Structuring of ZnTiO3/TiO2 Adsorbents for the Removal of Methylene Blue, Using Zeolite Precursor Clays as Natural Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Clay Purification
2.3. Synthesis of the ZnTiO3/TiO2 Semiconductor
2.4. Synthesis of Zeolite from Ecuadorian Clays
2.5. Preparation of Extruded Composites
2.6. Characterization
2.7. Adsorption Studies
2.7.1. Effect of pH
2.7.2. Isotherm Models
2.7.3. Kinetic Models
2.7.4. Reuse of the Adsorbents
3. Results
3.1. Characterization of the Compounds
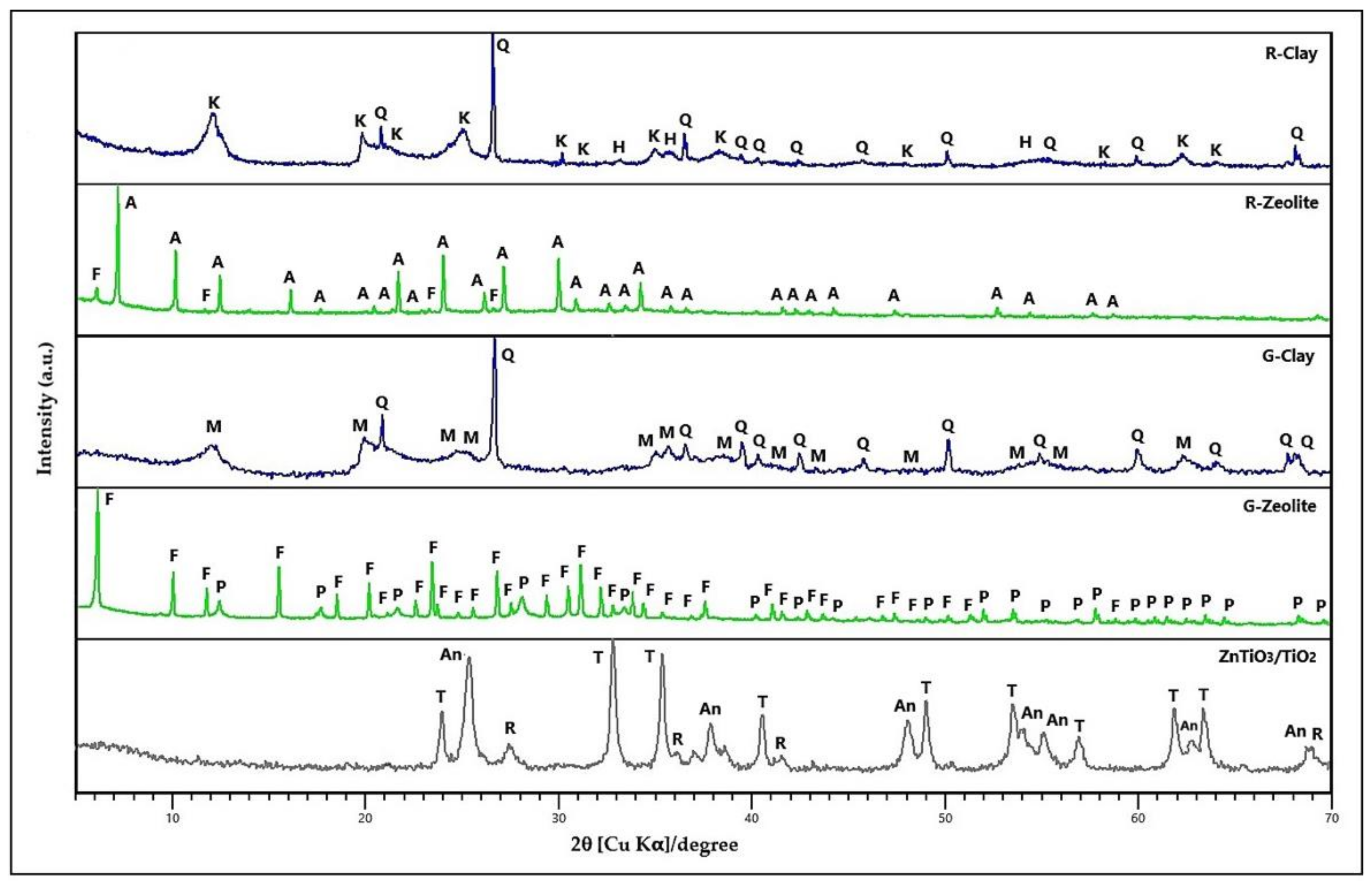
3.1.1. XRD Analysis
3.1.2. SEM-EDX Analysis
3.1.3. XRF Analysis
3.1.4. Specific Surface Area (SSA) Analysis
3.2. Adsorption of MB
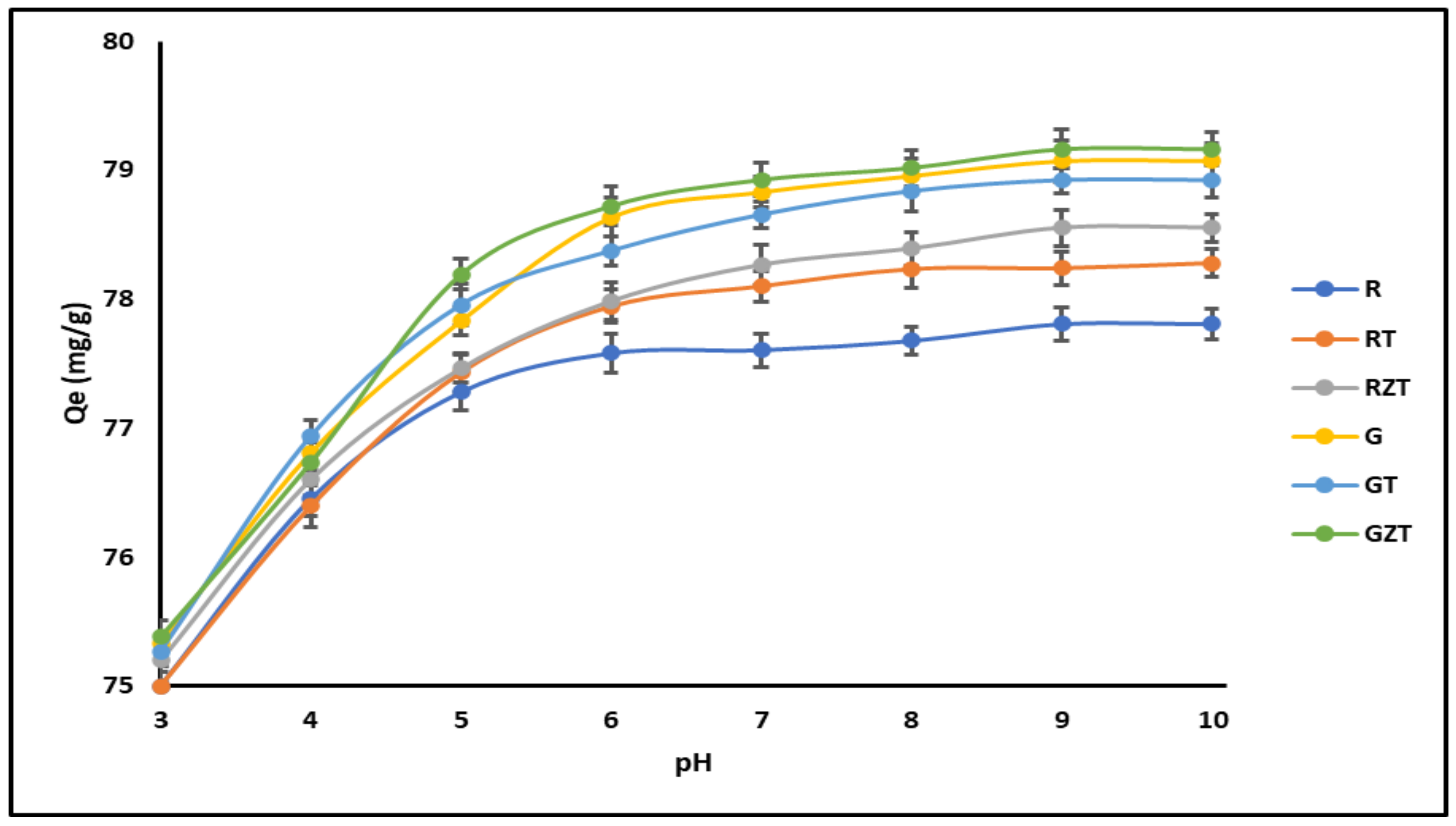
3.2.1. Effect of pH
3.2.2. Adsorption Isotherm
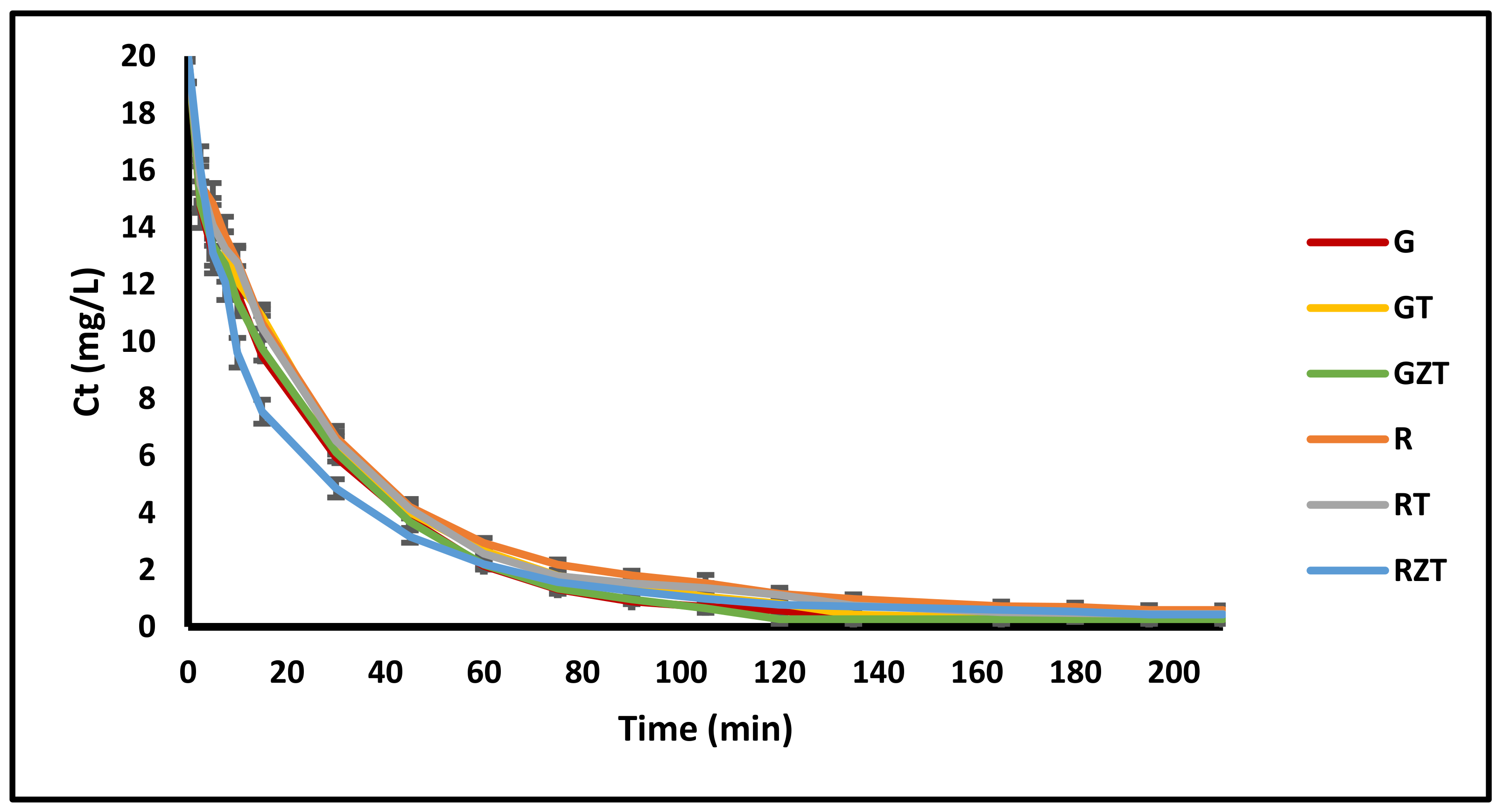
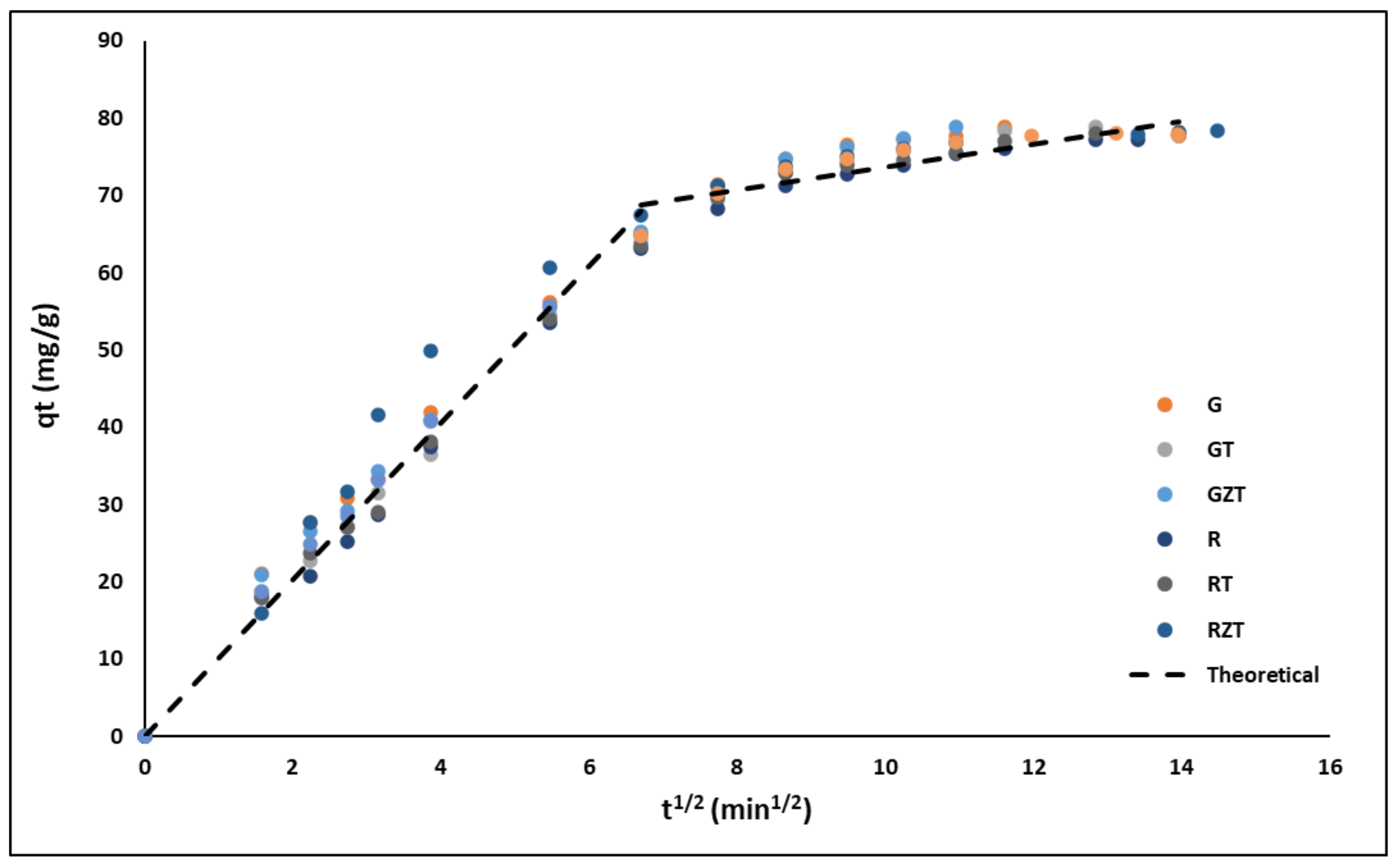
3.2.3. Adsorption Kinetics
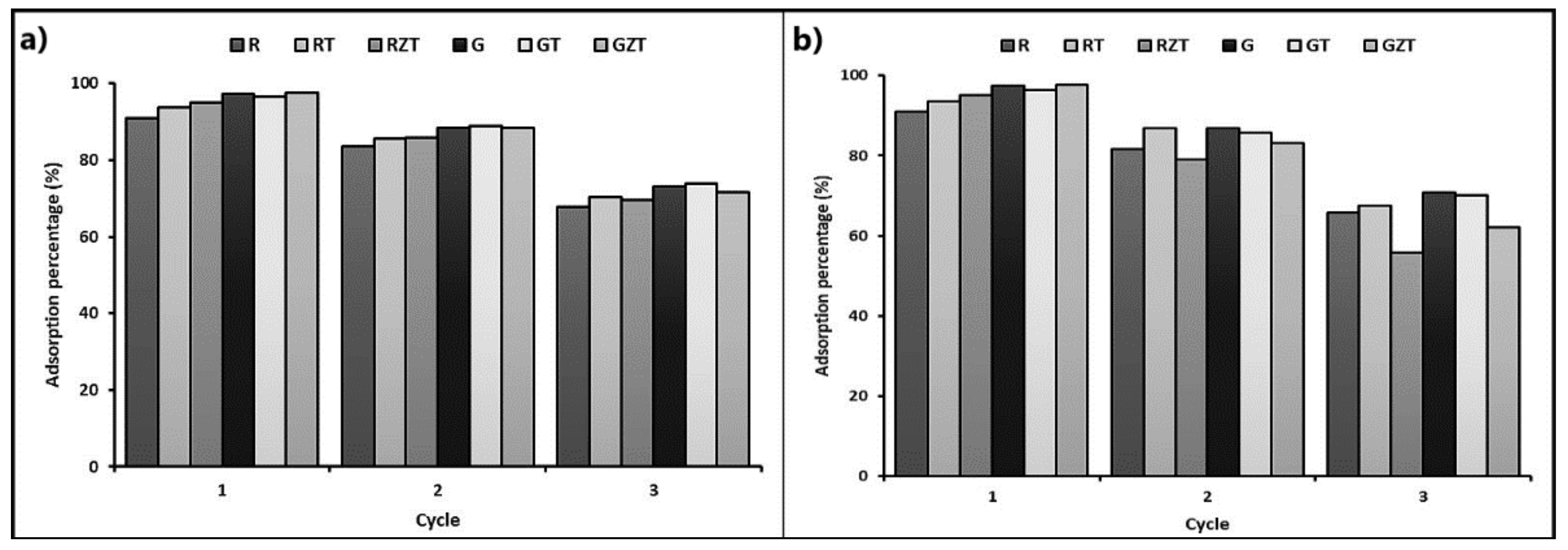
3.3. Reuse of the Adsorbents
4. Discussion
4.1. Synthesis and Characterization of Compounds
4.2. Adsorption of MB
4.2.1. Effect of pH
4.2.2. Adsorption Isotherm
4.2.3. Adsorption Kinetics
4.3. Reuse of the Adsorbents
4.4. MB Adsorption Capacity of the Synthesized Compounds Compared to Other Compounds Described in the Literature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sohrabnezhad, S.; Pourahmad, A. Comparison absorption of new methylene blue dye in zeolite and nanocrystal zeolite. Desalination 2010, 256, 84–89. [Google Scholar] [CrossRef]
- Cigdem, S.O. Adsorption and desorption kinetics behaviour of methylene blue onto activated carbon. Physicochem. Probl. Miner. Process. 2012, 48, 441–454. [Google Scholar] [CrossRef]
- Badeenezhad, A.; Azhdarpoor, A.; Bahrami, S.; Yousefinejad, S. Removal of methylene blue dye from aqueous solutions by natural clinoptilolite and clinoptilolite modified by iron oxide nanoparticles. Mol. Simul. 2019, 45, 564–571. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Hegazey, R.; El-Azabawy, R.E. Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents. J. Mater. Res. Technol. 2019, 8, 5301–5313. [Google Scholar] [CrossRef]
- Hosseini, S.; Khan, M.A.; Malekbala, M.R.; Cheah, W.; Choong, T.S. Carbon coated monolith, a mesoporous material for the removal of methyl orange from aqueous phase: Adsorption and desorption studies. Chem. Eng. J. 2011, 171, 1124–1131. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.; Albeniz, S.; Korili, S. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. J. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Muhammad, M.H.; Ismail, N. Izzati A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process. Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef]
- Isosaari, P.; Srivastava, V.; Sillanpää, M. Ionic liquid-based water treatment technologies for organic pollutants: Current status and future prospects of ionic liquid mediated technologies. Sci. Total Environ. 2019, 690, 604–619. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Jiang, X.; Liu, X.; Zhou, W.; Garba, Z.N.; Lawan, I.; Wang, L.; Yuan, Z. Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J. Clean. Prod. 2021, 284, 124773. [Google Scholar] [CrossRef]
- Liu, L.; Fan, S.; Li, Y. Removal Behavior of Methylene Blue from Aqueous Solution by Tea Waste: Kinetics, Isotherms and Mechanism. Int. J. Environ. Res. Public Health 2018, 15, 1321. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Evrendilek, G.A.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Nyankson, E.; Adjasoo, J.; Efavi, J.K.; Yaya, A.; Manu, G.; Kingsford, A.; Abrokwah, R.Y. Synthesis and kinetic adsorption characteristics of Zeolite/CeO2 nanocomposite. Sci. Afr. 2020, 7, e00257. [Google Scholar] [CrossRef]
