A new hybrid perovskite. J. Appl. Phys. 2020, 127, 204103. [Google Scholar] [CrossRef]
- Fatima, S.A.; Khan, M.N.; Shaheen, R.; Shahzad, K.; Iqbal, M. Temperature dependent AC-Conduction and relaxation mechanism of spinel MgCo2O4 system by impedance spectroscopy. Phys. B Condens. Matter 2021, 600, 412581. [Google Scholar] [CrossRef]
- Thakur, V.; Singh, A.; Awasthi, A.M.; Singh, L. Temperature dependent electrical transport characteristics of BaTiO3 modified lithium borate glasses. AIP Adv. 2015, 5, 87110. [Google Scholar] [CrossRef]
- Kolonelou, E.; Papathanassiou, A.N.; Sakellis, E. Pressure-induced electro-switching of polymer/nano-graphene composites. Mater. Chem. Phys. 2019, 232, 319–324. [Google Scholar] [CrossRef]
- Papathanassiou, A.; Mykhailiv, O.; Echegoyen, L.; Sakellis, I.; Plonska-Brzezinska, M.E. Electric properties of carbon nano-onion/polyaniline composites: A combined electric modulus and ac conductivity study. J. Phys. D Appl. Phys. 2016, 49, 285305. [Google Scholar] [CrossRef]
- Bashir, J.; Shaheen, R. Structural and complex AC impedance spectroscopic studies of A2CoNbO6 (A = Sr, Ba) ordered double perovskites. Solid State Sci. 2011, 13, 993–999. [Google Scholar] [CrossRef]

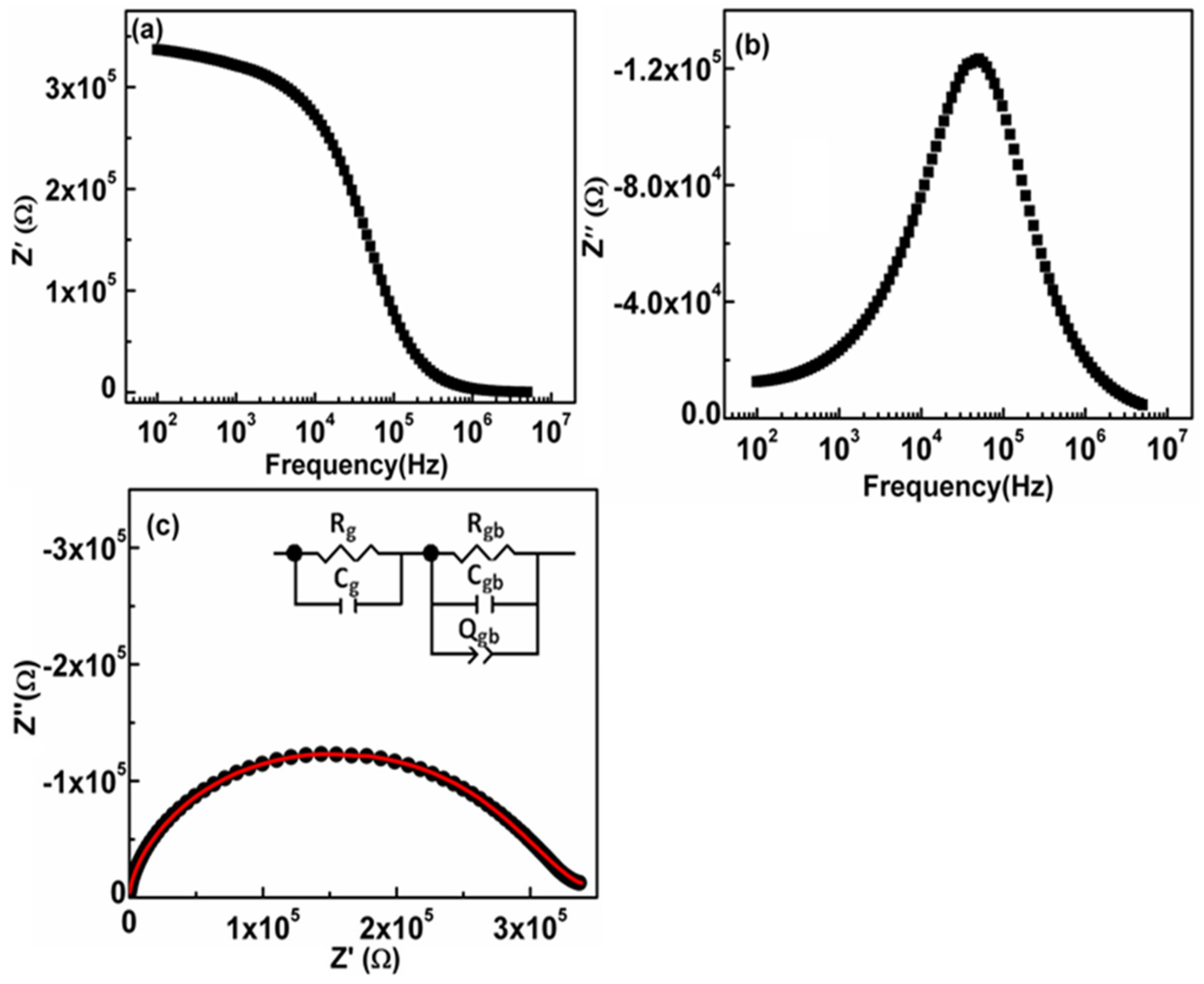
| σdc (S/m) | A | s |
|---|---|---|
| 2.76 × 10−5 | 7.2158 × 10−8 | 0.48 |
| Rg (Ω) | Cg (F) | Rgb (Ω) | Cgb (F) | Qgb | ngb |
|---|---|---|---|---|---|
