Nano-Magnetic NiFe2O4 and Its Photocatalytic Oxidation of Vanillyl Alcohol—Synthesis, Characterization, and Application in the Valorization of Lignin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Solvents, and Starting Materials
2.2. Preparation of the Plant Extract
2.3. Synthesis and Characterization of NiFe2O4 Nanoparticles
2.4. Mössbauer Spectroscopy
2.5. Photocatalytic Reaction
2.6. Experimental Procedure for Reusing of the Catalyst
3. Results and Discussion
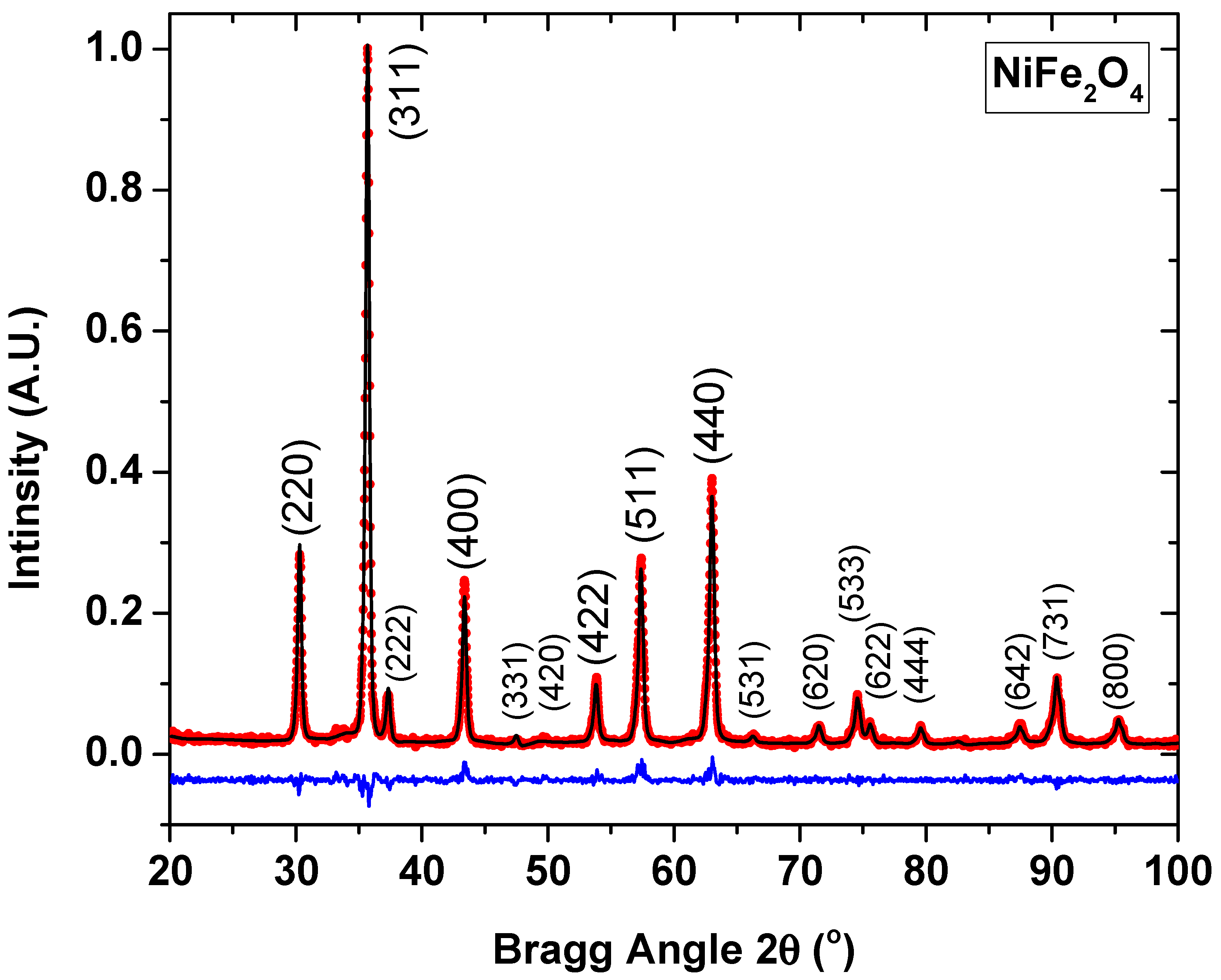
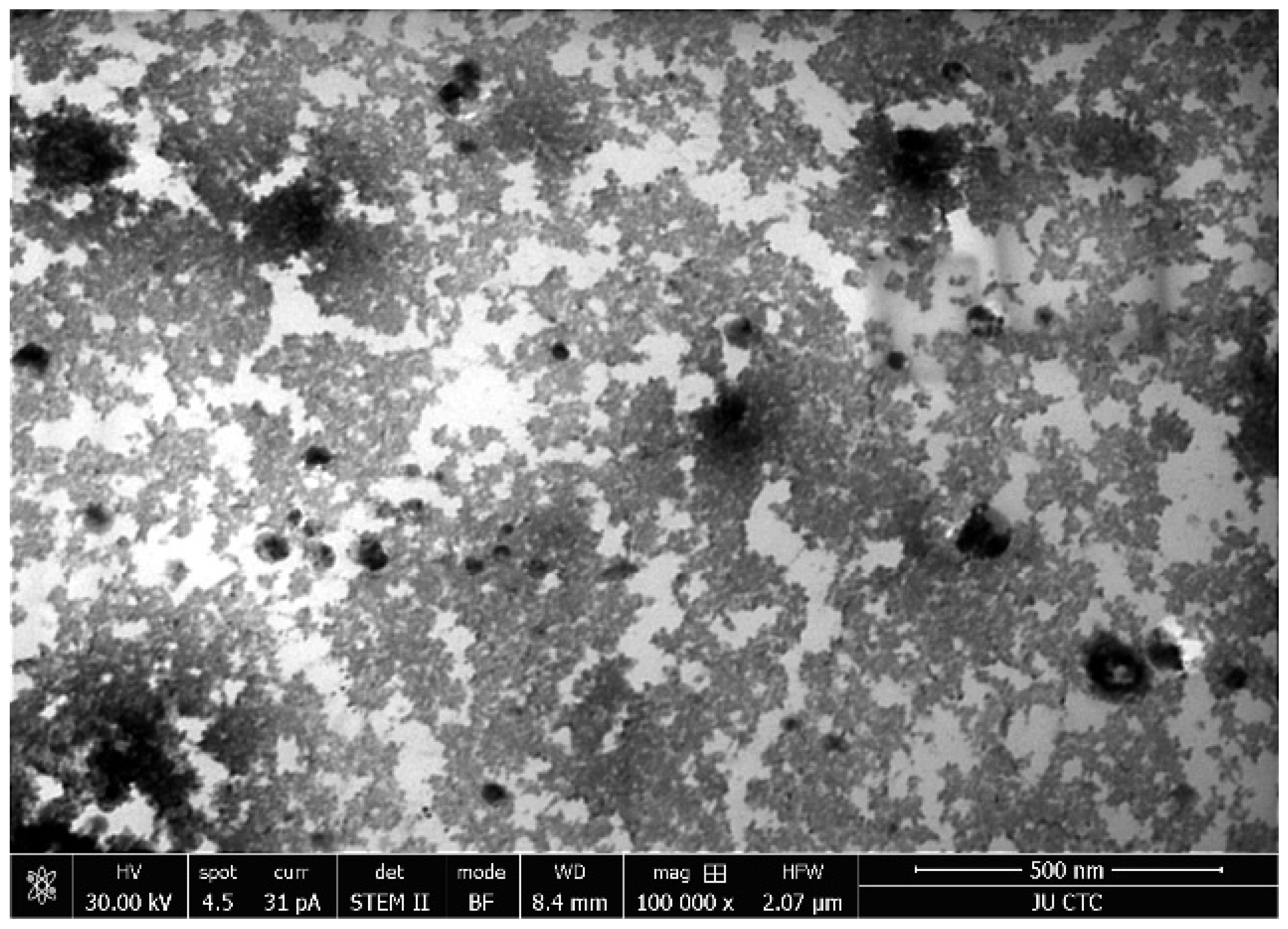
3.1. Structural Analysis
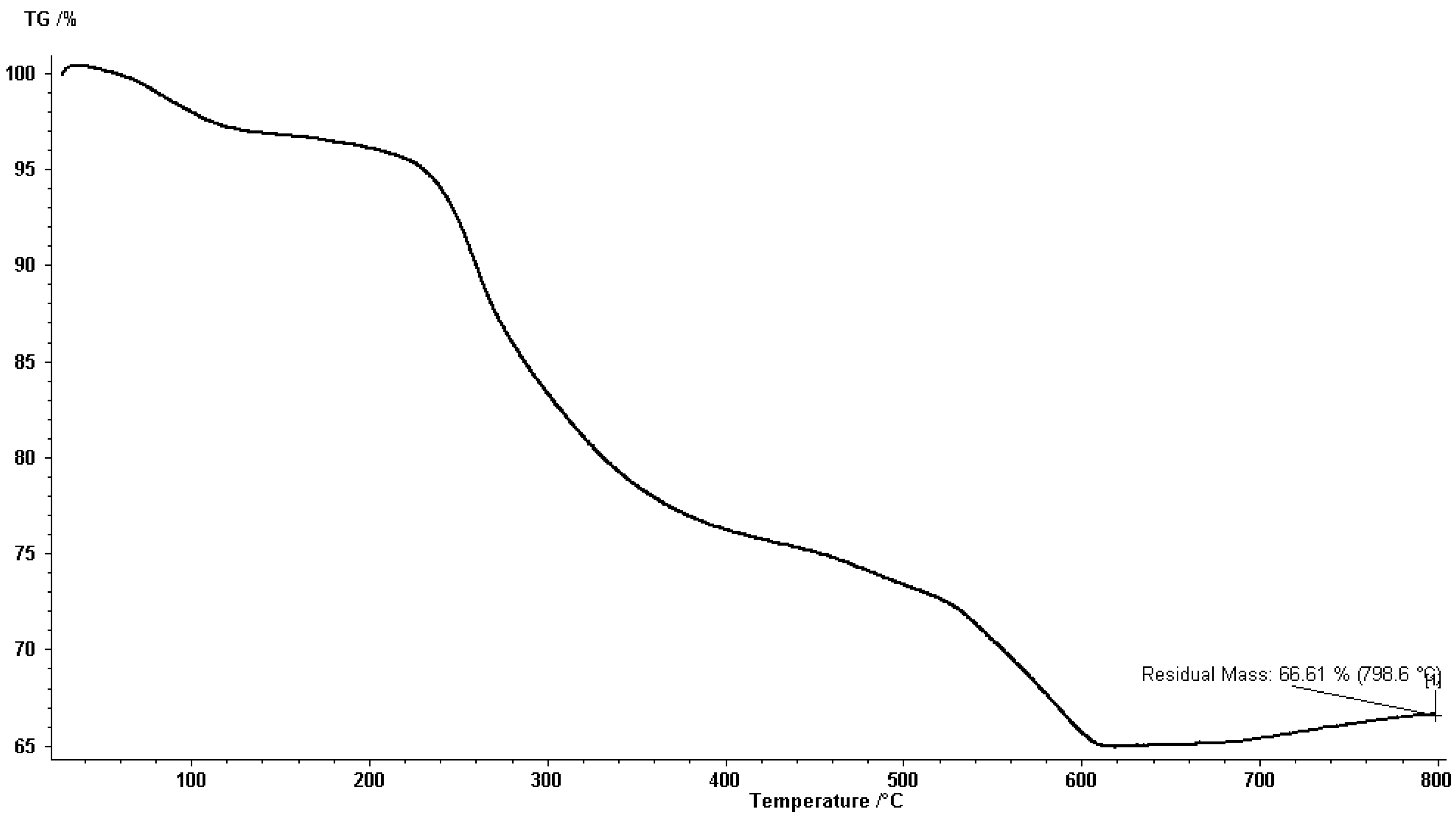
3.2. Thermo Gravimetric Analysis (TGA)
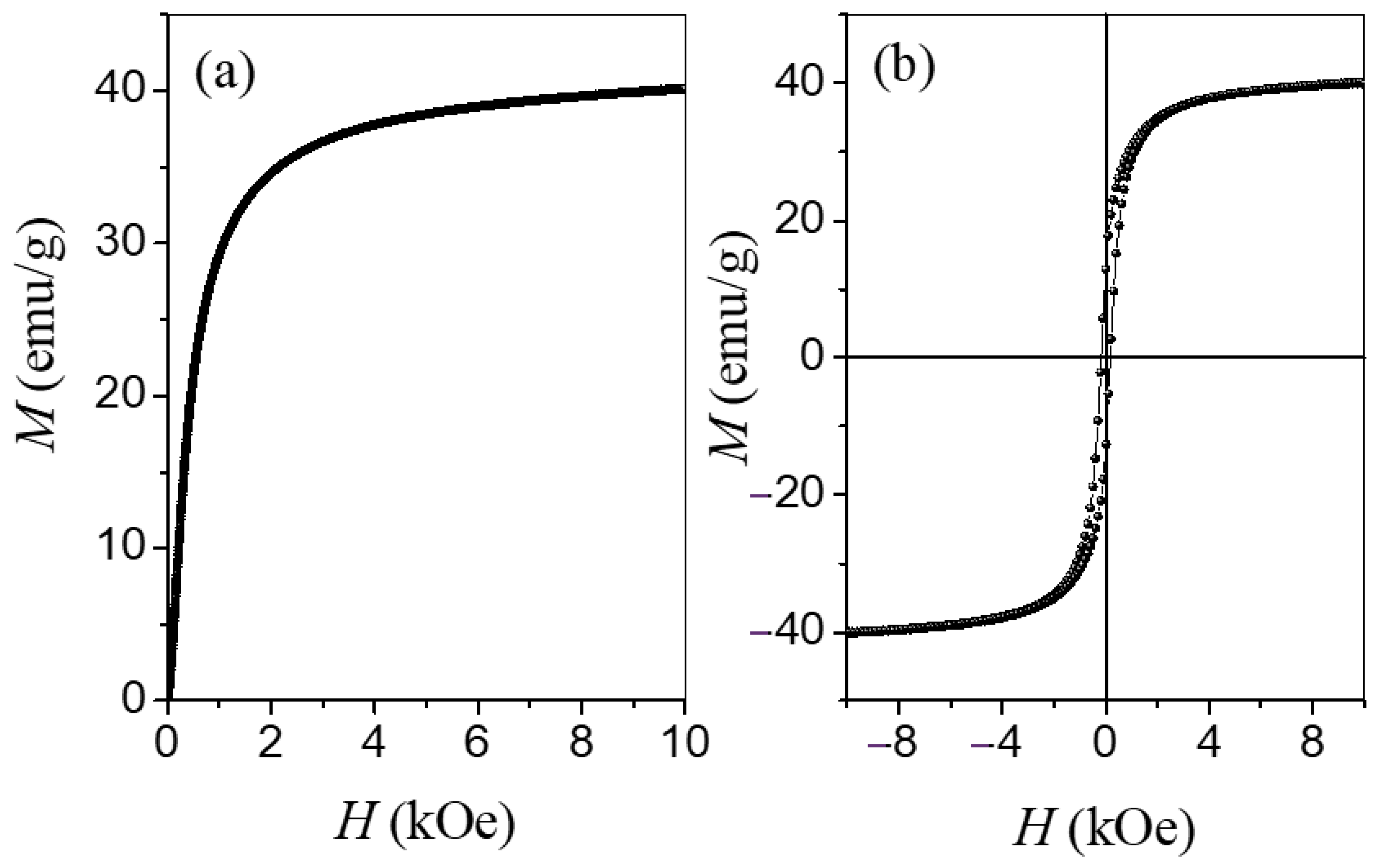
3.3. Magnetization
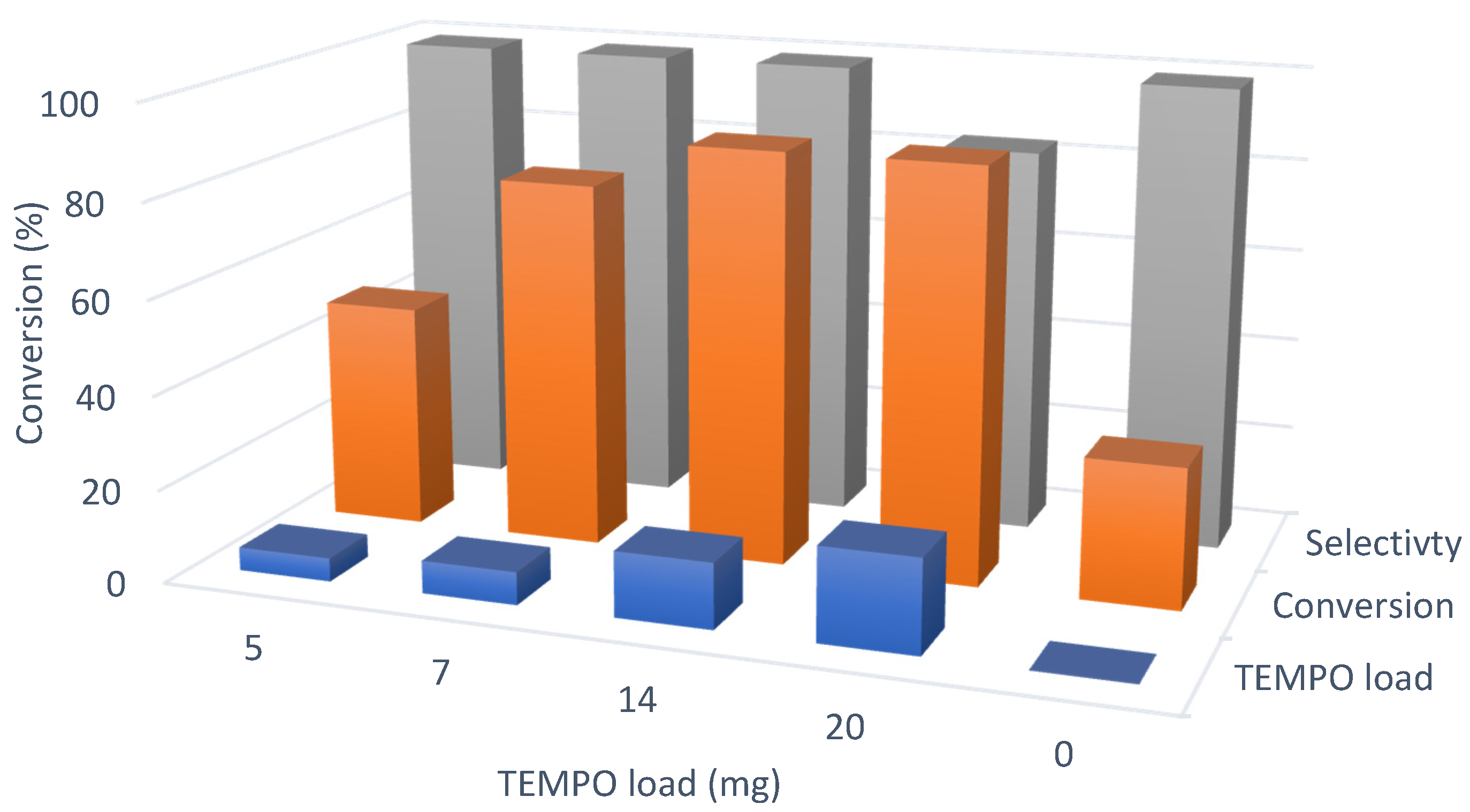
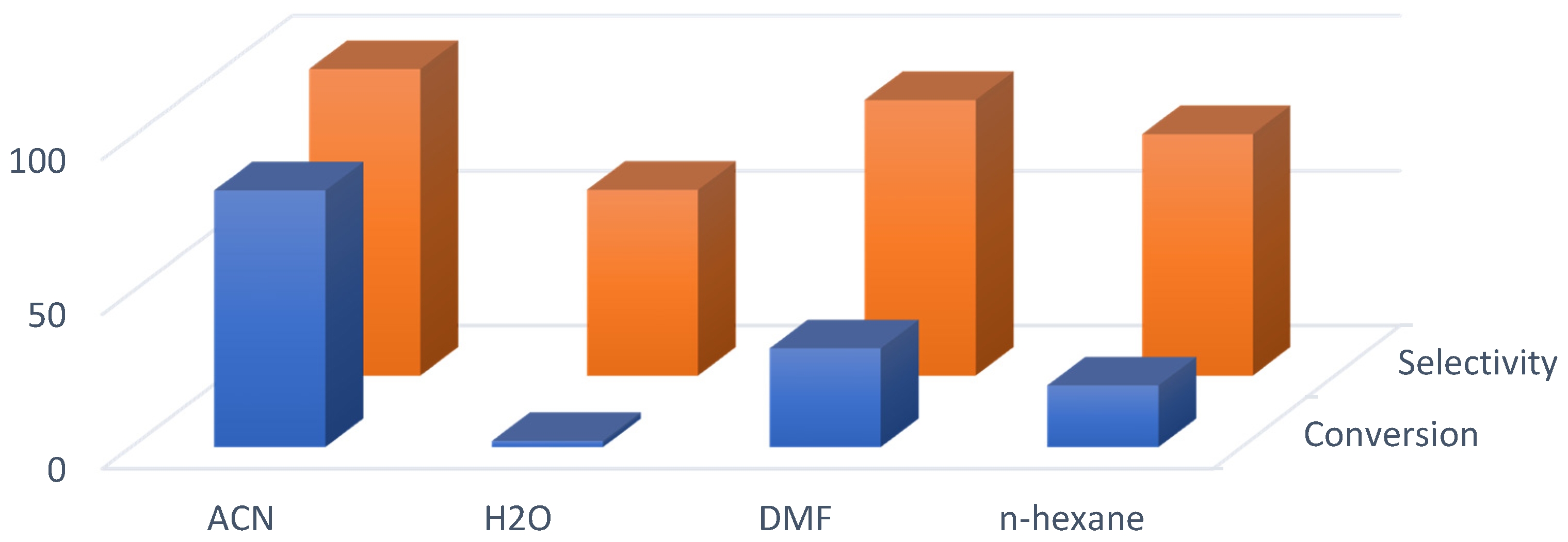
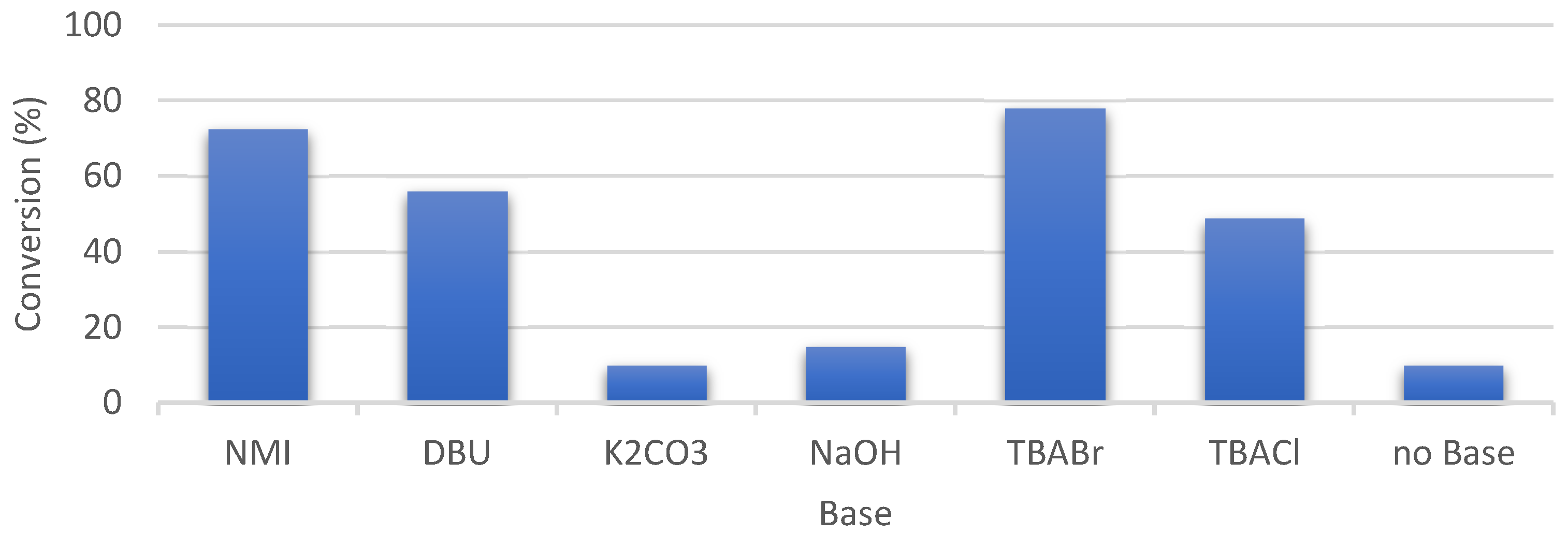
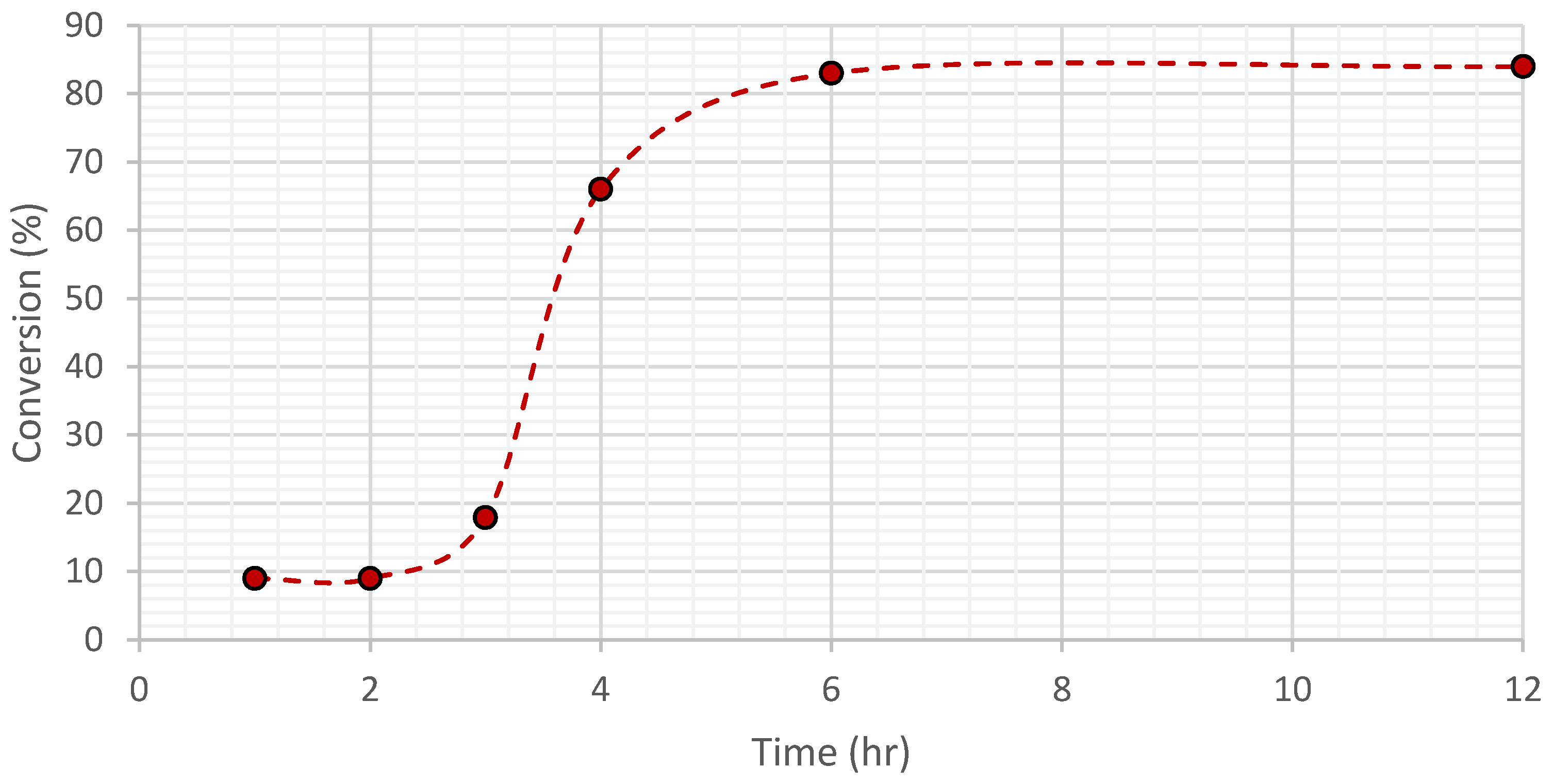
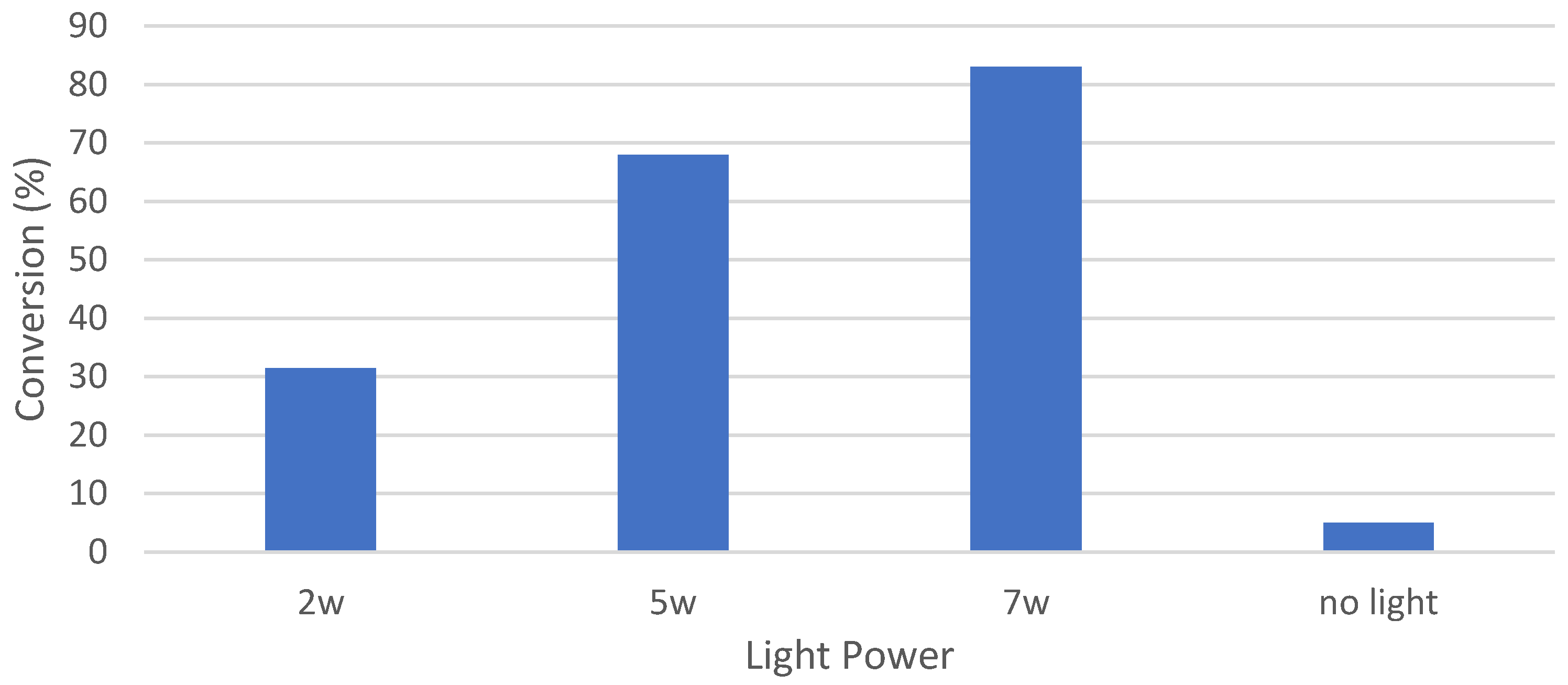
3.4. Catalytic Oxidation and Optimization
3.5. Comparative Studies
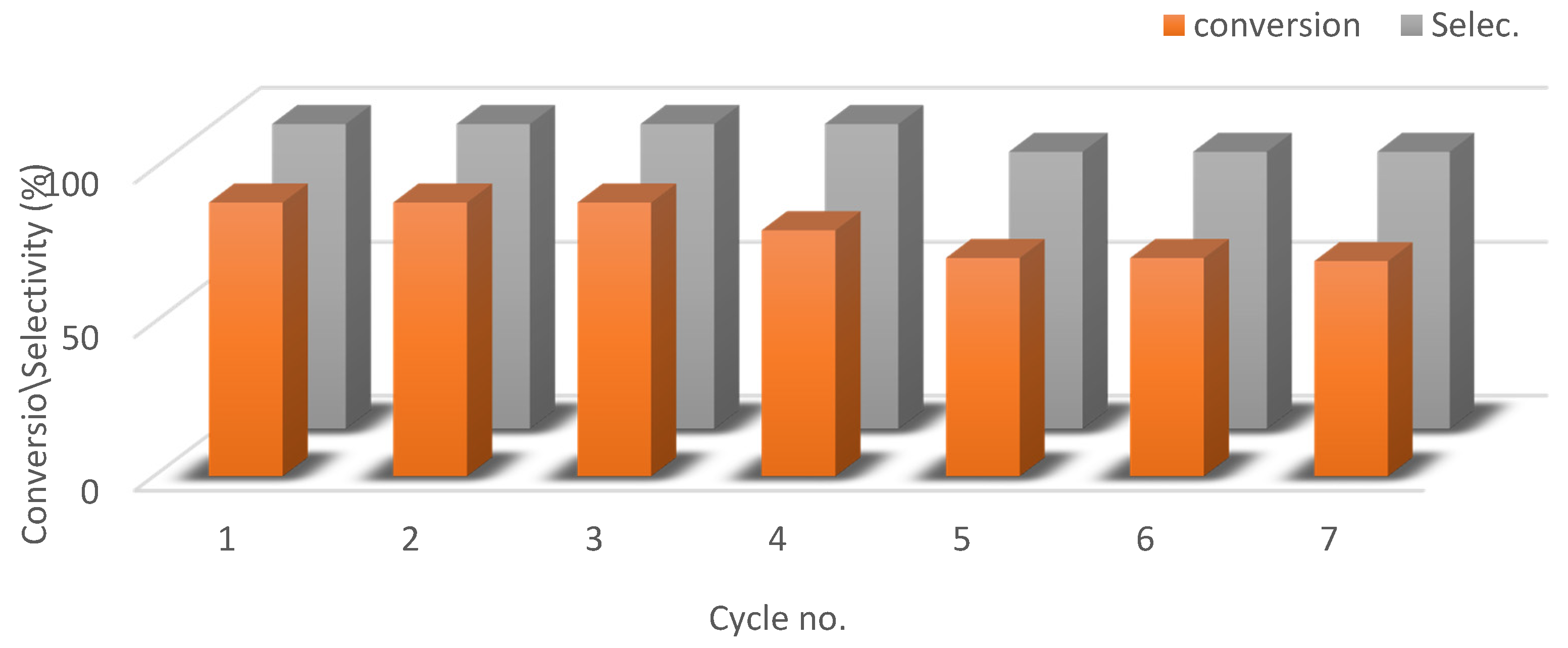
3.6. Recyclability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, J.H.; Luque, R.; Matharu, A.S. Green Chemistry, Biofuels, and Biorefinery. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 183–207. [Google Scholar] [CrossRef]
- Zakzesk, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Tunc, M.S.; van Heiningen, A.R. Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Carbohydr. Polym. 2011, 83, 8–13. [Google Scholar] [CrossRef]
- Rezanowich, A.; Goring, A. Polyelectrolyte expansion of a lignin sulfonate microgel. J. Colloid Sci. 1960, 15, 452–471. [Google Scholar] [CrossRef]
- Behling, R.; Valange, S.; Chatel, G. Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: What results? What limitations? What trends? Green Chem. 2016, 18, 1839–1854. [Google Scholar] [CrossRef]
- Perlack, R.D.; Wright, L.L.; Turhollow, A.F.; Graham, R.L.; Stokes, B.J.; Erbach, D.C. Biomass as feedstock for a bioenergy and bioproducts industry: The technical feasibility of a billion-ton annual supply. In DTIC Document; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2005. [Google Scholar]
