High-Performance-Based Perovskite-Supported Nanocomposite for the Development of Green Energy Device Applications: An Overview
Abstract
1. Introduction
2H+ + O2− -> H2O(I)
2. Electrode Surface Studies
3. Perovskite-Based Nanocomposite in Supercapacitor Applications
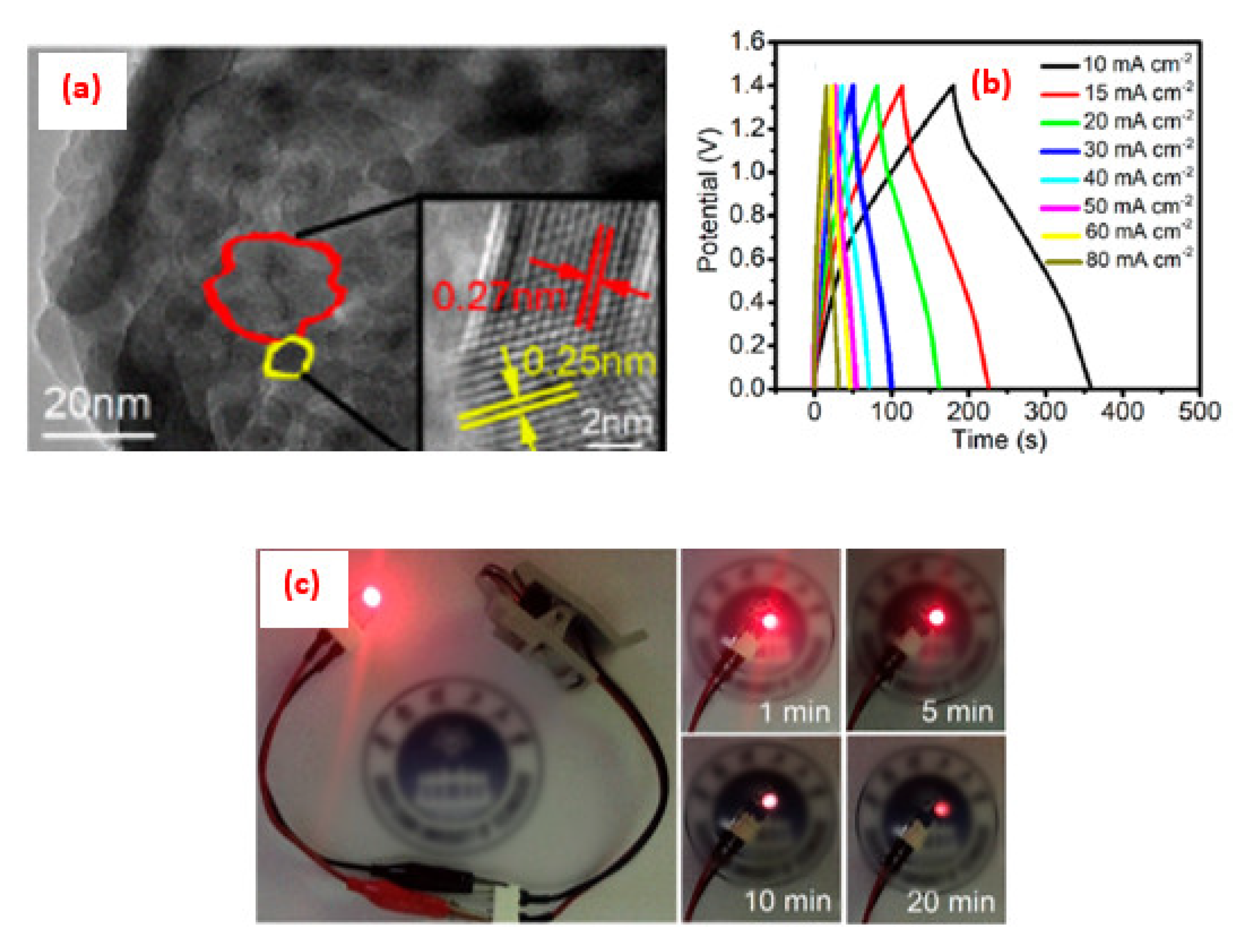
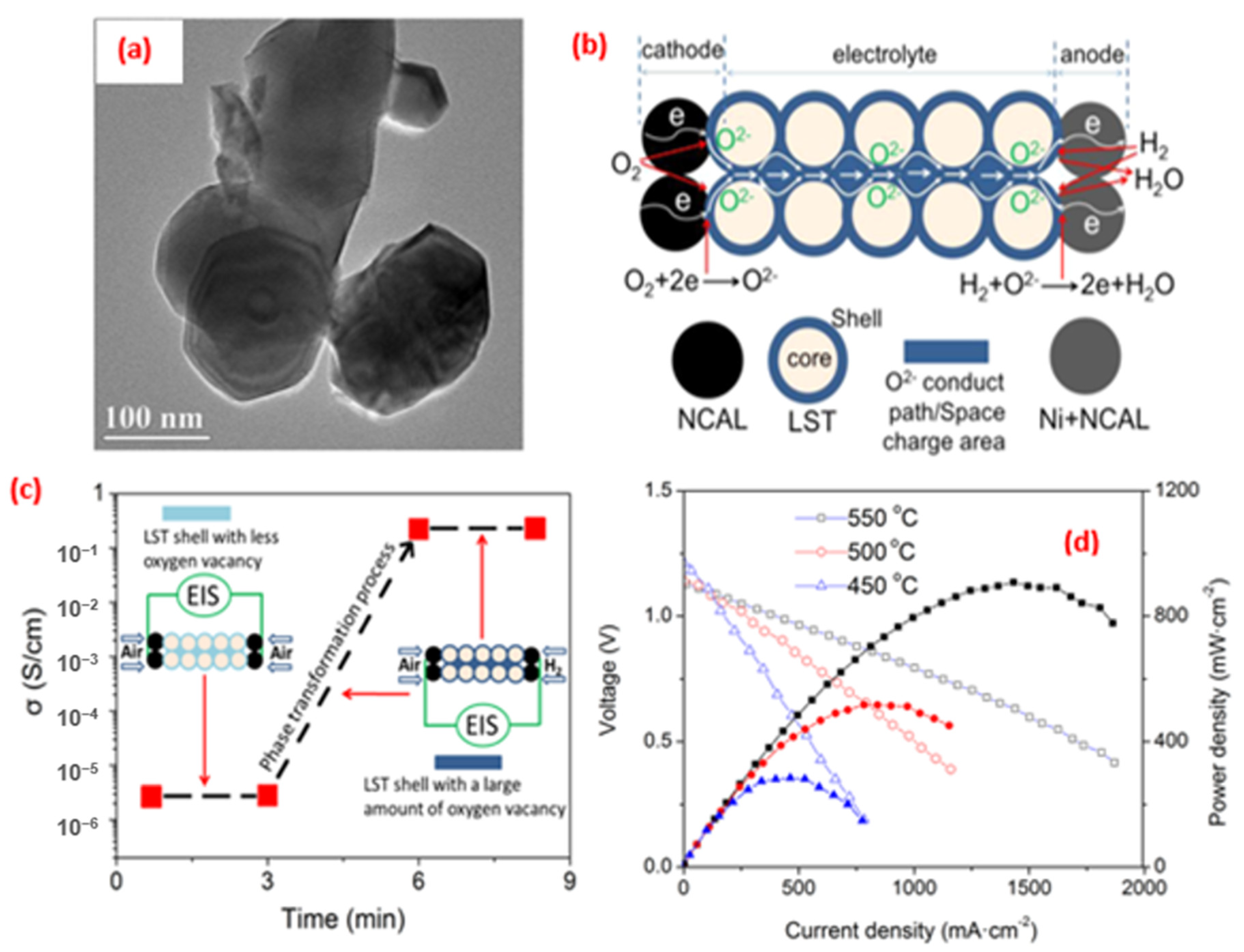
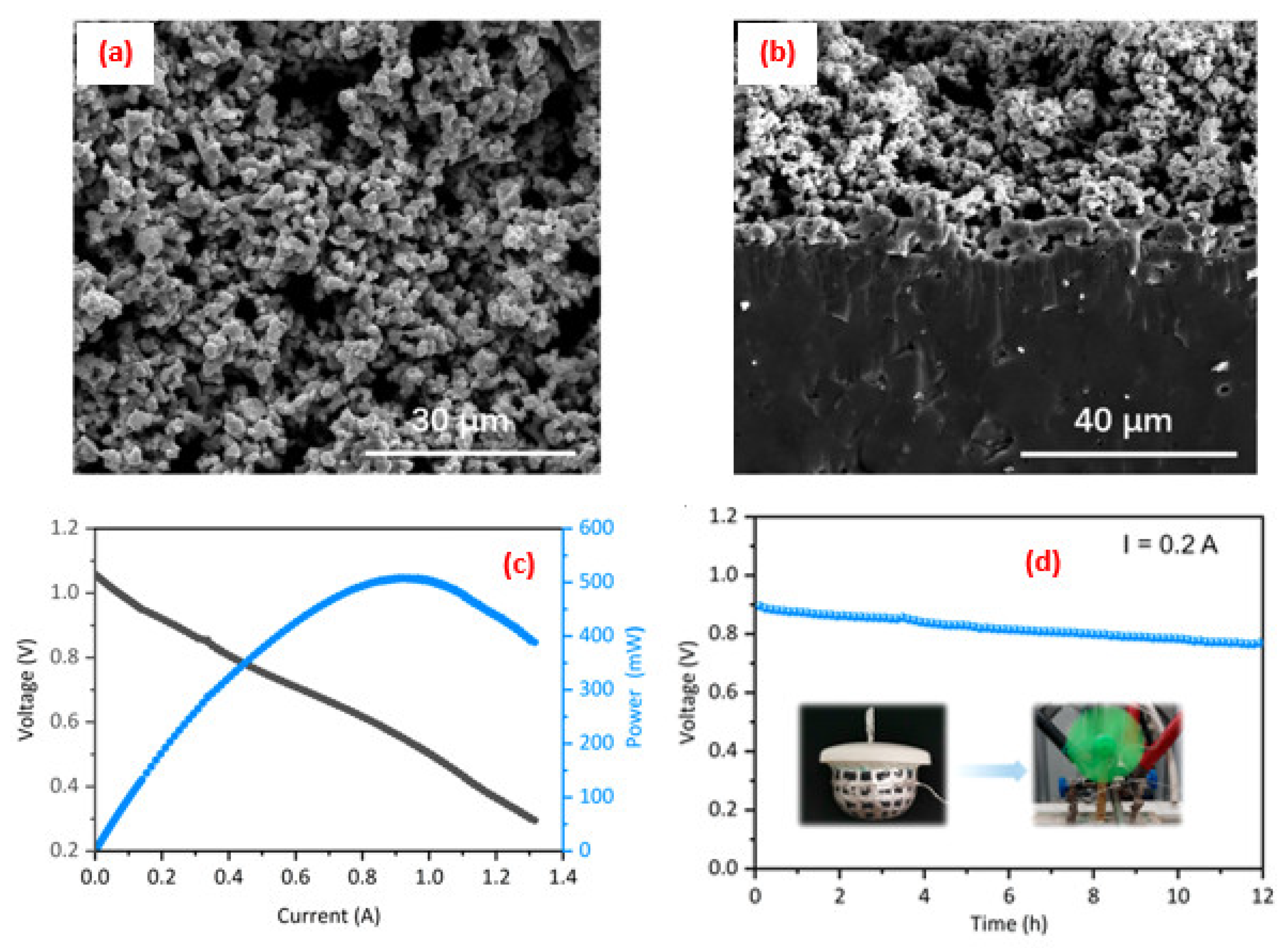
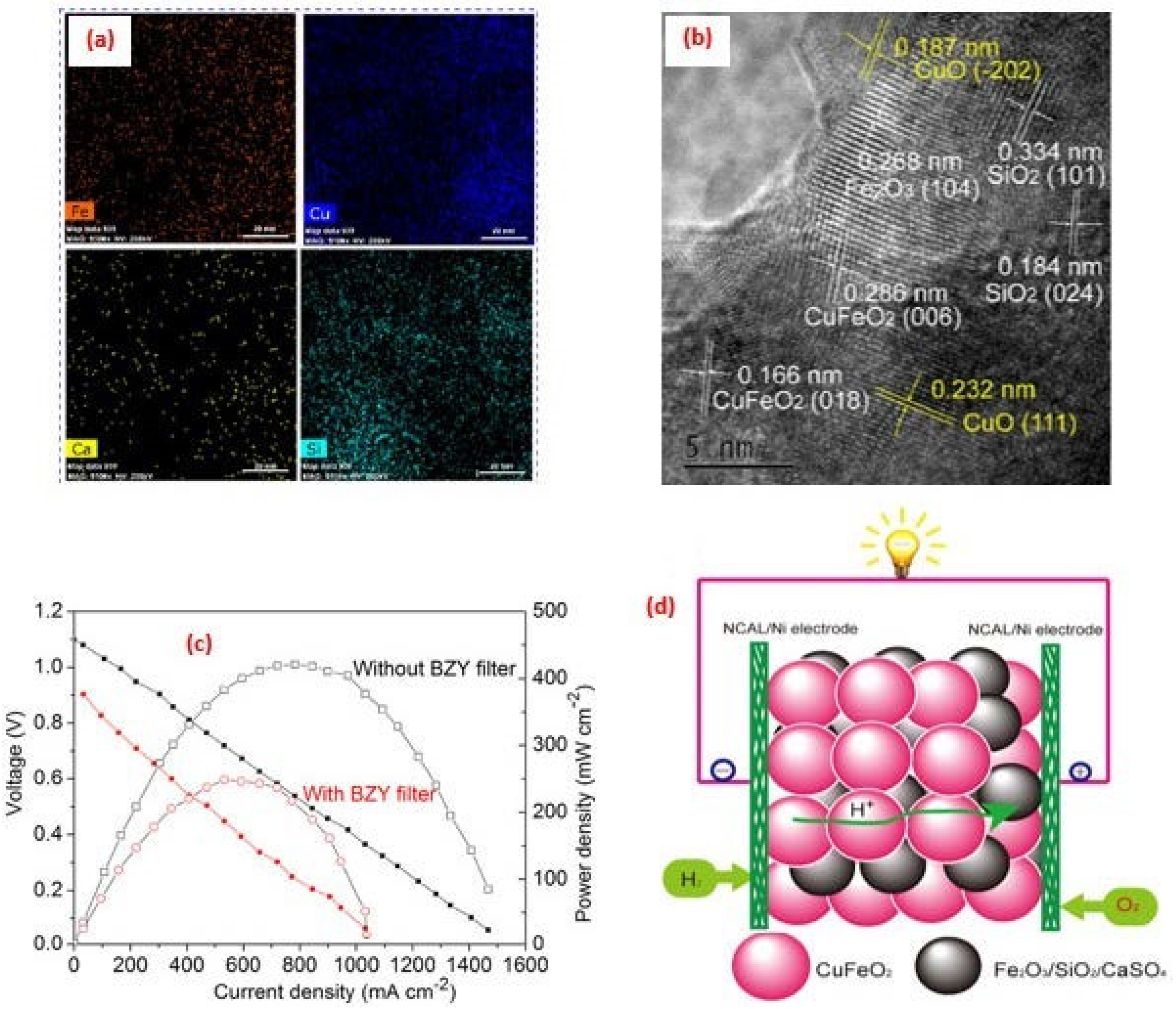
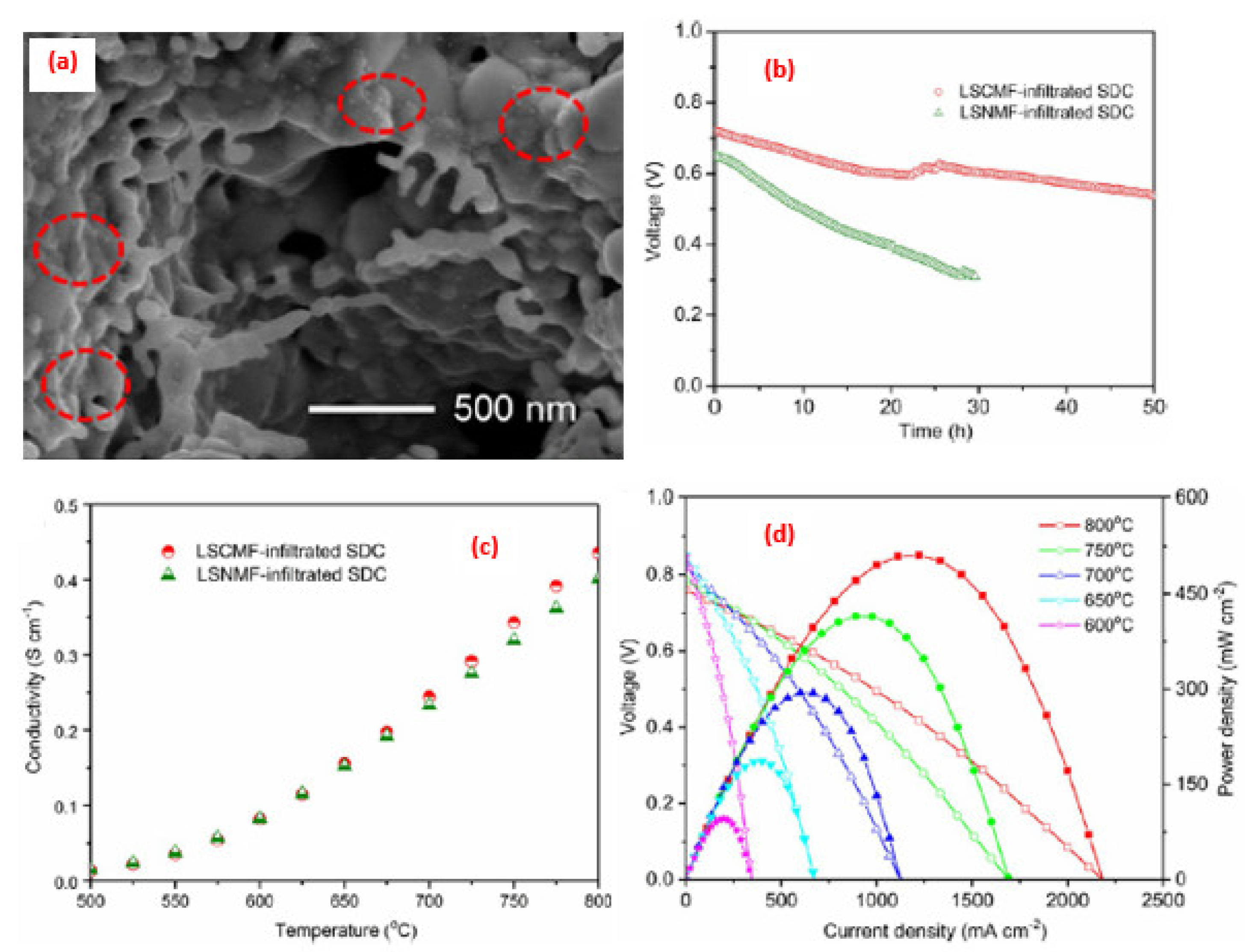
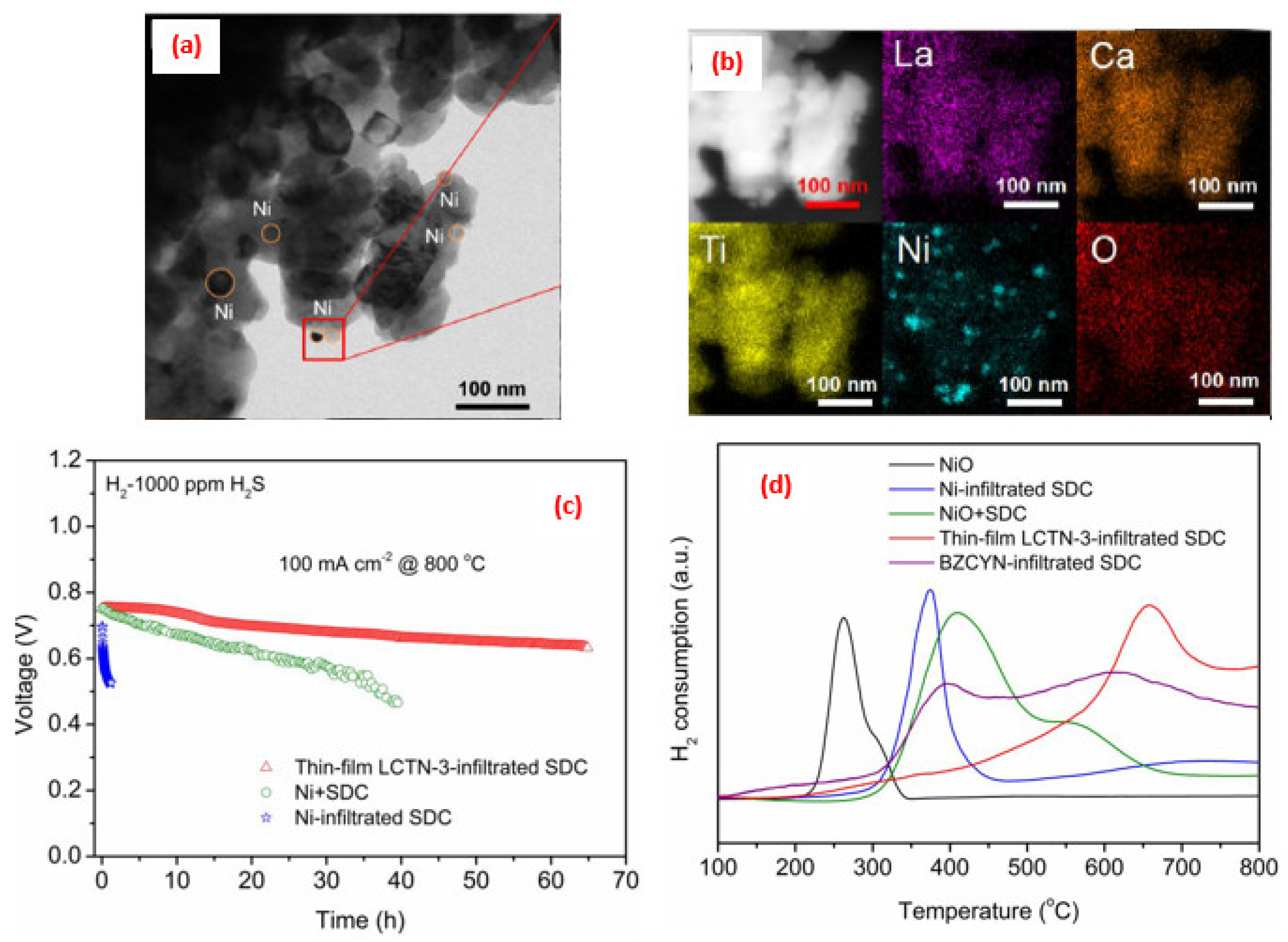
4. Application of Perovskite-Based Nanocomposites in Fuel Cells
5. Advance in the Power Density Level for SOFCs
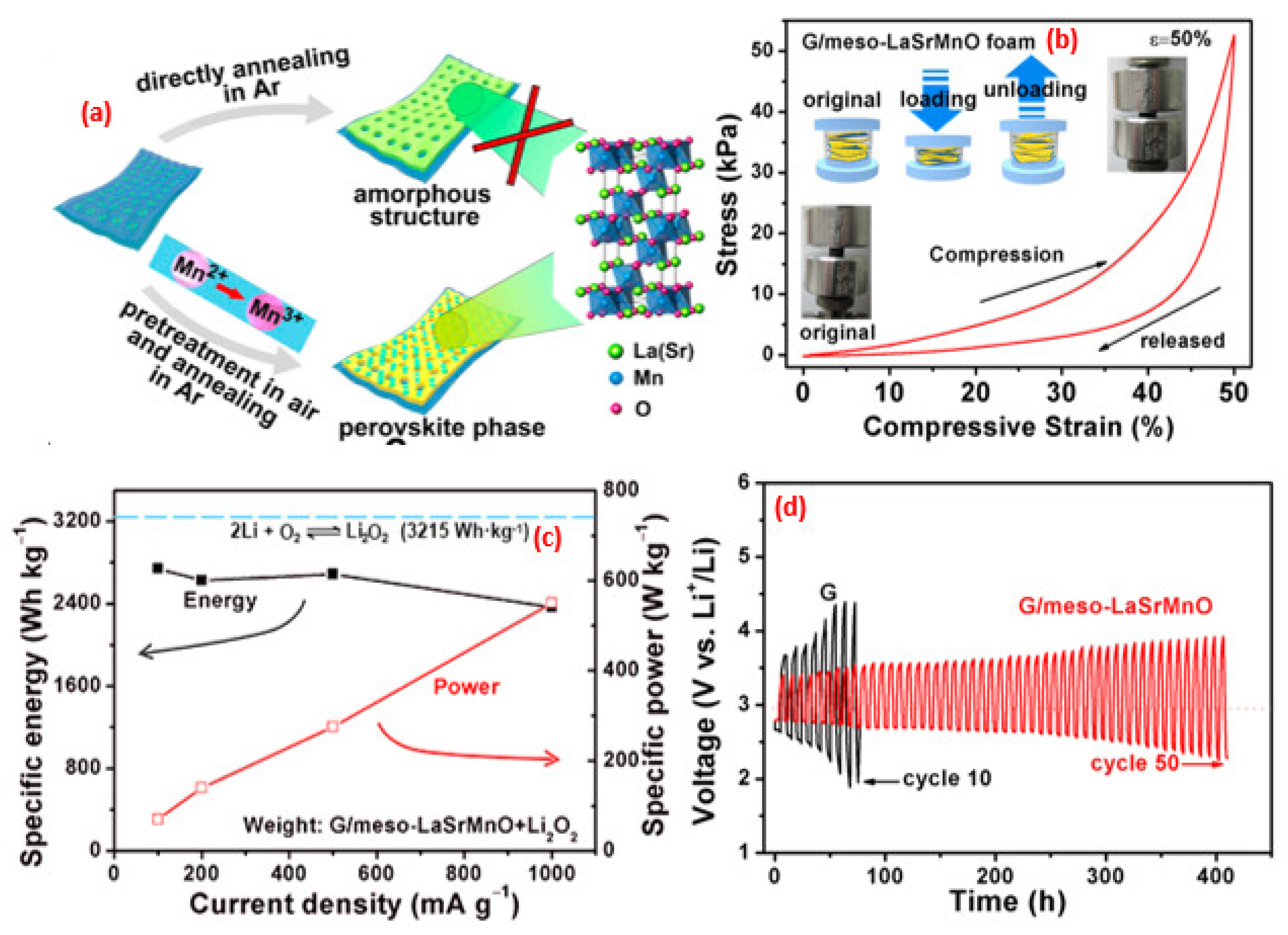
6. Perovskite-Based Nanocomposites in Battery Studies
7. Perovskite-Based Solar-Cell Studies
8. Electrochemical Measurements and Cyclic Stability
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wenk, H.R.; Bulakh, A. Mineral: Their Constitution and Origin; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Varma, P.C.R. Perovskite Photovoltaics, Basic Concepts and Implementation, Low-Dimensional Perovskite; Academic Press: Cambridge, MA, USA, 2018; pp. 197–229. [Google Scholar]
