Vanadium Pentoxide Nanofibers/Carbon Nanotubes Hybrid Film for High-Performance Aqueous Zinc-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of V2O5 Nanofibers
2.2. Preparation of V2O5/CNTs Hybrid Film Electrodes
2.3. Material Characterizations
2.4. Electrochemical Measurements
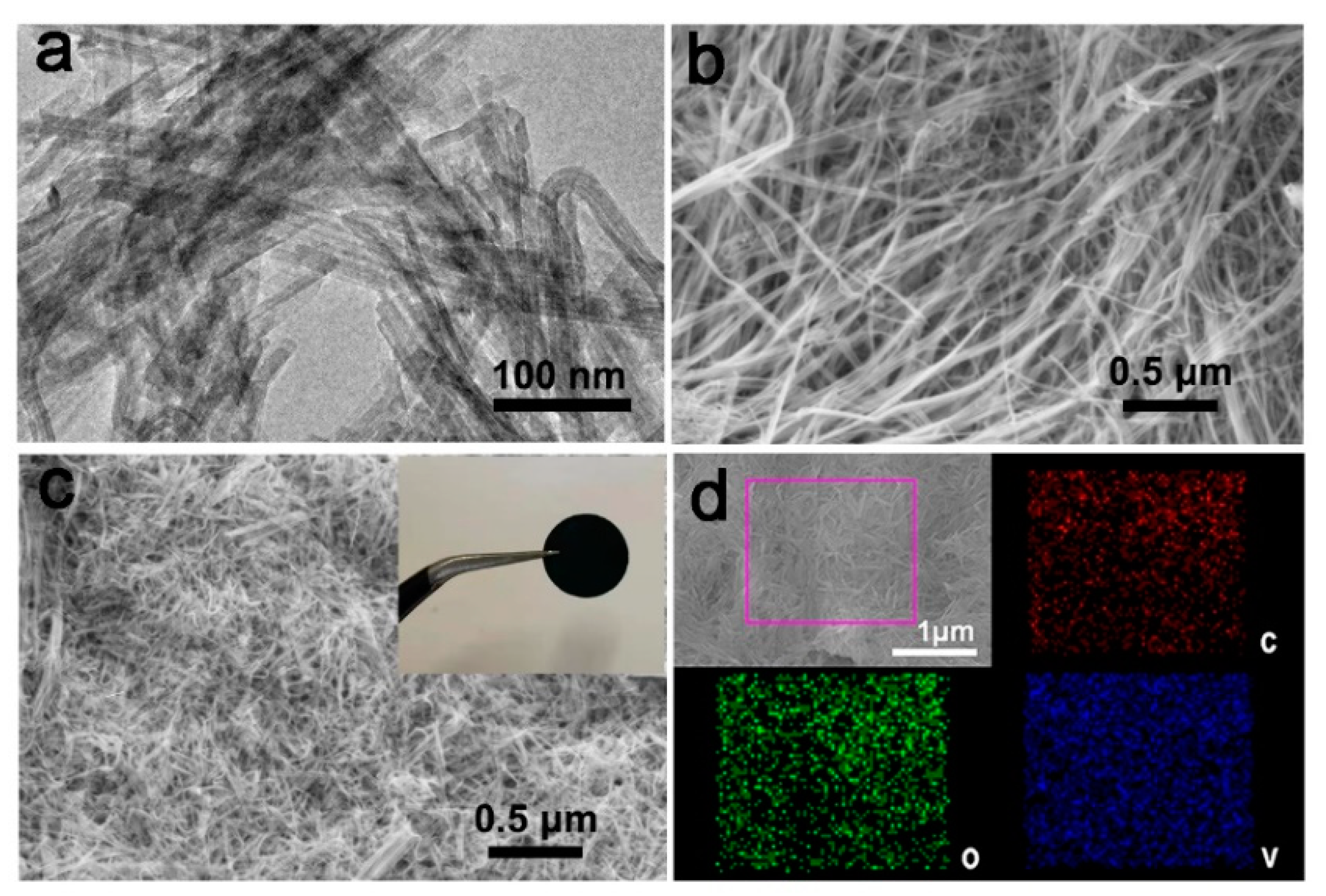
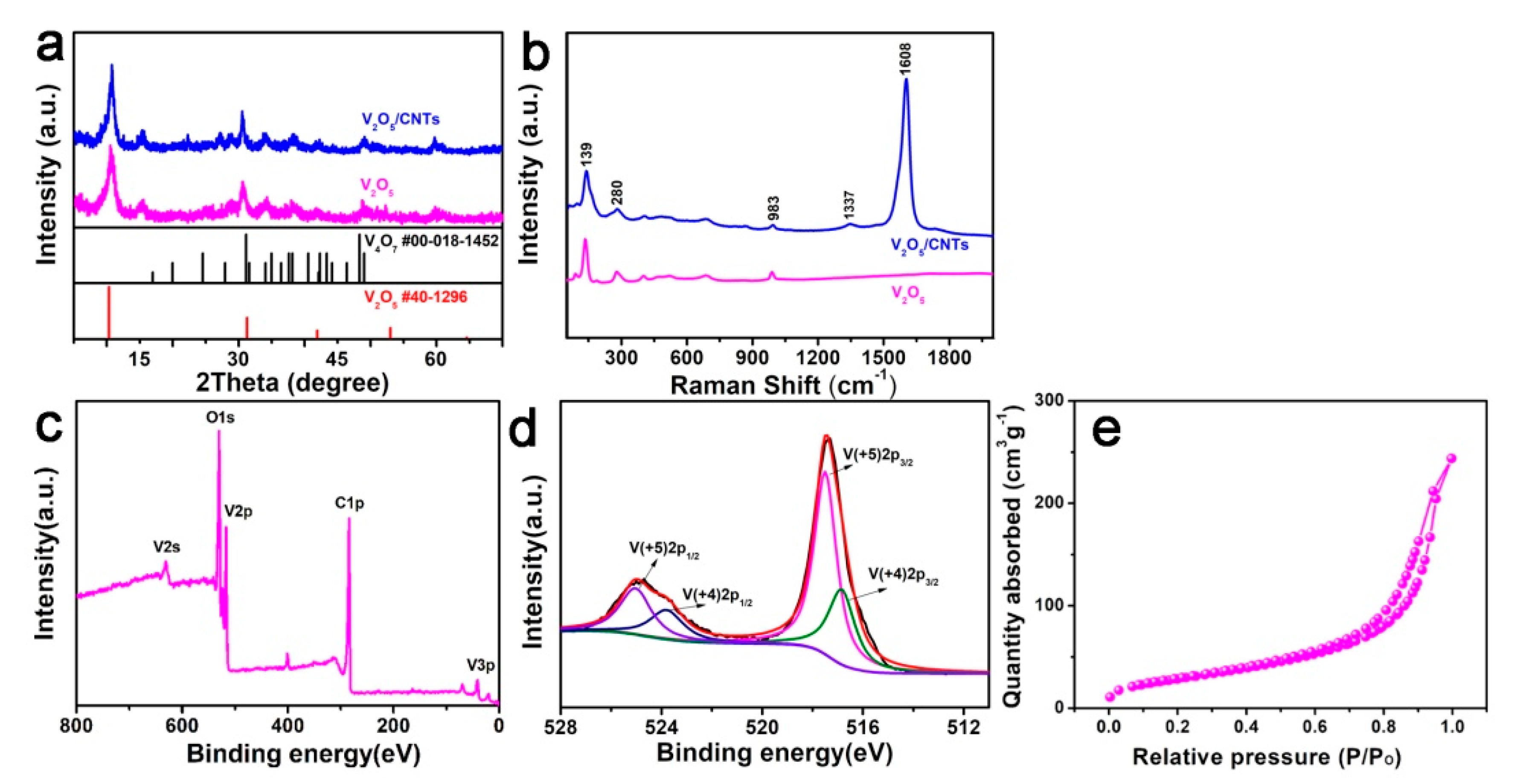
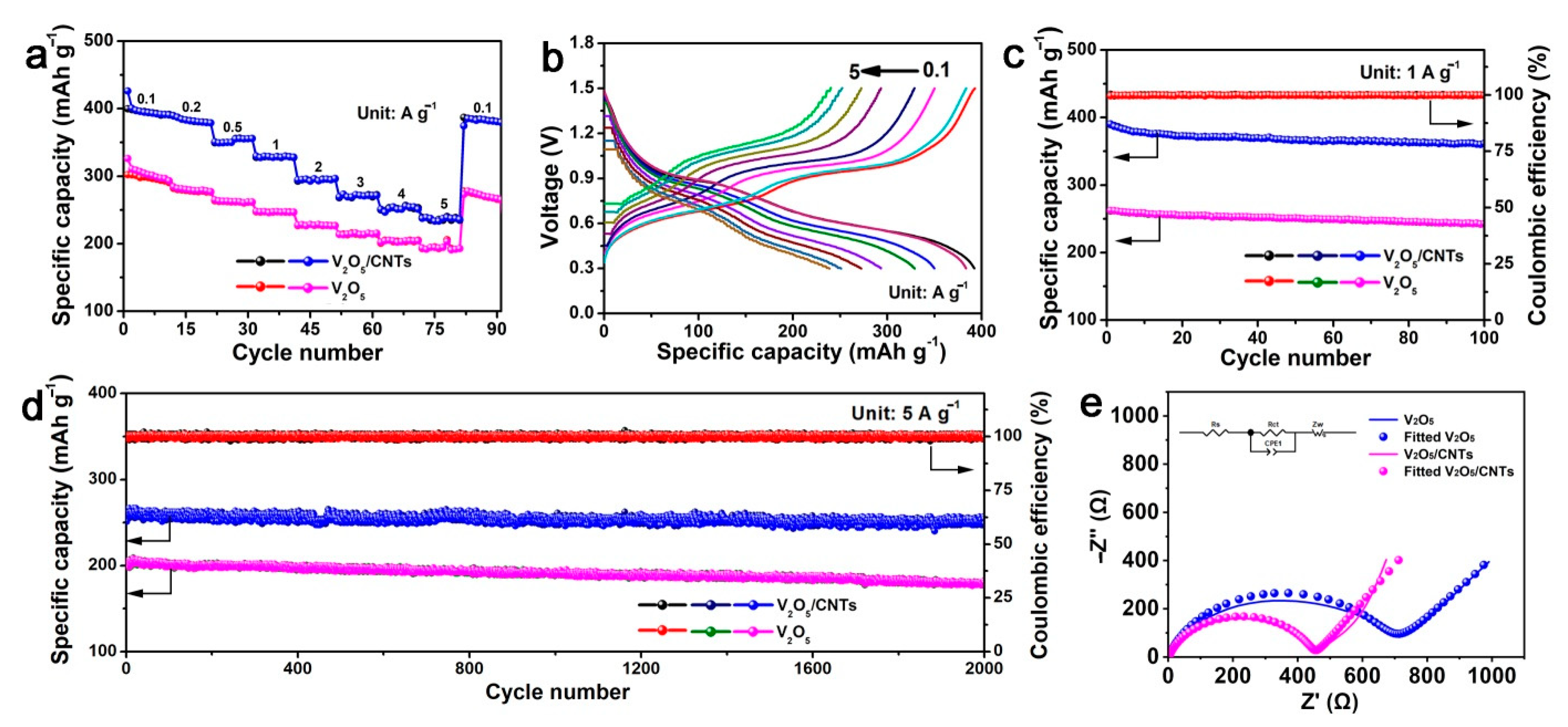
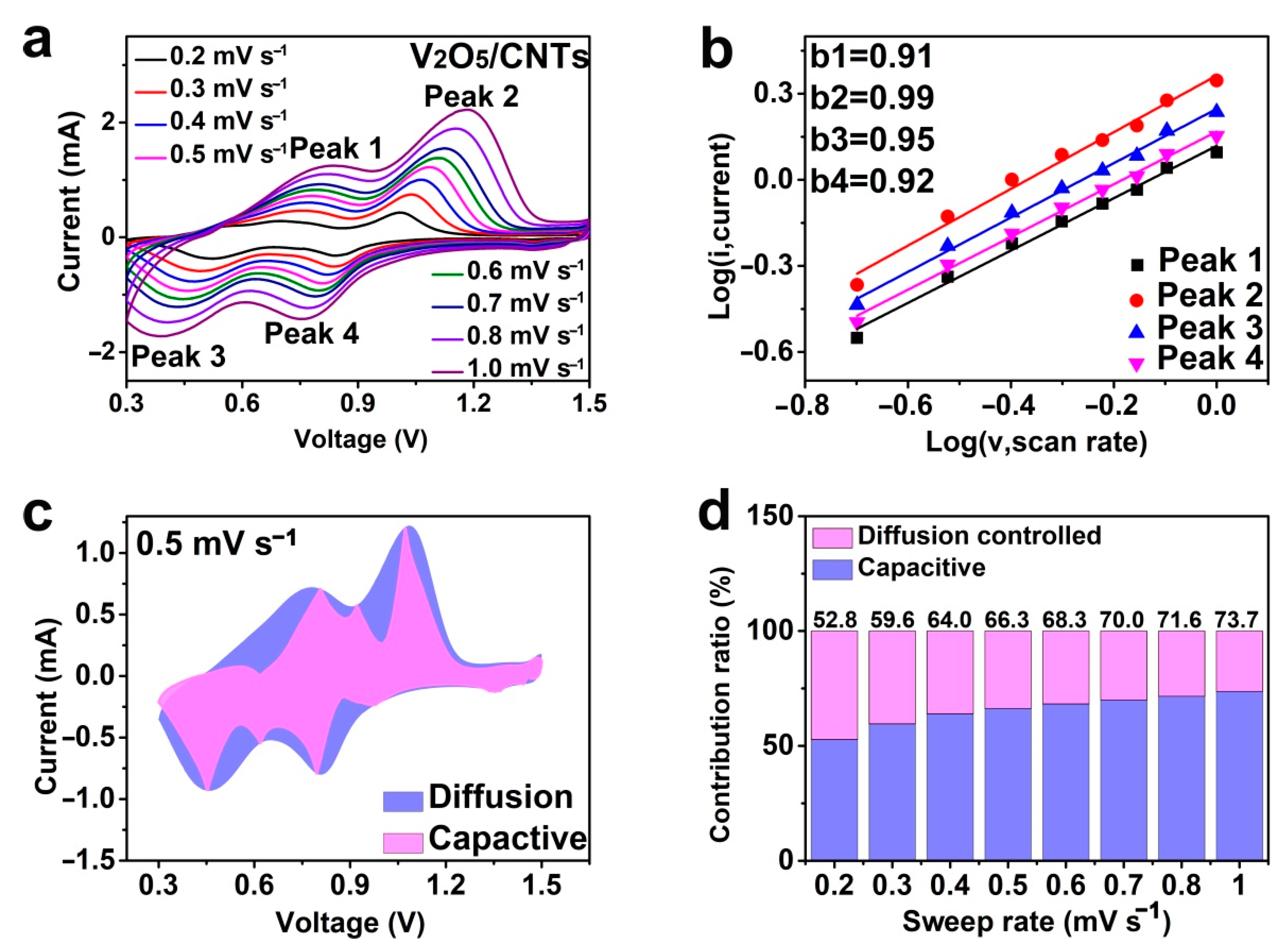
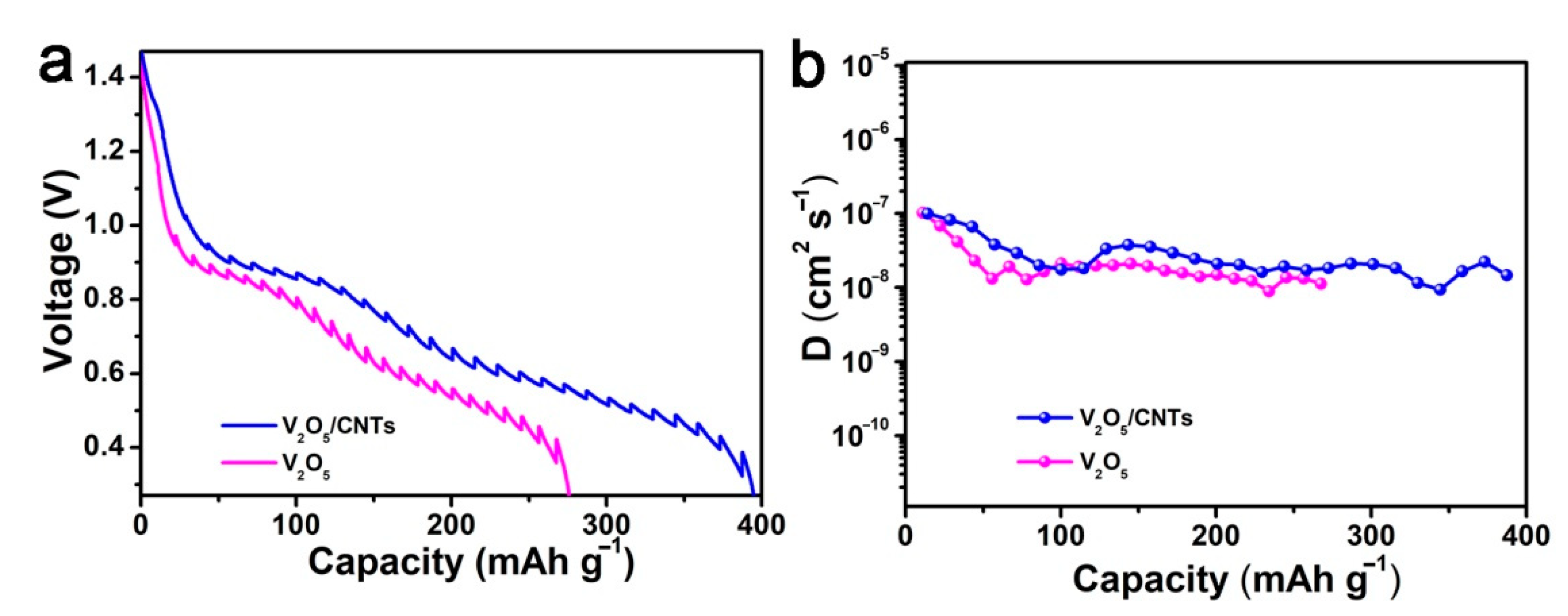
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Bao, Z.N.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Liu, J.Y.; Long, J.W.; Du, S.; Sun, B.; Zhu, S.G.; Li, J.J. Three-Dimensionally porous Li-ion and Li-S battery cathodes: A mini review for preparation methods and energy-storage performance. Nanomaterials 2019, 9, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Song, W.T.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Liu, C.C.; Lu, Q.Q.; Omar, A.; Mikhailova, D. A Facile Chemical Method Enabling Uniform Zn Deposition for Improved Aqueous Zn-Ion Batteries. Nanomaterials 2021, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, X.Y.; Yu, M.; Niu, Z.Q.; Cheng, F.Y.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.X.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Wang, Y.Y.; Wang, Y.X.; Li, J.J.; Xu, J.Z.; Liu, Y.C.; Jiao, L.F.; Cheng, F.Y. Ultrafast rechargeable zinc battery based on high-voltage graphite cathode and stable nonaqueous electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 32978–32986. [Google Scholar] [CrossRef]
