Hybrid Silica Materials Applied for Fuchsine B Color Removal from Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of the Platinum Colloid
2.3. Catalyzed Sol-Gel Method in Acid/Base Two Steps for Obtaining Silica Hybrid Material
2.4. Nitrogen Porosimetry
2.5. Small Angle Neutron Scattering (SANS)
2.6. Scanning Electron Microscopy
2.7. Atomic Force Microscopy
2.8. UV-VIS Spectroscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characterization
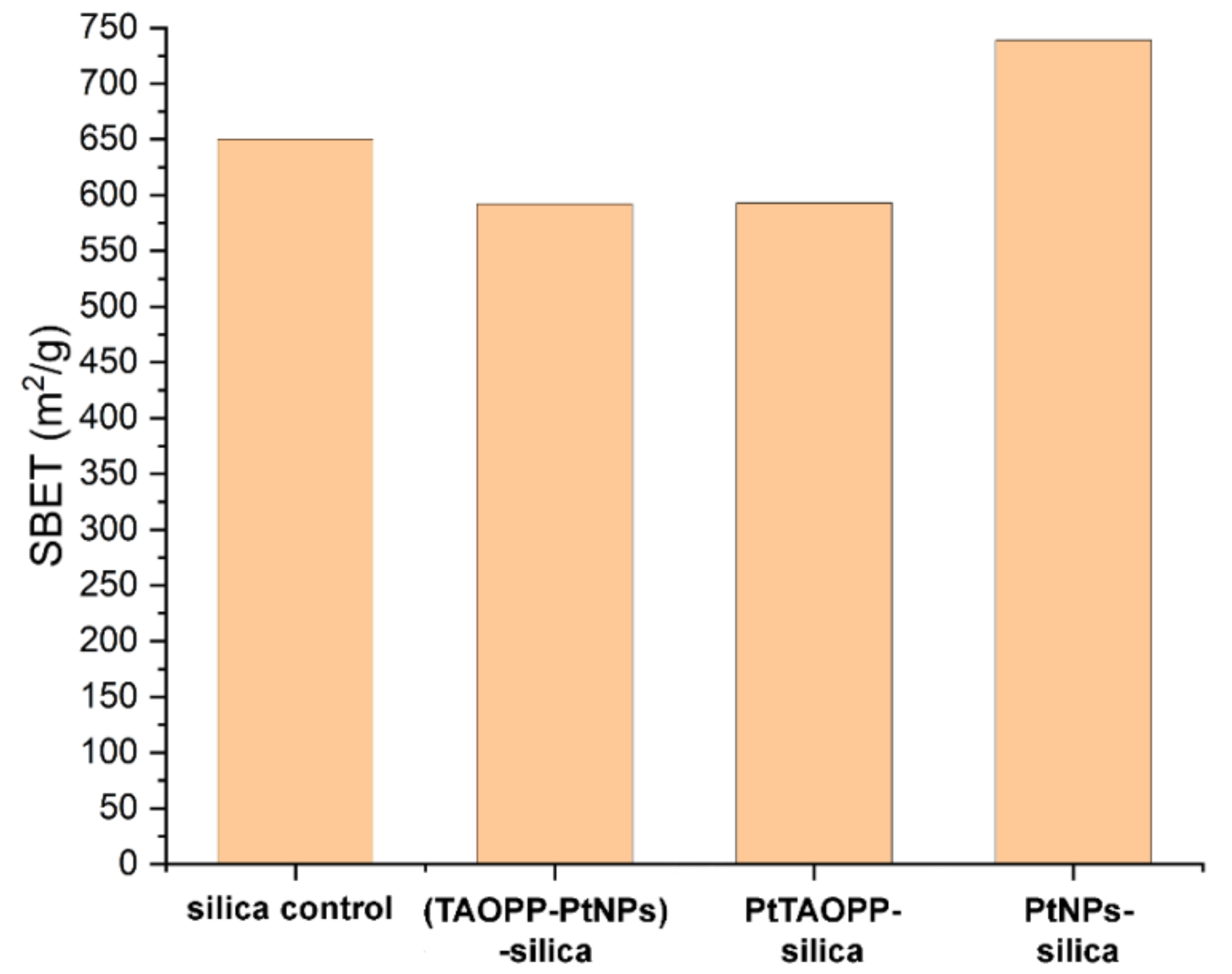
3.1.1. Nitrogen Porosimetry
3.1.2. Small Angle Neutron Scattering (SANS)
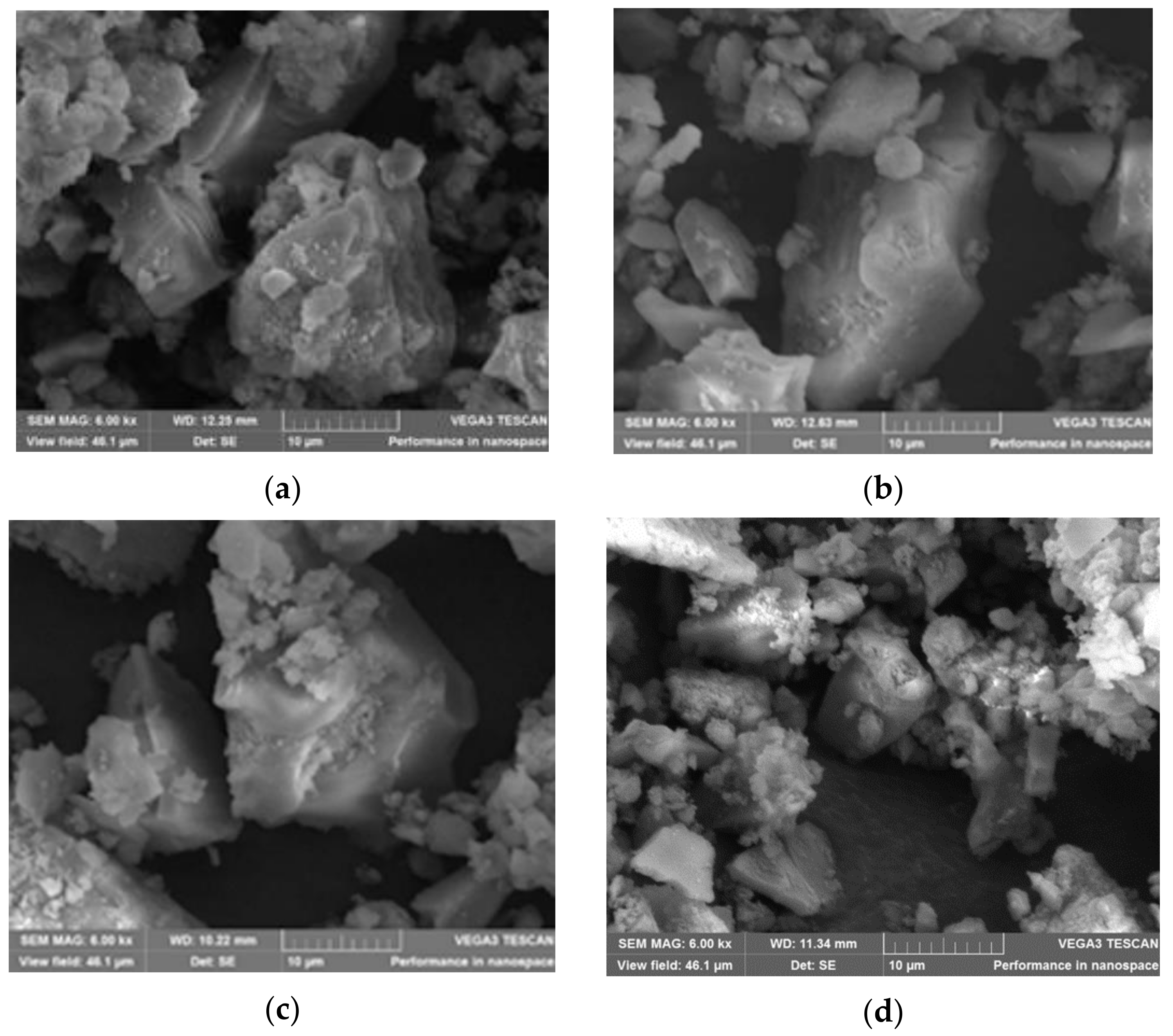
3.1.3. SEM Characterization of the Hybrid Silica Materials after Fuchsine B Adsorption
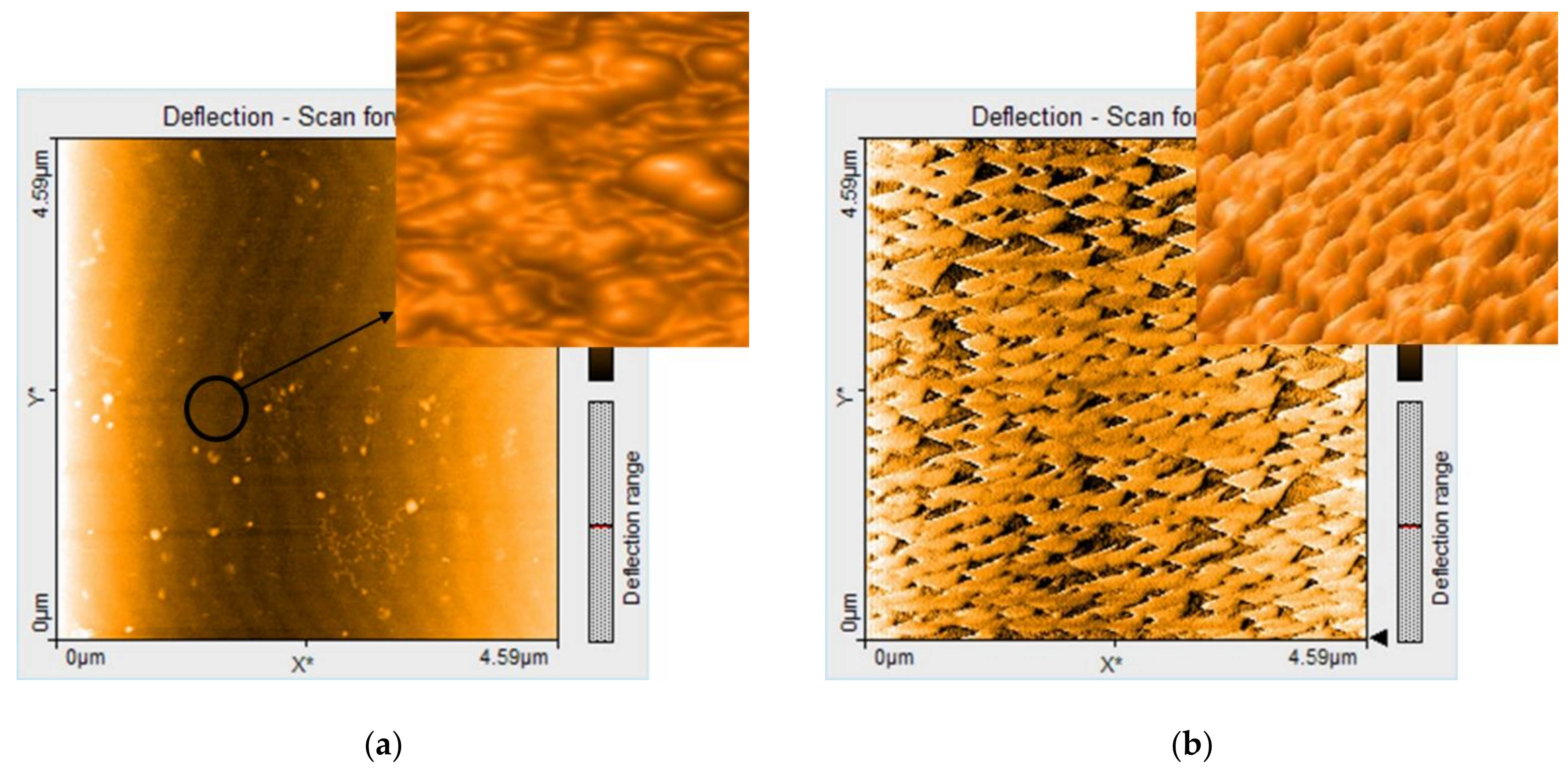
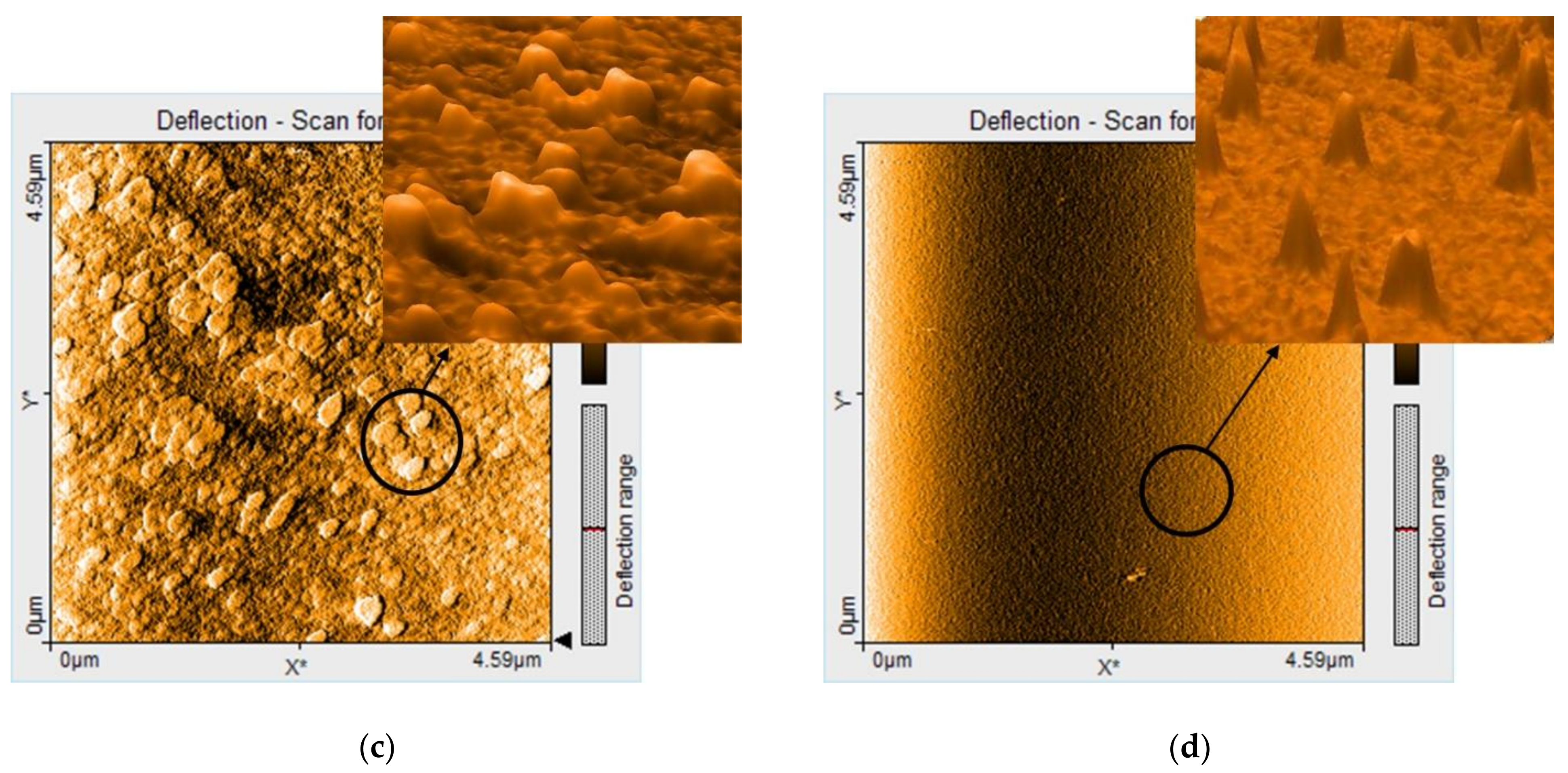
3.1.4. AFM Investigation of the Hybrid Silica Materials after Fuchsine B Adsorption
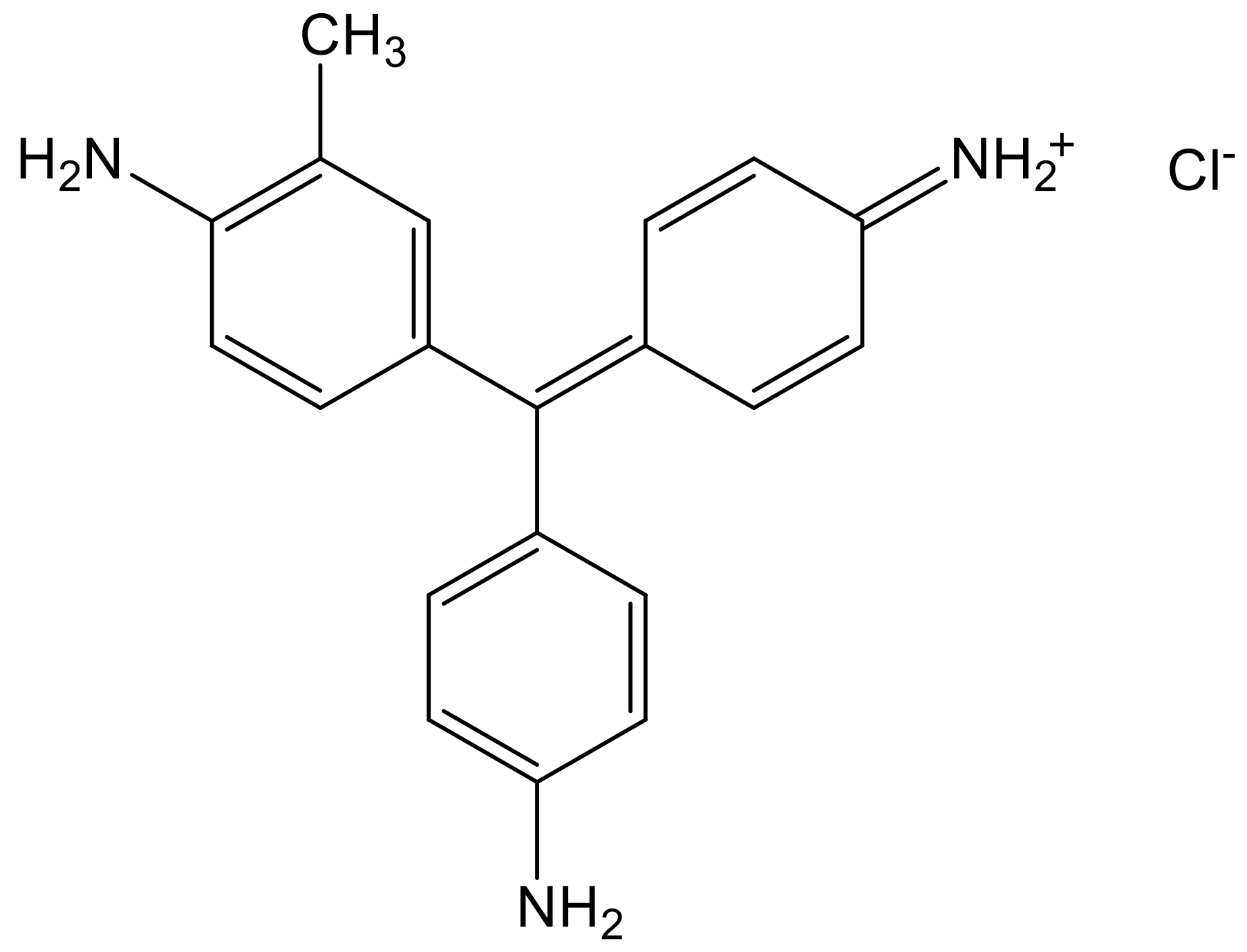
3.2. Adsorption of Fuchsine B
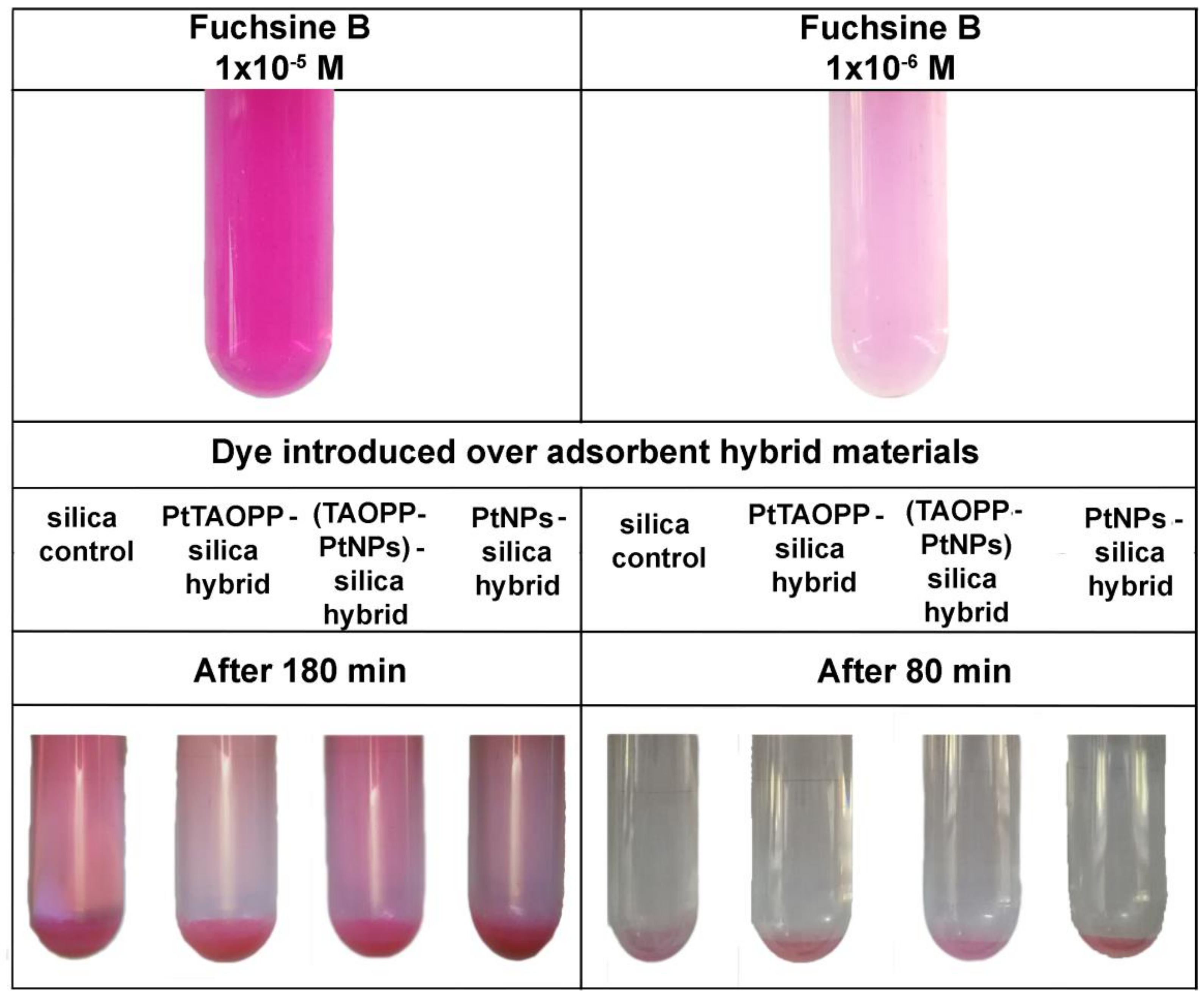
3.2.1. Testing of Impregnated-Silica Hybrid Materials and of Silica Control for Fuchsine B Removal from Wastewaters
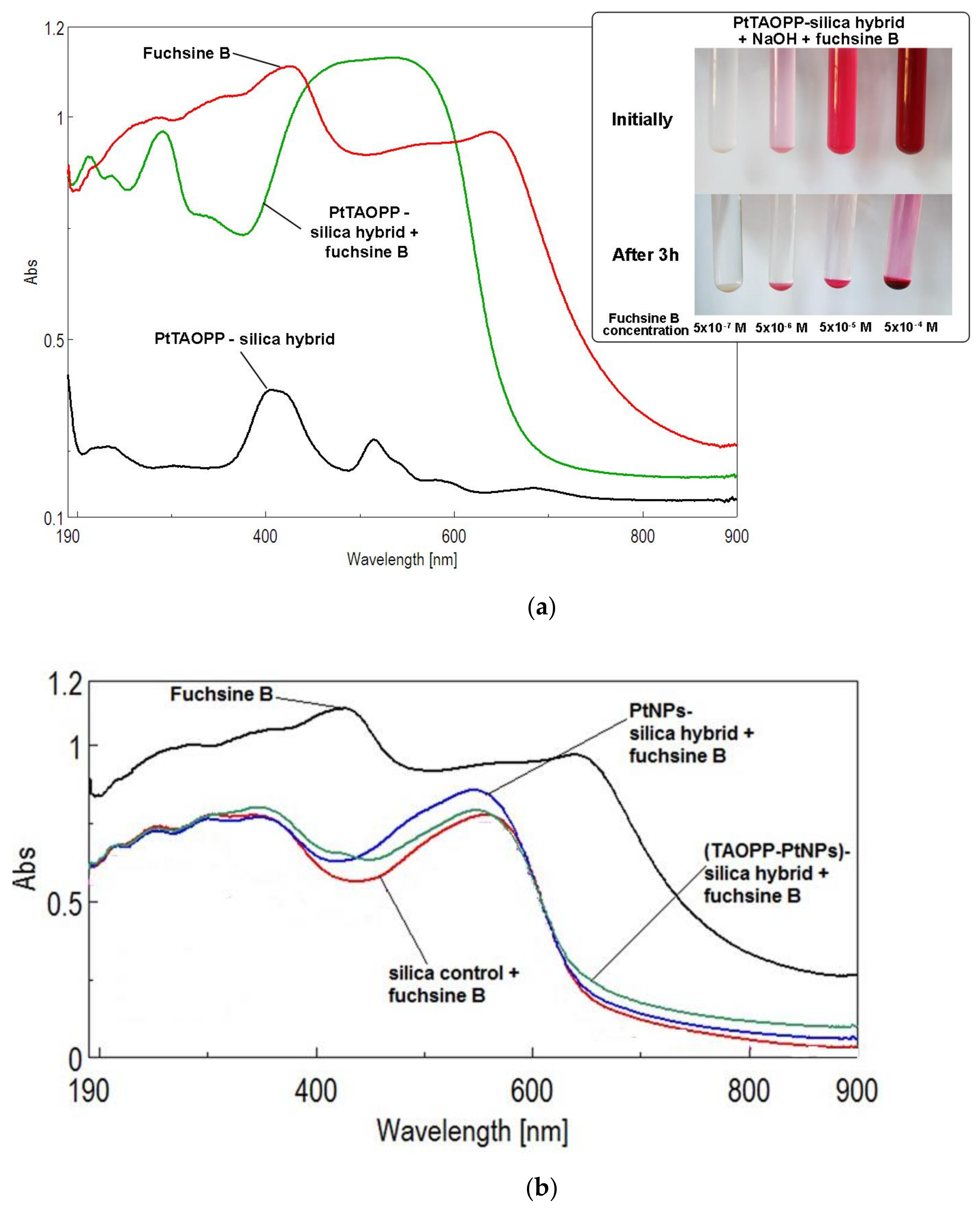
3.2.2. Method for Adsorption of Fuchsine B from Wastewaters, Monitored by Solid UV-Vis Spectra
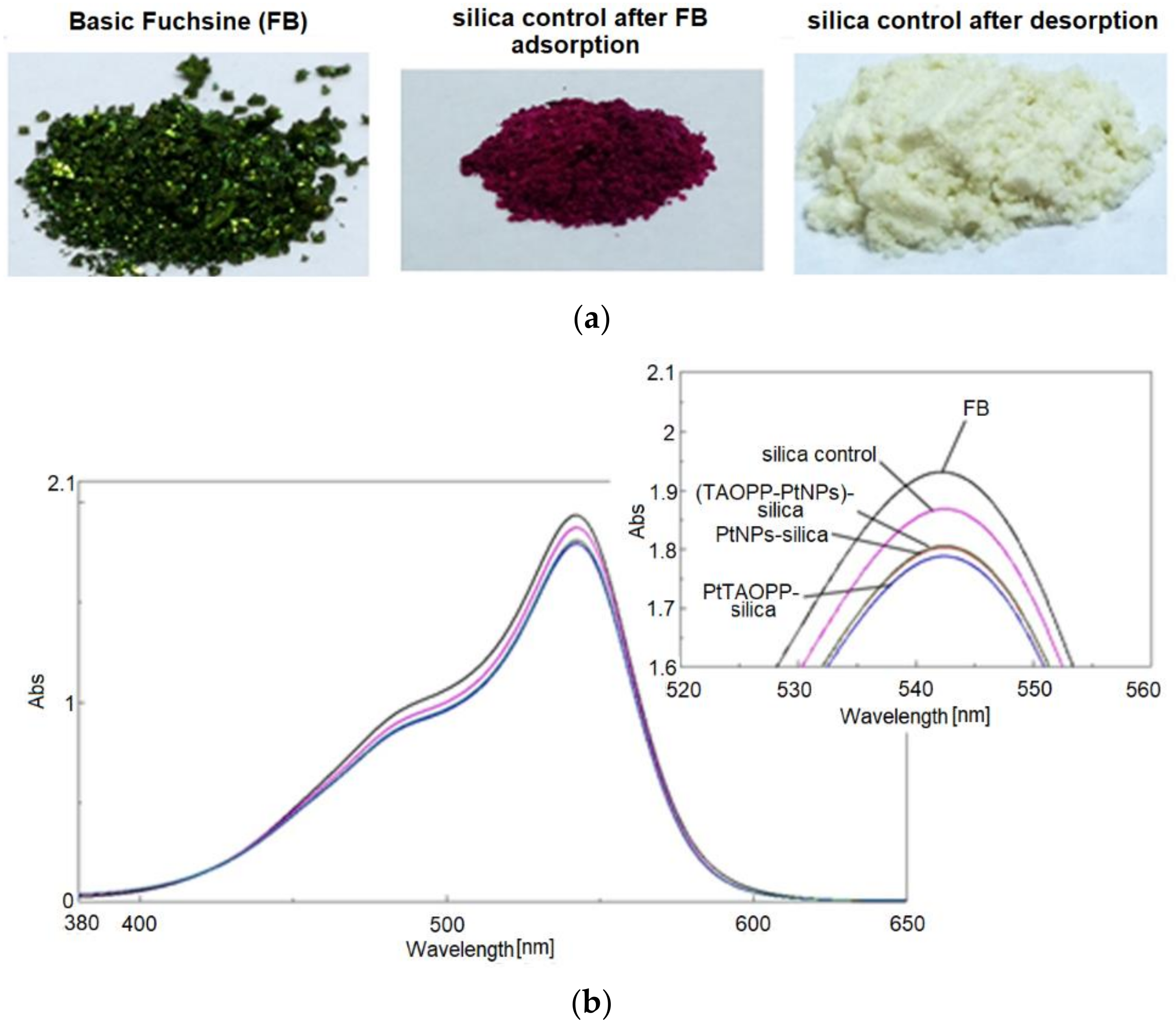
3.2.3. Adsorption of Fuchsine B from Wastewaters, Monitored by UV-Vis-Spectra in Water
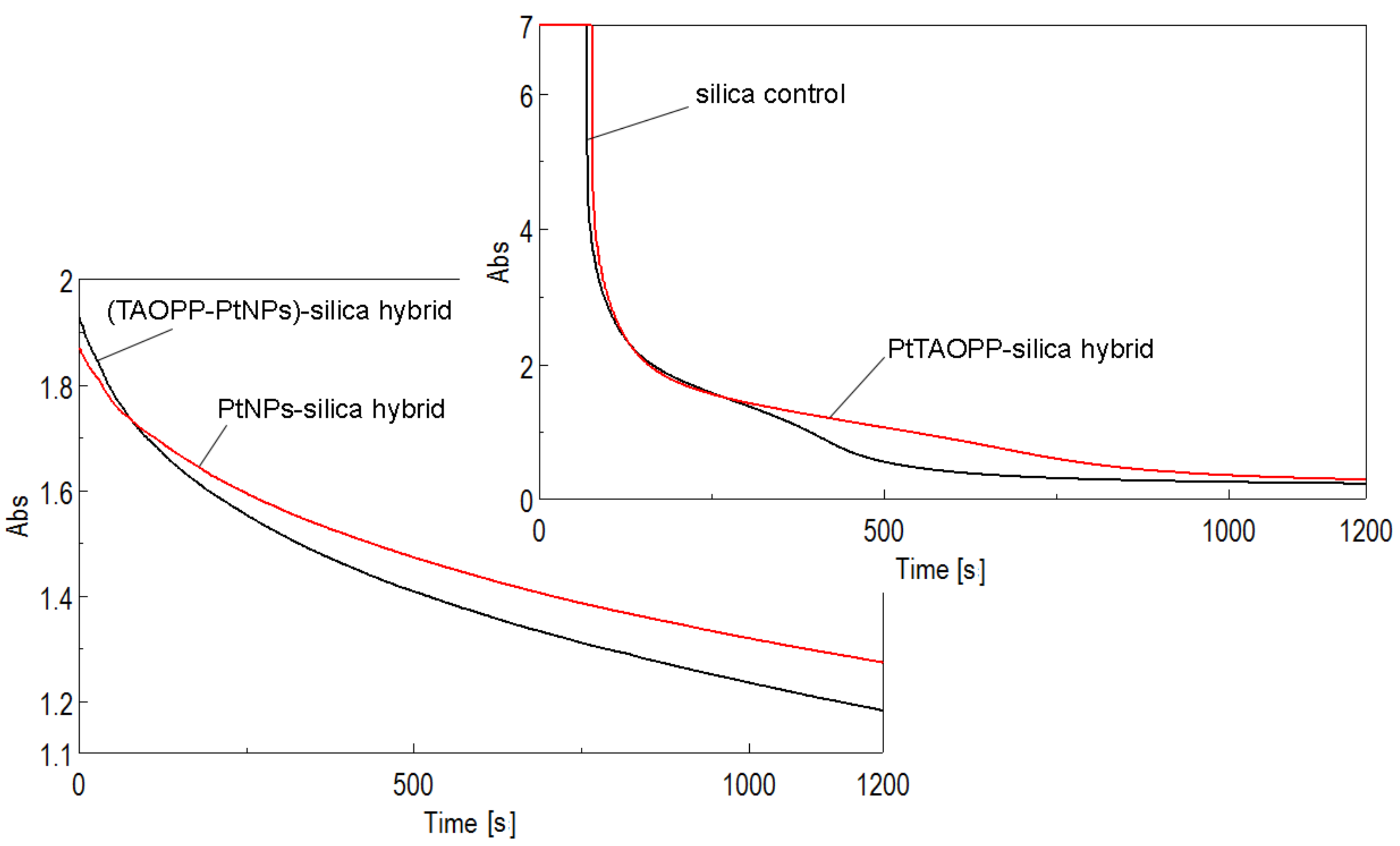
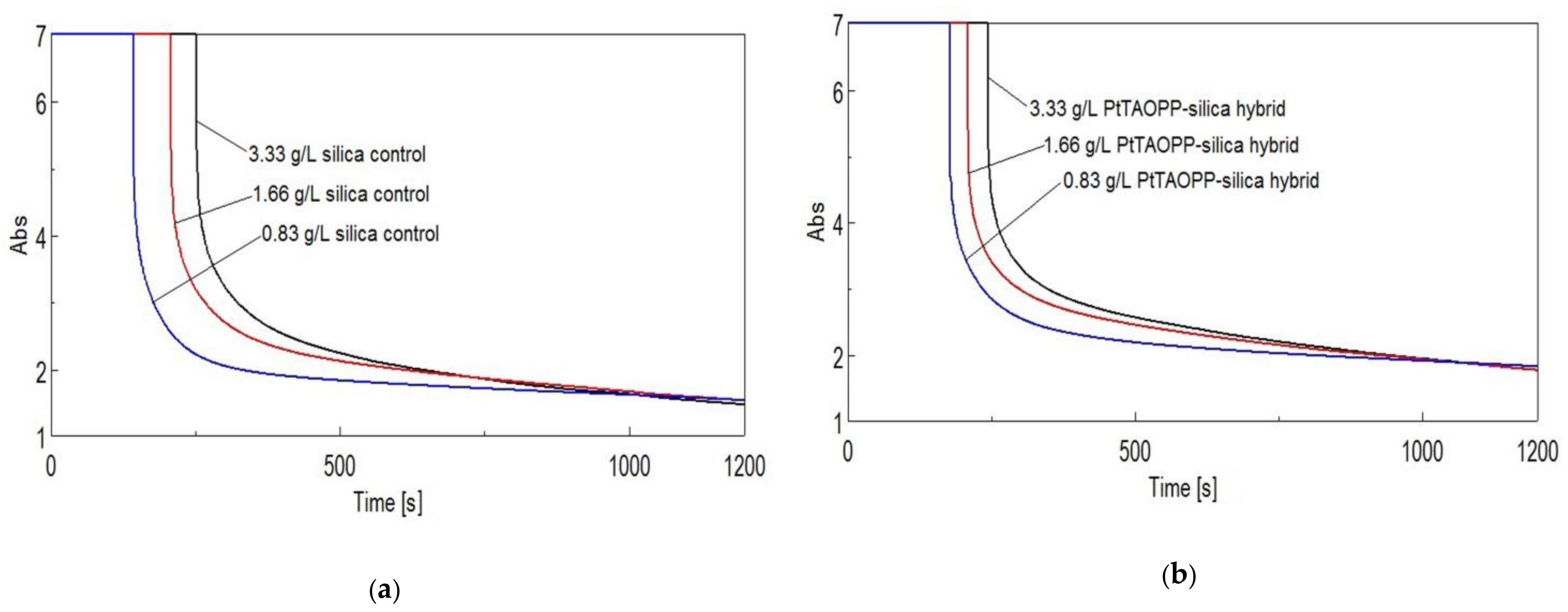
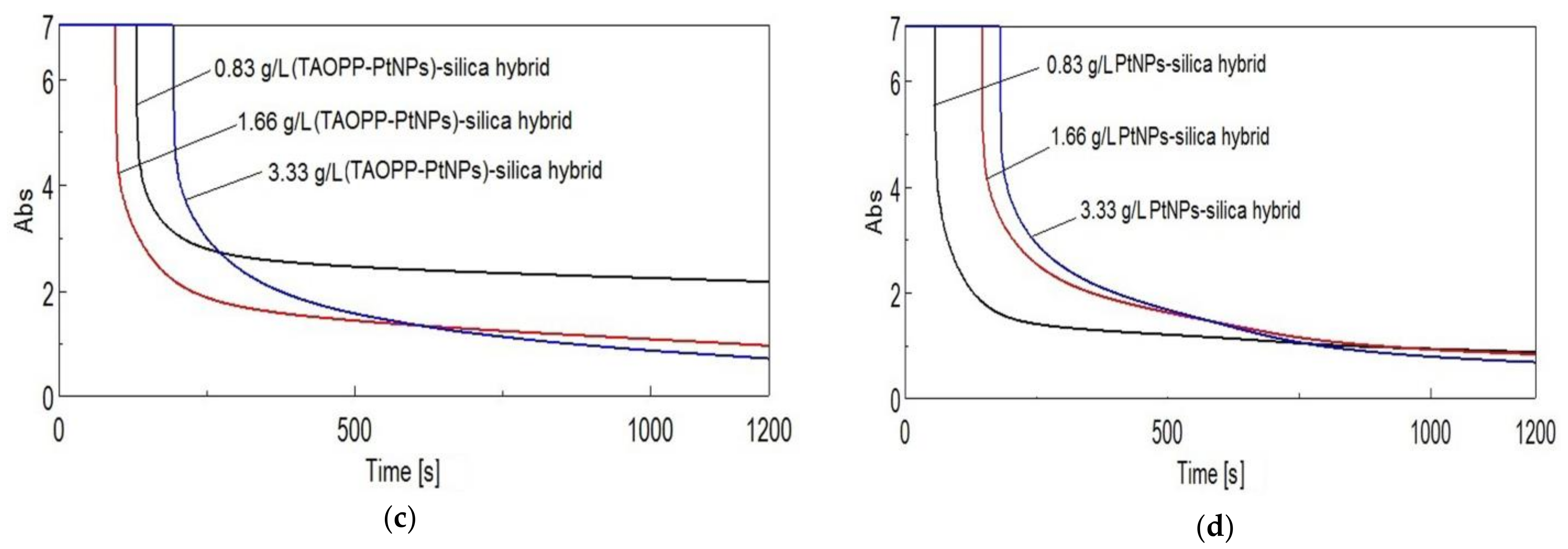
3.2.4. Time Course Measurements
3.2.5. The Effect of Adsorbent Quantity upon the Adsorption of Fuchsine B, for Optimization of the Adsorption Process
