Adsorption Geometry of Alizarin on Silver Nanoparticles: A Computational and Spectroscopic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Silver Nanoparticles Synthesis and Chemicals
2.2. Instruments
2.3. Computational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gettens, R.J.; Stout, G.L. Painting Materials, a Short Encyclopaedia; Dover Publications: New York, NY, USA, 1966. [Google Scholar]
- Berrie, B.H. An Improved method for indentifying red lakes on art and historical artifacts. Proc. Natl. Acad. Sci. USA 2009, 106, 15095–15096. [Google Scholar] [CrossRef]
- Kirby, J.; Spring, M.; Higgett, C. The Technology of Red Lake Pigment Manufacture: Study of the Dyestuff Substrate. Nat. Gall. Tech. Bull. 2005, 26, 71–88. [Google Scholar]
- Kirby, J.; van Bommel, M.; Verhecken, A. (Eds.) Natural Colorants for Dyeing and Lake Pigments: Practical Recipes and Their Historical Sources; Archetype Publications: London, UK, 2014. [Google Scholar]
- Osticioli, I.; Pagliai, M.; Comelli, D.; Schettino, V.; Nevin, A. Red lakes from Leonardo’s Last Supper and other Old Master Paintings: Micro-Raman spectroscopy of anthraquinone pigments in paint cross-sections. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117273. [Google Scholar] [CrossRef] [PubMed]
- Cardon, D. Natural Dyes: Sources, Tradition, Technology and Science; Archetype Publications: London, UK, 2007. [Google Scholar]
- Kirby, J.; White, R. The identification of red lake pigment dyestuffs and a discussion of their use. Nat. Gall. Tech. Bull. 1996, 17, 56–80. [Google Scholar]
- Wouters, J. High Performance Liquid Chromatography of Anthraquinones: Analysis of Plant and Insect Extracts and Dyed Textiles. Stud. Conserv. 1985, 30, 119–128. [Google Scholar] [CrossRef]
- Murcia-Mascarós, S.; Domingo, C.; Sanchez-Cortes, S.; Cañamares, M.V.; Garcia-Ramos, J.V. Spectroscopic identification of alizarin in a mixture of organic red dyes by incorporation in Zr-Ormosil. J. Raman Spectrosc. 2005, 36, 420–426. [Google Scholar] [CrossRef]
- Leona, M.; Stenger, J.; Ferloni, E. Application of surface-enhanced Raman scattering techniques to the ultrasensitive identification of natural dyes in works of art. J. Raman Spectrosc. 2006, 37, 981–992. [Google Scholar] [CrossRef]
