Morphological and Rheological Properties of PLA, PBAT, and PLA/PBAT Blend Nanocomposites Containing CNCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
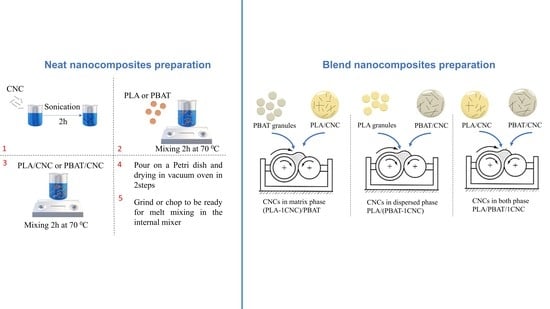
2.2. Single Polymer Matrix and Blend Nanocomposites Preparation
2.2.1. Single Polymer Matrix Nanocomposites Preparation
2.2.2. Blend Nanocomposites Preparation
2.3. Characterization
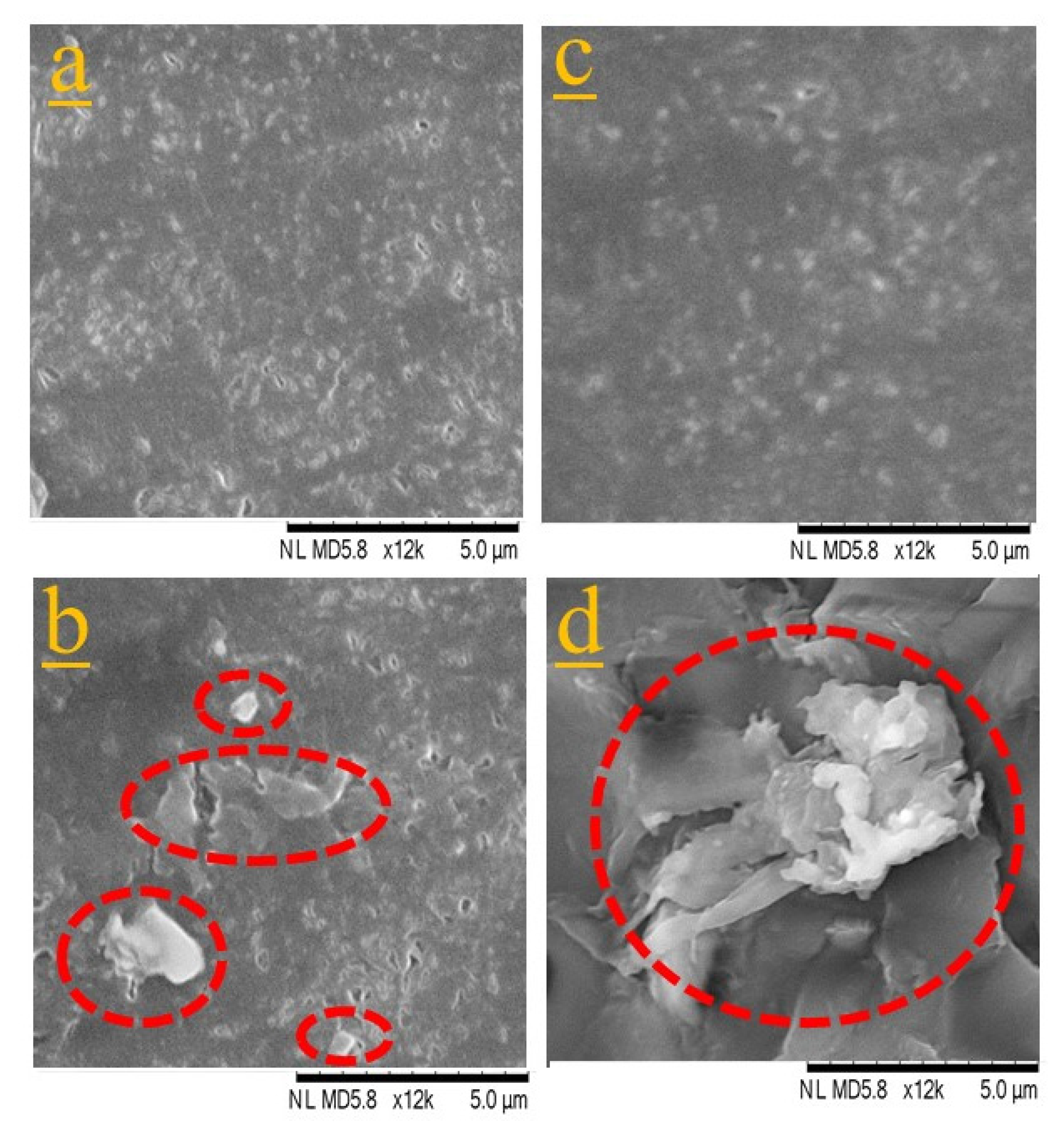
2.3.1. Scanning Electron Microscope (SEM)
2.3.2. Atomic Force Microscopy (AFM)
2.3.3. Rheometry
3. Results and Discussion
3.1. Neat PLA and BPAT Nanocomposites
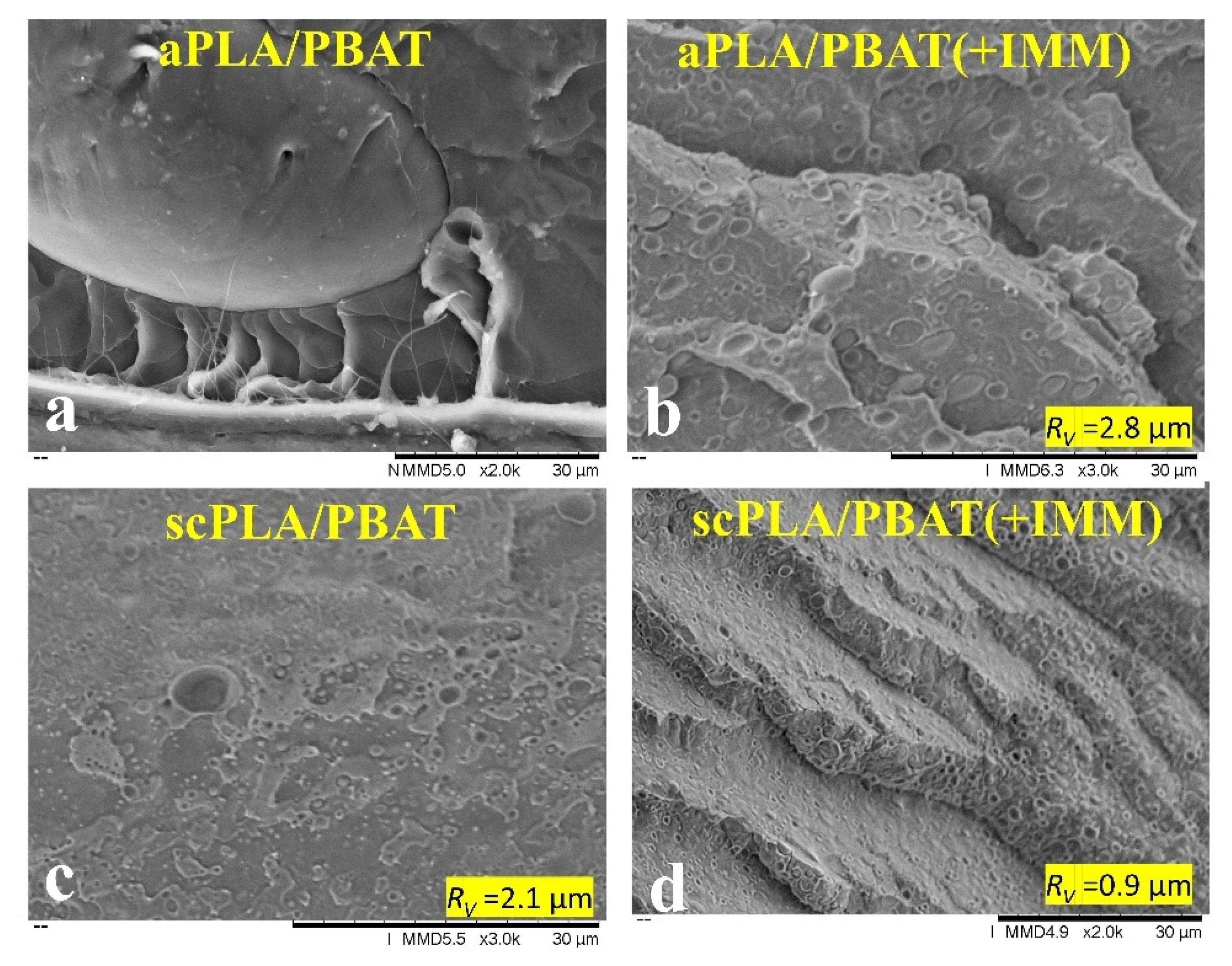
3.1.1. Dispersion of CNCs in PLA and PBAT Matrices
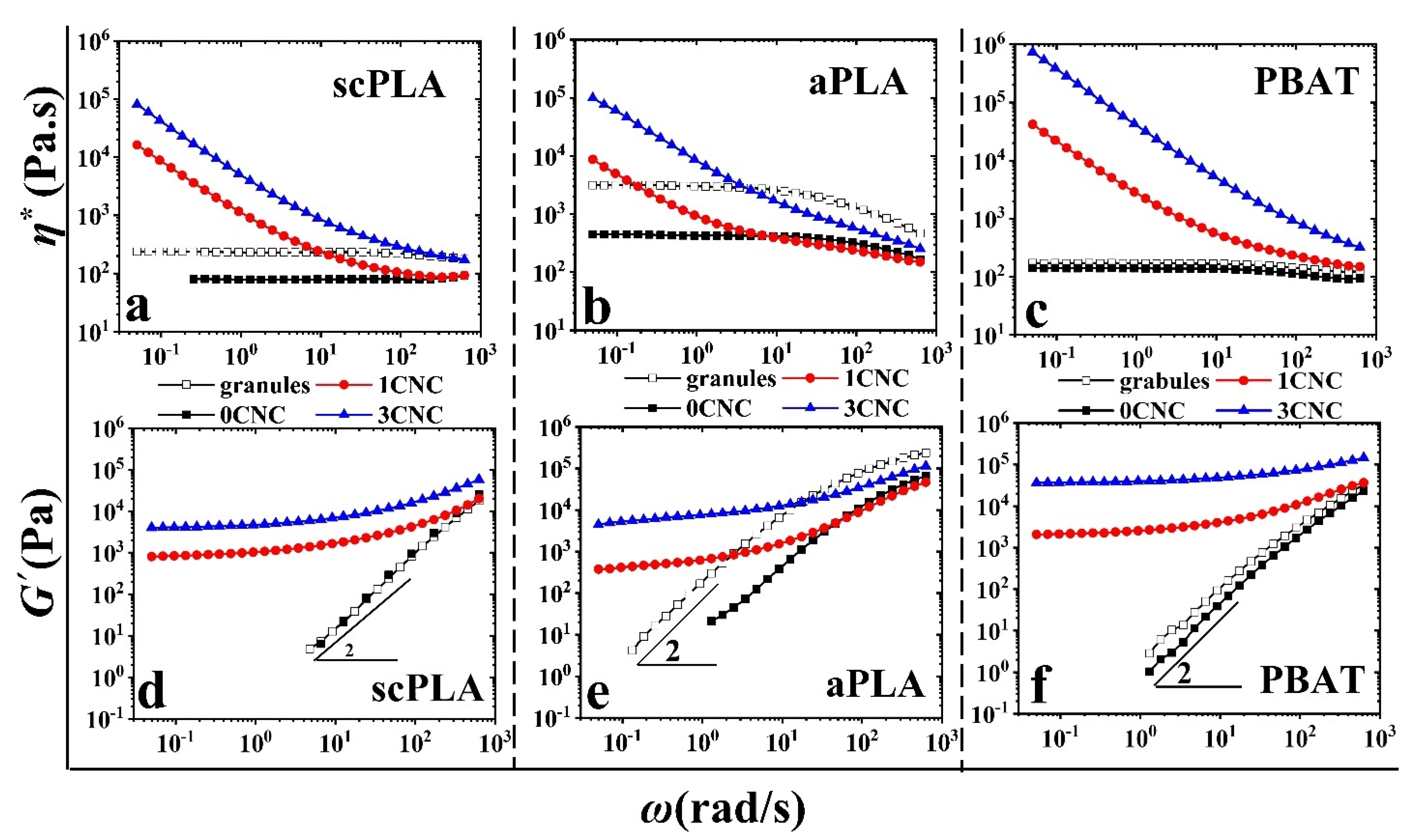
3.1.2. Rheology of Single Polymer Matrix Nanocomposites
3.2. PLA/PBAT Blend Nanocomposites
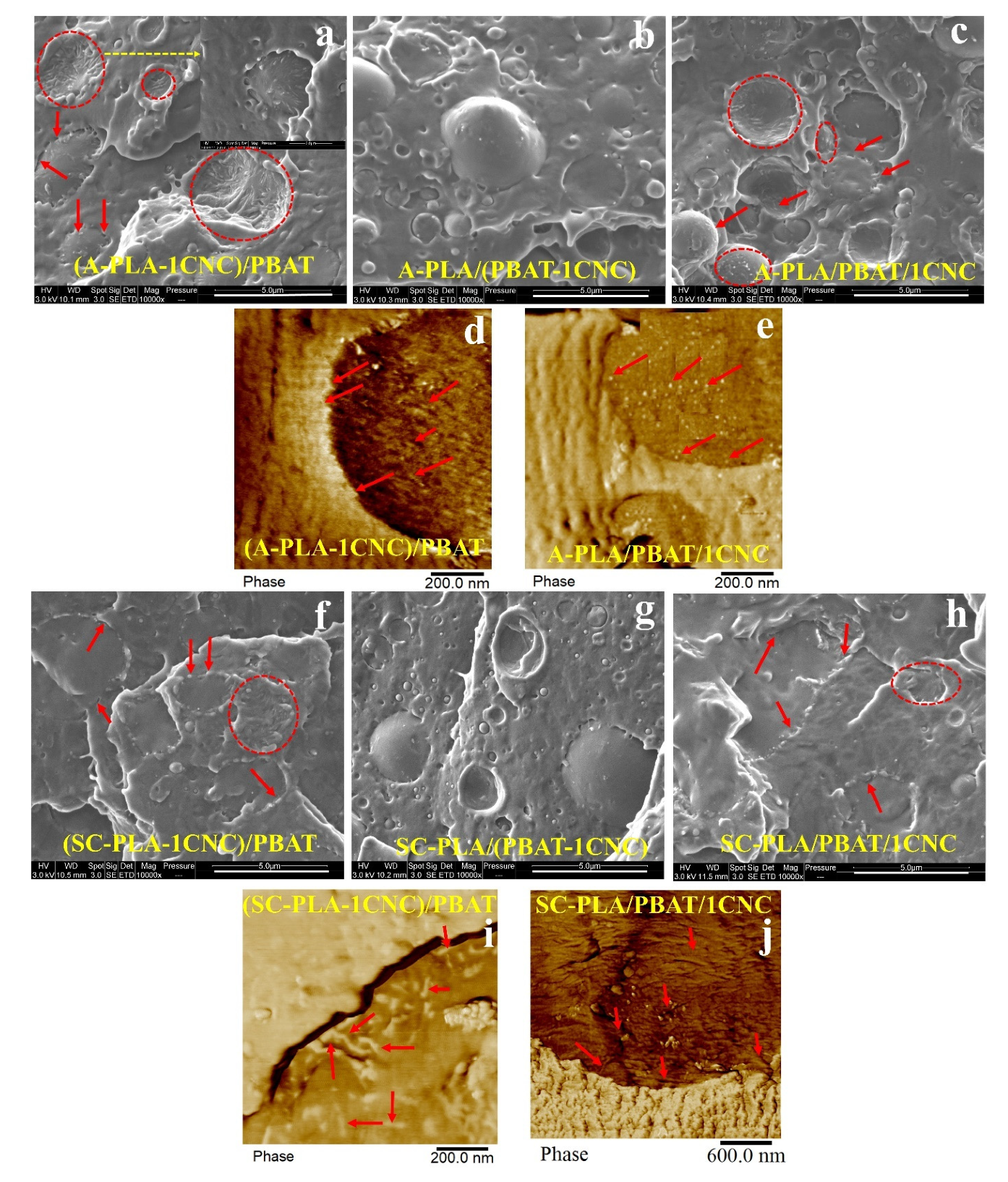
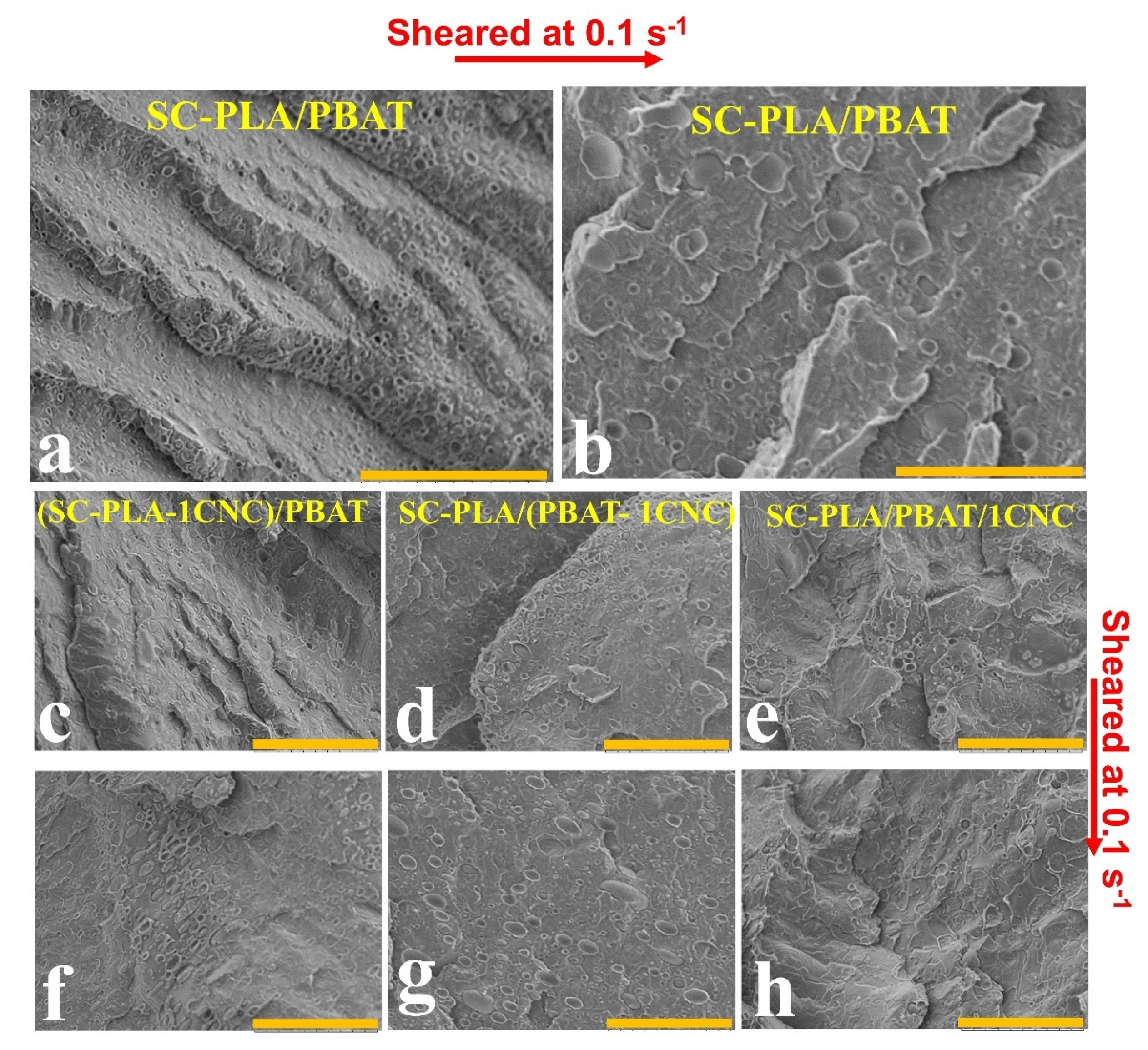
3.2.1. Morphology of Blend Nanocomposites
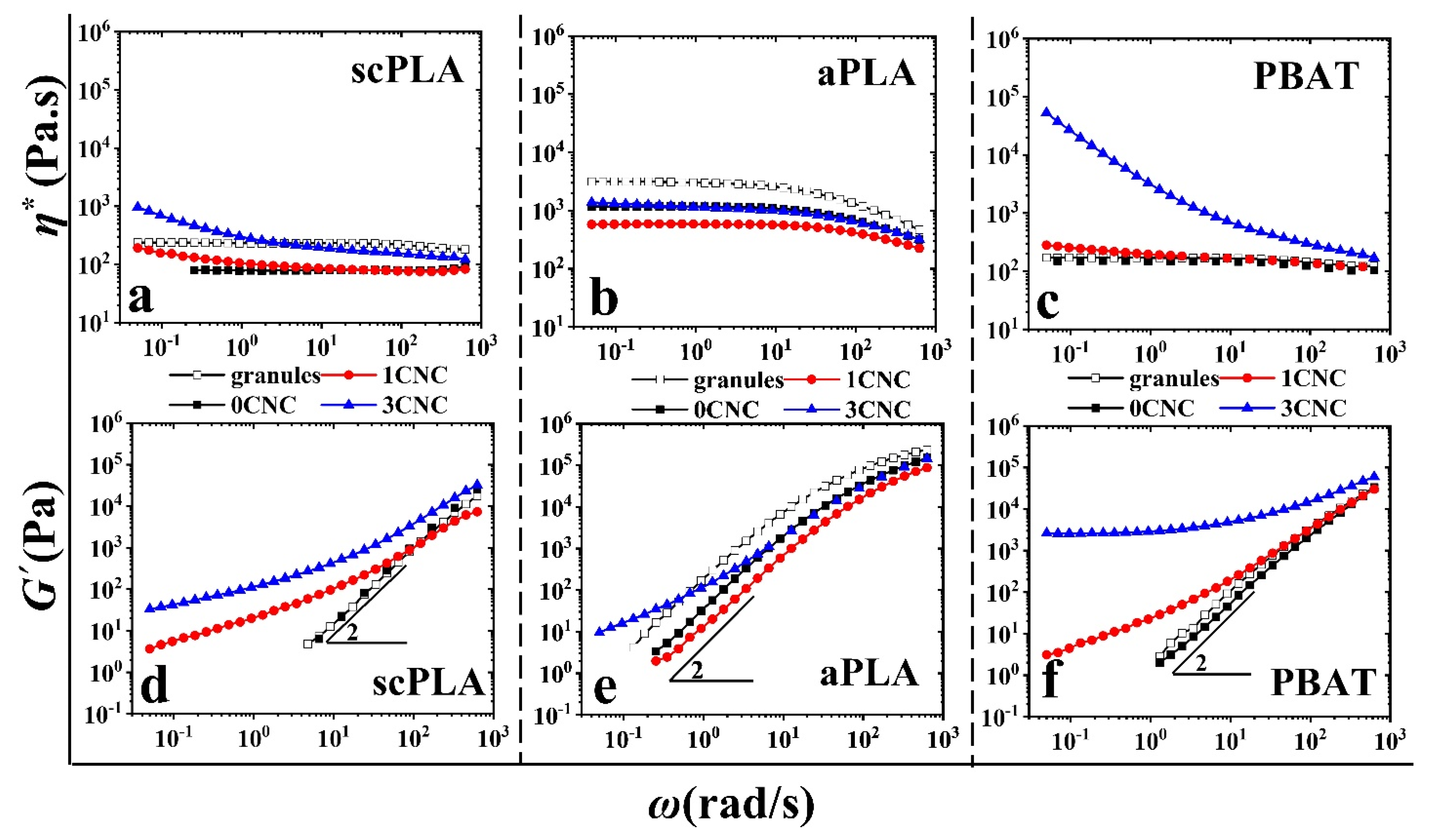
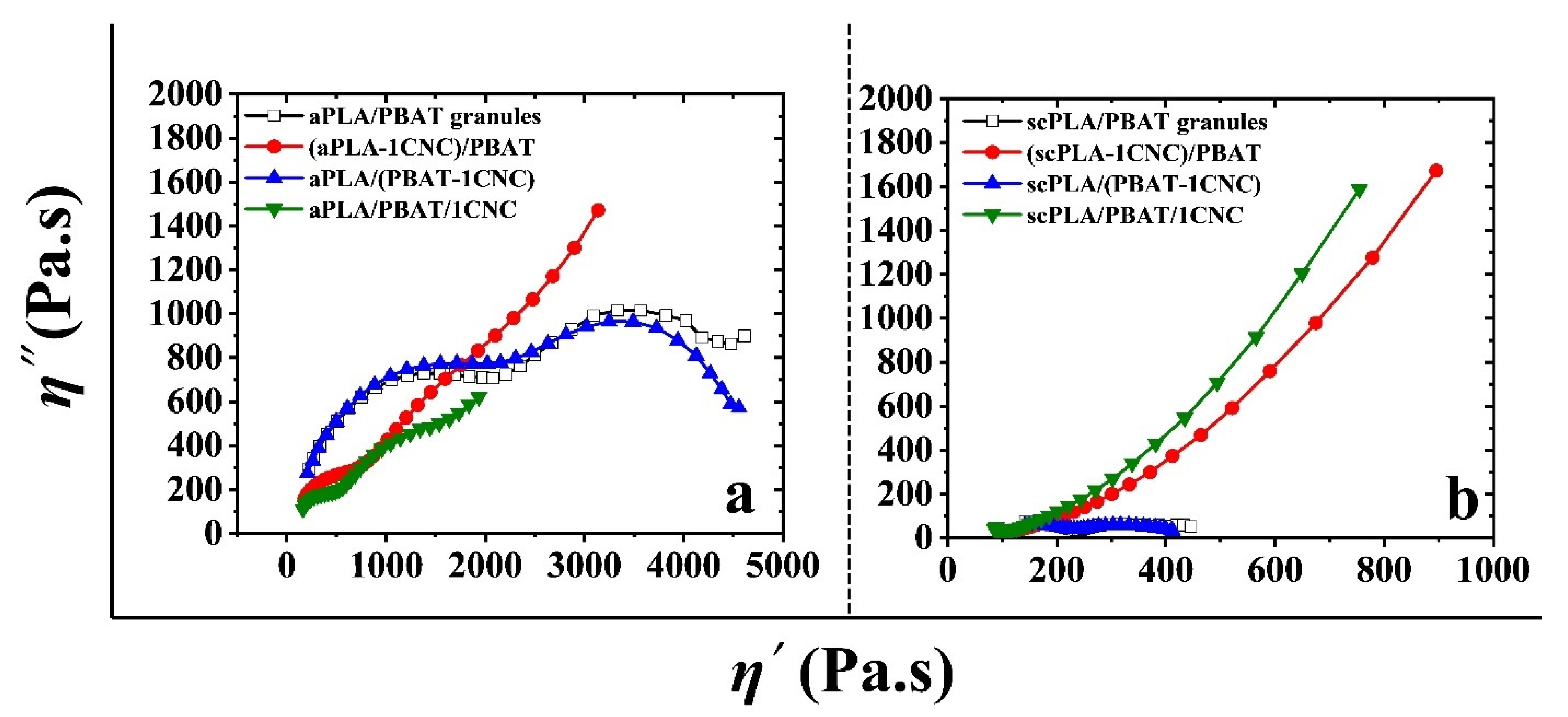
3.2.2. SAOS Behavior of PLA/PBAT/CNC Nanocomposites
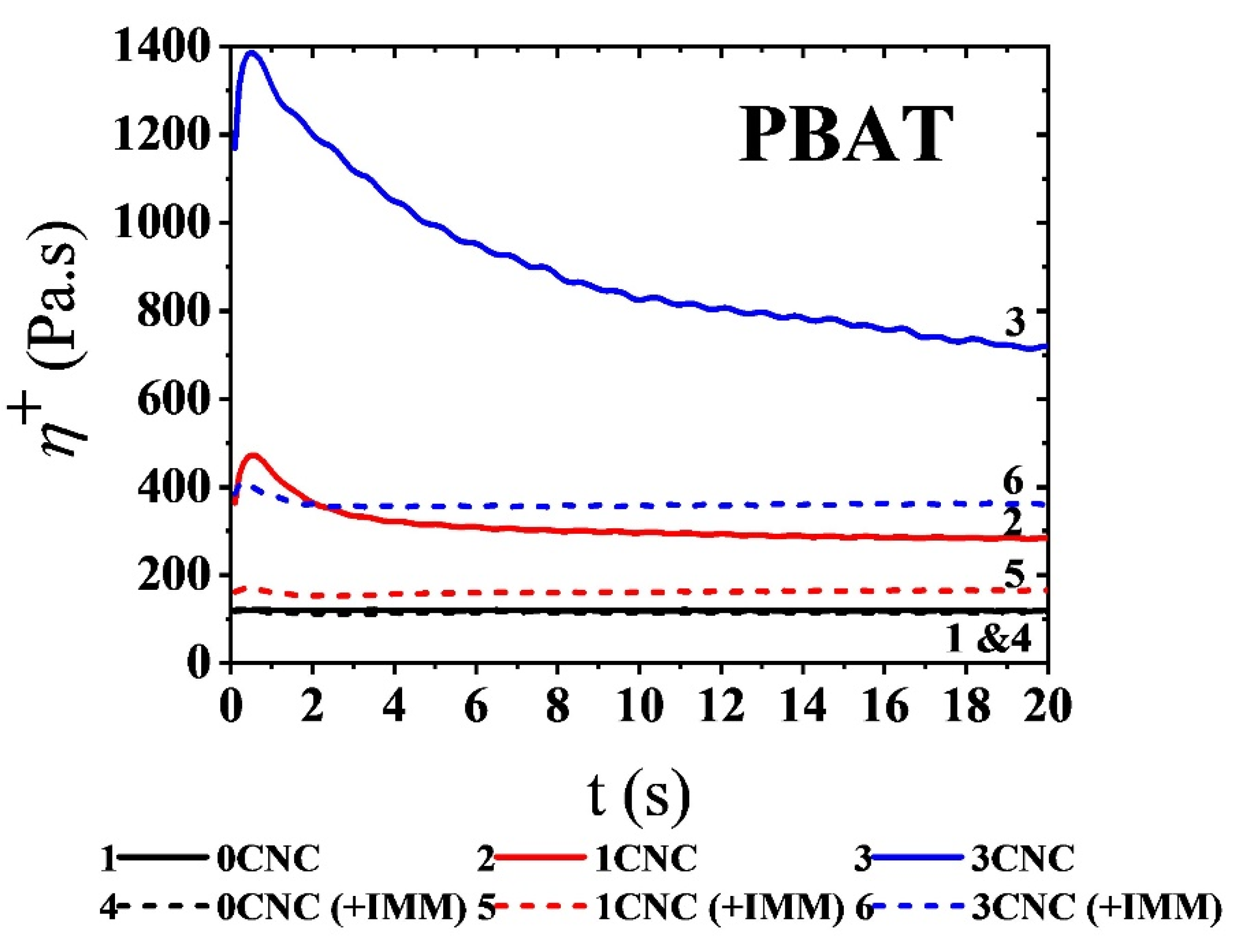
3.2.3. Stress Growth Behavior and Coalescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, Y.-D.; Zeng, J.-B. Progress in Toughening Poly(Lactic Acid) with Renewable Polymers. Polym. Rev. 2017, 57, 557–593. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2018, 125, 307–360. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Jalali Dil, E.; Carreau, P.J.; Favis, B.D. Morphology, miscibility and continuity development in poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. Polymer (Guildf) 2015, 68, 202–212. [Google Scholar] [CrossRef]
- Nofar, M.; Maani, A.; Sojoudi, H.; Heuzey, M.C.; Carreau, P.J. Interfacial and rheological properties of PLA/PBAT and PLA/PBSA blends and their morphological stability under shear flow. J. Rheol. 2015, 59, 317–333. [Google Scholar] [CrossRef]