- Lange, H.; Decina, S.; Crestini, C. Oxidative upgrade of lignin—Recent routes reviewed. Eur. Polym. J. 2013, 49, 1151–1173. [Google Scholar] [CrossRef]
- Azarpira, A.; Ralph, J.; Lu, F. Catalytic alkaline oxidation of lignin and its model compounds: A pathway to aromatic biochemicals. BioEnergy Res. 2014, 7, 78–86. [Google Scholar] [CrossRef]
- Dai, J.; Patti, A.F.; Saito, K. Recent developments in chemical degradation of lignin: Catalytic oxidation and ionic liquids. Tetrahedron Lett. 2016, 57, 4945–4951. [Google Scholar] [CrossRef]
- Pan, J.; Fu, J.; Lu, X. Microwave-assisted oxidative degradation of lignin model compounds with metal salts. Energy Fuels 2016, 29, 4503–4509. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 4888–4907. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Baerns, M. Aspects of Heterogeneous Catalysis and of Its Industrial and Environmental Practice. In Reference Module in Chemistry. Mol. Sci. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Gale, M.; Cai, C.C.; Leslie, K.; Abdul-Aziz, G. Heterogeneous Catalyst Design Principles for the Conversion of Lignin into High-Value Commodity Fuels and Chemicals. ChemSusChem 2020, 13, 1947–1966. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M.; Che, M. Applications of Photoluminescence Techniques to the Characterization of Solid Surfaces in Relation to Adsorption, Catalysis, and Photocatalysis. Adv. Catal. 1999, 44, 119–257. [Google Scholar]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein. J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Kolenˇcík, M.; Nˇemcová, Y.; Schröfel, A.; Peikertová, P.; Slabotinský, J.; Kratošová, G. Biosilica-nanogold composite: Easy-to-prepare catalyst for soman degradation. Arab. J. Chem. 2019, 12, 262–271. [Google Scholar]
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403. [Google Scholar] [CrossRef]
- Ye, Z.; Giraudon, J.M.; Nuns, N.; Simon Geyter, P.N.; De Morent, R.; Lamonier, J.F. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation. Appl. Catal. B 2018, 223, 154–166. [Google Scholar] [CrossRef]