- Casadio, F.; Leona, M.; Van Duyne, J.R.L.R. Identification of Organic Colorants in Fibers, Paints, and Glazes by Surface Enhanced Raman Spectroscopy. Acc. Chem. Res. 2010, 43, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Leona, M. Microanalysis of organic pigments and glazes in polychrome works of art by surface-enhanced resonance Raman scattering. Proc. Natl. Acad. Sci. USA 2009, 106, 14757–14762. [Google Scholar] [CrossRef] [PubMed]
- Whitney, A.V.; Casadio, F.; Van Duyne, R.P. Identification and Characterization of Artists’ Red Dyes and Their Mixtures by Surface-Enhanced Raman Spectroscopy. Appl. Spectrosc. 2007, 61, 994–1000. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Leona, M. Surface-enhanced Raman scattering study of the red dye laccaic acid. J. Raman Spectrosc. 2007, 38, 1259–1266. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J. Library of F-T Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation. Spectrochim. Acta 2001, 57A, 1491–1521. [Google Scholar] [CrossRef]
- Pagliai, M.; Osticioli, I.; Nevin, A.; Siano, S.; Cardini, G.; Schettino, V. DFT calculations of the IR and Raman spectra of anthraquinone dyes and lakes. J. Raman Spectrosc. 2018, 49, 668–683. [Google Scholar] [CrossRef]
- Baran, A.; Wrzosek, B.; Bukowska, J.; Proniewicz, L.M.; Baranska, M. Analysis of alizarin by surface-enhanced and FT-Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 436–441. [Google Scholar] [CrossRef]
- Cañamares, M.V.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Surface-enhanced Raman scattering study of the adsorption of the anthraquinone pigment alizarin on Ag nanoparticles. J. Raman Spectrosc. 2004, 35, 921–927. [Google Scholar] [CrossRef]
- Lofrumento, C.; Platania, E.; Ricci, M.; Becucci, M.; Castellucci, E.M. SERS Spectra of Alizarin Anion–Agn (n = 2, 4, 14) Systems: TDDFT Calculation and Comparison with Experiment. J. Phys. Chem. C 2016, 120, 12234–12241. [Google Scholar] [CrossRef]
- Whitney, A.V.; Van-Duyne, R.P.; Casadio, F. An innovative surface-enhanced Raman spectroscopy (SERS) method for the identification of six historical red lakes and dyestuffs. J. Raman Spectrosc. 2006, 37, 993–1002. [Google Scholar] [CrossRef]
- Cyrański, M.K.; Jamróz, M.H.; Rygula, A.; Dobrowolski, J.C.; Dobrzycki, L.; Baranska, M. On two alizarin polymorphs. CrystEngComm 2012, 14, 3667–3676. [Google Scholar] [CrossRef]
- Lofrumento, C.; Platania, E.; Ricci, M.; Mulana, C.; Becucci, M.; Castellucci, E.M. The SERS spectra of alizarin and its ionized species: The contribution of the molecular resonance to the spectral enhancement. J. Mol. Struct. 2015, 1090, 98–106. [Google Scholar] [CrossRef]
