Active Nanointerfaces Based on Enzyme Carbonic Anhydrase and Metal–Organic Framework for Carbon Dioxide Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MIL-160/Al2O3 Hybrids
2.3. Characterization of MOFs and Resulting Hybrids
2.4. CA/MIL-160/Al2O3 Membrane Preparation
2.5. Enzyme Loading Assessment
2.6. Enzyme Activity at the Designed Interfaces
2.7. Assessment of Enzyme-Based Conjugates
2.8. Performance of the CA/MIL-160/Al2O3 Membrane or CA/Al2O3 Filters
3. Results and Discussion
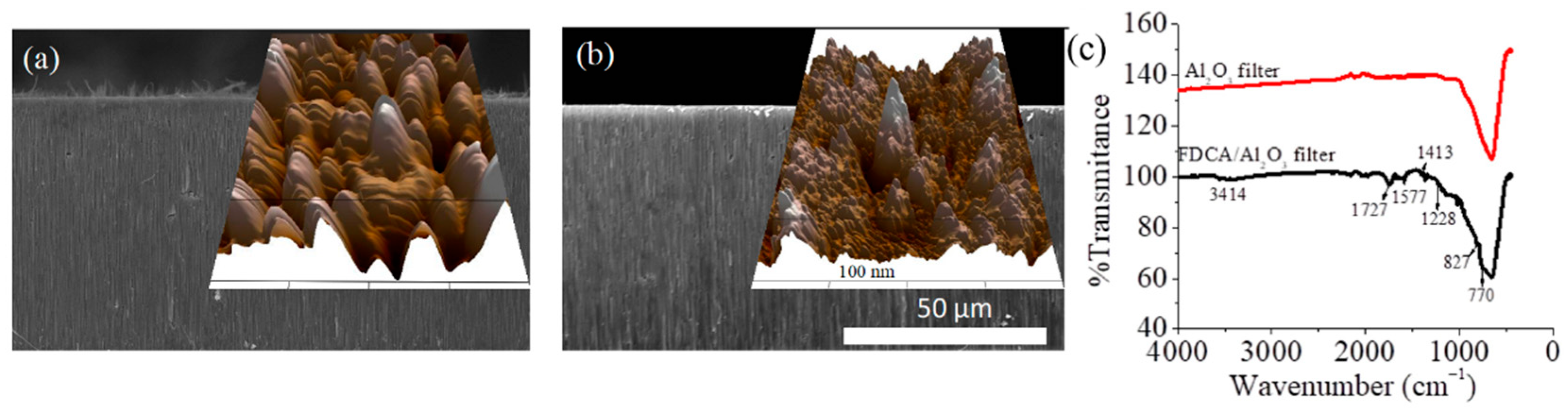
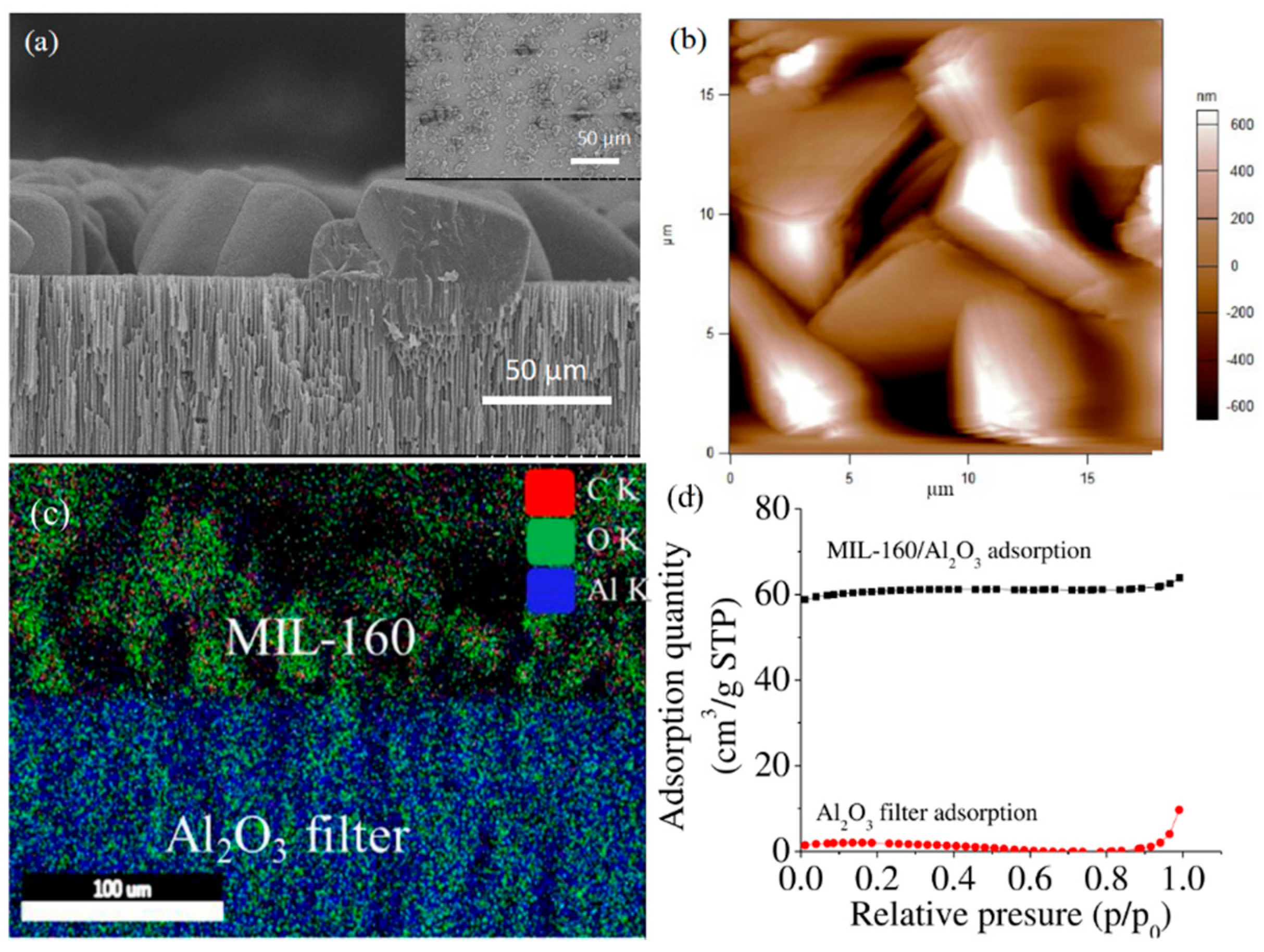
3.1. Synthesis and Characterization of the Components and the BioMembrane
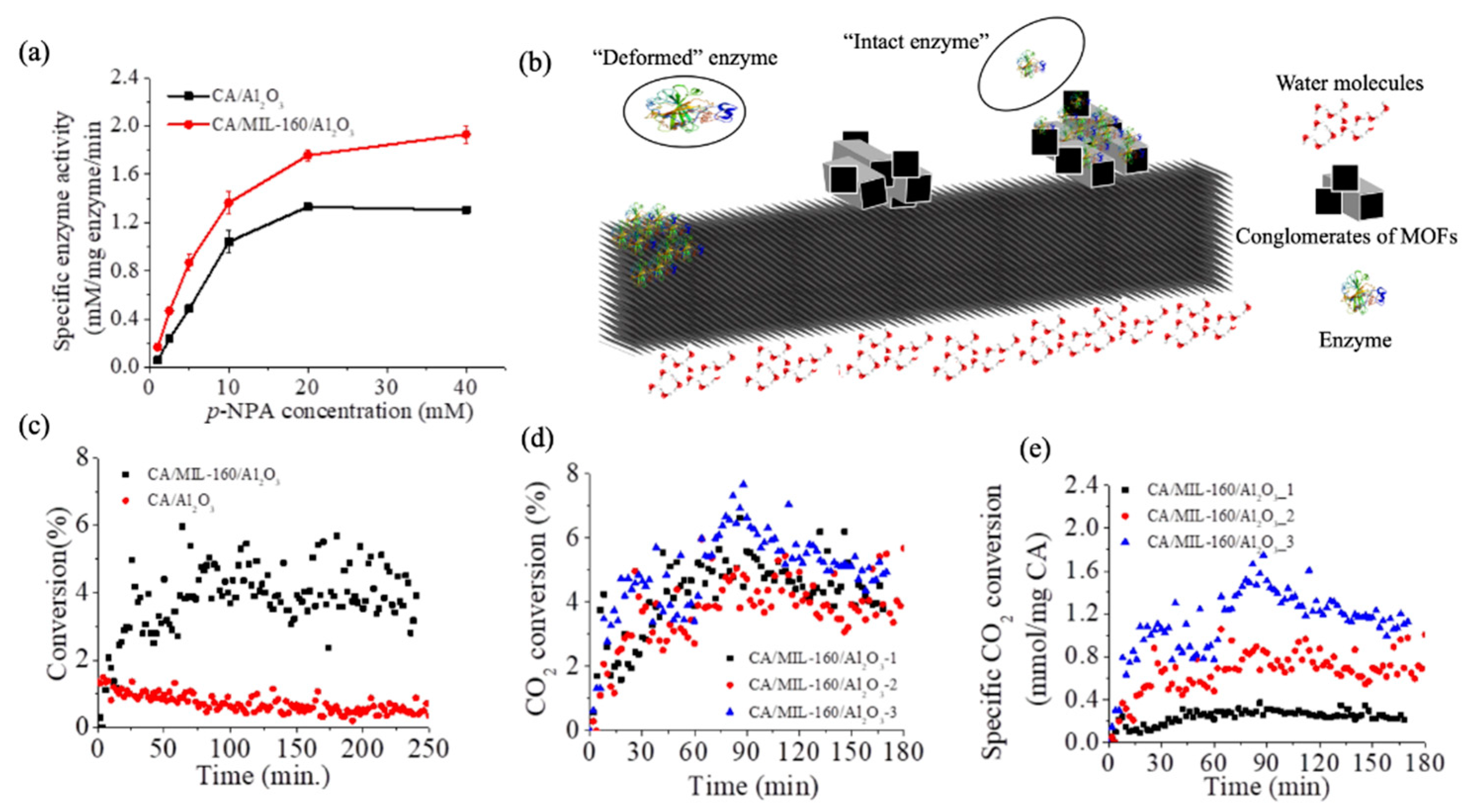
3.2. Functionality of the CA Enzyme at the BioMembrane Interface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Joel, A.S.; Ramshaw, C.; Eimer, D.; Musa, N.M. Process intensification for post-combustion CO2 capture with chemical absorption: A critical review. Appl. Energy 2015, 158, 275–291. [Google Scholar] [CrossRef]
- Williamson, P. Scrutinize CO2 removal methods. Nature 2016, 530, 153–155. [Google Scholar] [CrossRef]
- Ben Mariem, H.; Chaieb, M. Climate Change Impacts on the Distribution of Stipa Tenacissima L. Ecosystems in North African Arid Zone—A Case Study in Tunisia. Appl. Ecol. Environ. Res. 2017, 15, 67–82. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, G.; Wang, Y.; Song, X. Sensitive Indicators of Zonal Stipa Species to Changing Temperature and Precipitation in Inner Mongolia Grassland, China. Front. Plant Sci. 2016, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Barreca, A.; Clay, K.; Deschenes, O.; Greenstone, M.; Shapiro, J.S. Adapting to Climate Change: The Remarkable Decline in the US Temperature-Mortality Relationship over the Twentieth Century. J. Political-Econ. 2016, 124, 105–159. [Google Scholar] [CrossRef]
- Greene, L.A. United Nations Framework Convention on Climate Change. Environ. Health Persp. 2000, 108, A353. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-Based CO2 Capture Technology Development from the Beginning of 2013—A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, G. Conventional Amine Scrubbing for CO2 Capture; Woodhead Publishing Series: Cambridge, UK, 2016; Volume 101, pp. 35–67. [Google Scholar]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Shan, G.; Zhang, T.C.; Surampalli, R.Y. Surampalli CO2 Scrubbing Processes and Applications. In Carbon Capture and Storage; American Society of Civil Engineers: Reston, VA, USA, 2015; pp. 239–280. [Google Scholar]
