Atomic Layer Deposition of Ni-Co-O Thin-Film Electrodes for Solid-State LIBs and the Influence of Chemical Composition on Overcapacity
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Atomic Layer Deposition of Ni-Co-O Thin Films
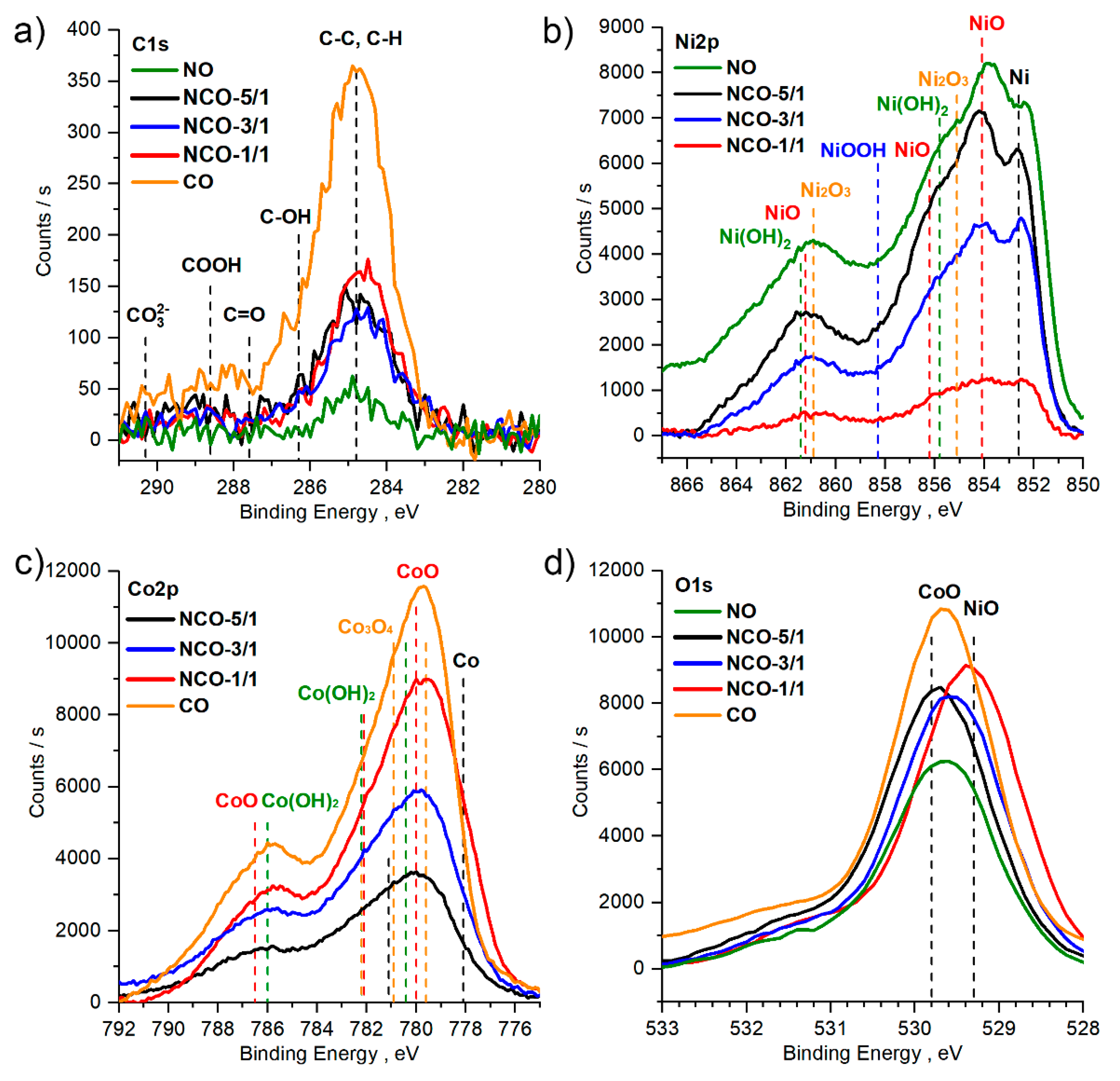
3.2. Chemical Composition of the Films
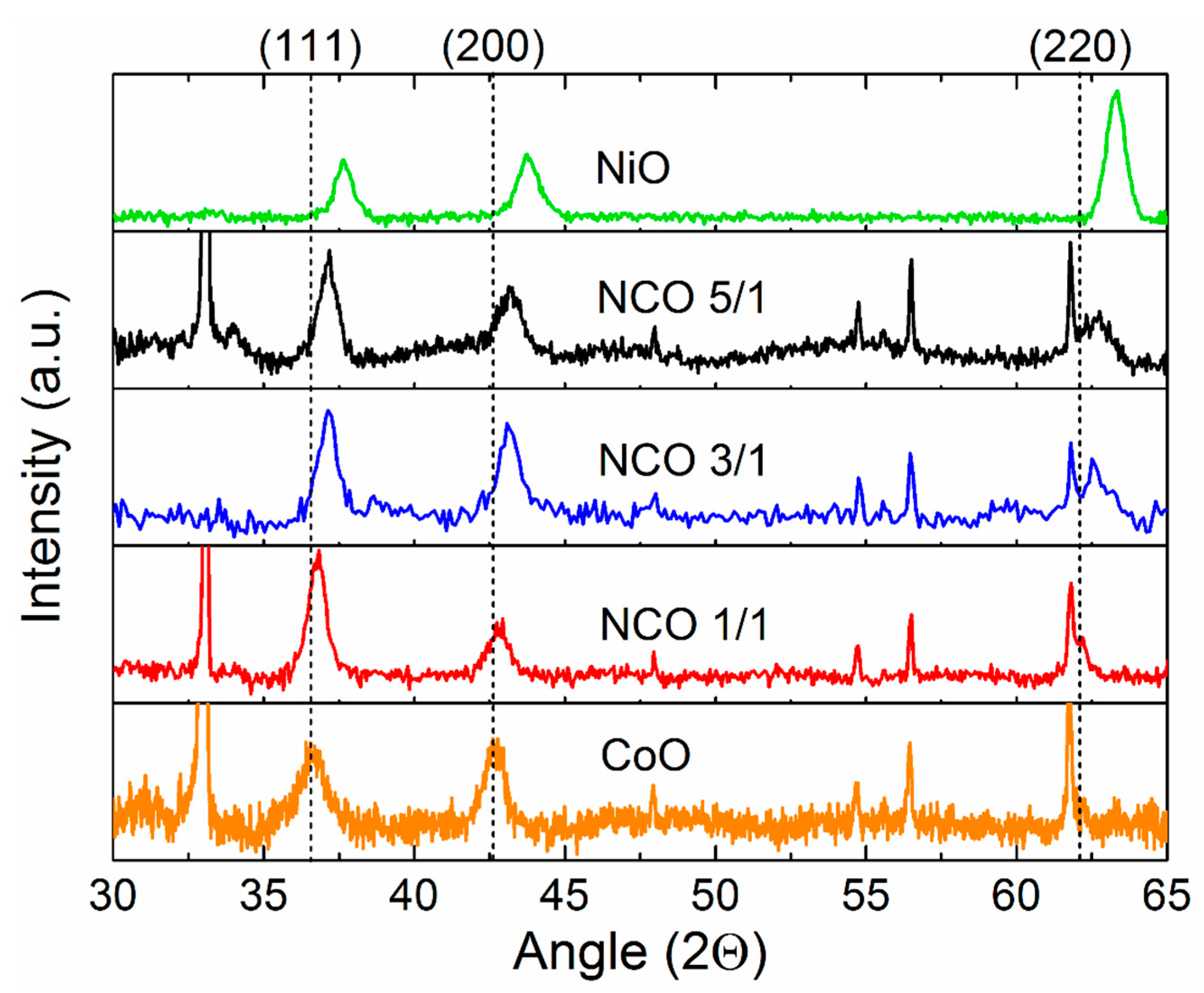
3.3. Crystal Structure
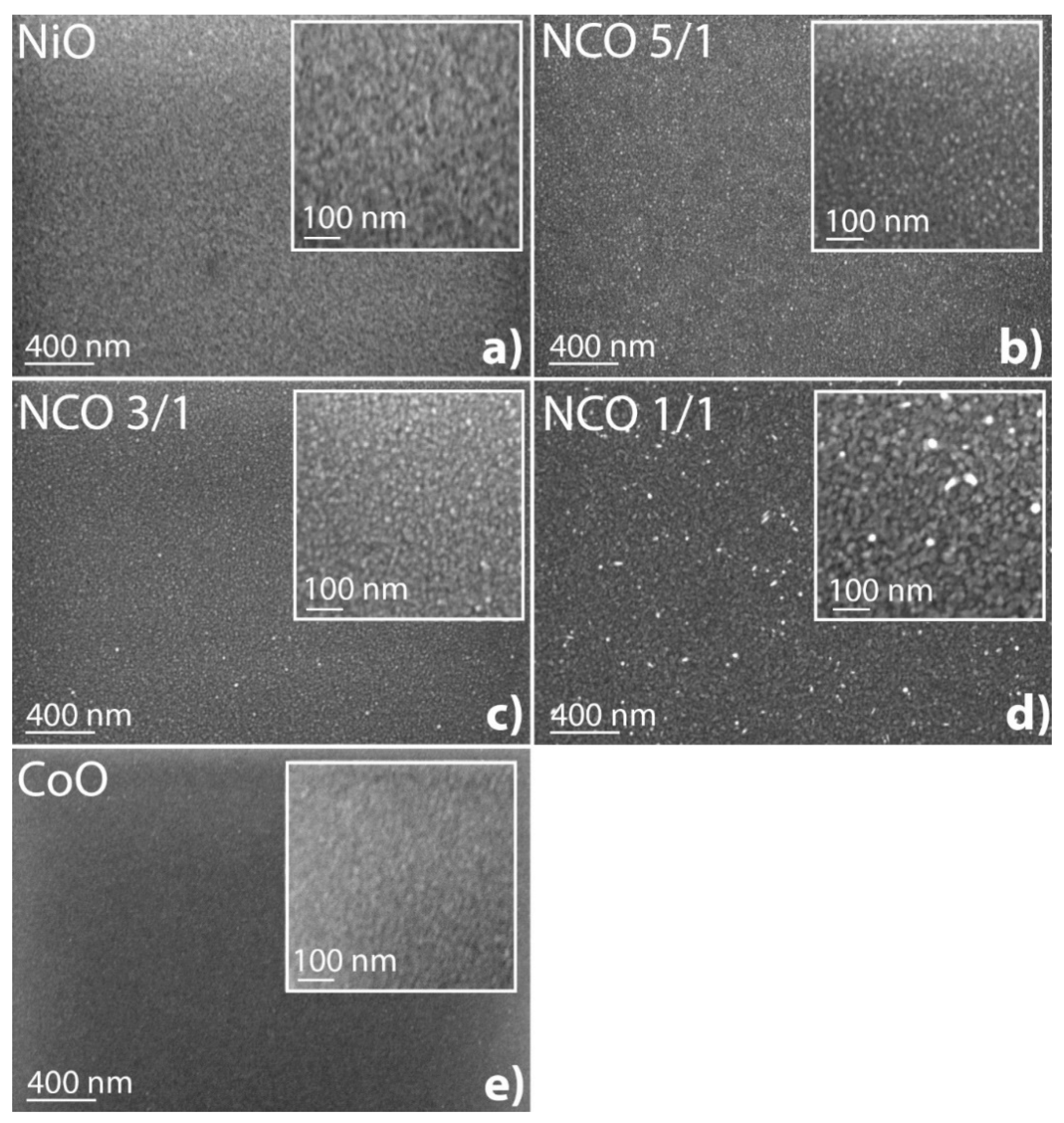
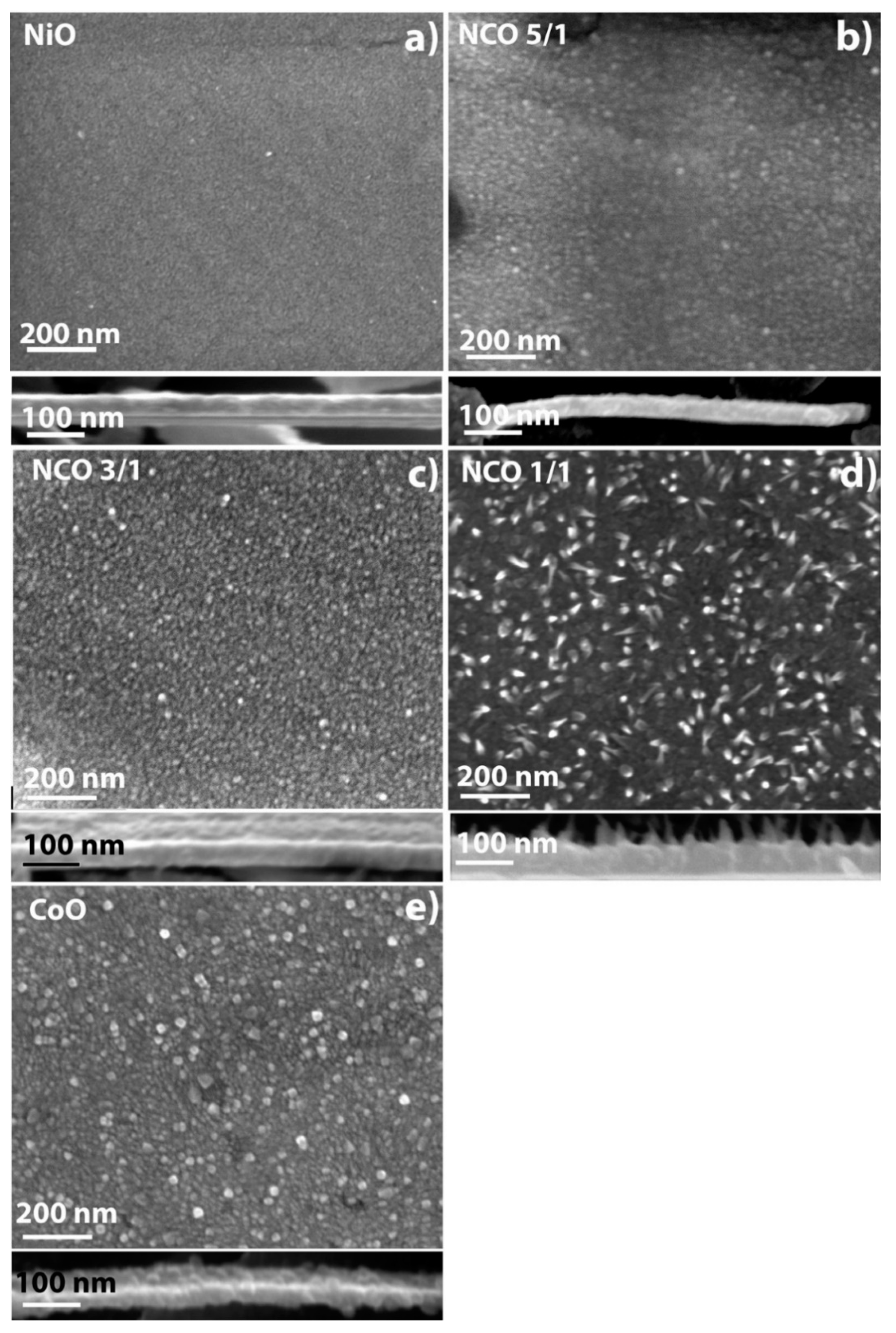
3.4. Morphology of Films on Si and Steel Substrates
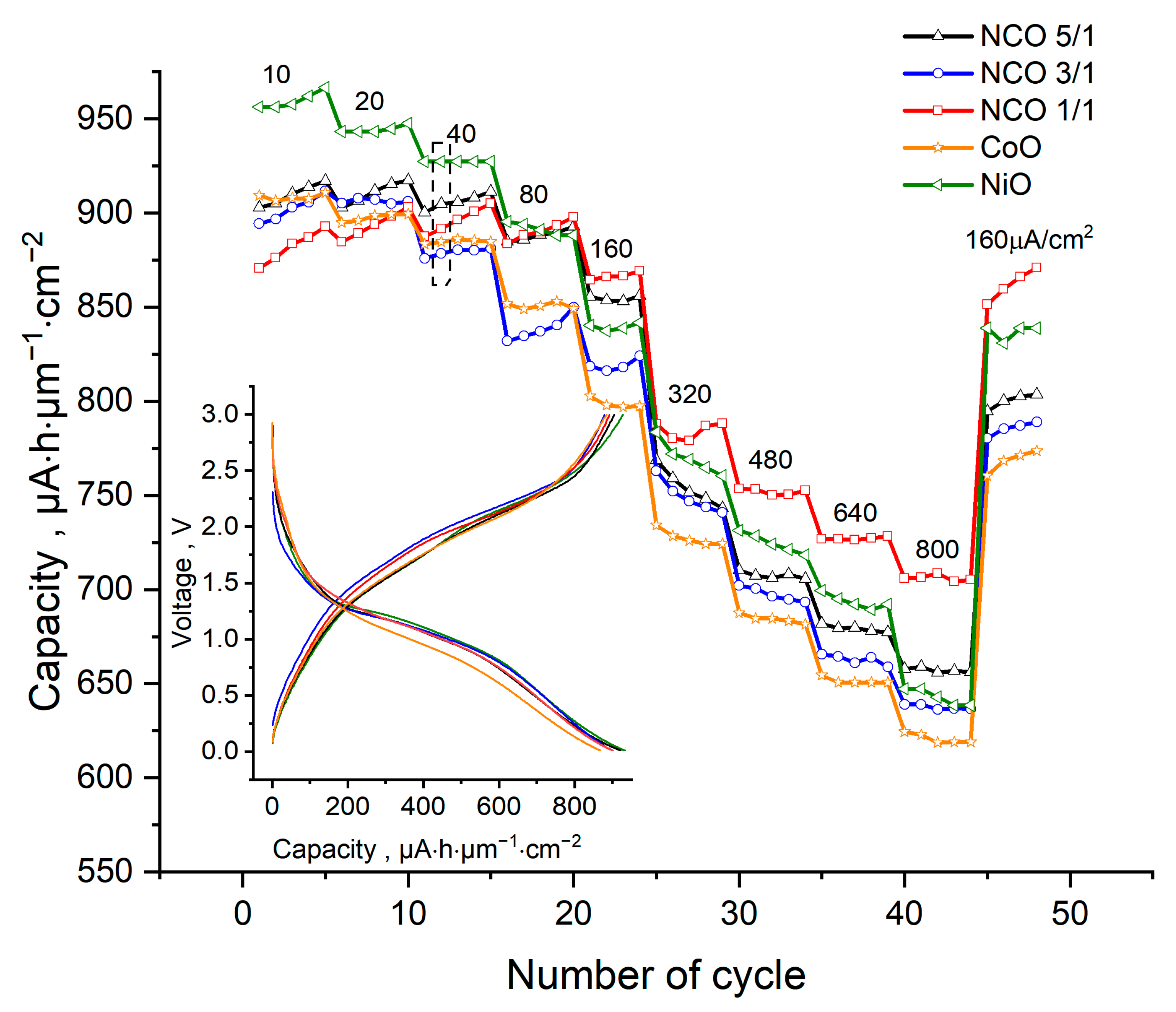
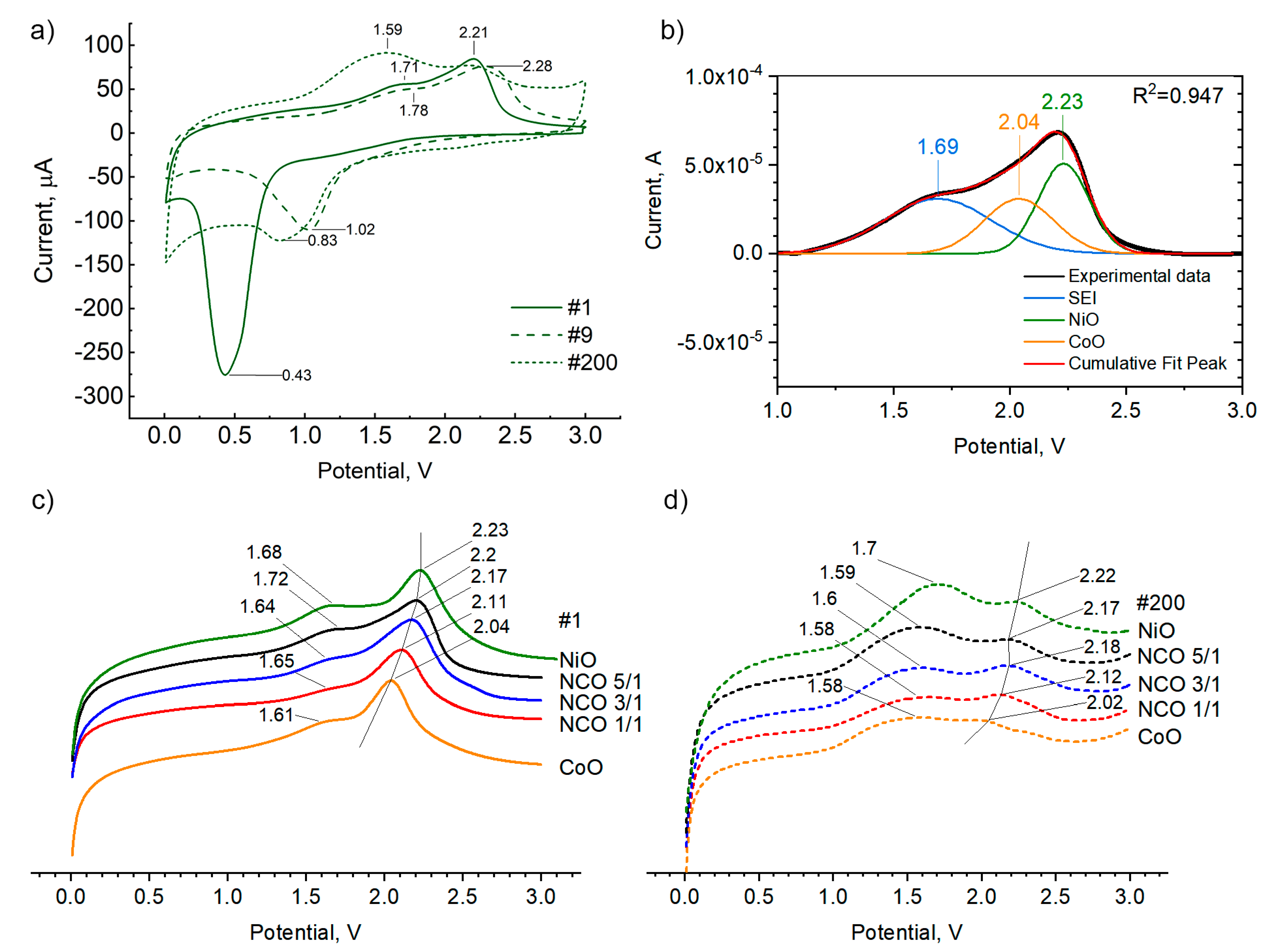
3.5. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goldstein, M. IEEE Computer Society Phoenix Chapter Internet of Things Innovations & Megatrends Update. Available online: https://www.slideshare.net/markgirc/ieee-computer-society-phoenix-chapter-internet-of-things-innovations-megatrends-update-121119 (accessed on 3 August 2020).
- CeraCharge™. Backup Battery for Real Time Clock; TDK Electronics AG Piezo & Protection Devices Business Group Multilayer Technology: Munich, Germany, 1 May 2020; Available online: https://www.tdk-electronics.tdk.com/download/2831894/a5932aa34a732bb2ad827025c862e61f/06-dl---real-time-clock.pdf (accessed on 17 June 2020).
- Shirvanimoghaddam, M.; Shirvanimoghaddam, K.; Abolhasani, M.M.; Farhangi, M.; Barsari, V.Z.; Liu, H.Y.; Dohler, M.; Naebe, M. Towards a Green and Self-Powered Internet of Things Using Piezoelectric Energy Harvesting. IEEE Access 2019, 7, 94533–94556. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.T.; Oh, S.M.; Sun, Y.K. Nickel-Rich and Lithium-Rich Layered Oxide Cathodes: Progress and Perspectives. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Sun, C.W.; Liu, J.; Gong, Y.D.; Wilkinson, D.P.; Zhang, J.J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.F.; Yan, B.; Xiong, D.B.; Li, D.J.; Lawes, S.; Sun, X.L. Recent Developments and Understanding of Novel Mixed Transition-Metal Oxides as Anodes in Lithium Ion Batteries. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Huang, Z.D.; Zhang, K.; Zhang, T.T.; Yang, X.S.; Liu, R.Q.; Li, Y.; Lin, X.J.; Feng, X.M.; Ma, Y.W.; Huang, W. Hierarchical NiCoO2 mesoporous microspheres as anode for lithium ion batteries with superior rate capability. Energy Storage Mater. 2016, 3, 36–44. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef]
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D Thin-Film Li-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 17. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Baggetto, L.; Notten, P.H.L. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Pearse, A.; Schmitt, T.; Sahadeo, E.; Stewart, D.M.; Kozen, A.; Gerasopoulos, K.; Talin, A.A.; Lee, S.B.; Rubloff, G.W.; Gregorczyk, K.E. Three-Dimensional Solid-State Lithium-Ion Batteries Fabricated by Conformal Vapor-Phase Chemistry. ACS Nano 2018, 12, 4286–4294. [Google Scholar] [CrossRef] [PubMed]