- Smith, E.; Dent, G. Modern Raman Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2019. [Google Scholar]
- Chen, K.; Leona, M.; Vo-Dinh, K.C.; Yan, F.; Wabuyele, M.B.; Vo-Dinh, T. Application of surface-enhanced Raman scattering (SERS) for the identification of anthraquinone dyes used in works of art. J. Raman Spectrosc. 2006, 37, 520–527. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Kneipp, K.; Moskovits, M.; Kneipp, H. Surface-Enhanced Raman Scattering—Physics and Applications; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Le Ru, E.C.; Etchegoin, P.G. Principles of Surface-Enhanced Raman Spectroscopy and Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Schlücker, S. Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Procházka, M. Surface-Enhanced Raman Spectroscopy, Bioanalytical, Biomolecular and Medical Applications; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Fasolato, C. Surface Enhanced Raman Spectroscopy for Biophysical Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A Unified View of Surface-Enhanced Raman Scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Cañamares, M.V.; Garcia-Ramos, J.V.; Gómez-Varga, J.D.; Domingo, C.; Sanchez-Cortes, S. Ag Nanoparticles Prepared by Laser Photoreduction as Substrates for in Situ Surface-Enhanced Raman Scattering Analysis of Dyes. Langmuir 2007, 23, 5210–5215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Retko, K.; Ropret, P.; Cerc Korošec, R.; Sanchez-Cortes, S.; Cañamares, M.V. Characterization of HPC-based photoreduced SERS substrates and detection of different organic dyes. J. Raman Spectrosc. 2018, 49, 1288–1300. [Google Scholar] [CrossRef]
- Marcaida, I.; Maguregui, M.; Morillas, H.; García-Florentino, C.; Pintus, V.; Aguayo, T.; Campos-Vallette, M.; Madariaga, J.M. Optimization of sample treatment for the identification of anthraquinone dyes by surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2017, 409, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, F.; Zaleski, S.; Casadio, F.; Van Duyne, R.P. SERS Discrimination of Closely Related Molecules: A Systematic Study of Natural Red Dyes in Binary Mixtures. J. Phys. Chem. C 2016, 120, 21017–21026. [Google Scholar] [CrossRef]