- Zhengab, X.; Zhengab, H.; Xionga, Z.; Zhaoa, R.; Liua, Y.; Zhaoab, C.; Zhenga, C. Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem. Eng. J. 2020, 392, 123706. [Google Scholar] [CrossRef]
- Zalani, N.M.; Kaleji, B.K.; Mazinani, B. Synthesis and characterisation of the mesoporous ZnO-TiO2 nanocomposite; Taguchi optimisation and photocatalytic methylene blue degradation under visible light. Mater. Technol. 2019, 35, 281–289. [Google Scholar] [CrossRef]
- Jose, M.; Elakiya, M.; Dhas, S.A.M.B. Structural and optical properties of nanosized ZnO/ZnTiO3 composite materials synthesized by a facile hydrothermal technique. J. Mater. Sci. Mater. Electron. 2017, 28, 13649–13658. [Google Scholar] [CrossRef]
- Chen, F.; Yu, C.; Wei, L.; Fan, Q.; Ma, F.; Zeng, J.; Yi, J.; Yang, K.; Ji, H. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total Environ. 2020, 706, 136026. [Google Scholar] [CrossRef]
- Al-Hajji, L. A Comparative Study on the Zinc Metatitanate Microstructure by Ball Milling and Solvothermal Approaches. J. Struct. Chem. 2019, 60, 830–837. [Google Scholar] [CrossRef]
- Baamran, K.S.; Tahir, M. Ni-embedded TiO2-ZnTiO3 reducible perovskite composite with synergistic effect of metal/support towards enhanced H2 production via phenol steam reforming. Energy Convers. Manag. 2019, 200, 112064. [Google Scholar] [CrossRef]
- Chuaicham, C.; Karthikeyan, S.; Song, J.T.; Ishihara, T.; Ohtani, B.; Sasaki, K. Importance of ZnTiO3 Phase in ZnTi-Mixed Metal Oxide Photocatalysts Derived from Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2020, 12, 9169–9180. [Google Scholar] [CrossRef] [PubMed]
- Surynek, M.; Spanhel, L.; Lapcik, L.; Mrazek, J. Tuning the photocatalytic properties of sol–gel-derived single, coupled, and alloyed ZnO–TiO2 nanoparticles. Res. Chem. Intermed. 2019, 45, 4193–4204. [Google Scholar] [CrossRef]
- Müllerová, J.; Sutta, P.; Medlín, R.; Netrvalová, M.; Novák, P. Optical properties of zinc titanate perovskite prepared by reactive RF sputtering. J. Electr. Eng. 2017, 68, 10–16. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Pérez, S.G.; Jaramillo, H.A.; Cabello, F.M. Synthesis of the ZnTiO3/TiO2 Nanocomposite Supported in Ecuadorian Clays for the Adsorption and Photocatalytic Removal of Methylene Blue Dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef]
- Xue, C.; Wei, X.; Zhang, Z.; Bai, Y.; Li, M.; Chen, Y. Synthesis and Characterization of LSX Zeolite/AC Composite from Elutrilithe. Materials 2020, 13, 3469. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodriguez, J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Wadhwa, S.; Mathur, A.; Pendurthi, R.; Singhal, U.; Khanuja, M.; Roy, S.S. Titania-based porous nanocomposites for potential environmental applications. Bull. Mater. Sci. 2020, 43, 47. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.; Garcia-Matamoros, J.B. Titania–clay heterostructures with solar photocatalytic applications. Appl. Catal. B Environ. 2015, 176–177, 278–287. [Google Scholar] [CrossRef]
- Whiting, G.T.; Chowdhury, A.D.; Oord, R.; Paalanen, P.; Weckhuysen, B.M. The curious case of zeolite–clay/binder interactions and their consequences for catalyst preparation. Faraday Discuss. 2015, 188, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Bingre, R.; Louis, B.; Nguyen, P. An Overview on Zeolite Shaping Technology and Solutions to Overcome Diffusion Limitations. Catalysts 2018, 8, 163. [Google Scholar] [CrossRef]
- Jing, G.; Sun, Z.; Ye, P.; Wei, S.; Liang, Y. Clays for heterogeneous photocatalytic decolorization of wastewaters contaminated with synthetic dyes: A review. Water Pract. Technol. 2017, 12, 432–443. [Google Scholar] [CrossRef]