- Notten, P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D integrated all-solid-state rechargeable batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Baggetto, L.; Niessen, R.A.H.; Roozeboom, F.; Notten, P.H.L. High energy density all-solid-state batteries: A challenging concept towards 3D integration. Adv. Funct. Mater. 2008, 18, 1057–1066. [Google Scholar] [CrossRef]
- Chernyaeva, O.Y.; Kyashkin, V.M.; Ivleva, A.Y.; Yrova, V.Y.; Solovyova, E.O. The influence of process conditions on the phase composition of the LiFePO4 film obtained by the atomic layer method. Polyhedron 2019, 157, 297–300. [Google Scholar] [CrossRef]
- Liu, J.; Banis, M.N.; Sun, Q.; Lushington, A.; Li, R.Y.; Sham, T.K.; Sun, X.L. Rational Design of Atomic-Layer-Deposited LiFePO4 as a High-Performance Cathode for Lithium-Ion Batteries. Adv. Mater. 2014, 26, 6472–6477. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, B.W.; Banis, M.N.; Li, R.Y.; Sham, T.K.; Sun, X.L. Atomic layer deposition of amorphous iron phosphates on carbon nanotubes as cathode materials for lithium-ion batteries. Electrochim. Acta 2015, 162, 275–281. [Google Scholar] [CrossRef]
- Donders, M.E.; Arnoldbik, W.M.; Knoops, H.C.M.; Kessels, W.M.M.; Notten, P.H.L. Atomic Layer Deposition of LiCoO2 Thin-Film Electrodes for All-Solid-State Li-Ion Micro-Batteries. J. Electrochem. Soc. 2013, 160, A3066–A3071. [Google Scholar] [CrossRef]
- Donders, M.E.; Knoops, H.C.M.; Kessels, W.M.M.; Notten, P.H.L. Remote Plasma Atomic Layer Deposition of Thin Films of Electrochemically Active LiCoO2. In Atomic Layer Deposition Applications 7; Elam, J.W., Londergan, A., VanDerStraten, O., Roozeboom, F., DeGendt, S., Bent, S.F., Delabie, A., Eds.; ECS Transactions: San Francisco, CA, USA, 2011; Volume 41, pp. 321–330. [Google Scholar]
- Miikkulainen, V.; Ruud, A.; Ostreng, E.; Nilsen, O.; Laitinen, M.; Sajavaara, T.; Fjellvag, H. Atomic Layer Deposition of Spinel Lithium Manganese Oxide by Film-Body-Controlled Lithium Incorporation for Thin-Film Lithium-Ion Batteries. J. Phys. Chem. C 2014, 118, 1258–1268. [Google Scholar] [CrossRef]
- Nieminen, H.E.; Miikkulainen, V.; Settipani, D.; Simonelli, L.; Honicke, P.; Zech, C.; Kayser, Y.; Beckhoff, B.; Honkanen, A.P.; Heikkila, M.J.; et al. Intercalation of Lithium Ions from Gaseous Precursors into beta-MnO2 Thin Films Deposited by Atomic Layer Deposition. J. Phys. Chem. C 2019, 123, 15802–15814. [Google Scholar] [CrossRef]
- Young, M.J.; Schnabel, H.D.; Holder, A.M.; George, S.M.; Musgrave, C.B. Band Diagram and Rate Analysis of Thin Film Spinel LiMn2O4 Formed by Electrochemical Conversion of ALD-Grown MnO. Adv. Funct. Mater. 2016, 26, 7895–7907. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Golabkan, V.; Pereira-Ramos, J.P.; Mantoux, A.; Lincot, D. A Raman study of the lithium insertion process in vanadium pentoxide thin films deposited by atomic layer deposition. J. Raman Spectrosc. 2002, 33, 631–638. [Google Scholar] [CrossRef]