3.3. Desorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jedynak, K.; Repelewicz, M. Adsorption of methylene blue and malachite green on micro-mesoporous carbon materials. Adsorp. Sci. Technol. 2017, 35, 499–506. [Google Scholar] [CrossRef]
- Tang, L.; Cheng, J. Nonporous silica nanoparticles for nanomedicine application. 2013, 8, 290–312. [Google Scholar] [CrossRef]
- Shirani, M.; Semnani, A.; Haddadi, H.; Habibollahi, S. Optimization of Simultaneous Removal of Methylene Blue, Crystal Violet, and Fuchsine from Aqueous Solutions by Magnetic NaY Zeolite Composite. Water Air Soil Pollut. 2014, 225, 2054. [Google Scholar] [CrossRef]
- Osica, I.; Imamura, G.; Shiba, K.; Ji, Q.; Shrestha, L.K.; Hill, J.P.; Kurzydlowski, K.J.; Yoshikawa, G.; Ariga, K. Highly Networked Capsular Silica–Porphyrin Hybrid Nanostructures as Efficient Materials for Acetone Vapor Sensing. ACS Appl. Mater. Interfaces 2017, 9, 9945–9954. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.; Zhu, Q.; Qiao, D.; Chen, K.; Tang, L.; Wang, D.; He, W.; Chen, Y.; Yu, T. CO2 Capture with Absorbents of Tertiary Amine Functionalized Nano–SiO2. Front. Chem. 2020, 8, 146. [Google Scholar] [CrossRef]
- Mak, C.A.; Pericas, M.A.; Fagadar-Cosma, E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal. Today 2018, 306, 268–275. [Google Scholar] [CrossRef]
- Tambe Patil, B.B. Wastewater treatment using nanoparticles. J. Adv. Chem. Eng 2015, 5. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Wawrzkiewicz, M.; Polska-Adach, E.; Fijałkowska, G.; Goncharuk, O. Nanosized silica–titanium oxide as a potential adsorbent for C.I. Acid Yellow 219 dye removal from textile baths and wastewaters. Appl. Nanosci. 2018, 8, 867–876. [Google Scholar] [CrossRef]
- Farias, R.S.; Buarque, H.L.; Cruz, M.R.; Cardoso, L.M.F.; Gondim, T.A.; Paulo, V.R. Adsorption of congo red dye from aqueous solution onto amino-functionalized silica gel. Eng. Sanit. Ambient. 2018, 23, 1053–1060. [Google Scholar] [CrossRef]