- Al-Kahlout, A. ZnO nanoparticles and porous coatings for dye-sensitized solar cell application: Photoelectrochemical characterization. Thin Solid Films 2012, 520, 1814–1820. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; Ben Ameur, S.; Da Costa, P.; Ben Zina, M.; Galvez, M.E. Photocatalytic decolorization of cationic and anionic dyes over ZnO nanoparticle immobilized on natural Tunisian clay. Appl. Clay Sci. 2018, 152, 148–157. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for the pH controlled release of drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef]
- Ferraz, E.; Andrejkovičová, S.; Velosa, A.L.; Silva, A.S.; Rocha, F. Synthetic zeolite pellets incorporated to air lime–metakaolin mortars: Mechanical properties. Constr. Build. Mater. 2014, 69, 243–252. [Google Scholar] [CrossRef]
- Akhtar, F.; Andersson, L.; Ogunwumi, S.; Hedin, N.; Bergström, L. Feature Article Structuring adsorbents and catalysts by processing of porous powders. J. Eur. Ceram. Soc. 2014, 34, 1643–1666. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Rozhina, E.; Konnova, S.; Kryuchkova, M.; Khaertdinov, N.; Fakhrullin, R. Organic-nanoclay composite materials as removal agents for environmental decontamination. RSC Adv. 2019, 9, 40553–40564. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Adsorption of methylene blue, crystal violet and congo red from binary and ternary systems with natural clay: Kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198–199, 219–227. [Google Scholar] [CrossRef]
- Youcef, L.D.; Belaroui, L.S.; López-Galindo, A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl. Clay Sci. 2019, 179, 105145. [Google Scholar] [CrossRef]
- Ravi; Pandey, L.M. Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Appl. Clay Sci. 2019, 169, 102–111. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Lackovičová, M.; Baranyaiová, T.; Bujdák, J. The chemical stabilization of methylene blue in colloidal dispersions of smectites. Appl. Clay Sci. 2019, 181, 105222. [Google Scholar] [CrossRef]
- Supelano, G.; Cuaspud, J.G.; Moreno-Aldana, L.C.; Ortiz, C.; Trujillo, C.; Palacio, C.; Vargas, C.P.; Gómez, J.M. Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue. Fuel 2020, 263, 116800. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and fast adsorption of methylene blue dye onto a nanosheet MFI zeolite. J. Solid State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A.; Aly, A.A.; Mohamed, T.Y. A facile synthesis of mordenite zeolite nanostructures for efficient bleaching of crude soybean oil and removal of methylene blue dye from aqueous media. J. Mol. Liq. 2017, 248, 302–313. [Google Scholar] [CrossRef]
- Aysan, H.; Edebali, S.; Ozdemir, C.; Karakaya, M.C.; Karakaya, N. Use of chabazite, a naturally abundant zeolite, for the investigation of the adsorption kinetics and mechanism of methylene blue dye. Microporous Mesoporous Mater. 2016, 235, 78–86. [Google Scholar] [CrossRef]
- Hor, K.Y.; Chee, J.M.C.; Chong, M.N.; Jin, B.; Saint, C.; Poh, P.E.; Aryal, R. Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as low-cost adsorbent of methylene blue dye from wastewater. J. Clean. Prod. 2016, 118, 197–209. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A. Hydrothermal tuning of the morphology and crystallite size of zeolite nanostructures for simultaneous adsorption and photocatalytic degradation of methylene blue dye. J. Mol. Liq. 2017, 242, 364–374. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Soofivand, F.; Sobhani-Nasab, A.; Shakouri-Arani, M.; Faal, A.Y.; Bagheri, S. Synthesis, characterization, and morphological control of ZnTiO3 nanoparticles through sol-gel processes and its photocatalyst application. Adv. Powder Technol. 2016, 27, 2066–2075. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Crystallization and Phase-Transition Characteristics of Sol–Gel-Synthesized Zinc Titanates. Chem. Mater. 2011, 23, 1496–1504. [Google Scholar] [CrossRef]