- Pagliai, M.; Caporali, S.; Muniz-Miranda, M.; Pratesi, G.; Schettino, V. SERS, XPS, and DFT Study of Adenine Adsorption on Silver and Gold Surfaces. J. Phys. Chem. Lett. 2012, 3, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, M.; Muniz-Miranda, F.; Schettino, V.; Muniz-Miranda, M. Competitive Solvation and Chemisorption in Silver Colloidal Suspensions. In UK Colloids 2011; Starov, V., Griffiths, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 39–44. [Google Scholar]
- Lawless, D.; Kapoor, S.; Kennepohl, P.; Meisel, D.; Serpone, N. Reduction and Aggregation of Silver Ions at the Surface of Colloidal Silica. J. Phys. Chem. 1994, 98, 9619–9625. [Google Scholar] [CrossRef]
- Xiong, Y.; Washio, I.; Chen, J.; Sadilek, M.; Xia, Y. Trimeric Clusters of Silver in Aqueous AgNO3 Solutions and Their Role as Nuclei in Forming Triangular Nanoplates of Silver. Angew. Chem. Int. Ed. 2007, 46, 4917–4921. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, M.; Pagliai, M.; Muniz-Miranda, F.; Schettino, V. Raman and computational study of solvation and chemisorption of thiazole in silver hydrosol. Chem. Commun. 2011, 47, 3138–3140. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Raman and Computational Study on the Adsorption of Xanthine on Silver Nanocolloids. ACS Omega 2018, 3, 13530–13537. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.R.; Golden, J.W.; Price, A.S.; Henry, W.A.; Barker, W.K.; Perry, D.A. Surface-Enhanced Vibrational Spectroscopy and Density Functional Theory Study of Isoniazid Layers Adsorbed on Silver Nanostructures. J. Phys. Chem. C 2014, 118, 28959–28969. [Google Scholar] [CrossRef]
- Jensen, L.; Aikens, C.M.; Schatz, G.C. Electronic structure methods for studying surface-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Otto, A. The ‘chemical’ (electronic) contribution to surface-enhanced Raman scattering. J. Raman Spectrosc. 2005, 36, 497–509. [Google Scholar] [CrossRef]
- Aranda, D.; Valdivia, S.; Soto, J.; López-Tocón, I.; Avila, F.J.; Otero, J.C. Theoretical Approaches for Modeling the Effect of the Electrode Potential in the SERS Vibrational Wavenumbers of Pyridine Adsorbed on a Charged Silver Surface. Front. Chem. 2019, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Furtak, T.E. Vibrational characteristics of silver clusters in surface-enhanced Raman scattering. Phys. Rev. B 1986, 34, 5111–5117. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, M.; Pagliai, M. Positively Charged Active Sites for the Adsorption of Five-Membered Heterocycles on Silver Colloids. J. Phys. Chem. C 2013, 117, 2328–2333. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Pagliai, M.; Cardini, G.; Schettino, V. Role of Surface Metal Clusters in SERS Spectra of Ligands Adsorbed on Ag Colloidal Nanoparticles. J. Phys. Chem. C 2008, 112, 762–767. [Google Scholar] [CrossRef]