- Song, Y.H.; Chen, S.M.; Ren, J.M.; Gao, Y.; Xu, H. Fuchsine Adsorption from Aqueous Solution by Epichlorohydrin Crosslinked Peanut Husk. Adv. Mat. Res. 2012, 549, 362–365. [Google Scholar] [CrossRef]
- Moosavi, A.; Amooey, A.A.; Alinejad mir, A.; Marzbali, M.H. Extraordinary Adsorption of Acidic Fuchsine and Malachite Green onto Cheap Nano-adsorbent derived from Eggshell. Chin. J. Chem. Eng. 2020, 28, 1591–1602. [Google Scholar] [CrossRef]
- Cotoruelo, L.M.; Marqués, M.D.; Rodríguez-Mirasol, J.; Rodríguez, J.J.; Cordero, T. Cationic dyes removal by multilayer adsorption on activated carbons from lignin. J. Porous Mater. 2010, 18, 693–702. [Google Scholar] [CrossRef]
- Dissanayake, M.R.E.A.; Wijesinghe, M.K.E.H.; Iqbal, S.S.; Priyantha, N.; Iqbal, M. Fuchsine biosorption using Asplenium nidus biosorbent-a mechanism using kinetic and isotherm data. RSC Adv. 2016, 6, 98682–98692. [Google Scholar] [CrossRef]
- El Haddad, M. Removal of Basic Fuchsin dye from water using mussel shellbiomass waste as an adsorbent: Equilibrium, kinetics, and thermodynamics. J. Taibah Univ. Sci. 2016, 10, 664–674. [Google Scholar] [CrossRef]
- Renita, A.A.; Amarnath, D.J.; Duraikannu, S.L. Synthesis of peanut-shell magnetized biocarbon for acid fuchsin dye removal. Mater. Today Proceedings 2021, 2214–7853. [Google Scholar] [CrossRef]
- Mukurala, N.; Mokurala, K.; Suman, S.; Kushwaha, A.K. Synthesis of porous Cu2FeSnS4 particles via solvothermal process for removal of organic acid fuchsin dye pollutant from wastewater. Nano-Struct. Nano-Objects 2021, 26, 100697. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Sayed, A.Z.; El-Wahab, H.A.; Sayah, M.M. Synthesis of a hydrogel by grafting of acrylamide-co-sodium methacrylate onto chitosan for effective adsorption of Fuchsin basic dye. Int. J. Biol. Macromol. 2020, 159, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Georgin, J.; Franco, D.; Drumm, F.C.; Grassi, P.; Netto, M.S.; Allasia, D.; Dotto, G.L. Powdered biosorbent from the mandacaru cactus (cereus jamacaru) for discontinuous and continuous removal of Basic Fuchsin from aqueous solutions. Powder Technol. 2020, 364, 584–592. [Google Scholar] [CrossRef]
- Mohammed, B.B.; Hsini, A.; Abdellaoui, Y.; Oualid, H.A.; Laabd, M.; Ouardi, M.E.; Addi, A.A.; Yamni, K.; Tijani, N. Fe-ZSM-5 zeolite for efficient removal of basic Fuchsin dye from aqueous solutions: Synthesis, characterization and adsorption process optimization using BBD-RSM modeling. J. Environ. Chem. Eng. 2020, 8, 104419. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, S.; Wang, X.; Sun, C.; Wang, Y.; Hu, L. Preparation of mesoporous Al-MCM-41 from natural palygorskite and its adsorption performance for hazardous aniline dye-basic fuchsin. Microporous Mesoporous Mater. 2018, 265, 266–274. [Google Scholar] [CrossRef]
- Kalita, S.; Pathak, M.; Devi, G.; Sarma, H.P.; Bhattacharyya, K.G.; Sarma, A.; Devi, A. Utilization of Euryale ferox Salisbury seed shell for removal of basic fuchsin dye from water: Equilibrium and kinetics investigation. RSC Adv. 2017, 7, 27248–27259. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, S.; Sun, C.; Yi, G.; Wu, X.; Chen, L.; Ma, X. Wet chemical extraction of silicon from natural palygorskite for preparing a mesoporous molecular sieve of Al-SBA-16. Chem. Zvesti. 2019, 73, 2655–2666. [Google Scholar] [CrossRef]