- Lopez, C.M.; Garcia, A.; Garcia, L.; Casanova, J.; Errico, L.; Ortega, N. Análisis Morfológico De Zeolitas Con Baja Relación Sial Preparadas A Partir De Arcillas Venezolanas. Acta Microsc. 2018, 27, 108–123. [Google Scholar]
- Cordova, C.M.L.; Castillo, J.M.; Valarezo, L.J.R.; Berfon, L.V.G.; Fierro, X.V.J.; López, A.L.G. Zeolitización de arcillas rojas de Ecuador y aplicación en la adsorción de CO2. Ing. Investig. Tecnol. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Bello, M.O.; Abdus-Salam, N.; Adekola, F.A.; Pal, U. Isotherm and kinetic studies of adsorption of methylene blue using activated carbon from ackee apple pods. Chem. Data Collect. 2021, 31, 100607. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Lebedynets, M.; Zbytniewski, R.; Namiesnik, J.; Buszewski, B. Ammonium removal from aqueous solution by natural zeolite, Transcarpathian mordenite, kinetics, equilibrium and column tests. Sep. Purif. Technol. 2005, 46, 155–160. [Google Scholar] [CrossRef]
- Lakiss, L.; Gilson, J.-P.; Valtchev, V.; Mintova, S.; Vicente, A.; Vimont, A.; Bedard, R.; Abdo, S.; Bricker, J. Zeolites in a good shape: Catalyst forming by extrusion modifies their performances. Microporous Mesoporous Mater. 2020, 299, 110114. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Selvam, P. Rational design, synthesis, characterization and catalytic properties of high-quality low-silica hierarchical FAU- and LTA-type zeolites. Sci. Rep. 2018, 8, 16291. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Lin, G.; Zhuang, Q.; Cui, Q.; Wang, H.; Yao, H. Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid. Chin. J. Chem. Eng. 2015, 23, 1768–1773. [Google Scholar] [CrossRef]
- Melo, C.C.A.; Melo, B.L.S.; Angélica, R.S.; Paz, S.P.A. Gibbsite-kaolinite waste from bauxite beneficiation to obtain FAU zeolite: Synthesis optimization using a factorial design of experiments and response surface methodology. Appl. Clay Sci. 2019, 170, 125–134. [Google Scholar] [CrossRef]
- Bunmai, K.; Osakoo, N.; Deekamwong, K.; Kosri, C.; Khemthong, P.; Wittayakun, J. Fast synthesis of zeolite NaP by crystallizing the NaY gel under microwave irradiation. Mater. Lett. 2020, 272, 127845. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; El-Reash, Y.A.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 2021, 401, 123813. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.I. The removal of colour from textile wastewater using whole bacterial cells: A review. Dyes Pigments 2003, 58, 179–196. [Google Scholar] [CrossRef]
- He, P.; Wang, Q.; Fu, S.; Wang, M.; Zhao, S.; Liu, X.; Jiang, Y.; Jia, D.; Zhou, Y. Hydrothermal transformation of geopolymers to bulk zeolite structures for efficient hazardous elements adsorption. Sci. Total Environ. 2021, 767, 144973. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Khalid, H.R.; Lee, H. Synthesis of mesoporous geopolymers containing zeolite phases by a hydrothermal treatment. Microporous Mesoporous Mater. 2016, 229, 22–30. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Huertas, F.J.; Lettino, A.; Ragone, P.; Fiore, S. The crystallisation of zeolite (X- and A-type) from fly ash at 25 °C in artificial sea water. Microporous Mesoporous Mater. 2012, 162, 115–121. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Shoumkova, A.; Stoyanova, V. Zeolites formation by hydrothermal alkali activation of coal fly ash from thermal power station “Maritsa 3”, Bulgaria. Fuel 2013, 103, 533–541. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Mańko, M.; Vittenet, J.; Rodriguez, J.; Cot, D.; Mendret, J.; Brosillon, S.; Makowski, W.; Galarneau, A. Synthesis of binderless zeolite aggregates (SOD, LTA, FAU) beads of 10, 70μm and 1mm by direct pseudomorphic transformation. Microporous Mesoporous Mater. 2013, 176, 145–154. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Salim, C.; Hinode, H. Synthesis of pure Na–X and Na–A zeolite from bagasse fly ash. Microporous Mesoporous Mater. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Porcher, F.; Dusausoy, Y.; Souhassou, M.; LeComte, C. Epitaxial growth of zeolite X on zeolite A and twinning in zeolite A: Structural and topological analysis. Miner. Mag. 2000, 64, 1–8. [Google Scholar] [CrossRef]
- Sanabria, N.; Avila, P.; Yates, M.; Rasmussen, S.; Molina, R.; Moreno, S. Mechanical and textural properties of extruded materials manufactured with AlFe and AlCeFe pillared bentonites. Appl. Clay Sci. 2010, 47, 283–289. [Google Scholar] [CrossRef]
- Grande, C.A.; Águeda, V.I.; Spjelkavik, A.; Blom, R. An efficient recipe for formulation of metal-organic Frameworks. Chem. Eng. Sci. 2015, 124, 154–158. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Fernández, A.G.; Cabeza, L.F.; Gómez, M.A. Influence of thermal treatments on the absorption and thermal properties of a clay mineral support used for shape-stabilization of fatty acids. J. Energy Storage 2021, 36, 102427. [Google Scholar] [CrossRef]
- Strejcová, K.; Tišler, Z.; Svobodová, E.; Velvarská, R. Characterization of Modified Natural Minerals and Rocks for Possible Adsorption and Catalytic Use. Molecules 2020, 25, 4989. [Google Scholar] [CrossRef]
- Elgamouz, A.; Tijani, N. Dataset in the production of composite clay-zeolite membranes made from naturally occurring clay minerals. Data Brief 2018, 19, 2267–2278. [Google Scholar] [CrossRef]
- An, F.; Liu, J.; Xu, Z.; Zheng, S. Efficient removal of three dyes using porous covalent triazine frameworks: Adsorption mechanism and role of pore distribution. Water Sci. Technol. 2020, 82, 3023–3031. [Google Scholar] [CrossRef]
- El-Kousy, S.M.; El-Shorbagy, H.G.; El-Ghaffar, M.A. Chitosan/montmorillonite composites for fast removal of methylene blue from aqueous solutions. Mater. Chem. Phys. 2020, 254, 123236. [Google Scholar] [CrossRef]
- Carrillo, A.M.; Urruchurto, C.M.; Carriazo, J.G.; Moreno, S.; Molina, R.A. Structural and textural characterization of a Colombian halloysite. Rev. Mex. Ing. Quim. 2014, 13, 563–571. [Google Scholar]
- Wang, G.; Li, G.; Huan, Y.; Hao, C.; Chen, W. Acrylic acid functionalized graphene oxide: High-efficient removal of cationic dyes from wastewater and exploration on adsorption mechanism. Chemosphere 2020, 261, 127736. [Google Scholar] [CrossRef]
- Malatji, N.; Makhado, E.; Ramohlola, K.E.; Modibane, K.D.; Maponya, T.C.; Monama, G.R.; Hato, M.J. Synthesis and characterization of magnetic clay-based carboxymethyl cellulose-acrylic acid hydrogel nanocomposite for methylene blue dye removal from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 44089–44105. [Google Scholar] [CrossRef]
- Al-Degs, Y.; El-Barghouthi, M.; El-Sheikh, A.; Walker, G. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Khraisheh, M.; Allen, S.; Ahmad, M. The removal of dyes from textile wastewater: A study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J. Environ. Manag. 2003, 69, 229–238. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, M.; Nishioka, H. Adsorption of methylene blue dye from aqueous solution by novel biomassEucalyptus sheathianabark: Equilibrium, kinetics, thermodynamics and mechanism. Desalination Water Treat. 2016, 57, 5858–5878. [Google Scholar] [CrossRef]