- Badot, J.C.; Mantoux, A.; Baffier, N.; Dubrunfaut, O.; Lincot, D. Electrical properties of V2O5 thin films obtained by atomic layer deposition (ALD). J. Mater. Chem. 2004, 14, 3411–3415. [Google Scholar] [CrossRef]
- Badot, J.C.; Mantoux, A.; Baffier, N.; Dubrunfaut, O.; Lincot, D. Submicro- and nanostructural eff ects on electrical properties of Li0.2V2O5 thin films obtained by atomic layer deposition (ALD). J. Phys. Chem. Solids 2006, 67, 1270–1274. [Google Scholar] [CrossRef]
- Prasadam, V.P.; Bahlawane, N.; Mattelaer, F.; Rampelberg, G.; Detavernier, C.; Fang, L.; Jiang, Y.; Martens, K.; Parkin, I.P.; Papakonstantinou, I. Atomic layer deposition of vanadium oxides: Process and application review. Mater. Today Chem. 2019, 12, 396–423. [Google Scholar] [CrossRef]
- Wang, B.Q.; Liu, J.; Sun, Q.; Xiao, B.W.; Li, R.Y.; Sham, T.K.; Sun, X.L. Titanium Dioxide/Lithium Phosphate Nanocomposite Derived from Atomic Layer Deposition as a High-Performance Anode for Lithium Ion Batteries. Adv. Mater. Interfaces 2016, 3, 8. [Google Scholar] [CrossRef]
- Kia, A.M.; Bonhardt, S.; Zybell, S.; Kuhnel, K.; Haufe, N.; Weinreich, W. Development of Rutile Titanium Oxide Thin Films as Battery Material Component Using Atomic Layer Deposition. Phys. Status Solidi 2020, 217, 1800769. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Maximov, M.Y.; Novikov, P.A.; Popovich, A.A.; Silin, A.O.; Smirnov, V.M.; Bobrysheva, N.P.; Osmolovskaya, O.M.; Osmolovsky, M.G.; Rumyantsev, A.M. Atomic layer deposition of tin oxide using tetraethyltin to produce high-capacity Li-ion batteries. J. Vac. Sci. Technol. A 2017, 35. [Google Scholar] [CrossRef]
- Maximov, M.Y.; Novikov, P.A.; Nazarov, D.V.; Rymyantsev, A.M.; Silin, A.O.; Zhang, Y.; Popovich, A.A. Characterization and Electrochemical Performance at High Discharge Rates of Tin Dioxide Thin Films Synthesized by Atomic Layer Deposition. J. Electron. Mater. 2017, 46, 6571–6577. [Google Scholar] [CrossRef]
- Zhao, B.; Mattelaer, F.; Kint, J.; Werbrouck, A.; Henderick, L.; Minjauw, M.; Dendooven, J.; Detavernier, C. Atomic layer deposition of ZnO-SnO2 composite thin film: The influence of structure, composition and crystallinity on lithium-ion battery performance. Electrochim. Acta 2019, 320, 13. [Google Scholar] [CrossRef]
- Kint, J.; Mattelaer, F.; Minjauw, M.; Zhao, B.; Detavernier, C. Atomic layer deposition of thin films as model electrodes: A case study of the synergistic effect in Fe2O3-SnO2. J. Vac. Sci. Technol. A 2019, 37. [Google Scholar] [CrossRef]