- Bessashia, W.; Berredjem, Y.; Hattab, Z.; Bououdina, M. Removal of Basic Fuchsin from water by using mussel powdered eggshell membrane as novel bioadsorbent: Equilibrium, kinetics, and thermodynamic studies. Environ. Res. 2020, 186, 109484. [Google Scholar] [CrossRef] [PubMed]
- Sharifpour, E.; Ghaedi, M.; Asfaram, A.; Farsadrooh, M.; Dil, E.A.; Javadian, H. Modeling and optimization of ultrasound-assisted high performance adsorption of Basic Fuchsin by starch-capped zinc selenide nanoparticles/AC as a novel composite using response surface methodology. Int. J. Biol. Macromol. 2020, 152, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. Int. J. Mol. Sci. 2020, 21, 4262. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Han, X.; Liu, J.; Zhang, Y.; Van Der Bruggen, B. Zr-Porphyrin Metal–Organic Framework-Based Photocatalytic Self-Cleaning Membranes for Efficient Dye Removal. Ind. Eng. Chem. Res. 2021, 60, 1850–1858. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Lu, Z.-Y. Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Micropor. Mesopor. Mat. 2020, 292, 109774. [Google Scholar] [CrossRef]
- Wu, G.W.; He, S.B.; Peng, H.P.; Deng, H.H.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. Citrate-Capped Platinum Nanoparticle as a Smart Probe for Ultrasensitive Mercury Sensing. Anal. Chem. 2014, 86, 10955–10960. [Google Scholar] [CrossRef]
- Len, A. Small Angle Neutron Scattering. In Research Instruments at the Budapest Neutron Centre: Handbook of the Central European Training School on Neutron Techniques; Füzi, J., Len, A., Bajnok, K., Eds.; KFKI: Budapest, Hungary, 2017; pp. 84–100. ISBN 978-963-12-8757-8. [Google Scholar]
- Kaushal, A.; Singh, S.K. Critical analysis of adsorption data statistically. Appl. Water Sci. 2017, 7, 3191–3196. [Google Scholar] [CrossRef]