- Fisher, A.E.; Pettingrew, K.A.; Rolison, D.R.; Stround, R.M.; Long, J.W. Incorporation of homogeneous nano-scale MnO2 within ultraporous carbon structures via self-limiting electroless deposition; implications for electrochemical capacitors. Nano Lett. 2007, 7, 281–286. [Google Scholar] [CrossRef]
- Duraisamy, K.; Archana, S.; Prasath, A.; Elumalai, P. High capacity and high stability lithium-ion battery using nano Sn/SnS decorated carbon leaf anode and LiCoO2 cathode for consumer electronics. Electrochim. Acta 2020, 338, 135863. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Solvent engineering for high peroformance inorganic-organic hybrid perovskite solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Javed, M.S.; Raza, R.; Ahsan, Z.; Rafique, M.S.; Shahzadi, S.S.; Shaukat, S.F.; Shaheen, N.; Khalid, M.S.; Chengou, H.; Zhou, B. Electrochemical studies of perovskite cathode materials fordirect natural gas fuel cells. Int. J. Hydrog. Energy 2016, 41, 3072–3078. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells; a comprehensive review. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Oraganometal halide perovskite as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Lett. 2009, 6, 4874–4875. [Google Scholar]
- Nan, H.S.; Hu, X.Y.; Tian, H.W. Recent advances in perovskite oxide for anio-intercalation supercapacitor; A review. Mater. Sci. Semicond. Process. 2019, 94, 35–50. [Google Scholar] [CrossRef]
- Tomar, A.K.; Signg, G.; Sharma, R.K. Charge storage characterstics of mesoporous strontium titanate perovskite aqueous as well as flexible solid-state supercapacitor cell. J. Power Sources 2019, 426, 223–232. [Google Scholar] [CrossRef]
- Vinuraj, T.N.; Hoskeri, P.A.; Muralidhara, H.B.; Manjunatha, C.R.; Yogeshkumar, K.; Raghu, M.S. Facile synthesis of perovskite lanthanum aluminate and its green reduced graphene oxide composite for high performance supercapacitors. J. Electroanaltical Chem. 2020, 858, 113830. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Xu, W.; Wang, M.; Shao, R.; Sun, Y.; Lin, B. Mesoporous LaFeO3 perovskite derived from MOF gel for all-solid state symmetric supercapacitor. Chem. Eng. J. 2020, 386, 124030. [Google Scholar] [CrossRef]
- Tan, Z.F.; Zhang, C.; Liu, P.K.; Reed, B.; Zhao, Y.J. Focus on fuel cell system in China. Rev. Sustain. Energy Rev. 2015, 47, 912–923. [Google Scholar]