- Bonhardt, S.; Kuhnel, K.; Kia, A.M.; Weinreich, W. Formation of highly conformal spinel lithium titanate thin films based on a novel three-step atomic layer deposition process. J. Vac. Sci. Technol. A 2019, 37, 6. [Google Scholar] [CrossRef]
- Meng, X.B.; Liu, J.; Li, X.F.; Banis, M.N.; Yang, J.L.; Li, R.Y.; Sun, X.L. Atomic layer deposited Li4Ti5O12 on nitrogen-doped carbon nanotubes. RSC Adv. 2013, 3, 7285–7288. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Nilsen, O.; Laitinen, M.; Sajavaara, T.; Fjellvag, H. Atomic layer deposition of LixTiyOz thin films. RSC Adv. 2013, 3, 7537–7542. [Google Scholar] [CrossRef]
- Kozen, A.C.; Pearse, A.J.; Lin, C.F.; Noked, M.; Rubloff, G.W. Atomic Layer Deposition of the Solid Electrolyte LiPON. Chem. Mater. 2015, 27, 5324–5331. [Google Scholar] [CrossRef]
- Put, B.; Mees, M.J.; Hornsveld, N.; Hollevoet, S.; Sepulveda, A.; Vereecken, P.M.; Kessels, W.M.M.; Creatore, M. Plasma-Assisted ALD of LiPO(N) for Solid State Batteries. J. Electrochem. Soc. 2019, 166, A1239–A1242. [Google Scholar] [CrossRef]
- Wang, B.Q.; Zhao, Y.; Banis, M.N.; Sun, Q.; Adair, K.R.; Li, R.Y.; Sham, T.K.; Sun, X.L. Atomic Layer Deposition of Lithium Niobium Oxides as Potential Solid-State Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1654–1661. [Google Scholar] [CrossRef]
- Ostreng, E.; Sonsteby, H.H.; Sajavaara, T.; Nilsen, O.; Fjellvag, H. Atomic layer deposition of ferroelectric LiNbO3. J. Mater. Chem. C 2013, 1, 4283–4290. [Google Scholar] [CrossRef]
- Atosuo, E.; Mantymaki, M.; Mizohata, K.; Heikkila, M.J.; Raisanen, J.; Ritala, M.; Leskela, M. Preparation of Lithium Containing Oxides by the Solid State Reaction of Atomic Layer Deposited Thin Films. Chem. Mater. 2017, 29, 998–1005. [Google Scholar] [CrossRef]
- Amirmaleki, M.; Cao, C.H.; Wang, B.Q.; Zhao, Y.; Cui, T.; Tam, J.; Sun, X.L.; Sun, Y.; Filleter, T. Nanomechanical elasticity and fracture studies of lithium phosphate (LPO) and lithium tantalate (LTO) solid-state electrolytes. Nanoscale 2019, 11, 18730–18738. [Google Scholar] [CrossRef]
- Liu, J.; Banis, M.N.; Li, X.F.; Lushington, A.; Cai, M.; Li, R.Y.; Sham, T.K.; Sun, X.L. Atomic Layer Deposition of Lithium Tantalate Solid-State Electrolytes. J. Phys. Chem. C 2013, 117, 20260–20267. [Google Scholar] [CrossRef]
- Li, X.F.; Liu, J.; Banis, M.N.; Lushington, A.; Li, R.Y.; Cai, M.; Sun, X.L. Atomic layer deposition of solid-state electrolyte coated cathode materials with superior high-voltage cycling behavior for lithium ion battery application. Energy Environ. Sci. 2014, 7, 768–778. [Google Scholar] [CrossRef]
- Comstock, D.J.; Elam, J.W. Mechanistic Study of Lithium Aluminum Oxide Atomic Layer Deposition. J. Phys. Chem. C 2013, 117, 1677–1683. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Nilsen, O.; Li, H.; King, S.W.; Laitinen, M.; Sajavaara, T.; Fjellvag, H. Atomic layer deposited lithium aluminum oxide: (In)dependency of film properties from pulsing sequence. J. Vac. Sci. Technol. A 2015, 33, 7. [Google Scholar] [CrossRef]