- Huang, R.; Zhao, L.B.; Wu, D.Y.; Tian, Z.Q. Tautomerization, Solvent Effect and Binding Interaction on Vibrational Spectra of Adenine–Ag+ Complexes on Silver Surfaces: A DFT Study. J. Phys. Chem. C 2011, 115, 13739–13750. [Google Scholar] [CrossRef]
- Yao, G.; Zhai, Z.; Zhong, J.; Huang, Q. DFT and SERS Study of 15N Full-Labeled Adenine Adsorption on Silver and Gold Surfaces. J. Phys. Chem. C 2017, 121, 9869–9878. [Google Scholar] [CrossRef]
- Cardini, G.; Muniz-Miranda, M.; Pagliai, M.; Schettino, V. A density functional study of the SERS spectra of pyridine adsorbed on silver clusters. Theor. Chem. Acc. 2007, 117, 451–458. [Google Scholar] [CrossRef]
- Pagliai, M.; Muniz-Miranda, M.; Cardini, G.; Schettino, V. Solvation Dynamics and Adsorption on Ag Hydrosols of Oxazole: A Raman and Computational Study. J. Phys. Chem. A 2009, 113, 15198–15205. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Giorgetti, E.; Marsili, P.; Giammanco, F.; Trigari, S.; Gellini, C.; Muniz-Miranda, M. Ag nanoparticles obtained by pulsed laser ablation in water: Surface properties and SERS activity. J. Raman Spectrosc. 2015, 46, 462–469. [Google Scholar] [CrossRef]
- Koo, T.W.; Chan, S.; Sun, L.; Su, X.; Zhang, J.; Berlin, A.A. Specific Chemical Effects on Surface-Enhanced Raman Spectroscopy for Ultra-Sensitive Detection of Biological Molecules. Appl. Spectrosc. 2004, 58, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised Basis Sets for the LANL Effective Core Potentials. J. Chem. Theory Comput. 2008, 4, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Baker, J. Molecular Structure and Vibrational Spectra. In Handbook of Computational Chemistry; Leszczynski, J., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 293–359. [Google Scholar] [CrossRef]
- Polavarapu, P.L. Ab initio vibrational Raman and Raman optical activity spectra. J. Phys. Chem. 1990, 94, 8106–8112. [Google Scholar] [CrossRef]