- Dong, Y.; Jia, M.; Wang, Y.Y.; Xu, J.Z.; Liu, Y.C.; Jiao, L.F.; Zhang, N. Long-life zinc/vanadium pentoxide battery enabled by a concentrated aqueous ZnSO4 electrolyte with proton and zinc ion co-intercalation. ACS Appl. Energy Mater. 2020, 3, 11183–11192. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Liu, C.C.; Du, Y.H.; Wang, X.Y.; Ding, L.; Omar, A.; Mikhailova, D. Uniform Zn Deposition Achieved by Ag Coating for Improved Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef]
- Zhu, M.S.; Hu, J.P.; Lu, Q.Q.; Dong, H.Y.; Karnaushenko, D.D.; Becker, C.; Karnaushenko, D.D.; Li, Y.; Tang, H.M.; Qu, Z.; et al. A patternable and in situ formed polymeric zinc blanket for a reversible zinc anode in a skin-mountable microbattery. Adv. Mater. 2021, 33, 2007497. [Google Scholar] [CrossRef]
- Wang, X.Y.; Qin, X.H.; Lu, Q.Q.; Han, M.M.; Omar, A.; Mikhailova, D. Mixed phase sodium manganese oxide as cathode for enhanced aqueous zinc-ion storage. Chin. J. Chem. Eng. 2020, 28, 2214–2220. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ma, L.W.; Zhang, P.C.; Wang, H.Y.; Li, S.; Ji, S.J.; Wen, Z.S.; Sun, J.C. Vanadium pentoxide nanosheets as cathodes for aqueous zinc-ion batteries with high rate capability and long durability. Appl. Surf. Sci. 2020, 502, 144207. [Google Scholar] [CrossRef]