| 89679 | 7.93 × 10−12 | 256380 | 3.92 × 10−11 | 7.63 × 10−9 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iram, S.; Mahmood, A.; Ehsan, M.F.; Mumtaz, A.; Sohail, M.; Sitara, E.; Bukhari, S.A.B.; Gul, S.; Fatima, S.A.; Khan, M.Z.; et al. Impedance Spectroscopic Study of Nickel Sulfide Nanostructures Deposited by Aerosol Assisted Chemical Vapor Deposition Technique. Nanomaterials 2021, 11, 1105. https://doi.org/10.3390/nano11051105
Iram S, Mahmood A, Ehsan MF, Mumtaz A, Sohail M, Sitara E, Bukhari SAB, Gul S, Fatima SA, Khan MZ, et al. Impedance Spectroscopic Study of Nickel Sulfide Nanostructures Deposited by Aerosol Assisted Chemical Vapor Deposition Technique. Nanomaterials. 2021; 11(5):1105. https://doi.org/10.3390/nano11051105
Chicago/Turabian StyleIram, Sadia, Azhar Mahmood, Muhammad Fahad Ehsan, Asad Mumtaz, Manzar Sohail, Effat Sitara, Syeda Aqsa Batool Bukhari, Sumia Gul, Syeda Arooj Fatima, Muhammad Zarrar Khan, and et al. 2021. "Impedance Spectroscopic Study of Nickel Sulfide Nanostructures Deposited by Aerosol Assisted Chemical Vapor Deposition Technique" Nanomaterials 11, no. 5: 1105. https://doi.org/10.3390/nano11051105
APA StyleIram, S., Mahmood, A., Ehsan, M. F., Mumtaz, A., Sohail, M., Sitara, E., Bukhari, S. A. B., Gul, S., Fatima, S. A., Khan, M. Z., Shaheen, R., Malik, S. N., & Malik, M. A. (2021). Impedance Spectroscopic Study of Nickel Sulfide Nanostructures Deposited by Aerosol Assisted Chemical Vapor Deposition Technique. Nanomaterials, 11(5), 1105. https://doi.org/10.3390/nano11051105