- Obregón, S.; Munoz-Batista, M.J.; Fernandez-Garcia, M.; Kubacka, A.; Colon, G. Cu–TiO2 systems for the photocatalytic H2 production: Influence of structural and surface support features. Appl. Catal. B 2015, 179, 468–478. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumara, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Langille, M.R.; Mirkin, C.A. Synthesis of silver nanorods by low energy excitation of spherical plasmonic seeds. Nano Lett. 2011, 11, 2495–2498. [Google Scholar]
- Davaran, S.; Kouhi, M.; Akbarzadeh, A. Bimetallic nanoparticles: Preparation, properties, and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 376–380. [Google Scholar]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, A.; Dinu-Pirvu, C.; Claudiu Fierascu, R.; Anuta, V.; Stefan Velescu, B.; Jinga, M.; Jinga, V. Short Overview of Recent Developments on Antimicrobial Coatings Based on Phyto synthesized Metal Nanoparticles. J. Coating 2019, 9, 787. [Google Scholar] [CrossRef]
- Laokul, P.; Amornkitbamrung, V.; Seraphin, S.; Maensiri, S. Characterization and Magnetic Properties of Nanocrystalline CuFe2O4, NiFe2O4, ZnFe2O4 Powders Prepared by the Aloe Vera Extract Solution. Curr. Appl. Phys. 2011, 11, 101–108. [Google Scholar] [CrossRef]
- Elavazhagan, T.; Arunachalam, K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed. 2011, 6, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green. Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Kavitha, K.S.; Baker, S.; Rakshith, D.; Kavitha, H.U.; Rao, H.C.Y.; Harini, B.P.; Satish, S. Plants as green source towards synthesis of nanoparticles. Int. Res. J. Biol. Sci. 2013, 2, 66–76. [Google Scholar]
- Narayanasamy, A.; Jeyadevan, B.; Chinnasamy, C.N.; Ponpandian, N.; Greneche, J.M. Structural, magnetic and electrical properties of spinel ferrite nanoparticles. In Proceedings of the Ninth International Conference on Ferrites, San Francisco, CA, USA, 3 January 2005; pp. 867–875. [Google Scholar]
- Zhou, Z.H.; Xue, J.M.; Chan, H.S.O.; Wang, J. Nanocomposites of NiFe2O4 in silica: Synthesis, magnetic and optical properties. Mater. Chem. Phys. 2002, 75, 181–185. [Google Scholar] [CrossRef]