- Zampardi, G.; La, M.F. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Glatz, H.; Lizundia, E.; Pacifico, F.; Kundu, D. An organic cathode based dual-ion aqueous zinc battery enabled by a cellulose membrane. ACS Appl. Energy Mater. 2019, 2, 1288–1294. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.Y.; Qiu, M.D.; Xu, J.Z.; Liu, Y.C.; Jiao, L.F.; Cheng, F.Y. Rechargeable aqueous Zn-V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ma, L.W.; Sun, J.C. Vanadium pentoxide nanosheets in-situ spaced with acetylene black as cathodes for high-performance zinc-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 41297–41303. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, Y.; Zhang, L.L.; Liu, D.B.; Wang, C.D.; Song, L.; Niu, Z.Q.; Chen, J. Reversible oxygen redox chemistry in aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2019, 58, 7062–7067. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.H.; Wang, X.Y.; Sun, J.C.; Lu, Q.Q.; Omar, A.; Mikhailova, D. Polypyrrole wrapped V2O5 nanowires composite for advanced aqueous zinc-ion batteries. Front. Energy Res. 2020, 8, 199. [Google Scholar] [CrossRef]
- Wu, J.B.; Gao, X.; Yu, H.M.; Ding, T.P.; Yan, Y.X.; Yao, B.; Yao, X.; Chen, D.C.; Liu, M.L.; Huang, L. A scalable free-standing V2O5/CNT film electrode for supercapacitors with a wide operation voltage (1.6 V) in an aqueous electrolyte. Adv. Funct. Mater. 2016, 26, 6114–6120. [Google Scholar] [CrossRef]
- Jiang, H.F.; Cai, X.Y.; Qian, Y.; Zhang, C.Y.; Zhou, L.J.; Liu, W.L.; Li, B.S.; Lai, L.F.; Huang, W. V2O5 embedded in vertically aligned carbon nanotube arrays as free-standing electrodes for flexible supercapacitors. J. Mater. Chem. A 2017, 5, 23727–23736. [Google Scholar] [CrossRef]