- Shi, J.-W.; Chen, S.-H.; Wang, S.-M.; Ye, Z.-L.; Wu, P.; Xu, B. Favorable recycling photocatalyst TiO2/CFA: Effects of calcination temperature on the structural property and photocatalytic activity. J. Mol. Catal. A Chem. 2010, 330, 41–48. [Google Scholar] [CrossRef]
- Huang, T.; Yan, M.; He, K.; Huang, Z.; Zeng, G.; Chen, A.; Peng, M.; Li, H.; Yuan, L.; Chen, G. Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. J. Colloid Interface Sci. 2019, 543, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, M.-Q.; Shan, X.-Q.; Pei, Z.-G.; Chen, Z. Adsorption of methylene blue and orange II onto unmodified and surfactant-modified zeolite. J. Colloid Interface Sci. 2008, 328, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-S.; Liang, R.; Sun, G. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Varmazyar, A.; Sedaghat, S.; Khalaj, M. Highly efficient removal of methylene blue by a synthesized TiO2/montmorillonite-albumin nanocomposite: Kinetic and isothermal analysis in water. RSC Adv. 2017, 7, 37214–37219. [Google Scholar] [CrossRef]
- Marsiezade, N.; Javanbakht, V. Novel hollow beads of carboxymethyl cellulose/ZSM-5/ZIF-8 for dye removal from aqueous solution in batch and continuous fixed bed systems. Int. J. Biol. Macromol. 2020, 162, 1140–1152. [Google Scholar] [CrossRef]
- Anantha, M.; Olivera, S.; Hu, C.; Jayanna, B.; Reddy, N.; Venkatesh, K.; Muralidhara, H.; Naidu, R. Comparison of the photocatalytic, adsorption and electrochemical methods for the removal of cationic dyes from aqueous solutions. Environ. Technol. Innov. 2020, 17, 100612. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Guo, D.-X.; Li, S.-F.; Cao, J.-P.; Wei, X.-Y. Removal of methylene blue by NaX zeolites synthesized from coal gasification fly ash using an alkali fusion-hydrothermal method. Desalination Water Treat. 2020, 185, 355–363. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Reghioua, A.; Yaseen, Z.M. Zwitterion composite chitosan-epichlorohydrin/zeolite for adsorption of methylene blue and reactive red 120 dyes. Int. J. Biol. Macromol. 2020, 163, 756–765. [Google Scholar] [CrossRef]
- Xu, R.; Mao, J.; Peng, N.; Luo, X.; Chang, C. Chitin/clay microspheres with hierarchical architecture for highly efficient removal of organic dyes. Carbohydr. Polym. 2018, 188, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Bée, A.; Obeid, L.; Mbolantenaina, R.; Welschbillig, M.; Talbot, D. Magnetic chitosan/clay beads: A magsorbent for the removal of cationic dye from water. J. Magn. Magn. Mater. 2017, 421, 59–64. [Google Scholar] [CrossRef]
- Marrakchi, F.; Bouaziz, M.; Hameed, B. Activated carbon–clay composite as an effective adsorbent from the spent bleaching sorbent of olive pomace oil: Process optimization and adsorption of acid blue 29 and methylene blue. Chem. Eng. Res. Des. 2017, 128, 221–230. [Google Scholar] [CrossRef]
- Badri, A.; Alvarez-Serrano, I.; López, M.L.; Ben Amara, M. Sol-gel synthesis, magnetic and methylene blue adsorption properties of lamellar iron monophosphate KMgFe(PO4)2. Inorg. Chem. Commun. 2020, 121, 108217. [Google Scholar] [CrossRef]
- Woolard, C.; Strong, J.; Erasmus, C. Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams. Appl. Geochem. 2002, 17, 1159–1164. [Google Scholar] [CrossRef]
- El-Mekkawi, D.M.; Ibrahim, F.A.; Selim, M.M. Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. J. Environ. Chem. Eng. 2016, 4, 1417–1422. [Google Scholar] [CrossRef]
- Ge, S.; Geng, W.; He, X.; Zhao, J.; Zhou, B.; Duan, L.; Wu, Y.; Zhang, Q. Effect of framework structure, pore size and surface modification on the adsorption performance of methylene blue and Cu2+ in mesoporous silica. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 154–162. [Google Scholar] [CrossRef]