- Taguet, A.; Cassagnau, P.; Lopez-Cuesta, J.M. Structuration, selective dispersion and compatibilizing effect of (nano)fillers in polymer blends. Prog. Polym. Sci. 2014, 39, 1526–1563. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Filippone, G. Effects of nanoparticles on the morphology of immiscible polymer blends—Challenges and opportunities. Eur. Polym. J. 2016, 79, 198–218. [Google Scholar] [CrossRef]
- Fenouillot, F.; Cassagnau, P.; Majesté, J.-C. Uneven distribution of nanoparticles in immiscible fluids: Morphology development in polymer blends. Polymer (Guildf) 2009, 50, 1333–1350. [Google Scholar] [CrossRef]
- Yuan, Z.; Favis, B.D. Coarsening of immiscible co-continuous blends during quiescent annealing. AIChE J. 2005, 51, 271–280. [Google Scholar] [CrossRef]
- Jalali Dil, E.; Arjmand, M.; Otero Navas, I.; Sundararaj, U.; Favis, B.D. Interface Bridging of Multiwalled Carbon Nanotubes in Polylactic Acid/Poly (butylene adipate-co-terephthalate): Morphology, Rheology, and Electrical Conductivity. Macromolecules 2020, 53, 10267–10277. [Google Scholar] [CrossRef]
- Jalali Dil, E.; Favis, B.D. Localization of micro- and nano-silica particles in heterophase poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. Polymer (Guildf) 2015, 76, 295–306. [Google Scholar] [CrossRef]
- Göldel, A.; Kasaliwal, G.; Pötschke, P. Selective Localization and Migration of Multiwalled Carbon Nanotubes in Blends of Polycarbonate and Poly(styrene-acrylonitrile). Macromol. Rapid Commun. 2009, 30, 423–429. [Google Scholar] [CrossRef]
- Salehiyan, R.; Sinha Ray, S. Processing of Polymer Blends, Emphasizing: Melt Compounding; Influence of Nanoparticles on Blend Morphology and Rheology; Reactive Processing in Ternary Systems; Morphology–Property Relationships; Performance and Application Challenges; and Opportunities. In Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2018; Volume 278, pp. 167–197. [Google Scholar]