- Mazari, S.A.; Ali, B.S.; Jan, B.M.; Saeed, I.M.; Nizamuddin, S. An overview of solvent management and emissions of amine-based CO2 capture technology. Int. J. Greenh. Gas Control 2015, 34, 129–140. [Google Scholar] [CrossRef]
- Khakharia, P.; Brachert, L.; Mertens, J.; Huizinga, A.; Schallert, B.; Schaber, K.; Vlugt, T.J.; Goetheer, E. Investigation of aerosol based emission of MEA due to sulphuric acid aerosol and soot in a Post Combustion CO2 Capture process. Int. J. Greenh. Gas Control 2013, 19, 138–144. [Google Scholar] [CrossRef]
- Huang, K.; Chen, F.-F.; Tao, D.-J.; Dai, S. Ionic liquid–formulated hybrid solvents for CO2 capture. Curr. Opin. Green Sustain. Chem. 2017, 5, 67–73. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 absorption studies in amino acid-anion based ionic liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Chen, F.-F.; Huang, K.; Zhou, Y.; Tian, Z.-Q.; Zhu, X.; Tao, D.; Jiang, D.; Dai, S. Multi-Molar Absorption of CO2 by the Activation of Carboxylate Groups in Amino Acid Ionic Liquids. Angew. Chem. Int. Ed. 2016, 55, 7166–7170. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, G.; Chen, E.; Freeman, S.; Van Wagener, D.; Xu, Q.; Voice, A. Aqueous piperazine as the new standard for CO2 capture technology. Chem. Eng. J. 2011, 171, 725–733. [Google Scholar] [CrossRef]
- Du, Y.; Yuan, Y.; Rochelle, G.T. Capacity and absorption rate of tertiary and hindered amines blended with piperazine for CO2 capture. Chem. Eng. Sci. 2016, 155, 397–404. [Google Scholar] [CrossRef]
- Mathias, P.M.; Zheng, F.; Heldebrant, D.J.; Zwoster, A.; Whyatt, G.; Freeman, C.M.; Bearden, M.D.; Koech, P. Measuring the Absorption Rate of CO2 in Nonaqueous CO2-Binding Organic Liquid Solvents with a Wetted-Wall Apparatus. ChemSusChem 2015, 8, 3617–3625. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Efficient CO2 absorption and low temperature desorption with non-aqueous solvents based on 2-amino-2-methyl-1-propanol (AMP). Int. J. Greenh. Gas Control 2013, 16, 217–223. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, J.; Hu, L.; Liu, J.; Zhou, J.; Cen, K. Phase-changing solution PZ/DMF for efficient CO2 capture and low corrosiveness to carbon steel. Fuel 2018, 216, 418–426. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Velardi, U.V.; Brenna, A.; Mele, A.; Pedeferri, M.; Ormellese, M. Compatibility of Imidazolium-Based Ionic Liquids for CO2 Capture with Steel Alloys: A Corrosion Perspective. Electrochim. Acta 2016, 192, 414–421. [Google Scholar] [CrossRef]
- Dai, C.; Wei, W.; Lei, Z.; Li, C.; Chen, B. Absorption of CO2 with methanol and ionic liquid mixture at low temperatures. Fluid Phase Equilibria 2015, 391, 9–17. [Google Scholar] [CrossRef]