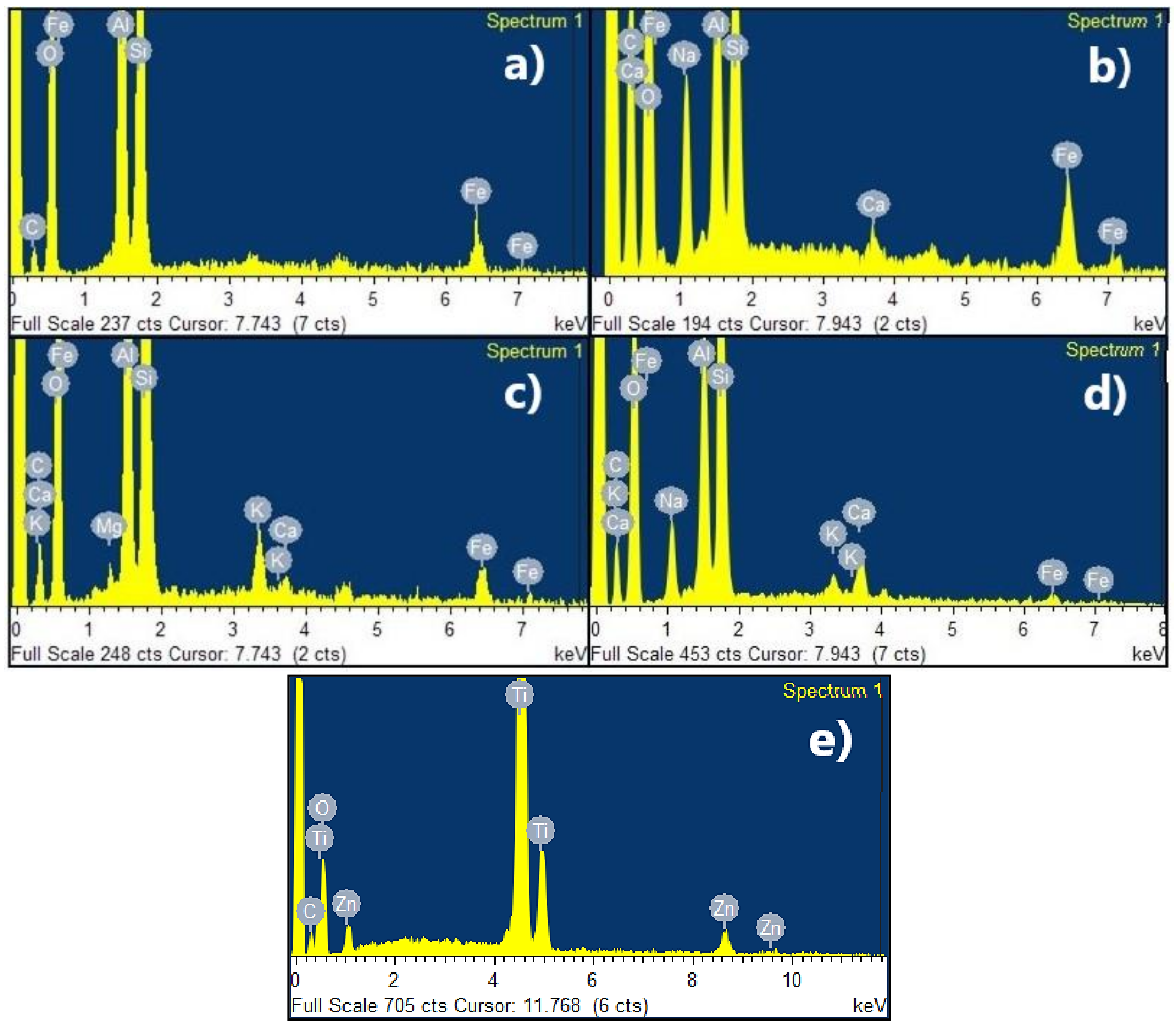



| C | O | Si | Al | Fe | Na | Ca | K | Mg | Ti | Zn | O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R-Clay | 12.10 | 47.71 | 19.08 | 15.40 | 5.72 | - | - | - | - | - | - | - |
| R-Zeolite | 32.43 | 47.89 | 6.49 | 5.01 | 3.91 | 3.79 | 0.48 | - | - | - | - | - |
| G-Clay | 16.99 | 46.46 | 18.17 | 12.22 | 3.27 | - | 0.54 | 1.84 | 0.53 | - | - | - |
| G-Zeolite | 20.77 | 50.91 | 14.44 | 6.77 | 1.88 | 2.70 | 1.88 | 0.65 | - | - | - | - |
| ZnTiO3/TiO2 | 5.42 | - | - | - | - | - | - | - | - | 54.85 | 6.13 | 33.60 |
| Compound | Al2O3 | SiO2 | K2O | CaO | TiO2 | MgO | Fe2O3 | ZnO |
|---|---|---|---|---|---|---|---|---|
| G-Clay | 21.20 (±0.85) | 45.60 (±0.68) | 1.63 (±0.03) | 0.22 (±0.01) | 0.39 (±0.01) | 0.16 (±0.00) | 1.94 (±0.01) | 0.01 (±0.00) |
| G-Zeolite | 19.60 (±0.79) | 41.30 (±0.63) | 0.49 (±0.02) | 4.11 (±0.02) | 0.19 (±0.01) | 0.07 (±0.00) | 0.70 (±0.01) | 0.06 (±0.00) |
| GT | 11.60 (±1.56) | 12.20 (±0.53) | 0.32 (±0.03) | 1.59 (±0.02) | 51.30 (±0.13) | 0.04 (±0.01) | 3.64 (±0.03) | 18.95 (±0.05) |
| GZT | 18.00 (±1.20) | 34.60 (±0.68) | 1.58 (±0.04) | 2.67 (±0.03) | 28.10 (±0.08) | 0.07 (±0.01) | 2.38 (±0.02) | 12.29 (±0.03) |
| R-Clay | 27.10 (±0.98) | 39.40 (±0.65) | 0.78 (±0.02) | 0.12 (±0.01) | 1.01 (±0.01) | 0.09 (±0.00) | 7.81 (±0.02) | 0.01 (±0.00) |
| R-Zeolite | 18.60 (±0.99) | 27.60 (±0.59) | - | 1.52 (±0.02) | 1.09 (±0.01) | 0.08 (±0.00) | 6.72 (±0.02) | 0.08 (±0.00) |
| RT | 8.88 (±1.62) | 13.90 (±0.58) | 1.19 (±0.04) | 1.69 (±0.02) | 51.40 (±0.14) | - | 2.09 (±0.03) | 20.66 (±0.06) |
| RZT | 10.10 (±1.31) | 15.60 (±0.58) | 0.38 (±0.59) | 2.20 (±0.50) | 31.30 (±1.82) | 0.06 (±0.16) | 7.87 (±0.69) | 16.30 (±0.07) |
| Adsorbent | Composition | Form | SSA (m2/g) |
|---|---|---|---|
| R-Clay | Red clay | Powder | 48.8 |
| R-Clay (R) | Red clay | Extrudate | 29.3 |
| R-Zeolite | LTA/FAU zeolites | Powder | 104 |
| R-Composite | Red clay + ZnTiO3/TiO2 | Powder | 82.5 |
| R-Composite (RT) | Red clay + ZnTiO3/TiO2 | Extrudate | 29.5 |
| R-Composite | Red clay + LTA/FAU zeolites + ZnTiO3/TiO2 | Powder | 84.1 |
| R-Composite (RZT) | Red clay + LTA/FAU zeolites + ZnTiO3/TiO2 | Extrudate | 31.1 |
| G-Clay | Gray clay | Powder | 42.8 |
| G-Clay (G) | Gray clay | Extrudate | 25.5 |
| G-Zeolite | FAU/Na-P1 zeolites | Powder | 349 |
| G-Composite | Gray clay + ZnTiO3/TiO2 | Powder | 184 |
| G-Composite (GT) | Gray clay + ZnTiO3/TiO2 | Extrudate | 25.8 |
| G-Composite | Gray clay + FAU/Na-P1 zeolites + ZnTiO3/TiO2 | Powder | 188 |
| G-Composite (GZT) | Gray clay + FAU/Na-P1 zeolites + ZnTiO3/TiO2 | Extrudate | 26.4 |
| Isotherm Parameters | R | RT | RZT | G | GT | GZT | |
|---|---|---|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 29.14 (±0.97) | 37.59 (±0.98) | 42.00 (±1.16) | 45.88 (±1.65) | 43.40 (±1.33) | 49.81 (±1.33) |
| KL (L mg−1) | 0.82 (±0.15) | 1.00 (±0.14) | 0.77 (±0.11) | 0.56 (±0.09) | 0.99 (±0.17) | 0.43 (±0.05) | |
| RL | 0.06 | 0.05 | 0.06 | 0.08 | 0.05 | 0.10 | |
| χ2 | 2.00 | 2.11 | 2.34 | 3.36 | 3.38 | 1.60 | |
| R2 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |
| Freundlich | KF (L mg−1) | 11.98 (±0.92) | 15.88 (±1.46) | 16.16 (±1.55) | 15.26 (±1.62) | 18.80 (±1.58) | 16.12 (±1.59) |
| n | 3.30 (±0.32) | 3.25 (±0.39) | 2.96 (±0.34) | 2.62 (±0.30) | 3.30 (±0.37) | 2.66 (±0.29) | |
| 1/n | 0.30 | 0.31 | 0.34 | 0.38 | 0.30 | 0.38 | |
| χ2 | 3.81 | 9.84 | 11.10 | 12.40 | 11.50 | 11.10 | |
| R2 | 0.97 | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | |
| Kinetic Parameters | R | RT | RZT | G | GT | GZT | |