- Jalali Dil, E.; Virgilio, N.; Favis, B.D. The effect of the interfacial assembly of nano-silica in poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends on morphology, rheology and mechanical properties. Eur. Polym. J. 2016, 85, 635–646. [Google Scholar] [CrossRef]
- Ko, S.W.; Hong, M.K.; Park, B.J.; Gupta, R.K.; Choi, H.J.; Bhattacharya, S.N. Morphological and rheological characterization of multi-walled carbon nanotube/PLA/PBAT blend nanocomposites. Polym. Bull. 2009, 63, 125–134. [Google Scholar] [CrossRef]
- Urquijo, J.; Aranburu, N.; Dagréou, S.; Guerrica-Echevarría, G.; Eguiazábal, J.I. CNT-induced morphology and its effect on properties in PLA/PBAT-based nanocomposites. Eur. Polym. J. 2017, 93, 545–555. [Google Scholar] [CrossRef]
- Girdthep, S.; Komrapit, N.; Molloy, R.; Lumyong, S.; Punyodom, W.; Worajittiphon, P. Effect of plate-like particles on properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate) blend: A comparative study between modified montmorillonite and graphene nanoplatelets. Compos. Sci. Technol. 2015, 119, 115–123. [Google Scholar] [CrossRef]
- Garg, M.S.; Srivastava, D. Effect of glycidyl methacrylate (GMA) content on thermal and mechanical properties of ternary blend systems based on cardanol-based vinyl ester resin, styrene and glycidyl methacrylate. Prog. Org. Coat. 2014, 77, 1208–1220. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility improvements of PLA-based binary and ternary blends with controlled morphology using PBAT, PBSA, and nanoclay. Compos. Part B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Nofar, M.; Heuzey, M.C.; Carreau, P.J.; Kamal, M.R. Nanoparticle Interactions and Molecular Relaxation in PLA/PBAT/Nanoclay Blends. Exp. Results 2020, 1, e47. [Google Scholar] [CrossRef]