- Yan, M.Y.; He, P.; Chen, Y.; Wang, S.Y.; Wei, Q.L.; Zhao, K.N.; Xu, X.; An, Q.Y.; Shuang, Y.; Shao, Y.; et al. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Tang, Y.; Liang, S.Q.; Wu, Z.X.; Fang, G.Z.; Cao, X.X.; Wang, C.; Lin, T.Q.; Pan, A.Q.; Zhou, J. Transition metal ion-preintercalated V2O5 as high-performance aqueous zinc-ion battery cathode with broad temperature adaptability. Nano Energy 2019, 61, 617–625. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Putro, D.Y.; Soundharrajan, V.; Sambandam, B.; Jo, J.; Park, S.; Lee, S.; Mathew, V.; Kim, J. K+ intercalated V2O5 nanorods with exposed facets as advanced cathodes for high energy and high rate zinc-ion batteries. J. Mater. Chem. A 2019, 7, 20335–20347. [Google Scholar] [CrossRef]
- Pang, Q.; Sun, C.L.; Yu, Y.H.; Zhao, K.N.; Zhang, Z.Y.; Voyles, P.M.; Chen, G.; Wei, Y.J.; Wang, X.D. H2V3O8 Nanowire/Graphene electrodes for aqueous rechargeable zinc ion batteries with high rate capability and large capacity. Adv. Energy Mater. 2018, 8, 1800144. [Google Scholar] [CrossRef]
- Du, Y.H.; Wang, X.Y.; Man, J.Z.; Sun, J.C. A novel organic-inorganic hybrid V2O5@polyaniline as high-performance cathode for aqueous zinc-ion batteries. Mater. Lett. 2020, 272, 127813. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Wang, Z.L.; Liu, B.T.; Cai, X.; Mai, W.J. 