- Rincón-Granados, K.L.; Vázquez-Olmos, A.R.; Vega-Jiménez, A.; Ruiz, F.; Garibay-Febles, V.; Ximénez-Fyvie, L. Preparation, characterization and photocatalytic activity of NiO, Fe2O3 and NiFe2O4. Materialia 2021, 15, 177–182. [Google Scholar]
- Colmenares, J.C. Selective redox photocatalysis: Is there any chance for solar bio-refineries. Curr. Opin. Green Sustain. Chem. 2019, 15, 38–46. [Google Scholar] [CrossRef]
- Kombaiah, K.; Judith Vijaya, J.; John Kennedy, L.; Kaviyarasu, K. Catalytic studies of NiFe2O4 nanoparticles prepared by conventional and microwave combustion method. Mater. Chem. Phys. 2019, 221, 11–28. [Google Scholar] [CrossRef]
- Tolba, R.; Tian, M.; Wen, J.; Jiang, Z.H.; Chen, A. Electrochemical oxidation of lignin at IrO2-based oxide electrodes. J. Electroanal. Chem. 2010, 649, 9–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Fatehi, P. Lignin-derived platform molecules through TEMPO catalytic oxidation strategies. Prog. Energy Combust. Sci. 2019, 72, 59–89. [Google Scholar]
- Stahl, S.S.; Coon, J.; Rahimi, A.; Ulbrich, A. Selective CeO Bond Cleavage of Oxidized Lignin and Lignin-Type Materials into Simple Aromatic Compounds. U.S. Patent No. 9,359,391, 7 January 2016. [Google Scholar]
- Jha, A.; Patil, K.R.; Rode, C.V. Mixed Co–Mn oxide-catalysed selective aerobic oxidation of vanillyl alcohol to vanillin in base free conditions. ChemPlusChem 2013, 78, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Kumar, M.; Chhoker, S.; Srivastava, G.; Jewariya, M.; Singh, V.N. Structural, magnetic, dielectric and optical properties of nickel ferrite nanoparticles synthesized by co-precipitation method. J. Mol. Struct. 2014, 1076, 55–62. [Google Scholar] [CrossRef]
- Sainsbury, P.D.; Hardiman, E.M.; Ahmad, M.; Otani, H.; Seghezzi, N.; Eltis, L.D.; Bugg, T.D.H. Breaking down lignin to high-value chemicals: The conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem. Biol. 2013, 8, 2151–2156. [Google Scholar] [CrossRef]
- Chen, H.P.; Chow, M.; Liu, C.C.; Lau, A.; Liu, J.; Eltis, L.D. Vanillin catabolism in Rhodococcus jostii RHA1. Appl. Environ. Microbiol. 2012, 78, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chen, P.; Zhang, S.; Li, S.; Yan, X.; Wang, N.; Liang, N.; Li, H. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci. Rep. 2016, 6, 34644. [Google Scholar] [CrossRef]