|---|---|---|---|---|---|---|---|
| Pseudo-first-order | qmax (mg g−1) | 75.47 (±1.15) | 75.84 (±1.35) | 75.50 (±1.04) | 76.43 (±1.73) | 76.57 (±1.42) | 76.37 (±1.87) |
| k1 (L mg−1) | 0.05 (±3.28 × 10–3) | 0.05 (±3.90 × 10–3) | 0.07 (±4.73 × 10–3) | 0.06 (±5.75 × 10–3) | 0.05 (±3.99 × 10–3) | 0.06 (±5.75 × 10–3) | |
| χ2 | 11.20 | 14.30 | 10.10 | 22.60 | 15.50 | 22.60 | |
| R2 | 0.98 | 0.98 | 0.98 | 0.97 | 0.98 | 0.97 | |
| Pseudo-second-order | qmax (mg g−1) | 85.45 (±1.20) | 85.90 (±1.41) | 82.92 (±0.48) | 85.99 (±1.64) | 87.01 (±1.44) | 87.16 (±1.84) |
| k2 (L mg−1) | 6.89 × 10–4 (±5.29 × 10–5) | 7.19 × 10–4 (±6.31 × 10–5) | 1.16 × 10–3 (±3.97 × 10–5) | 8.66 × 10–4 (±8.82 × 10–5) | 6.97 × 10–4 (±6.09 × 10–5) | 8.02 × 10–4 (±8.57 × 10–5) | |
| χ2 | 5.04 | 6.45 | 1.14 | 8.78 | 6.40 | 8.74 | |
| R2 | 0.99 | 0.99 | 1.00 | 0.99 | 0.99 | 0.99 | |
| Intraparticle diffusion | k3 (mg g−1 min−1/2) | 5.50 (±0.40) | 5.72 (±0.28) | 4.92 (±0.36) | 6.61 (±0.29) | 6.28 (±0.26) | 7.10 (±0.79) |
| A | 13.58 (±1.75) | 13.55 (±1.25) | 21.13 (±1.57) | 12.47 (±1.27) | 11.35 (±1.16) | 10.34 (±3.49) | |
| R2 | 0.90 | 0.90 | 0.81 | 0.94 | 0.93 | 0.95 | |
| External-film diffusion | Df (m2 min−1) | 1.10 × 10–11 | 1.21 × 10–11 | 1.13 × 10–11 | 1.38 × 10–11 | 1.25 × 10–11 | 1.42 × 10–11 |
| R2 | 0.99 | 0.98 | 0.99 | 0.99 | 0.98 | 0.99 | |
| Internal-pore diffusion | Dp (m2 min−1) | 1.3 × 10–17 | 1.4 × 10–17 | 1.3 × 10–17 | 1.6 × 10–17 | 1.4 × 10–17 | 1.6 × 10–17 |
| R2 | 0.98 | 0.97 | 0.98 | 0.98 | 0.97 | 0.98 | |
| Material | qe (mg g–1) | References |
|---|---|---|
| R | 85.45 | This study |
| RT | 85.90 | This study |
| RZT | 82.92 | This study |
| G | 85.99 | This study |
| GT | 87.01 | This study |
| GZT | 87.16 | This study |
| Magnetic graphene oxide modified zeolite | 97.35 | [91] |
| Raw zeolite | 6.10 | [92] |
| Activated lignin-chitosan composite extrudates | 36.25 | [93] |
| Poly (dopamine hydrochloride) (PDA) microspheres | 90.70 | [94] |
| TiO2/montmorillonite-albumin nanocomposite | 18.18 | [95] |
| Carboxymethyl cellulose/ZSM-5/ZIF-8 | 10.49 | [96] |
| TiO2 nanoparticles | 88.10 | [97] |
| ZSM-5 zeolite | 105.82 | [48] |
| NaX zeolite | 127.13 | [98] |
| Chitosan-epichlorohydrin/zeolite | 156.10 | [99] |
| Chitin/clay microspheres | 152.20 | [100] |
| Magnetic chitosan/clay beads | 82.00 | [101] |
| Activated carbon-clay composite | 178.64 | [102] |
| KMgFe(PO4)2 | 22.83 | [103] |
| Hydroxysodalite | 10.82 | [104] |
| Kaolin | 21.41 | [105] |
| Nonporous silica | 91.10 | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Fierro, X.; González, S.; Montesdeoca-Mendoza, F.; Medina, F. Structuring of ZnTiO3/TiO2 Adsorbents for the Removal of Methylene Blue, Using Zeolite Precursor Clays as Natural Additives. Nanomaterials 2021, 11, 898. https://doi.org/10.3390/nano11040898
Jaramillo-Fierro X, González S, Montesdeoca-Mendoza F, Medina F. Structuring of ZnTiO3/TiO2 Adsorbents for the Removal of Methylene Blue, Using Zeolite Precursor Clays as Natural Additives. Nanomaterials. 2021; 11(4):898. https://doi.org/10.3390/nano11040898
Chicago/Turabian StyleJaramillo-Fierro, Ximena, Silvia González, Fernando Montesdeoca-Mendoza, and Francesc Medina. 2021. "Structuring of ZnTiO3/TiO2 Adsorbents for the Removal of Methylene Blue, Using Zeolite Precursor Clays as Natural Additives" Nanomaterials 11, no. 4: 898. https://doi.org/10.3390/nano11040898
APA StyleJaramillo-Fierro, X., González, S., Montesdeoca-Mendoza, F., & Medina, F. (2021). Structuring of ZnTiO3/TiO2 Adsorbents for the Removal of Methylene Blue, Using Zeolite Precursor Clays as Natural Additives. Nanomaterials, 11(4), 898. https://doi.org/10.3390/nano11040898