- Shen, S.; Bian, Y.; Zhao, Y. Energy-efficient CO2 capture using potassium prolinate/ethanol solution as a phase-changing absorbent. Int. J. Greenh. Gas Control 2017, 56, 1–11. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Carradori, S.; Mollica, A.; De Monte, C.; Granese, A.; Supuran, C.T. Nitric Oxide Donors and Selective Carbonic Anhydrase Inhibitors: A Dual Pharmacological Approach for the Treatment of Glaucoma, Cancer and Osteoporosis. Molecules 2015, 20, 5667–5679. [Google Scholar] [CrossRef]
- Strong, S.A.; Hirji, N.; Quartilho, A.; Kalitzeos, A.; Michaelides, M. Retrospective cohort study exploring whether an association exists between spatial distribution of cystoid spaces in cystoid macular oedema secondary to retinitis pigmentosa and response to treatment with carbonic anhydrase inhibitors. Br. J. Ophthalmol. 2019, 103, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Durante, M.; Di Leva, F.S.; Cosconati, S.; Masini, E.; Scozzafava, A.; Novellino, E.; Supuran, C.T.; Carta, F. Monothiocarbamates Strongly Inhibit Carbonic Anhydrases in Vitro and Possess Intraocular Pressure Lowering Activity in an Animal Model of Glaucoma. J. Med. Chem. 2016, 59, 5857–5867. [Google Scholar] [CrossRef]
- Supuran, C.T.; Winum, J.-Y. Carbonic anhydrase IX inhibitors in cancer therapy: An update. Future Med. Chem. 2015, 7, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin. Ther. Targets 2015, 19, 1689–1704. [Google Scholar] [CrossRef]
- Supuran, C.T. Legionella pneumophila Carbonic Anhydrases: Underexplored Antibacterial Drug Targets. Pathogens 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Tobal, J.M.; Balieiro, M.E.D.S.F. Role of carbonic anhydrases in pathogenic micro-organisms: A focus on Aspergillus fumigatus. J. Med. Microbiol. 2014, 63, 15–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Supuran, C.T. Carbonic anhydrases: From biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J. Enzym. Inhib. Med. Chem. 2013, 28, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, E.; Pronina, N.; Los, D. Carbonic anhydrase—A universal enzyme of the carbon-based life. Photosynthetica 2017, 55, 3–19. [Google Scholar] [CrossRef]
- Bagchi, S.; Sengupta, S.; Mondal, S. Development and Characterization of Carbonic Anhydrase-Based CO2 Biosensor for Primary Diagnosis of Respiratory Health. IEEE Sens. J. 2017, 17, 1384–1390. [Google Scholar] [CrossRef]
- Scarabelli, S.; Tan, K.T.; Griss, R.; Hovius, R.; D’Alessandro, P.L.; Vorherr, T.; Johnsson, K. Evaluating Cellular Drug Uptake with Fluorescent Sensor Proteins. ACS Sens. 2017, 2, 1191–1197. [Google Scholar] [CrossRef]
- Supuran, C.T.; Capasso, C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin. Ther. Patents 2018, 28, 745–754. [Google Scholar] [CrossRef]