- Keresztury, G.; Holly, S.; Besenyei, G.; Varga, J.; Wang, A.; Durig, J. Vibrational spectra of monothiocarbamates-II. IR and Raman spectra, vibrational assignment, conformational analysis and ab initio calculations of S-methyl-N,N-dimethylthiocarbamate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1993, 49, 2007–2026. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Keresztury, G.; Sundius, T.; Seshadri, S. Density functional theory study of vibrational spectra and assignment of fundamental vibrational modes of 1-methyl-4-piperidone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, M.; Caporali, S. Surface-enhanced Raman scattering of ‘push–pull’ molecules: Disperse orange 3 adsorbed on Au and Ag nanoparticles. J. Opt. 2015, 17, 114005. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Cardini, G.; Pagliai, M.; Schettino, V. DFT investigation on the SERS band at ∼1025 cm−1 of pyridine adsorbed on silver. Chem. Phys. Lett. 2007, 436, 179–183. [Google Scholar] [CrossRef]
- Van Elslande, E.; Lecomte, S.; Le Hô, A.S. Micro-Raman spectroscopy (MRS) and surface-enhanced Raman scattering (SERS) on organic colourants in archaeological pigments. J. Raman Spectrosc. 2008, 39, 1001–1006. [Google Scholar] [CrossRef]
- Van Dyck, C.; Fu, B.; Van Duyne, R.P.; Schatz, G.C.; Ratner, M.A. Deducing the Adsorption Geometry of Rhodamine 6G from the Surface-Induced Mode Renormalization in Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2018, 122, 465–473. [Google Scholar] [CrossRef]
- Ungurean, A.; Oltean, M.; David, L.; Leopold, N.; Prates Ramalho, J.P.; Chiş, V. Adsorption of sulfamethoxazole molecule on silver colloids: A joint SERS and DFT study. J. Mol. Struct. 2014, 1073, 71–76. [Google Scholar] [CrossRef]
- Reckien, W.; Kirchner, B.; Janetzko, F.; Bredow, T. Theoretical Investigation of Formamide Adsorption on Ag(111) Surfaces. J. Phys. Chem. C 2009, 113, 10541–10547. [Google Scholar] [CrossRef]
- Zhao, L.L.; Jensen, L.; Schatz, G.C. Pyridine-Ag20 Cluster: A Model System for Studying Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2006, 128, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Jensen, L.; Schatz, G.C. Surface-Enhanced Raman Scattering of Pyrazine at the Junction between Two Ag20 Nanoclusters. Nano Lett. 2006, 6, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Zhao, L.L.; Schatz, G.C. Size-Dependence of the Enhanced Raman Scattering of Pyridine Adsorbed on Agn (n = 2–8, 20) Clusters. J. Phys. Chem. C 2007, 111, 4756–4764. [Google Scholar] [CrossRef]