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries. Nano Energy 2020, 70, 104573. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.L.; Dai, X.; Wang, X.Y.; Niu, Z.Q.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Alfaruqi, M.H.; Putro, D.Y.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.K.; Kim, J. Na2V6O16·3H2O barnesite nanorod: An open door to display a stable and high energy for aqueous rechargeable Zn-ion batteries as cathodes. Nano Lett. 2018, 18, 2402–2410. [Google Scholar] [CrossRef]
- Sambandam, B.; Soundharrajan, V.; Kim, S.; Alfaruqi, M.H.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.K.; Kim, J. K2V6O16·2.7H2O nanorod cathode: An advanced intercalation system for high energy aqueous rechargeable Zn-ion batteries. J. Mater. Chem. A 2018, 6, 15530–15539. [Google Scholar] [CrossRef]
- Cai, Y.S.; Liu, F.; Luo, Z.G.; Fang, G.Z.; Zhou, J.; Pan, A.Q.; Liang, S.Q. Pilotaxitic Na1.1V3O7.9 nanoribbons/graphene as high-performance sodium ion battery and aqueous zinc ion battery cathode. Energy Storage Mater. 2018, 13, 168–174. [Google Scholar] [CrossRef]
- Li, Y.K.; Huang, Z.; Kalambate, P.K.; Zhong, Y.; Huang, Z.; Xie, M.; Shen, Y.; Huang, Y.H. V2O5 nanopaper as a cathode material with high capacity and long cycle life for rechargeable aqueous zinc-ion battery. Nano Energy 2019, 60, 752–759. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, S.; Ke, K.; Xiong, T.; Wang, Y.; Lim, B.K.D.; Lee, W.S.V.; Wang, Z.; Xue, J. Binder-free V2O5/CNT paper electrode for high rate performance zinc ion battery. Nanoscale 2019, 11, 19723–19728. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Qin, H.G.; Chen, L.L.; Wu, J.; Yang, Z.H. V2O5@CNTs as cathode of aqueous zinc ion battery with high rate and high stability. J. Alloys Compd. 2020, 842, 155912. [Google Scholar] [CrossRef]
- He, P.; Yan, M.Y.; Zhang, G.B.; Sun, R.M.; Chen, L.N.; An, Q.Y.; Mai, L.Q. Layered VS2 nanosheet-based aqueous Zn ion battery cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Islam, S.; Pham, D.T.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Electrochemical zinc intercalation in lithium vanadium oxide: A high-capacity zinc-ion battery cathode. Chem. Mater. 2017, 29, 1684–1694. [Google Scholar] [CrossRef]
- He, P.; Zhang, G.B.; Liao, X.B.; Yan, M.Y.; Xu, X.; An, Q.Y.; Liu, J.; Mai, L.Q. Sodium ion stabilized vanadium oxide nanowire cathode for high-performance zinc-ion batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Chen, L.; Zhou, X.F.; Liu, Z.P. Morphology-dependent electrochemical performance of zinc hexacyanoferrate cathode for zinc-ion battery. Sci. Rep. 2015, 5, 18263. [Google Scholar] [CrossRef]
- Li, G.L.; Yang, Z.; Jiang, Y.; Jin, C.H.; Huang, W.; Ding, X.L.; Huang, Y.H. Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 2016, 25, 211–217. [Google Scholar] [CrossRef]
- Bin, D.; Huo, W.C.; Yuan, Y.B.; Huang, J.H.; Liu, Y.; Zhang, Y.X.; Dong, F.; Wang, Y.G.; Xia, Y.Y. Organic-inorganic-induced polymer intercalation into layered composites for aqueous zinc-ion battery. Chem 2020, 6, 968–984. [Google Scholar] [CrossRef]