- Zhu, B.; Raza, R.; Abbas Ghazanfar Singh, M. An electrolyte-free fuel cell constructed from one homogeneous layer with mixed conductivity. Adv. Fun. Mater. 2011, 21, 2465–2469. [Google Scholar] [CrossRef]
- Singh, R.; Sui, P.C.; Wong, K.; Kjeang, E.; Knights, S.; Djilali, N. Modeling the effect of chemical membrane degradation on PEMFC performance. J. Electrochem. Soc. 2018, 165, F3328–F3336. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Wang, W.; Zhuo, C.; Zhong, Y.; Yang, G.; Zhou, W.; Liu, M.; Shao, Z. Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Jule 2019, 3, 2842–2853. [Google Scholar] [CrossRef]
- Yuvari, Z.; Noroozifar, M.; Parvizi, T. Performance evaluation of anodic nanocatalyst for direct methanol alkaline fuel cell. Environ. Prog. Sustain. Energy 2018, 37, 597–604. [Google Scholar]
- Popoola, I.K.; Gondal, M.A.; Qahtan, T.F. Recent progress in flexible perovskite solar cells; Materials, mechanical tolerance and stability. Renew. Sustain. Energy Rev. 2018, 82, 3127–3151. [Google Scholar] [CrossRef]
- Ava, T.T.; Mamun, A.A.; Marsillac, S.; Namkoong, G. A review; Thermal stability of methyl ammonium lead halide based perovskite solar cells. Appl. Sci. 2019, 9, 188. [Google Scholar] [CrossRef]