- Kaar, J.L.; Oh, H.-I.; Russell, A.J.; Federspiel, W.J. Towards improved artificial lungs through biocatalysis. Biomaterials 2007, 28, 3131–3139. [Google Scholar] [CrossRef]
- Yong, J.K.; Stevens, G.W.; Caruso, F.; Kentish, S.E. In situ layer-by-layer assembled carbonic anhydrase-coated hollow fiber membrane contactor for rapid CO2 absorption. J. Membr. Sci. 2016, 514, 556–565. [Google Scholar] [CrossRef]
- Hou, J.; Zulkifli, M.Y.; Mohammad, M.; Zhang, Y.; Razmjou, A.; Chen, V. Biocatalytic gas-liquid membrane contactors for CO2 hydration with immobilized carbonic anhydrase. J. Membr. Sci. 2016, 520, 303–313. [Google Scholar] [CrossRef]
- Abdelrahim, M.Y.M.; Martins, C.F.; Neves, L.; Capasso, C.; Supuran, C.T.; Coelhoso, I.M.; Crespo, J.G.; Barboiu, M. Supported ionic liquid membranes immobilized with carbonic anhydrases for CO2 transport at high temperatures. J. Membr. Sci. 2017, 528, 225–230. [Google Scholar] [CrossRef]
- Demontigny, D.; Tontiwachwuthikul, P.; Chakma, A. Using polypropylene and polytetrafluoroethylene membranes in a membrane contactor for CO2 absorption. J. Membr. Sci. 2006, 277, 99–107. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Wang, R.; Liang, D.T.; Tay, J.H. Theoretical and experimental studies of membrane wetting in the membrane gas–liquid contacting process for CO2 absorption. J. Membr. Sci. 2008, 308, 162–170. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y. Surfactants Facilitating Carbonic Anhydrase Enzyme-Mediated CO2 Absorption into a Carbonate Solution. Environ. Sci. Technol. 2017, 51, 8537–8543. [Google Scholar] [CrossRef] [PubMed]
- Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Zhai, H.; Rubin, E. Membrane properties required for post-combustion CO2 capture at coal-fired power plants. J. Membr. Sci. 2016, 511, 250–264. [Google Scholar] [CrossRef]
- Liu, Q.; Chapman, J.; Huang, A.; Williams, K.C.; Wagner, A.; Garapati, N.; Sierros, K.A.; Dinu, C.Z. User-Tailored Metal–Organic Frameworks as Supports for Carbonic Anhydrase. ACS Appl. Mater. Interfaces 2018, 10, 41326–41337. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Liu, T.-F.; Su, J.; Bosch, M.; Wei, Z.; Wan, W.; Yuan, D.; Chen, Y.-P.; Wang, X.; Wang, K.; et al. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015, 6, 5979. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Lian, X.Z.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.L.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef]
- Lian, X.; Chen, Y.-P.; Liu, T.-F.; Zhou, H.-C. Coupling two enzymes into a tandem nanoreactor utilizing a hierarchically structured MOF. Chem. Sci. 2016, 7, 6969–6973. [Google Scholar] [CrossRef]