- Du, Y.H.; Wang, X.Y.; Sun, J.C. Tunable oxygen vacancy concentration in vanadium oxide as mass-produced cathode for aqueous zinc-ion batteries. Nano Res. 2021, 14, 754–761. [Google Scholar] [CrossRef]
- Zhang, N.; Jia, M.; Dong, Y.; Wang, Y.Y.; Xu, J.Z.; Liu, Y.C.; Jiao, L.F.; Cheng, F.Y. Hydrated layered vanadium oxide as a highly teversible cathode for rechargeable aqueous zinc batteries. Adv. Funct. Mater. 2019, 29, 1807331. [Google Scholar] [CrossRef]

| Cathodes | Rate (A g−1) | Capacity Retention | Final Capacity (mAh g−1) | Reference |
|---|---|---|---|---|
| V2O5/CNTs | 5 | 94% (2000 cycles) | 251 | This work |
| V2O5·nH2O | 6 | 71.0% (900 cycles) | 213 | [21] |
| Cu2+-V2O5 | 10 | 88.0% (5000 cycles) | 180 | [22] |
| K+-V2O5 | 8 | 96.0% (1500 cycles) | 172 | [23] |
| Graphene/H2V3O8 | 6 | 87.0% (2000 cycles) | 240 | [24] |
| V2O5@PANI | 5 | 93.8% (1000 cycles) | 201 | [25] |
| 2D V2O5 | 20 | 68.2% (500 cycles) | 117 | [26] |
| Zn0.25V2O5·nH2O | 2.4 | 80.0% (1000 cycles) | 208 | [27] |
| NaV3O8·1.5H2O | 4 | 82.0% (1000 cycles) | 120 | [28] |
| Na2V6O16·3H2O | 14 | 85% (1000 cycles) | 129 | [29] |
| K2V6O16·2.7H2O | 5 | 88% (229 cycles) | 139 | [30] |
| Na1.1V3O7.9/rGO | 1 | 93% (500 cycles) | 85 | [31] |
| Cathodes | Specific Capacity | Capacity Retention | Reference |
|---|---|---|---|
| V2O5/CNTs | 399 mAh g−1 (0.1 A g−1) 327 mAh g−1 (1 A g−1) | 5A g−1: 94% (2000 cycles) | This work |