- Kaushal, A.; Singh, S.K. Removal of Zn (II) from aqueous solutions using agro-based adsorbents. Imp. J. Interdiscip. Res. 2016, 2, 1215–1218. [Google Scholar]
- Joshi, M.; Kremling, A.; Seidel-Morgenstern, A. Model based statistical analysis of adsorption equilibrium data. Chem. Eng. Sci. 2006, 61, 7805–7818. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhen, L.; Wang, X.; Li, N.; Tong, Q. Degradation of acid fuchsine by a modified electro-Fenton system with magnetic stirring as oxygen supplying. J. Env. Sci. 2010, 22, 547–554. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Dudas, Z.; Enache, C.; Fagadar-Cosma, G.; Armeanu, I.; Fagadar-Cosma, E. Hybrid silica-porphyrin materials with tailored pore sizes. Mater. Res. Bull. 2010, 45, 1150–1156. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Dudás, Z.; Birdeanu, M.; Almásy, L. Hybrid organic–silica nanomaterials based on novel A3B mixed substituted porphyrin. Mater. Chem. Phys. 2014, 148, 143–152. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Ashraf, A.; Issa, A.A. Eco-Structured Biosorptive Removal of Basic Fuchsin Using Pistachio Nutshells: A Definitive Screening Design—Based Approach. Appl. Sci. 2019, 9, 4855. [Google Scholar] [CrossRef]
- Oyelude, E.O.; Frimpong, F.; Dawson, D. Studies on the removal of basic fuchsin dye from aqueous solution by HCl treated malted sorghum mash. J. Mater. Environ. Sci. 2015, 6, 1126–1136. [Google Scholar]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dyes Pigm. 2001, 51, 25–40. [Google Scholar] [CrossRef]

| Adsorbent for Fuchsine Dyes | Adsorbtion Capacity (mg/g) | Surface Area (m2/g) | Reference |
|---|---|---|---|
| Calcined Mussel Shell | 141.65 | - | [14] |