- Migliardini, F.; De Luca, V.; Carginale, V.; Rossi, M.; Corbo, P.; Supuran, C.T.; Capasso, C. Biomimetic CO2 capture using a highly thermostable bacterial α-carbonic anhydrase immobilized on a polyurethane foam. J. Enzym. Inhib. Med. Chem. 2014, 29, 146–150. [Google Scholar] [CrossRef]
- Al-Dhrub, A.H.A.; Sahin, S.; Ozmen, I.; Tunca, E.; Bulbul, M. Immobilization and characterization of human carbonic anhydrase I on amine functionalized magnetic nanoparticles. Process. Biochem. 2017, 57, 95–104. [Google Scholar] [CrossRef]
- Khameneh, H.P.; Bolouri, T.G.; Nemati, F.; Rezvani, F.; Attar, F.; Saboury, A.A.; Falahati, M. A spectroscopic study on the absorption of carbonic anhydrase onto the nanoporous silica nanoparticle. Int. J. Biol. Macromol. 2017, 99, 739–745. [Google Scholar] [CrossRef]
- Fei, X.; Chen, S.; Liu, D.; Huang, C.; Zhang, Y. Comparison of amino and epoxy functionalized SBA-15 used for carbonic anhydrase immobilization. J. Biosci. Bioeng. 2016, 122, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Adewole, J.; Ahmad, A.; Ismail, S.; Leo, C. Current challenges in membrane separation of CO2 from natural gas: A review. Int. J. Greenh. Gas Control 2013, 17, 46–65. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Cao, C.; Liu, H.; Hou, Z.; Mehmood, F.; Liao, J.; Feng, W. A Review of CO2 Storage in View of Safety and Cost-Effectiveness. Energies 2020, 13, 600. [Google Scholar] [CrossRef]
- Chang, F.; Zhou, J.; Chen, P.; Chen, Y.; Jia, H.; Saad, S.M.I.; Gao, Y.; Cao, X.; Zheng, T. Microporous and mesoporous materials for gas storage and separation: A review. Asia-Pac. J. Chem. Eng. 2013, 8, 618–626. [Google Scholar] [CrossRef]
- Gatti, M.; Martelli, E.; Di Bona, D.; Gabba, M.; Scaccabarozzi, R.; Spinelli, M.; Viganò, F.; Consonni, S. Preliminary Performance and Cost Evaluation of Four Alternative Technologies for Post-Combustion CO2 Capture in Natural Gas-Fired Power Plants. Energies 2020, 13, 543. [Google Scholar] [CrossRef]
- Streb, A.; Hefti, M.; Gazzani, M.; Mazzotti, M. Novel Adsorption Process for Co-Production of Hydrogen and CO2 from a Multicomponent Stream. Ind. Eng. Chem. Res. 2019, 58, 17489–17506. [Google Scholar] [CrossRef]
- Schaef, H.T.; Davidson, C.L.; Owen, A.T.; Miller, Q.R.; Loring, J.S.; Thompson, C.J.; Bacon, D.H.; Glezakou, V.A.; McGrail, B.P. CO2 Utilization and Storage in Shale Gas Reservoirs: Experimental Results and Economic Impacts. Energy Procedia 2014, 63, 7844–7851. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Jones, C.W. CO2 Capture from Dilute Gases as a Component of Modern Global Carbon Management. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 31–52. [Google Scholar] [CrossRef]