| V2O5/CNTs nanopaper | 375 mAh g−1 (0.5 A g−1) | 10A g−1: 80.0% (500 cycles) | [32] |
| V2O5/CNTs (VCP) | 312 mAh g−1 (1 A g−1) | 1 A g−1: 81% (2000 cycles) | [33] |
| V2O5@CNTs | 293 mAh g−1 (0.3 A g−1) | 5 A g−1: 72.0% (6000 cycles) | [34] |
| Scan rate (mV s−1) | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 1.0 |
|---|---|---|---|---|---|---|---|---|
| Capacitive contribution (%) | 52.8 | 59.6 | 64.0 | 66.3 | 68.3 | 70.0 | 71.6 | 73.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ma, L.; Du, Y.; Lu, Q.; Yang, A.; Wang, X. Vanadium Pentoxide Nanofibers/Carbon Nanotubes Hybrid Film for High-Performance Aqueous Zinc-Ion Batteries. Nanomaterials 2021, 11, 1054. https://doi.org/10.3390/nano11041054
Liu X, Ma L, Du Y, Lu Q, Yang A, Wang X. Vanadium Pentoxide Nanofibers/Carbon Nanotubes Hybrid Film for High-Performance Aqueous Zinc-Ion Batteries. Nanomaterials. 2021; 11(4):1054. https://doi.org/10.3390/nano11041054
Chicago/Turabian StyleLiu, Xianyu, Liwen Ma, Yehong Du, Qiongqiong Lu, Aikai Yang, and Xinyu Wang. 2021. "Vanadium Pentoxide Nanofibers/Carbon Nanotubes Hybrid Film for High-Performance Aqueous Zinc-Ion Batteries" Nanomaterials 11, no. 4: 1054. https://doi.org/10.3390/nano11041054
APA StyleLiu, X., Ma, L., Du, Y., Lu, Q., Yang, A., & Wang, X. (2021). Vanadium Pentoxide Nanofibers/Carbon Nanotubes Hybrid Film for High-Performance Aqueous Zinc-Ion Batteries. Nanomaterials, 11(4), 1054. https://doi.org/10.3390/nano11041054