- Converti, A.; Aliakbarian, B.; Domínguez, J.M.; Vázquez, G.B.; Perego, P. Microbial production of biovanillin. Braz. J. Microbiol. 2010, 41, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Abd Hamid, S.B. Nanosized spinel Cu-Mn mixed oxide catalyst prepared via solvent evaporation for liquid phase oxidation of vanillyl alcohol using air and H2O2. RSC Adv. 2016, 6, 96314–96326. [Google Scholar] [CrossRef]
- Wu, M.; Pang, J.H.; Song, P.P.; Peng, J.J.; Xu, F.; Li, Q.; Zhang, X.M. Visible light-driven oxidation of vanillyl alcohol in air with Au–Pd bimetallic nanoparticles on phosphorylated hydrotalcite. New J. Chem. 2019, 43, 1964–1971. [Google Scholar] [CrossRef]
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, G.; Pan, Q.; Wang, H.; Shen, Z.; Peng, B.; Busser, G.W.; Wang, X.; Muhler, M. Highly Selective Anaerobic Oxidation of Alcohols Over Fe-doped SrTiO3 Under Visible Light. J. Catal. 2009, 266, 279. [Google Scholar]
- Zhang, B.; Li, J.; Gao, Y.; Chong, R.; Wang, Z.; Guo, L.; Zhang, X.; Li, C. To boost photocatalytic activity in selective oxidation of alcohols on ultrathin Bi2MoO6 nanoplates with Pt nanoparticles as cocatalyst. J. Catal. 2017, 345, 96–103. [Google Scholar] [CrossRef]
- Higashimoto, S.; Okada, K.; Morisugi, T.; Azuma, M.; Ohue, H.; Kim, T.H.; Matsuoka, M.; Anpo, M. Effect of Surface Treatment on the Selective Photocatalytic Oxidation of Benzyl Alcohol into Benzaldehyde by O2 on TiO2 Under Visible Light. Top. Catal. 2010, 53, 578. [Google Scholar] [CrossRef]
- Du, M.; Zeng, G.; Hui, C.; Ye, C.; Jin, H.; Huang, J.; Sun, D.H.; Li, Q.B.; Chen, B.; Li, X. Solvent-free photo-thermocatalytic oxidation of benzyl alcohol on Pd/TiO2 (B) nanowires. Mol. Catal. 2020, 483, 110771. [Google Scholar] [CrossRef]
- Al-Hunaiti, A.; Mohaidat, Q.; Bsoul, I.; Mahmood, S.H.; Taher, D.; Hussein, T. Synthesis and Characterization of Novel Phyto-Mediated Catalyst, and Its Application for a Selective Oxidation of (VAL) into Vanillin under Visible Light. Catalysts 2020, 10, 839. [Google Scholar] [CrossRef]