- Ozcan, A.; Semino, R.; Maurin, G.; Yazaydin, A.O. Modeling of Gas Transport through Polymer/MOF Interfaces: A Microsecond-Scale Concentration Gradient-Driven Molecule Dynamics Study. Chem. Mater. 2020, 32, 1288–1296. [Google Scholar] [CrossRef]
- Liu, Q.; Dordick, J.S.; Dinu, C.Z. Metal–Organic Framework-Based Composite for Photocatalytic Detection of Prevalent Pollutant. ACS Appl. Mater. Interfaces 2019, 11, 31049–31059. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, N.; Caro, J.; Huang, A. Bio-Inspired Polydopamine: A Versatile and Powerful Platform for Covalent Synthesis of Molecular Sieve Membranes. J. Am. Chem. Soc. 2013, 135, 17679–17682. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, J.; Liang, Y.; Zhang, H.; Zhang, X.; Shen, W.; Wang, H. Zeolitic Imidazolate Framework/Graphene Oxide Hybrid Nanosheets as Seeds for the Growth of Ultrathin Molecular Sieving Membranes. Angew. Chem. Int. Ed. 2016, 55, 2048–2052. [Google Scholar] [CrossRef]
- Miao, L.; Fan, Q.; Zhao, L.; Qiao, Q.; Zhang, X.; Hou, C.; Xu, J.; Luo, Q.; Liu, J. The construction of functional protein nanotubes by small molecule-induced self-assembly of cricoid proteins. Chem. Commun. 2016, 52, 4092–4095. [Google Scholar] [CrossRef]
- Hayden, E.Y.; Conovaloff, J.L.; Mason, A.; Bitan, G.; Teplow, D.B. Preparation of pure populations of covalently stabilized amyloid beta-protein oligomers of specific sizes. Anal. Biochem. 2017, 518, 78–85. [Google Scholar] [CrossRef]
- Ma, X.; Kumar, P.; Mittal, N.; Khlyustova, A.; Daoutidis, P.; Mkhoyan, K.A.; Tsapatsis, M. Zeolitic imidazolate framework membranes made by ligand-induced permselectivation. Science 2018, 361, 1008–1011. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Van Assche, T.; Campagnol, N.; Fransaer, J.; Denayer, J.; Tan, J.-C.; Falcaro, P.; De Vos, D.; Ameloot, R. Electrochemical Film Deposition of the Zirconium Metal–Organic Framework UiO-66 and Application in a Miniaturized Sorbent Trap. Chem. Mater. 2015, 27, 1801–1807. [Google Scholar] [CrossRef]
- Huang, B.; Liu, Q.; Caro, J.; Huang, A. Iso-butanol dehydration by pervaporation using zeolite LTA membranes prepared on 3-aminopropyltriethoxysilane-modified alumina tubes. J. Membr. Sci. 2014, 455, 200–206. [Google Scholar] [CrossRef]
- Moscatelli, D.; Gelosa, S.; Yogesh, H.; Storti, G. Kinetic Study of the Effect of Catalyst in the Polycondensation of Lactic Acid to Produce Low Molecular Weight Polymers; AIDIC. 2007. Available online: https://folk.ntnu.no/skoge/prost/proceedings/icheap8-pres07/icheap8webpapers/104%20Moscatelli.pdf (accessed on 15 April 2021).