- Aaltonen, T.; Alnes, M.; Nilsen, O.; Costelle, L.; Fjellvag, H. Lanthanum titanate and lithium lanthanum titanate thin films grown by atomic layer deposition. J. Mater. Chem. 2010, 20, 2877–2881. [Google Scholar] [CrossRef]
- Wang, B.Q.; Liu, J.; Banis, M.N.; Sun, Q.; Zhao, Y.; Li, R.Y.; Sham, T.K.; Sun, X.L. Atomic Layer Deposited Lithium Silicates as Solid-State Electrolytes for All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2017, 9, 31786–31793. [Google Scholar] [CrossRef]
- Aaltonen, T.; Nilsen, O.; Magraso, A.; Fjellvag, H. Atomic Layer Deposition of Li2O-Al2O3 Thin Films. Chem. Mater. 2011, 23, 4669–4675. [Google Scholar] [CrossRef]
- Kazyak, E.; Chen, K.H.; Davis, A.L.; Yu, S.; Sanchez, A.J.; Lasso, J.; Bielinski, A.R.; Thompson, T.; Sakamoto, J.; Siegel, D.J.; et al. Atomic layer deposition and first principles modeling of glassy Li3BO3-Li2CO3 electrolytes for solid-state Li metal batteries. J. Mater. Chem. A 2018, 6, 19425–19437. [Google Scholar] [CrossRef]
- Kazyak, E.; Chen, K.H.; Wood, K.N.; Davis, A.L.; Thompson, T.; Bielinski, A.R.; Sanchez, A.J.; Wang, X.; Wane, C.M.; Sakamoto, J.; et al. Atomic Layer Deposition of the Solid Electrolyte Garnet Li7La3Zr2O12. Chem. Mater. 2017, 29, 3785–3792. [Google Scholar] [CrossRef]
- Hofstetter, K.; Samson, A.J.; Dai, J.Q.; Gritton, J.E.; Hu, L.B.; Wachsman, E.D.; Thangadurai, V. Electrochemical Stability of Garnet-Type Li7La2.75Ca0.25Zr1.75Nb0.25O12 with and without Atomic Layer Deposited-Al2O3 under CO2 and Humidity. J. Electrochem. Soc. 2019, 166, A1844–A1852. [Google Scholar] [CrossRef]
- Nisula, M.; Shindo, Y.; Koga, H.; Karppinen, M. Atomic Layer Deposition of Lithium Phosphorus Oxynitride. Chem. Mater. 2015, 27, 6987–6993. [Google Scholar] [CrossRef]
- Shibata, S. Thermal Atomic Layer Deposition of Lithium Phosphorus Oxynitride as a Thin-Film Solid Electrolyte. J. Electrochem. Soc. 2016, 163, A2555–A2562. [Google Scholar] [CrossRef]
- Hagen, D.J.; Tripathi, T.S.; Karppinen, M. Atomic layer deposition of nickel-cobalt spinel thin films. Dalton Trans. 2017, 46, 4796–4805. [Google Scholar] [CrossRef]
- Koshtyal, Y.; Nazarov, D.; Ezhov, I.; Mitrofanov, I.; Kim, A.; Rymyantsev, A.; Lyutakov, O.; Popovich, A.; Maximov, M. Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries. Coatings 2019, 9, 301. [Google Scholar] [CrossRef]
- Maximov, M.; Nazarov, D.; Rumyantsev, A.; Koshtyal, Y.; Ezhov, I.; Mitrofanov, I.; Kim, A.; Medvedev, O.; Popovich, A. Atomic Layer Deposition of Lithium-Nickel-Silicon Oxide Cathode Material for Thin-Film Lithium-Ion Batteries. Energies 2020, 13, 2345. [Google Scholar] [CrossRef]