- Wang, H.; Cao, S.; Yang, B.; Li, H.; Wang, M.; Hu, X.; Sun, K.; Zang, Z. NH4Cl-modified ZnO for high performance CsPbIBr2 perovskite solar cells via low-temperature process. Sol. Rrl 2019, 4, 1900363. [Google Scholar] [CrossRef]
- Wang, P.C.; Govindan, V.; Chiang, C.H.; Wu, C.G. Room-temperature-processed fullerene/TiO2 nanocomposite electron transporting layer for high efficiency rigid and flexible planar perovskite solar cells. Sol. Rrl 2020, 4, 2000247. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Wu, Y.; Ran, R.; Shao, Z. Progress in solid oxide fuel cells with nickel-based anodes operating on methane and related fuels. Chem. Rev. 2013, 113, 8104–8151. [Google Scholar] [CrossRef]
- Song, Y.; Wang, W.; Ge, L.; Xu, X.; Zhang, Z.; Juliao, P.S.B.; Zhou, W.; Shao, Z. Rational design of a water-storable hierarchical architecture decorated with amorphous barium oxide and nickel nanoparticles as a solid oxide fuel cell anode with excellent sulphur tolerance. Adv. Sci. 2004, 4, 1700337. [Google Scholar] [CrossRef]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; Mcevoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef]