- Lee, H.W.; Insyani, R.; Prasetyo, D.; Prajitno, H.; Sitompul, J.P. Molecular Weight and Structural Properties of Biodegradable PLA Synthesized with Different Catalysts by Direct Melt Polycondensation. J. Eng. Technol. Sci. 2015, 47, 364–373. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, C.; Mejía-Centeno, I.; Fuentes, G.A.; Jentys, A.; Lercher, J.A. Comparison of kinetics and reaction pathways for hydrodeoxygenation of C3 alcohols on Pt/Al2O3. Catal. Today 2012, 183, 3–9. [Google Scholar] [CrossRef]
- Ghasempour, F.; Azimirad, R.; Amini, A.; Akhavan, O. Visible light photoinactivation of bacteria by tungsten oxide nanostructures formed on a tungsten foil. Appl. Surf. Sci. 2015, 338, 55–60. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Grafmueller, A.; Huang, X.; Liedel, C.; Algara-Siller, G.; Willinger, M.; Yang, C.; Fu, Y.; Wang, X.; et al. Self-assembled carbon nitride for photocatalytic hydrogen evolution and degradation of p-nitrophenol. Appl. Catal. B Environ. 2017, 205, 1–10. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L. A facile and effective method for preparation of 2.5-furandicarboxylic acid via hydrogen peroxide direct oxidation of 5-hydroxymethylfurfural. Pol. J. Chem. Technol. 2017, 19, 11–16. [Google Scholar] [CrossRef]
- Permyakova, A.; Skrylnyk, O.; Courbon, E.; Affram, M.; Wang, S.; Lee, U.-H.; Valekar, A.H.; Nouar, F.; Mouchaham, G.; Devic, T.; et al. Synthesis Optimization, Shaping, and Heat Reallocation Evaluation of the Hydrophilic Metal-Organic Framework MIL-160(Al). ChemSusChem 2017, 10, 1419–1426. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Jeong, S.K.; Yoon, Y.I.; Nam, S.C. Capture and Sequestration of CO2 by Human Carbonic Anhydrase Covalently Immobilized onto Amine-Functionalized SBA-15. J. Phys. Chem. C 2011, 115, 20209–20216. [Google Scholar] [CrossRef]
- Lykourinou, V.; Chen, Y.; Wang, X.-S.; Meng, L.; Hoang, T.; Ming, L.-J.; Musselman, R.L.; Ma, S. Immobilization of MP-11 into a Mesoporous Metal–Organic Framework, MP-11@mesoMOF: A New Platform for Enzymatic Catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385. [Google Scholar] [CrossRef]
- Zhou, Z.; Taylor, R.K.; Kullmann, S.; Bao, H.; Hartmann, M. Mesoporous Organosilicas With Large Cage-Like Pores for High Efficiency Immobilization of Enzymes. Adv. Mater. 2011, 23, 2627–2632. [Google Scholar] [CrossRef]
- Cadiau, A.; Lee, J.S.; Borges, D.D.; Fabry, P.; Devic, T.; Wharmby, M.T.; Martineau, C.; Foucher, D.; Taulelle, F.; Jun, C.-H.; et al. Design of Hydrophilic Metal Organic Framework Water Adsorbents for Heat Reallocation. Adv. Mater. 2015, 27, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.; Wang, Z.; Slocik, J.; Naik, R.R.; Singamaneni, S. Effect of size and curvature on the enzyme activity of bionanoconjugates. Nanoscale 2017, 9, 15666–15672. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.S.; Dong, C.; Meng, F.; Hardinger, J.; Perhinschi, G.; Wu, N.; Dinu, C.Z. Enzyme Catalytic Efficiency: A Function of Bio–Nano Interface Reactions. ACS Appl. Mater. Interfaces 2014, 6, 5393–5403. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.S.; Jose, M.V.; Marx, S.; Cornelius, S.; Koepsel, R.R.; Islam, M.F.; Russell, A.J. Improved power density of an enzymatic biofuel cell with fibrous supports of high curvature. RSC Adv. 2016, 6, 10150–10158. [Google Scholar] [CrossRef]