- Mitrofanov, I.; Ezhov, I.; Yury, K.; Nazarov, D.; Kim, A.; Rumyantsev, A.; Popovich, A.; Maximov, M. Synthesis of lithium and cobalt oxides systems by the ALD method to obtain lithium cobalt oxide cathode for thin-film LIBs. In Proceedings of the Nanocon 2019 11th International Conference on Nanomaterials Research & Application, Brno, Czech Republic, 16–18 October 2019; pp. 251–256. [Google Scholar]
- Mackus, A.J.M.; Schneider, J.R.; MacIsaac, C.; Baker, J.G.; Bent, S.F. Synthesis of Doped, Ternary, and Quaternary Materials by Atomic Layer Deposition: A Review. Chem. Mater. 2019, 31, 1142–1183. [Google Scholar] [CrossRef]
- Wang, Y.P.; Ding, Z.J.; Zhu, B.; Liu, W.J.; Zhang, D.W.; Ding, S.J. Atomic layer deposition of amorphous Ni-Ta-N films for Cu diffusion barrier. J. Vac. Sci. Technol. A 2018, 36. [Google Scholar] [CrossRef]
- Qian, S.B.; Wang, Y.P.; Shao, Y.; Liu, W.J.; Ding, S.J. Plasma-Assisted Atomic Layer Deposition of High-Density Ni Nanoparticles for Amorphous In-Ga-Zn-O Thin Film Transistor Memory. Nanoscale Res. Lett. 2017, 12, 138. [Google Scholar] [CrossRef]
- Marrani, A.G.; Novelli, V.; Sheehan, S.; Dowling, D.P.; Dini, D. Probing the Redox States at the Surface of Electroactive Nanoporous NiO Thin Films. ACS Appl. Mater. Interfaces 2014, 6, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-K.; Rhee, S.-W. Chemical vapor deposition of nickel oxide films from Ni(C5H5)2/O2. Thin Solid Films 2001, 391, 57–61. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Chaistain, J., Ed.; Perkin-Elmer Corporation, Physical Electronics Devision: Eden Prairie Minnesota, MN, USA, 1995. [Google Scholar]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Adhikari, H.; Marshall, A.F.; Goldthorpe, I.A.; Chidsey, C.E.D.; McIntyre, P.C. Metastability of Au-Ge liquid nanocatalysts: Ge vapor-liquid-solid nanowire growth far below the bulk eutectic temperature. ACS Nano 2007, 1, 415–422. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, T.; Meng, X. Gallium-assisted growth of InSb nanowire. Rev. Mex. Fis. 2019, 65, 601–606. [Google Scholar] [CrossRef]
- Dao, K.A.; Dao, D.K.; Nguyen, T.D.; Phan, A.T.; Do, H.M. The effects of Au surface diffusion to formation of Au droplets/clusters and nanowire growth on GaAs substrate using VLS method. J. Mater. Sci. Mater. Electron. 2012, 23, 2065–2074. [Google Scholar] [CrossRef]
- Yu, H.K.; Lee, J.L. Growth mechanism of metal-oxide nanowires synthesized by electron beam evaporation: A self-catalytic vapor-liquid-solid process. Sci. Rep. 2014, 4, 6589. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.L.; Zou, L.F.; Schreiber, D.K.; Baer, D.R.; Bruemmer, S.M.; Zhou, G.W.; Wang, C.M. In-situ transmission electron microscopy study of surface oxidation for Ni-10Cr and Ni-20Cr alloys. Scr. Mater. 2016, 114, 129–132. [Google Scholar] [CrossRef]