- Nofar, M.; Heuzey, M.C.; Carreau, P.J.; Kamal, M.R. Effects of nanoclay and its localization on the morphology stabilization of PLA/PBAT blends under shear flow. Polymer (Guildf) 2016, 98, 353–364. [Google Scholar] [CrossRef]
- Adrar, S.; Habi, A.; Ajji, A.; Grohens, Y. Synergistic effects in epoxy functionalized graphene and modified organo-montmorillonite PLA/PBAT blends. Appl. Clay Sci. 2018, 157, 65–75. [Google Scholar] [CrossRef]
- Salehiyan, R.; Nofar, M.; Malkappa, K.; Ray, S.S. Effect of nanofillers characteristics and their selective localization on morphology development and rheological properties of melt-processed polylactide/poly(butylene adipate-co-terephthalate) blend composites. Polym. Eng. Sci. 2020, 60, 2749–2760. [Google Scholar] [CrossRef]
- Bagheriasl, D.; Carreau, P.J.; Riedl, B.; Dubois, C.; Hamad, W.Y. Shear rheology of polylactide (PLA)–cellulose nanocrystal (CNC) nanocomposites. Cellulose 2016, 23, 1885–1897. [Google Scholar] [CrossRef]
- Bagheriasl, D.; Carreau, P.J.; Riedl, B.; Dubois, C. Enhanced properties of polylactide by incorporating cellulose nanocrystals. Polym. Compos. 2018, 39, 2685–2694. [Google Scholar] [CrossRef]
- Bagheriasl, D.; Safdari, F.; Carreau, P.J.; Dubois, C.; Riedl, B. Development of cellulose nanocrystal-reinforced polylactide: A comparative study on different preparation methods. Polym. Compos. 2019, 40, E342–E349. [Google Scholar] [CrossRef]
- Bagheriasl, D.; Carreau, P.J. Polymer–Cellulose Nanocrystal (CNC) Nanocomposites. In Processing of Polymer Nanocomposites; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2019; pp. 371–393. [Google Scholar]