| Magnetized bio carbon from peanut shell | 19.64 In acid form | - | [15] |
| Porous Cu2FeSnS particles | 123.12 In acid form | 53.5 | [16] |
| (Acrylamide-co-Sodium methacrylate)-graft-Chitosan gel | 6.07 | spongeous surface with pores of around 10 μm | [17] |
| Powdered biosorbent from the mandacaru cactus (cereus jamacaru) leaves | 324.5 | surface with cavities and protrusions | [18] |
| Zeolite made from sodium metasilicate as silicon precursor and impregnated with Fe(III) cations using tetrapropylammonium bromide | 205.83 | 399 | [19] |
| Mesoporous Si−Al material, prepared by alkali calcination leaching of natural palygorskite, and sequent hydrothermal synthesis coupled with calcination | 54.4 | 995 | [20] |
| Hard shell of Euryale ferox seeds | 19.48 | - | [21] |
| Mesoporous silica materials prepared via co-condensation of silicon from natural mineral palygorskite by alkali-melting extraction (SBA-16) | 39.61 | 687.23 | [22] |
| SBA-16 functionalized with aluminium | 70.08 | 514.39 | [22] |
| amorphous eggshell membrane porous powder | 47.85 | 11.56 | [23] |
| Starch-capped zinc selenide nanoparticles loaded on activated carbon | 222.7 | - | [24] |
| Silica hybrid materials impregnated with platinum nanoparticles (PtNPs) | 197.28 | 739 ± 19 | This work |
| Silica hybrid materials impregnated with Pt-metalloporphyrin (PtTAOPP) | 190.46± | 593±15 | This work |
| Adsorbent Material | Fuchsine B Concentration 1 × 10−5 M | Fuchsine B Concentration 1 × 10−6 M | ||
|---|---|---|---|---|
| qe (mg\g) | η (%) | qe (mg\g) | η (%) | |
| Silica control | 6.2 ± 0.01 | 60.7 | 1.02 ± 0.002 | 100 |
| PtTAOPP-silica hybrid | 7.9 ± 0.01 | 76.6 | ||
| (TAOPP-PtNPs)-silica hybrid | 7.7 ± 0.01 | 75.7 | ||