- Smit, J.; Wijn, H.P.J. Ferrites; Wiley: New York, NY, USA, 1959. [Google Scholar]
- Mahmood, S.H. Properties and Synthesis of Hexaferrites. In Hexaferrite Permanent Magnetic Materials; Mahmood, S.H., Abu-Aljarayesh, I., Eds.; Materials Research Forum LLC: Millersville, PA, USA, 2016; Volume 4, pp. 74–110. [Google Scholar]
- Mahmood, S.H. Magnetic anisotropy in fine magnetic particles. J. Magn. Magn. Mater. 1993, 118, 359–364. [Google Scholar] [CrossRef]
- Mahmood, S.H.; Bsoul, I. Hopkinson peak and superparamagnetic effects in BaFe12-xGaxO19 nanoparticles. In Proceedings of the EPJ Web of Conferences, Dubna, Russia, 3–7 July 2012; Volume 29, p. 00039. [Google Scholar]
- Aneesh Kumar, K.S.; Bhowmik, R.N.; Mahmood, S.H. Role of pH value during chemical reaction, and site occupancy of Ni2+ and Fe3+ ions in spinel structure for tuning room temperature magnetic properties of Ni1.5Fe1.5O4 ferrite. J. Magn. Magn. Mater. 2016, 406, 60–71. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Gubbala, S.; Kale, A.; Egelhoff, W.F., Jr. A comparison of the magnetic characteristics of nanocrystalline nickel, zinc, and manganese ferrites synthesized by reverse micelle technique. Mater. Sci. Eng. B 2004, 111, 164–174. [Google Scholar] [CrossRef]
- Lehlooh, A.F.; Mahmood, S.H. Mössbauer spectroscopy of Fe3O4 ultrafine particles. J. Magn. Magn. Mater. 1995, 151, 163–166. [Google Scholar] [CrossRef]
- Shilpy, M.; Ali, M.; Ehsan, M.; Hussein, T.; Bee Abd Hamid, A.S.; Eaqub Ali, M. Performance of cobalt titanate towards H2O2 based catalytic oxidation of lignin model compound. RSC Adv. 2015, 5, 79644–79653. [Google Scholar] [CrossRef]
- Mate, V.R.; Jha, A.; Joshi, U.D.; Patil, K.R.; Shirai, M.; Rode, C.V. Effect of preparation parameters on characterization and activity of Co3O4 catalyst in liquid phase oxidation of lignin model substrates. Appl. Catal. A 2014, 487, 130–138. [Google Scholar] [CrossRef]
- Karkas, M.D.; Bosque, I.; Matsuura, B.S.; Stephenson, C.R.J. Photocatalytic Oxidation of Lignin Model Systems by Merging Visible-Light Photoredox and Palladium Catalysis. Org. Lett. 2016, 18, 5166–5169. [Google Scholar] [CrossRef]