- Mohammadi, M.; Bruel, C.; Heuzey, M.C.; Carreau, P.J. CNC dispersion in PLA and PBAT using two solvents: Morphological and rheological properties. Cellulose 2020, 27, 9877–9892. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. Multifunctional PLA-PHB/cellulose nanocrystal films: Processing, structural and thermal properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crops Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Reinforcement effect of poly(butylene succinate) (PBS)-grafted cellulose nanocrystal on toughened PBS/polylactic acid blends. Carbohydr. Polym. 2016, 140, 374–382. [Google Scholar] [CrossRef]
- Pracella, M.; Haque, M.M.U.; Puglia, D. Morphology and properties tuning of PLA/cellulose nanocrystals bio-nanocomposites by means of reactive functionalization and blending with PVAc. Polymer (Guildf) 2014, 55, 3720–3728. [Google Scholar] [CrossRef]
- Bitinis, N.; Verdejo, R.; Bras, J.; Fortunati, E.; Kenny, J.M.; Torre, L.; Lopez-Manchado, M.A. Poly(lactic acid)/natural rubber/cellulose nanocrystal bionanocomposites Part I. Processing and morphology. Carbohydr. Polym. 2013, 96, 611–620. [Google Scholar] [CrossRef]
- Bitinis, N.; Fortunati, E.; Verdejo, R.; Bras, J.; Kenny, J.M.; Torre, L.; López-Manchado, M.A. Poly(lactic acid)/natural rubber/cellulose nanocrystal bionanocomposites. Part II: Properties evaluation. Carbohydr. Polym. 2013, 96, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Sarul, D.S.; Arslan, D.; Vatansever, E.; Kahraman, Y.; Durmus, A.; Salehiyan, R.; Nofar, M. Preparation and characterization of PLA/PBAT/CNC blend nanocomposites. Colloid Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Heshmati, V.; Kamal, M.R.; Favis, B.D. Cellulose nanocrystal in poly(lactic acid)/polyamide11 blends: Preparation, morphology and co-continuity. Eur. Polym. J. 2018, 98, 11–20. [Google Scholar] [CrossRef]
- Heshmati, V.; Kamal, M.R.; Favis, B.D. Tuning the localization of finely dispersed cellulose nanocrystal in poly (lactic acid)/bio-polyamide11 blends. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 576–587. [Google Scholar] [CrossRef]