- Cao, F.; Jia, S.F.; Zheng, H.; Zhao, L.L.; Liu, H.H.; Li, L.; Zhao, L.G.; Hu, Y.M.; Gu, H.S.; Wang, J.B. Thermal-induced formation of domain structures in CuO nanomaterials. Phys. Rev. Mater. 2017, 1, 053401. [Google Scholar] [CrossRef]
- Xu, X.; Dong, B.T.; Ding, S.J.; Xiao, C.H.; Yu, D.M. Hierarchical NiCoO2 nanosheets supported on amorphous carbon nanotubes for high-capacity Lithium-ion batteries with a long cycle life. J. Mater. Chem. A 2014, 2, 13069–13074. [Google Scholar] [CrossRef]
- Wei, Y.H.; Yan, F.L.; Tang, X.; Luo, Y.Z.; Zhang, M.; Wei, W.F.; Chen, L.B. Solvent-Controlled Synthesis of NiO-CoO/Carbon Fiber Nanobrushes with Different Densities and Their Excellent Properties for Lithium Ion Storage. ACS Appl. Mater. Interfaces 2015, 7, 21703–21711. [Google Scholar] [CrossRef]
- Liu, Y.G.; Zhao, Y.Y.; Yu, Y.L.; Ahmad, M.; Sun, H.Y. Facile synthesis of single-crystal mesoporous CoNiO2 nanosheets assembled flowers as anode materials for lithium-ion batteries. Electrochim. Acta 2014, 132, 404–409. [Google Scholar] [CrossRef]
- Cao, K.Z.; Jin, T.; Yang, L.; Jiao, L.F. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]

| Deposited Oxides | Reagent | Co-Reactant | Pulse/Purge Times, Sec t1/t2/t3/t4 | Reagent Temperature, °C | Reactor Temperature, °C | GPC Å/Cycle |
|---|---|---|---|---|---|---|
| NiO | NiCp2 | O2 plasma | 1/10/15/5 | 110 | 300 | 0.12 |
| CoO | CoCp2 | 150 | 0.22 |
| Sample | Ratio of Cycles | Number of Cycles/Supercycles | Ellipsometry, Å | XRR, Å | Calculation, Å | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NiO | CoO | h | GPSC | GPC | h | GPSC | GPC | h | GPSC | GPC | ||
| NO | 1 | 0 | 2308 | 272 | - | 0.12 | 280 | - | 0.12 | - | - | - |
| NCO-5/1 | 5 | 1 | 310/1860 | 284 | 0.92 | 0.15 | 261 | 0.84 | 0.15 | 254 | 0.82 | 0.14 |
| NCO-3/1 | 3 | 1 | 470/1880 | 338 | 0.72 | 0.18 | 300 | 0.64 | 0.16 | 273 | 0.58 | 0.15 |
| NCO-1/1 | 1 | 1 | 900/1800 | 346 | 0.38 | 0.19 | 322 | 0.36 | 0.18 | 306 | 0.34 | 0.17 |
| CO | 0 | 1 | 500 | 110 | - | 0.22 | 174 | - | 0.34 | - | - | - |
| Sample | Sputtering Time, s | C | O | Ni | Co | Ni + Co-O | Ni/Co |
|---|---|---|---|---|---|---|---|
| at.% | |||||||
| NO | 90 | 1.7 | 43.3 | 55.0 | 0 | 55.0 | – |
| NCO-5/1 | 90 | 3.8 | 43.4 | 35.1 | 17.6 | 9.3 | 1.99 |
| NCO-3/1 | 90 | 3.2 | 42.9 | 23.1 | 30.8 | 10.9 | 0.75 |
| NCO-1/1 | 90 | 3.5 | 47.1 | 7.5 | 41.9 | 2.4 | 0.18 |
| CO | 45 | 3.9 | 45.3 | 0 | 50.7 | 5.3 | – |
| Sample | a, Å | Density, g/cm3 | Roughness, nm |
|---|---|---|---|
| NO | 4.161 | 6.60 | 0.63 |
| NCO-5/1 | 4.196 | 6.40 | 1.78 |
| NCO-3/1 | 4.198 | 6.32 | 2.38 |
| NCO-1/1 | 4.232 | 6.19 | 3.23 |
| CO | 4.245 | 6.31 | 1.12 |
| Sample | Thickness on Si, nm (Ellipsometry) | Thickness on Steel, nm (SEM) | Thickness on Steel/Thickness on Si | Inclusion Size on Si, nm | Inclusion Size on Steel, nm |
|---|---|---|---|---|---|
| NO | 27 | 28–30 | 1.1 | - | 15–20 |