- Figueiredo, C.; Arán-Aís, M.; Feliu, R.; Kontturi, J.M.; Kallio, K.; Tanja, K. Pt catalysts modified with Bi: Enhancement of the catalytic activity for alcohols oxidation in alkaline media. J. Catal. A 2009, 366, 227–231. [Google Scholar] [CrossRef]
- Zheng, M.; Lin, K.A.; Lin, C. TEMPO-Functionalized Silica as an Efficient and Recyclable Oxidation Catalyst for Conversion of a Lignin Model Compound to Value-Added Products. Waste Biomass Valor. 2020, 11, 6917–6928. [Google Scholar] [CrossRef]
- Zope, B.N.; Hibbitts, D.D.; Neurock, M.; Davis, R.J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–78. [Google Scholar] [CrossRef]
- Bellardita, M.; Yurdakal, S.; Tek, B.S.; Degirmenci, C.; Palmisano, G.; Loddo, V.; Plamisano, L.; Soria, J.; Sanz, J.; Augugliaro, V. Tuning the selectivity to aldehyde via pH regulation in the photocatalytic oxidation of 4-methoxybenzyl alcohol and vanillyl alcohol by TiO2 catalysts. J. Environ. Chem. Eng. 2021, 9, 105308–105310. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size specific catalytic activity of polymer-stabilized gold nanoclusters for aerobic alcohol oxidation in water. J. Am. Chem. Soc. 2005, 127, 9374–9375. [Google Scholar] [CrossRef] [PubMed]
- Tsunoyama, H.; Tsukuda, T.; Sakurai, H. Synthetic application of PVP-stabilized Au nanocluster catalyst to aerobic oxidation of alcohols in aqueous solution under ambient conditions. Chem. Lett. 2007, 36, 212–221. [Google Scholar] [CrossRef]
- Fargues, C.; Mathias, A.; Rodrigues, A. Kinetics of vanillin production from kraft lignin oxidation. Ind. Eng. Chem. Res. 2016, 35, 28–36. [Google Scholar] [CrossRef]
- Gladysz, J.A. The experimental assay of catalyst recovery: General concepts. In Recoverable and Recyclable Catalysts; Benaglia, M., Ed.; Wiley: Chichester, UK, 2009; Chapter 1; pp. 1–14. [Google Scholar]

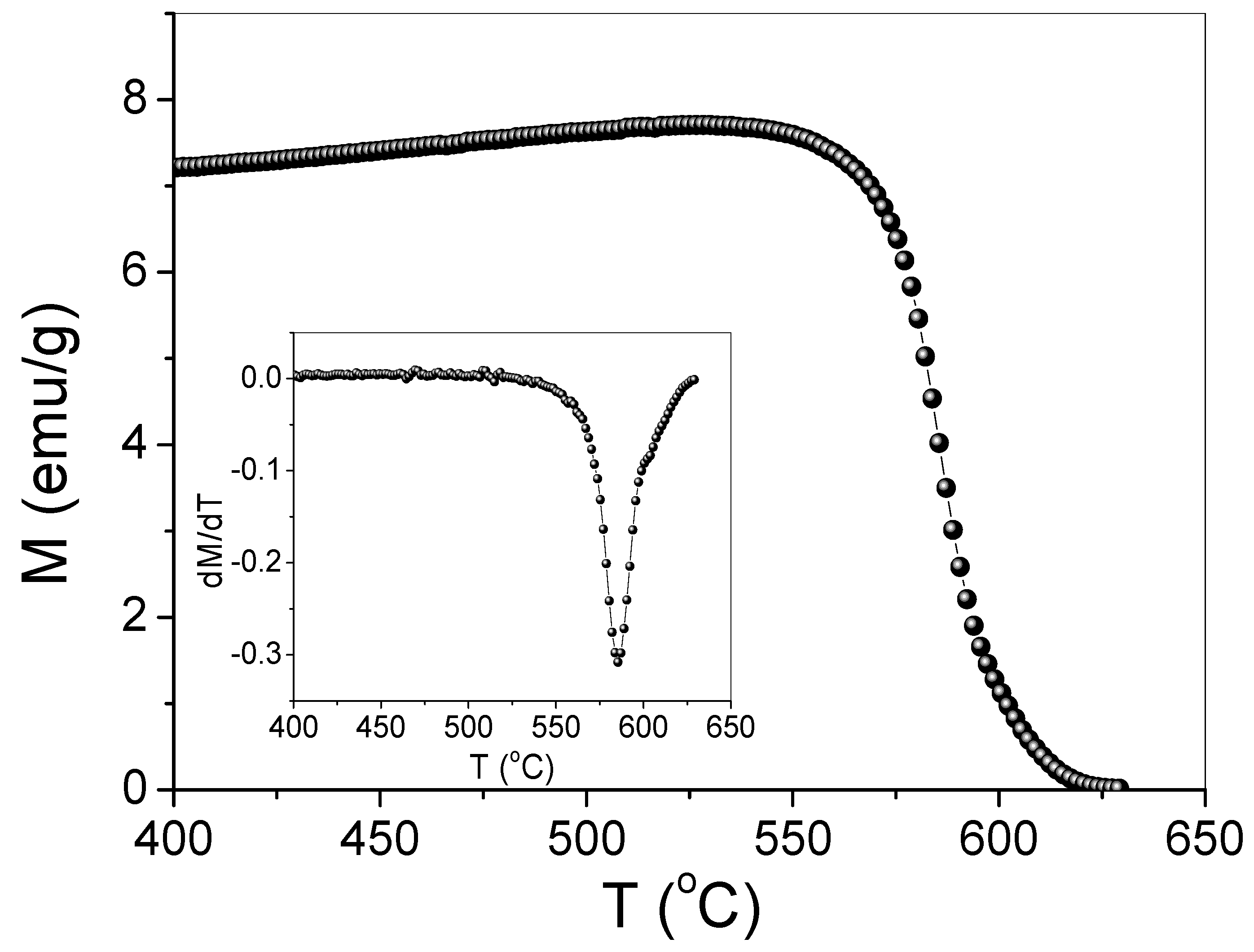
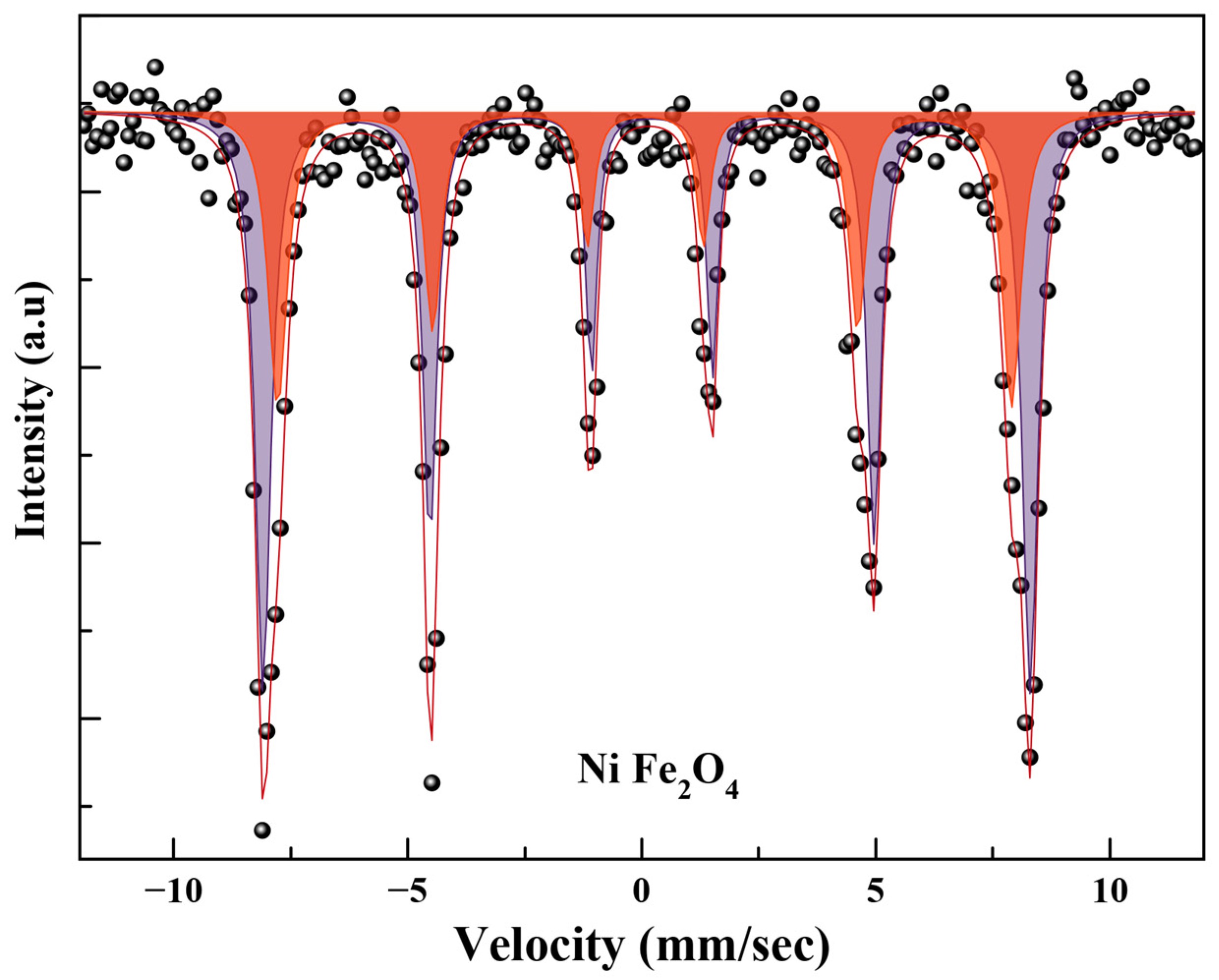
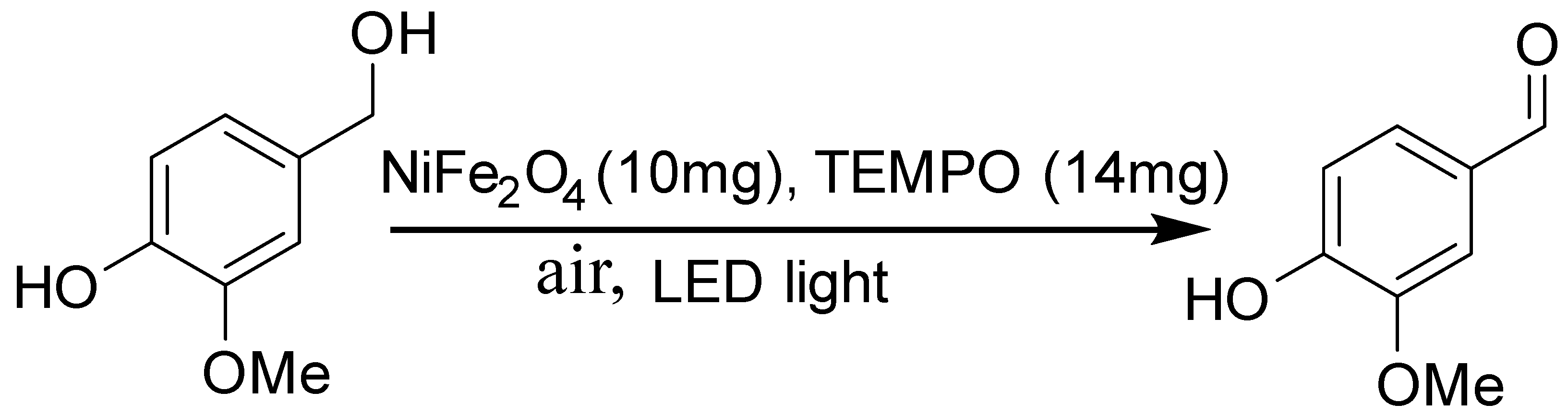
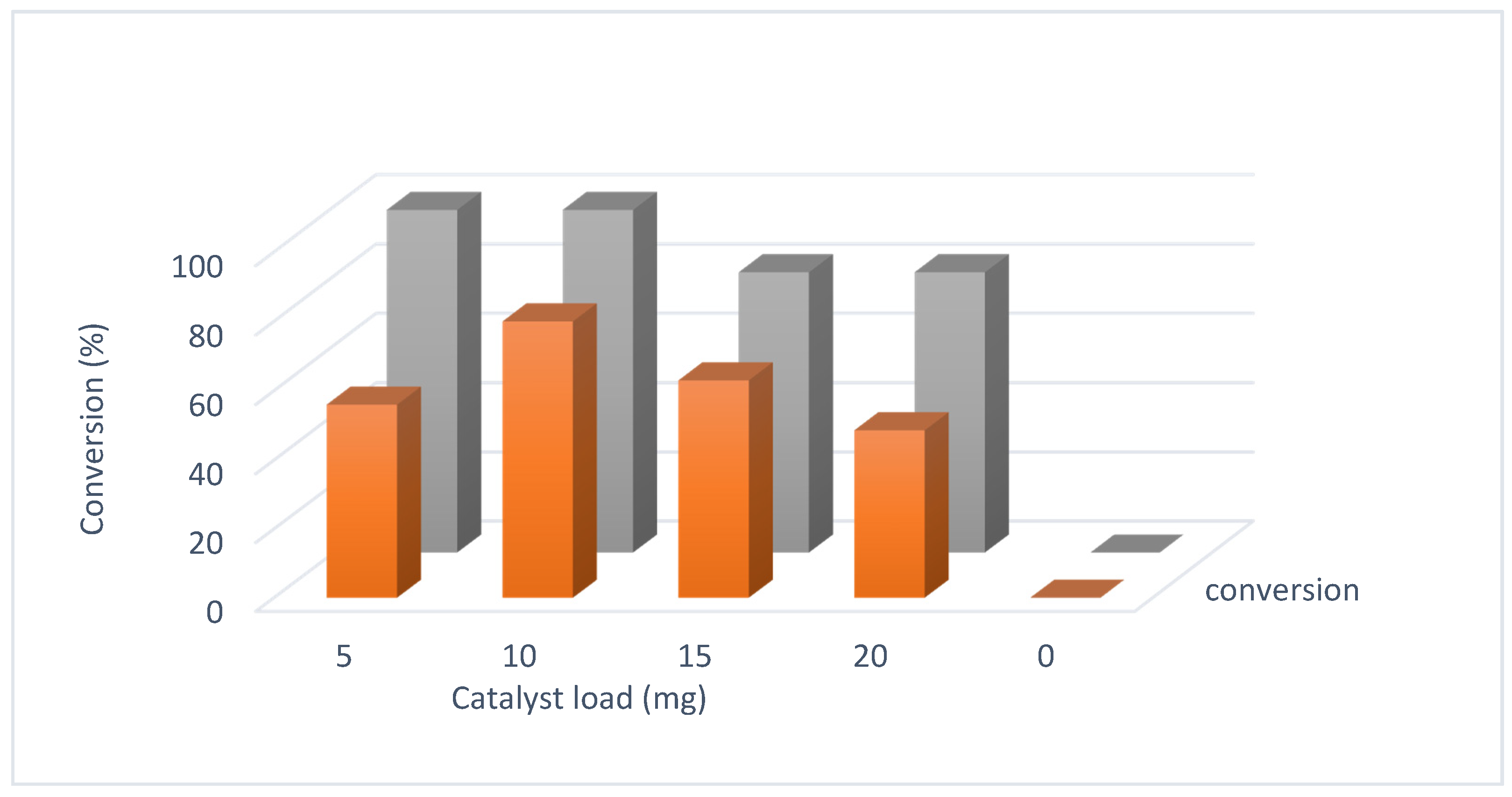
| Ms (emu/g) | Hc (Oe) | Ds (nm) | Dl (nm) | K (erg/cm3) | Dc (nm) |
|---|---|---|---|---|---|