- Birke, R.L.; Znamenskiy, V.; Lombardi, J.R. A charge-transfer surface enhanced Raman scattering model from time-dependent density functional theory calculations on a Ag10-pyridine complex. J. Chem. Phys. 2010, 132, 214707. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, T.; Iwasa, T.; Taketsugu, T. Roles of silver nanoclusters in surface-enhanced Raman spectroscopy. J. Chem. Phys. 2019, 151, 094102. [Google Scholar] [CrossRef] [PubMed]
- Arenas, J.F.; Soto, J.; Tocón, I.L.; Fernández, D.J.; Otero, J.C.; Marcos, J.I. The role of charge-transfer states of the metal-adsorbate complex in surface-enhanced Raman scattering. J. Chem. Phys. 2002, 116, 7207–7216. [Google Scholar] [CrossRef]
- Avila, F.; Ruano, C.; Lopez-Tocon, I.; Arenas, J.F.; Soto, J.; Otero, J.C. How the electrode potential controls the selection rules of the charge transfer mechanism of SERS. Chem. Commun. 2011, 47, 4213–4215. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.; Fernandez, D.J.; Arenas, J.F.; Otero, J.C.; Soto, J. Modelling the effect of the electrode potential on the metal–adsorbate surface states: Relevant states in the charge transfer mechanism of SERS. Chem. Commun. 2011, 47, 4210–4212. [Google Scholar] [CrossRef] [PubMed]
- Diller, K.; Maurer, R.J.; Müller, M.; Reuter, K. Interpretation of X-ray absorption spectroscopy in the presence of surface hybridization. J. Chem. Phys. 2017, 146, 214701. [Google Scholar] [CrossRef] [PubMed]

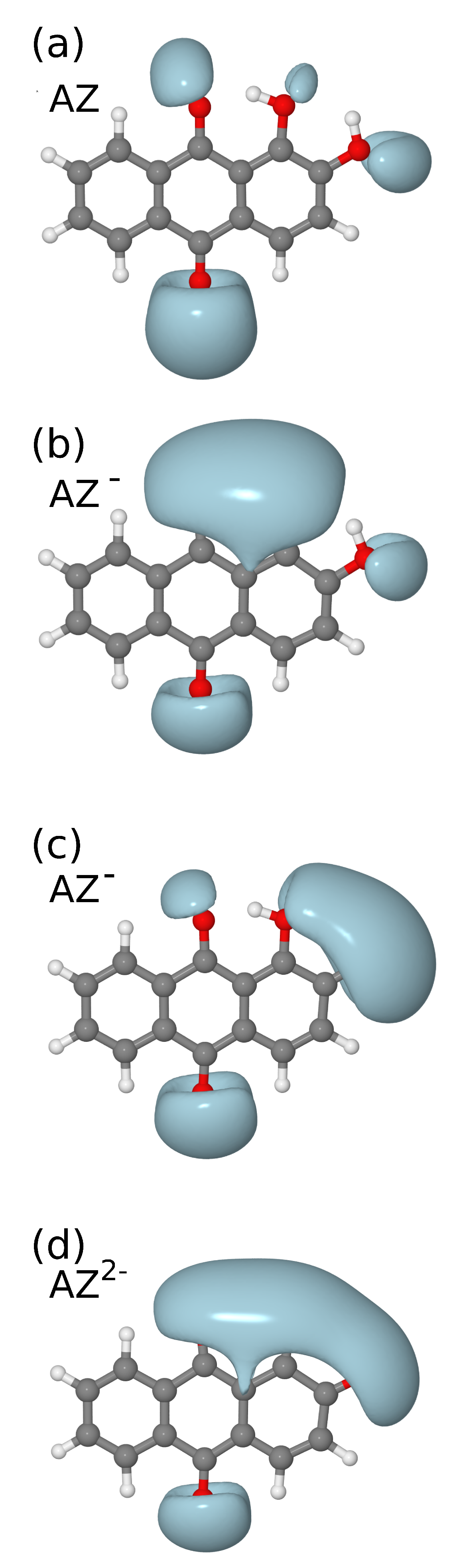


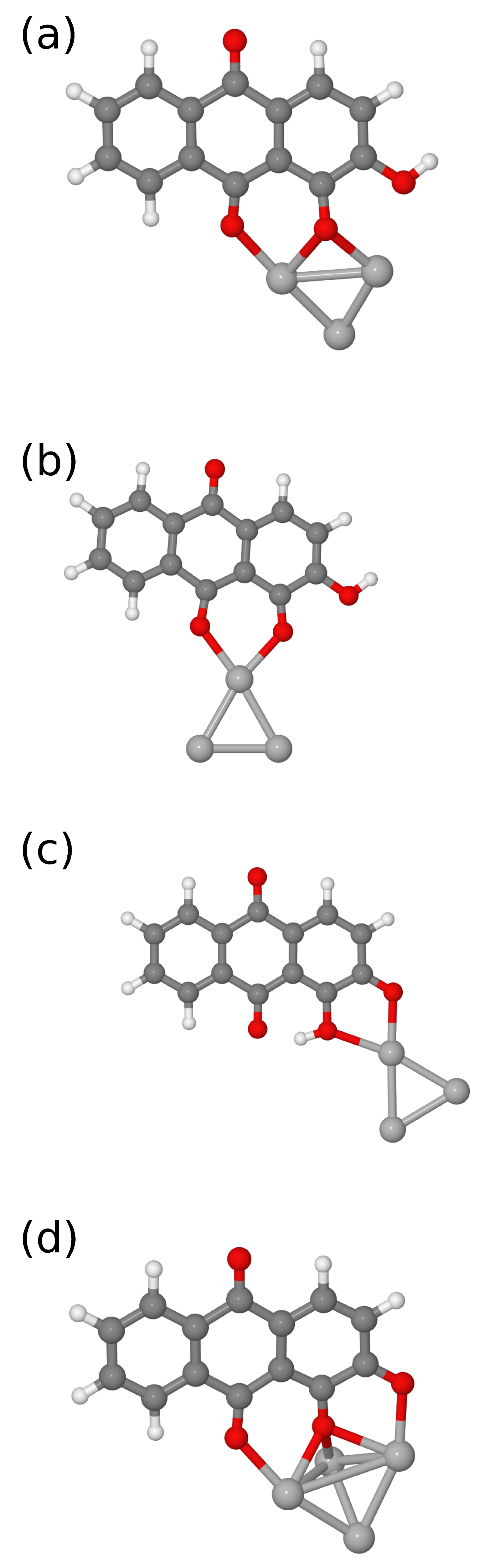
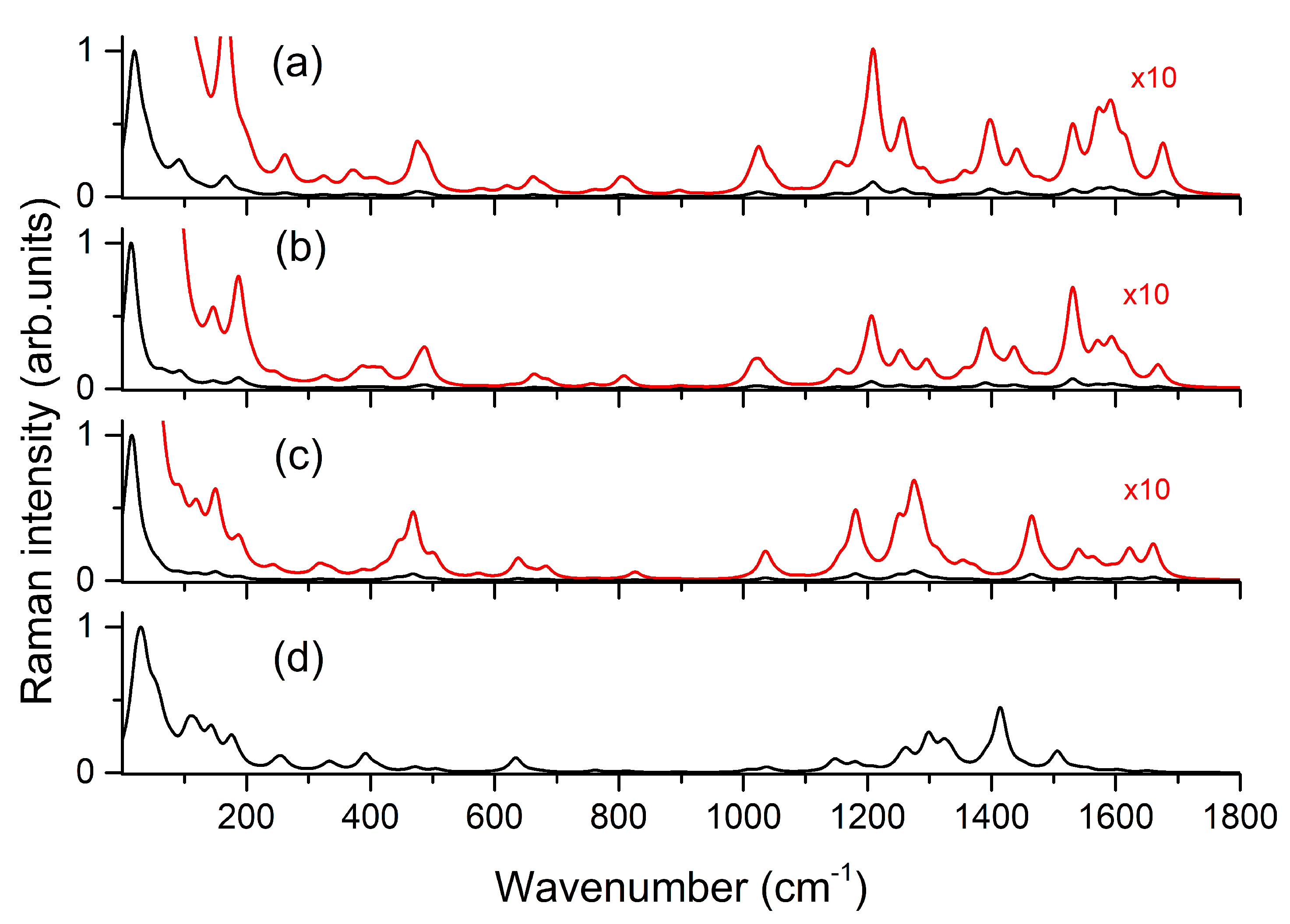
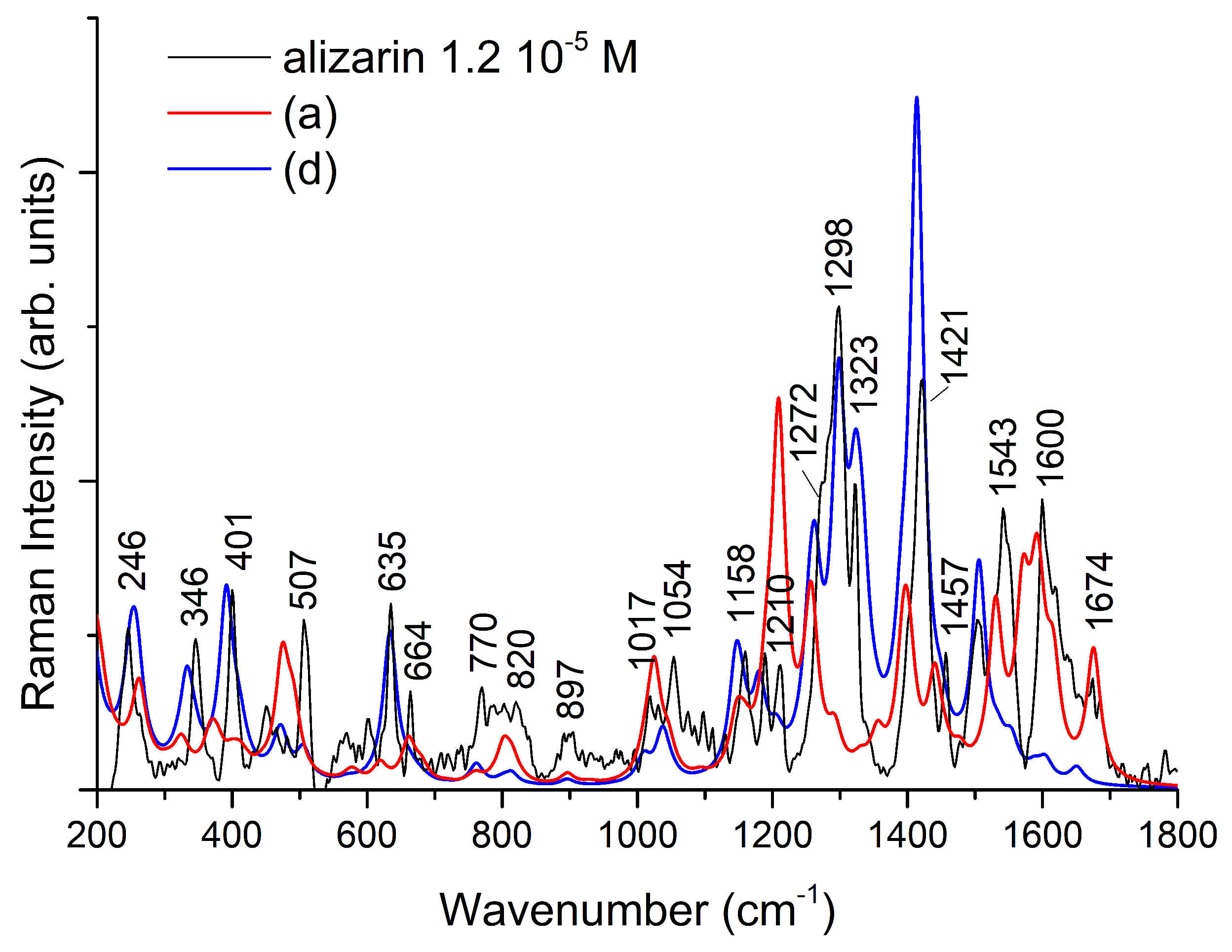
| sym | B3LYP/6-31G(d) [16] | B3LYP/6-311++G(d,p) | IR | Raman | Assignment [16] | |
|---|---|---|---|---|---|---|
| 1 | 47 | 43 | ||||
| 2 | 93 | 89 | ||||
| 3 | 123 | 116 | ||||
| 4 | 139 | 134 | ||||
| 5 | 178 | 176 | 182 | |||
| 6 | 192 | 192 | 193 | |||
| 7 | 250 | 245 | 261 | |||
| 8 | 283 | 285 | 296 | |||
| 9 | 320 | 321 | ||||
| 10 | 329 | 325 | ||||
| 11 | 345 | 342 | 347 | |||
| 12 | 385 | 386 | 392 | |||
| 13 | 417 | 416 | 419 | |||
| 14 | 417 | 417 | 419 | |||
| 15 | 444 | 440 | ||||
| 16 | 462 | 453 | ||||
| 17 | 475 | 465 | 470 | |||
| 18 | 478 | 477 | 486 | 486 | ||
| 19 | 499 | 488 | 499 | 501 | ||
| 20 | 562 | 564 | ||||
| 21 | 568 | 573 | 579 | |||
| 22 | 608 | 615 | 620 | 620 | ||
| 23 | 653 | 659 | 646 | |||
| 24 | 656 | 666 | 660 | 662 | ||
| 25 | 678 | 686 | 678 | 682 | ||
| 26 | 684 | 691 | 700 | |||
| 27 | 712 | 719 | 712 | 710 | ||
| 28 | 744 | 753 | 736 | |||