- Boukamp, B.A. Fuel cells: The amazing perovskite anode. Nat. Mater. 2003, 2, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Park, J.B.; Sun, Y.K.; Wu, F.; Amine, K. 2014, Aprotic and aqueous Li-O2 batteries. Chem. Rev. 2014, 114, 5611–5640. [Google Scholar] [CrossRef]
- Xue, Y.; Yan, S.; Huang, H.; Liu, Z. A nano-architectured metal-oxide/perovskite hybrid material as electrode catalyst for the oxygen reduction reaction in aluminium-air batteries. ACS Appl. Nano Mater. 2018. [Google Scholar] [CrossRef]
- Yang, W.; Salim, J.; Li, S.; Sun, C.; Chen, L.; Goodenough, J.B.; Kim, Y. Perovskite Sr0.95Ce0.05CoO3-δ loaded with copper nanoparticle as a bi-functional catalyst for lithium-air batteries. J. Mater. Chem. 2012, 22, 18902–18907. [Google Scholar] [CrossRef]
- Druce, J.; Tellez, H.; Burriel, M.; Sharpe, M.D.; Fawqult, L.J.; Cook, S.N.; Mcphail, D.S.; Ishihara, T.; Brongersma, H.H.; Kilner, J.A. Surface termination and subsurface restricting of perovskite-based solid oxide electrode materials. Energy Environ. Sci. 2014, 7, 3593–3599. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zheng, W.; Zheng, Y.; Chen, J.; Yu, B.; Chen, Y.; Liu, M. Controlling cation segregation in perovskite-based electrodes for high electrode-catalytic activity and durability. Chem. Soc. Rev. 2017, 46, 6345–6378. [Google Scholar] [CrossRef]