- Sanfins, E.; Augustsson, C.; Dahlbäck, B.; Linse, S.; Cedervall, T. Size-Dependent Effects of Nanoparticles on Enzymes in the Blood Coagulation Cascade. Nano Lett. 2014, 14, 4736–4744. [Google Scholar] [CrossRef]
- Mazurenko, I.; Monsalve, K.; Infossi, P.; Giudici-Orticoni, M.-T.; Topin, F.; Mano, N.; Lojou, E. Impact of substrate diffusion and enzyme distribution in 3D-porous electrodes: A combined electrochemical and modelling study of a thermostable H2/O2enzymatic fuel cell. Energy Environ. Sci. 2017, 10, 1966–1982. [Google Scholar] [CrossRef]
- Morthensen, S.T.; Meyer, A.S.; Jørgensen, H.; Pinelo, M. Significance of membrane bioreactor design on the biocatalytic performance of glucose oxidase and catalase: Free vs. immobilized enzyme systems. Biochem. Eng. J. 2017, 117, 41–47. [Google Scholar] [CrossRef]
- Wang, J.; He, B.; Xie, L.; Bei, K.; Li, G.; Chen, Z.; Chou, I.-M.; Lin, C.; Pan, Z. Determination of CO2 Solubility in Water and NaCl Solutions under Geological Sequestration Conditions Using a Fused Silica Capillary Cell with in Situ Raman Spectroscopy. J. Chem. Eng. Data 2019, 64, 2484–2496. [Google Scholar] [CrossRef]
- Yamada, K.; Iizawa, Y.; Yamada, J.-I.; Hirata, M. Retention of activity of urease immobilized on grafted polymer films. J. Appl. Polym. Sci. 2006, 102, 4886–4896. [Google Scholar] [CrossRef]
- Caruso, F.; Schüler, C. Enzyme Multilayers on Colloid Particles: Assembly, Stability, and Enzymatic Activity. Langmuir 2000, 16, 9595–9603. [Google Scholar] [CrossRef]
- Yong, J.K.J.; Cui, J.; Cho, K.L.; Stevens, G.W.; Caruso, F.; Kentish, S.E. Surface Engineering of Polypropylene Membranes with Carbonic Anhydrase-Loaded Mesoporous Silica Nanoparticles for Improved Carbon Dioxide Hydration. Langmuir 2015, 31, 6211–6219. [Google Scholar] [CrossRef]
- Hou, J.; Ji, C.; Dong, G.; Xiao, B.; Ye, Y.; Chen, V. Biocatalytic Janus membranes for CO2 removal utilizing carbonic anhydrase. J. Mater. Chem. A 2015, 3, 17032–17041. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Chew, N.G.P.; Malde, C.; Wang, R. Biocatalytic PVDF composite hollow fiber membranes for CO2 removal in gas-liquid membrane contactor. J. Membr. Sci. 2019, 572, 532–544. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Bai, X.; Pham, H.; Hu, J.; Dinu, C.Z. Active Nanointerfaces Based on Enzyme Carbonic Anhydrase and Metal–Organic Framework for Carbon Dioxide Reduction. Nanomaterials 2021, 11, 1008. https://doi.org/10.3390/nano11041008
Liu Q, Bai X, Pham H, Hu J, Dinu CZ. Active Nanointerfaces Based on Enzyme Carbonic Anhydrase and Metal–Organic Framework for Carbon Dioxide Reduction. Nanomaterials. 2021; 11(4):1008. https://doi.org/10.3390/nano11041008
Chicago/Turabian StyleLiu, Qian, Xinwei Bai, Huy Pham, Jianli Hu, and Cerasela Zoica Dinu. 2021. "Active Nanointerfaces Based on Enzyme Carbonic Anhydrase and Metal–Organic Framework for Carbon Dioxide Reduction" Nanomaterials 11, no. 4: 1008. https://doi.org/10.3390/nano11041008
APA StyleLiu, Q., Bai, X., Pham, H., Hu, J., & Dinu, C. Z. (2021). Active Nanointerfaces Based on Enzyme Carbonic Anhydrase and Metal–Organic Framework for Carbon Dioxide Reduction. Nanomaterials, 11(4), 1008. https://doi.org/10.3390/nano11041008