| 29 | 768 | 767 | 748 | |||
| 30 | 779 | 775 | 765 | 763 | ||
| 31 | 788 | 794 | 792 | 795 | ||
| 32 | 825 | 828 | 828 | 830 | ||
| 33 | 840 | 846 | 848 | |||
| 34 | 881 | 889 | 858 | |||
| 35 | 896 | 899 | 895 | 895 | ||
| 36 | 945 | 959 | 931 | |||
| 37 | 965 | 984 | 955 | 960 | ||
| 38 | 985 | 1000 | 972 | |||
| 39 | 1006 | 1007 | 1012 | 1012 | ||
| 40 | 1024 | 1026 | 1031 | 1030 | ||
| 41 | 1043 | 1046 | 1048 | 1048 | ||
| 42 | 1085 | 1092 | 1102 | 1102 | ||
| 43 | 1144 | 1149 | 1150 | 1150 | ||
| 44 | 1156 | 1160 | 1160 | 1164 | ||
| 45 | 1179 | 1182 | 1175 | |||
| 46 | 1193 | 1193 | 1198 | 1191 | ||
| 47 | 1227 | 1221 | 1220 | 1216 | ||
| 48 | 1259 | 1261 | 1266 | 1270 | ||
| 49 | 1284 | 1282 | 1295 | 1295 | ||
| 50 | 1298 | 1293 | 1300 | 1300 | ||
| 51 | 1327 | 1319 | 1330 | |||
| 52 | 1337 | 1332 | 1332 | 1332 | ||
| 53 | 1359 | 1350 | 1350 | 1350 | ||
| 54 | 1415 | 1405 | 1398 | 1399 | ||
| 55 | 1454 | 1454 | 1429 | |||
| 56 | 1465 | 1458 | 1452 | 1451 | ||
| 57 | 1475 | 1476 | 1465 | 1463 | ||
| 58 | 1484 | 1486 | 1477 | 1481 | ||
| 59 | 1578 | 1578 | 1571 | 1574 | ||
| 60 | 1594 | 1592 | 1587 | |||
| 61 | 1597 | 1595 | 1589 | |||
| 62 | 1602 | 1601 | 1595 | |||
| 63 | 1647 | 1641 | 1633 | 1632 | ||
| 64 | 1692 | 1685 | 1663 | 1658 | ||
| 65 | 3098 | 3111 | ||||
| 66 | 3112 | 3125 | ||||
| 67 | 3120 | 3130 | ||||
| 68 | 3130 | 3141 | ||||
| 69 | 3131 | 3143 | ||||
| 70 | 3133 | 3147 | ||||
| 71 | 3136 | 3224 | ||||
| 72 | 3568 | 3684 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gellini, C.; Macchiagodena, M.; Pagliai, M. Adsorption Geometry of Alizarin on Silver Nanoparticles: A Computational and Spectroscopic Study. Nanomaterials 2021, 11, 860. https://doi.org/10.3390/nano11040860
Gellini C, Macchiagodena M, Pagliai M. Adsorption Geometry of Alizarin on Silver Nanoparticles: A Computational and Spectroscopic Study. Nanomaterials. 2021; 11(4):860. https://doi.org/10.3390/nano11040860
Chicago/Turabian StyleGellini, Cristina, Marina Macchiagodena, and Marco Pagliai. 2021. "Adsorption Geometry of Alizarin on Silver Nanoparticles: A Computational and Spectroscopic Study" Nanomaterials 11, no. 4: 860. https://doi.org/10.3390/nano11040860
APA StyleGellini, C., Macchiagodena, M., & Pagliai, M. (2021). Adsorption Geometry of Alizarin on Silver Nanoparticles: A Computational and Spectroscopic Study. Nanomaterials, 11(4), 860. https://doi.org/10.3390/nano11040860