| NCO-5/1 | 28 | 33–37 | 1.2 | 10–13 | 10–15 |
| NCO-3/1 | 34 | 42–47 | 1.2 | 10–13 | 10–15 |
| NCO-1/1 | 35 | 56–60 | 1.8 | 25–30 | 20–30 |
| CO | 11 | 32 | 2.9 | - | 25–35 |
| Active Material | Density, g/cm3 | Specific Capacity, mAh/g | Specific Volume Capacity µAh/cm2/µm | Current Density, mA/g|µA/cm2|C-Rate | Link Source |
|---|---|---|---|---|---|
| NiO | 6.72 | 718 | 482.2 | - | Theoretical (Faraday Law) |
| CoO | 6.44 | 715 | 460.7 | - | |
| NiCoO2 | 6.58 * | 717 | 471.4 | - | |
| NiO thin films | 6.6 | 1460 981 | 967 647 | 32|20|0.3 2550|800|33 | This Research |
| NCO-5/1 thin films | 6.40 | 1430 1028 | 917 658 | 32|20|0.3 2550|800|33 | |
| NCO-3/1 thin films | 6.32 | 1442 1010 | 912 639 | 32|20|0.3 2550|800|33 | |
| NCO-1/1 thin films | 6.19 | 1440 1140.4 | 893 706 | 32|20|0.3 2550|800|33 | |
| CoO thin films | 6.31 | 1441.0 990 | 909 625 | 32|20|0.3 2550|800|33 | |
| NiCoO2 Hierarchical mesoporous microspheres | 6.58 * | 845 397 | 555.7 261.0 | 90|-|- 4000|-|- | [7] |
| NiCoO2 nanotubes with Nanosheets | 6.58 * | 1130 300 | 743.5 197.4 | 200|-|- 800|-|- | [71] |
| NiCoO2@CNT1 Composites | 6.58 * | 1150 920 | 756.7 605.4 | 200|-|- 800|-|- | [71] |
| NCO2 carbon fiber nano-brushes | 6.58 * | 1250 300 | 822.5 197.4 | 200|-|- 2000|-|- | [72] |
| NiO–CoO nanosphere | 6.58 * | 1100 | 723.8 | 200|-|- | [72] |
| Mesoporous CoNiO2 hierarchical micro flowers | 6.58 * | 600 200 | 394.8 131.6 | 100|-|- 1000|-|- | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshtyal, Y.; Mitrofanov, I.; Nazarov, D.; Medvedev, O.; Kim, A.; Ezhov, I.; Rumyantsev, A.; Popovich, A.; Maximov, M.Y. Atomic Layer Deposition of Ni-Co-O Thin-Film Electrodes for Solid-State LIBs and the Influence of Chemical Composition on Overcapacity. Nanomaterials 2021, 11, 907. https://doi.org/10.3390/nano11040907
Koshtyal Y, Mitrofanov I, Nazarov D, Medvedev O, Kim A, Ezhov I, Rumyantsev A, Popovich A, Maximov MY. Atomic Layer Deposition of Ni-Co-O Thin-Film Electrodes for Solid-State LIBs and the Influence of Chemical Composition on Overcapacity. Nanomaterials. 2021; 11(4):907. https://doi.org/10.3390/nano11040907
Chicago/Turabian StyleKoshtyal, Yury, Ilya Mitrofanov, Denis Nazarov, Oleg Medvedev, Artem Kim, Ilya Ezhov, Aleksander Rumyantsev, Anatoly Popovich, and Maxim Yu. Maximov. 2021. "Atomic Layer Deposition of Ni-Co-O Thin-Film Electrodes for Solid-State LIBs and the Influence of Chemical Composition on Overcapacity" Nanomaterials 11, no. 4: 907. https://doi.org/10.3390/nano11040907
APA StyleKoshtyal, Y., Mitrofanov, I., Nazarov, D., Medvedev, O., Kim, A., Ezhov, I., Rumyantsev, A., Popovich, A., & Maximov, M. Y. (2021). Atomic Layer Deposition of Ni-Co-O Thin-Film Electrodes for Solid-State LIBs and the Influence of Chemical Composition on Overcapacity. Nanomaterials, 11(4), 907. https://doi.org/10.3390/nano11040907