| 42.2 | 180 | 9.0 | 11.1 | 7.84 × 105 | 7.8 |
| Entry | Substrate | Yield (Ald. Selectivity) (%) 1 |
| 1 | Vanillyl alcohol | 88 (99) |
| 2 | Veratryl alcohol | 90 (99) |
| 3 | Cinnamyl alcohol | 84 (95) |
| 4 | Furfaryl alcohol | 85 (99) |
| Entry | Catalyst | Conditions | Main Products | Yield (%) | Refrences |
|---|---|---|---|---|---|
| 1 | CoTiO4/H2O2 | 298 K, MeOH, CH3COOH, 6 h | VN, Vanillic acid | 68 and 31 | [61] |
| 2 | Co3O4 | 413 K, 0.689–4 MPa, H2O, 7 h | Vanillic acid, VN | 69 and 9 | [62] |
| 3 | TEMPO@SiO2/Cu/air | 303 K, IPA, NMI, 1 h | VN | 92.6 | [65] |
| 4 | 2.3%TiO2/fluorescent lamps | 353 K, NaOH pH 13 | VN | 44 | [66] |
| 5 | Au–Pd@HT visible light, air | 298 K, dioxan, 24 h | VN | 52.8 | [47] |
| 6 | NiFe2O4/TEMPO/visible light, air | 298 K, TBABr, airACN, 12 h | VN | 89 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hunaiti, A.; Ghazzy, A.; Sweidan, N.; Mohaidat, Q.; Bsoul, I.; Mahmood, S.; Hussein, T. Nano-Magnetic NiFe2O4 and Its Photocatalytic Oxidation of Vanillyl Alcohol—Synthesis, Characterization, and Application in the Valorization of Lignin. Nanomaterials 2021, 11, 1010. https://doi.org/10.3390/nano11041010
Al-Hunaiti A, Ghazzy A, Sweidan N, Mohaidat Q, Bsoul I, Mahmood S, Hussein T. Nano-Magnetic NiFe2O4 and Its Photocatalytic Oxidation of Vanillyl Alcohol—Synthesis, Characterization, and Application in the Valorization of Lignin. Nanomaterials. 2021; 11(4):1010. https://doi.org/10.3390/nano11041010
Chicago/Turabian StyleAl-Hunaiti, Afnan, Asma Ghazzy, Nuha Sweidan, Qassem Mohaidat, Ibrahim Bsoul, Sami Mahmood, and Tareq Hussein. 2021. "Nano-Magnetic NiFe2O4 and Its Photocatalytic Oxidation of Vanillyl Alcohol—Synthesis, Characterization, and Application in the Valorization of Lignin" Nanomaterials 11, no. 4: 1010. https://doi.org/10.3390/nano11041010
APA StyleAl-Hunaiti, A., Ghazzy, A., Sweidan, N., Mohaidat, Q., Bsoul, I., Mahmood, S., & Hussein, T. (2021). Nano-Magnetic NiFe2O4 and Its Photocatalytic Oxidation of Vanillyl Alcohol—Synthesis, Characterization, and Application in the Valorization of Lignin. Nanomaterials, 11(4), 1010. https://doi.org/10.3390/nano11041010