- Hamad, W.Y.; Hu, T.Q. Structure-process-yield interrelations in nanocrystalline cellulose extraction. Can. J. Chem. Eng. 2010, 88, 392–402. [Google Scholar] [CrossRef]
- Wu, D.; Yuan, L.; Laredo, E.; Zhang, M.; Zhou, W. Interfacial properties, viscoelasticity, and thermal behaviors of poly(butylene succinate)/polylactide blend. Ind. Eng. Chem. Res. 2012, 51, 2290–2298. [Google Scholar] [CrossRef]
- Koch, P. Equivalent diameters of rectangular and oval ducts. Build. Serv. Eng. Res. Technol. 2008, 29, 341–347. [Google Scholar] [CrossRef]
- Grace, H.P. Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]
- Saha, S.; Hemraz, U.D.; Boluk, Y. The Effects of High Pressure and High Temperature in Semidilute Aqueous Cellulose Nanocrystal Suspensions. Biomacromolecules 2020, 21, 1031–1035. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Structural Reorganization of CNC in Injection-Molded CNC/PBAT Materials under Thermal Annealing. Langmuir 2016, 32, 10093–10103. [Google Scholar] [CrossRef]
- Sapkota, J.; Kumar, S.; Weder, C.; Foster, E.J. Influence of Processing Conditions on Properties of Poly (Vinyl acetate)/Cellulose Nanocrystal Nanocomposites. Macromol. Mater. Eng. 2015, 300, 562–571. [Google Scholar] [CrossRef]
- Kamal, M.R.; Khoshkava, V. Effect of cellulose nanocrystals (CNC) on rheological and mechanical properties and crystallization behavior of PLA/CNC nanocomposites. Carbohydr. Polym. 2015, 123, 105–114. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of cellulose nanocrystals (CNC) particle morphology on dispersion and rheological and mechanical properties of polypropylene/CNC nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 8146–8157. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, E.; Arslan, D.; Sarul, D.S.; Kahraman, Y.; Gunes, G.; Durmus, A.; Nofar, M. Development of CNC-reinforced PBAT nanocomposites with reduced percolation threshold: A comparative study on the preparation method. J. Mater. Sci. 2020, 55, 15523–15537. [Google Scholar] [CrossRef]