| PtNPs-silica hybrid | 5.7 ± 0.01 | 56.63 | ||
| Adsorbent Material | Mass of Sorbent (g/L) | |||||
|---|---|---|---|---|---|---|
| 0.83 | 1.66 | 3.33 | ||||
| q20min (mg/g) | η (%) | q20min (mg/g) | η (%) | q20min (mg/g) | η (%) | |
| Silica control | 192.55 ± 0.2 | 94.62 | 96.29 ± 0.1 | 94.64 | 48.10 ± 0.06 | 94.54 |
| PtTAOPP-silica hybrid | 190.46 ± 0.15 | 93.59 | 95.43 ± 0.1 | 93.79 | 47.57 ± 0.06 | 93.79 |
| (TAOPP-PtNPs)-silica hybrid | 168.10 ± 0.1 | 86.43 | 98.38 ± 0.1 | 96.69 | 49.47 ± 0.05 | 98.91 |
| PtNPs-silica hybrid | 197.28 ± 0.1 | 96.94 | 98.81 ± 0.1 | 97.11 | 49.52 ± 0.05 | 98.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fratilescu, I.; Dudás, Z.; Birdeanu, M.; Epuran, C.; Anghel, D.; Fringu, I.; Lascu, A.; Len, A.; Fagadar-Cosma, E. Hybrid Silica Materials Applied for Fuchsine B Color Removal from Wastewaters. Nanomaterials 2021, 11, 863. https://doi.org/10.3390/nano11040863
Fratilescu I, Dudás Z, Birdeanu M, Epuran C, Anghel D, Fringu I, Lascu A, Len A, Fagadar-Cosma E. Hybrid Silica Materials Applied for Fuchsine B Color Removal from Wastewaters. Nanomaterials. 2021; 11(4):863. https://doi.org/10.3390/nano11040863
Chicago/Turabian StyleFratilescu, Ion, Zoltán Dudás, Mihaela Birdeanu, Camelia Epuran, Diana Anghel, Ionela Fringu, Anca Lascu, Adél Len, and Eugenia Fagadar-Cosma. 2021. "Hybrid Silica Materials Applied for Fuchsine B Color Removal from Wastewaters" Nanomaterials 11, no. 4: 863. https://doi.org/10.3390/nano11040863
APA StyleFratilescu, I., Dudás, Z., Birdeanu, M., Epuran, C., Anghel, D., Fringu, I., Lascu, A., Len, A., & Fagadar-Cosma, E. (2021). Hybrid Silica Materials Applied for Fuchsine B Color Removal from Wastewaters. Nanomaterials, 11(4), 863. https://doi.org/10.3390/nano11040863