- Arslan, D.; Vatansever, E.; Sarul, D.S.; Kahraman, Y.; Gunes, G.; Durmus, A.; Nofar, M. Effect of preparation method on the properties of polylactide/cellulose nanocrystal nanocomposites. Polym. Compos. 2020, 41, 4170–4180. [Google Scholar] [CrossRef]
- Favis, B.D.; Chalifoux, J.P. The effect of viscosity ratio on the morphology of polypropylene/polycarbonate blends during processing. Polym. Eng. Sci. 1987, 27, 1591–1600. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, Z.; Xie, W.; Wu, D. Cellulose nanofibers reinforced biodegradable polyester blends: Ternary biocomposites with balanced mechanical properties. Carbohydr. Polym. 2020, 233, 115845. [Google Scholar] [CrossRef] [PubMed]
- Favis, B.D. Polymer alloys and blends: Recent advances. Can. J. Chem. Eng. 1991, 69, 619–625. [Google Scholar] [CrossRef]
- Graebling, D.; Muller, R.; Palierne, J.F. Linear Viscoelastic Behavior of Some Incompatible Polymer Blends in the Melt. Interpretation of Data with a Model of Emulsion of Viscoelastic Liquids. Macromolecules 1993, 26, 320–329. [Google Scholar] [CrossRef]



| Notation | Mixing Steps | Real Final Composition, wt% Poly (Lactic Acid)/ Poly (Butylene Adipate-Co-Terephthalate)/Cellulose Nanocrystals (PLA/PBAT/CNC) |
|---|---|---|
| PLA/PBAT granules | Mixing the neat PLA and PBAT granules using the internal mixer to prepare neat blends | 75/25/0 |
| PLA/PBAT | Mixing the neat PLA and PBAT granules using the solution casting to prepare neat blends | 75/25/0 |
| PLA/PBAT (+IMM) | Mixing the neat PLA and PBAT from solution casting followed by melt mixing via the internal mixer to prepare neat blends | 75/25/0 |
| (PLA-1CNC)/PBAT (Mixing strategy 1) | Mixing PLA/1.4CNC with PBAT granules via the internal mixer. CNCs were initially mixed with PLA | 74.95/25/1.05 |
| PLA/(PBAT-1CNC) (Mixing strategy 2) | Mixing PBAT/4CNC with PLA granules via the internal mixer CNCs were initially mixed with the PBAT | 75/24/1.0 |
| PLA/PBAT/1CNC (Mixing strategy 3) | Mixing PLA/1CNC and PBAT/1CNC. via the internal mixer CNCs were initially mixed with both PLA and PBAT | 74.25/24.75/1.0 |
| Non-Sheared, Rv or Rv-eq | Sheared at 0.1 s−1, Rv or Rv-eq | |
|---|---|---|
| scPLA/PBAT | 0.90 µm | 1.0–3.0 µm |
| (scPLA-1CNC)/PBAT | PBAT droplets are slightly elongated. 1.3 µm | PBAT droplets are slightly elongated. 1.4 µm |
| scPLA/(PBAT-1CNC) | 0.8 | 0.8 µm |
| scPLA/PBAT/1CNC | PBAT droplets are slightly elongated. 1.4 µm | PBAT droplets are slightly elongated. 1.4 µm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, M.; Heuzey, M.-C.; Carreau, P.J.; Taguet, A. Morphological and Rheological Properties of PLA, PBAT, and PLA/PBAT Blend Nanocomposites Containing CNCs. Nanomaterials 2021, 11, 857. https://doi.org/10.3390/nano11040857
Mohammadi M, Heuzey M-C, Carreau PJ, Taguet A. Morphological and Rheological Properties of PLA, PBAT, and PLA/PBAT Blend Nanocomposites Containing CNCs. Nanomaterials. 2021; 11(4):857. https://doi.org/10.3390/nano11040857
Chicago/Turabian StyleMohammadi, Mojtaba, Marie-Claude Heuzey, Pierre J. Carreau, and Aurélie Taguet. 2021. "Morphological and Rheological Properties of PLA, PBAT, and PLA/PBAT Blend Nanocomposites Containing CNCs" Nanomaterials 11, no. 4: 857. https://doi.org/10.3390/nano11040857
APA StyleMohammadi, M., Heuzey, M.-C., Carreau, P. J., & Taguet, A. (2021). Morphological and Rheological Properties of PLA, PBAT, and PLA/PBAT Blend Nanocomposites Containing CNCs. Nanomaterials, 11(4), 857. https://doi.org/10.3390/nano11040857