- Patel, N.K.; Utter, R.G.; Das, D.; Pecht, M. Surface degradation of stronstium-based perovskite electrodes of solid oxide fuel cells. J. Power Sources 2019, 438, 227040. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhong, Y.; Xu, X.; Veder, J.P.M.; Rowles, M.R.; Saunders, M.; Ran, R.; Shao, Z. Activation-free supercapacitor electrode based on surface modified Sr2CoMo1-xO6-δ perovskite. Chem. Eng. J. 2020, 390, 124645. [Google Scholar] [CrossRef]
- Jin, J.; Li, J.; Tai, Q.; Chen, Y.; Mishra, D.D.; Deng, W.; Xin, J.; Guo Xiao, B.; Wang, X. Efficient and stable flexible perovskite solar cells based on graphene-AgNWs substrate and carbon electrode without hole transport materials. J. Power Sources 2021, 482, 228953. [Google Scholar] [CrossRef]
- Kang, J.; Han, K.; Sun, X.; Zhang, L.; Huang, R.; Ismail, I.; Wang, Z.; Ding, C.; Zha, W.; Li, F.; et al. Suppression of Ag migration by low-temperature sol-gel Zinc oxide in the Ag nanowires transparent electrode-based flexible perovskite solar cells. Org. Electron. 2020, 82, 105714. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 10, 6446–6473. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkuvic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3rd M(Ni, Co, Fe, Mn) hydr(oxy)oxide catalyst. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancement in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Liu, Y.; Dinh, J.; Tade, M.O.; Shao, Z. Design of perovskite oxide as anion-intercalation-type electrodes for supercapacitors; Cation leaching effect. ACS Appl. Mater. Interfaces 2016, 8, 23774–23783. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shin, J.; Jang, I.C.; Ju, Y.W. Hydrothermal synthesis of three-dimensional perovskite NiMnO3 oxide and application in supercapacitor electrode. Energies 2020, 13, 36. [Google Scholar] [CrossRef]
- Alexander, C.T.; Mefford, J.T.; Saunders, J.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Anion based psedocapacitance of the perovskite library La1-xSrBO3-δ (B = Fe, Mn, Co). ACS Appl. Mater. Interfaces 2019, 11, 5084–5094. [Google Scholar] [CrossRef]
- Samdani, K.J.; Joh, D.W.; Lee, K.T. Molybdenum carbide nanoparticle-decorated 3Dnitrogen-doped carbon flowers as an efficient electrode for high-performance, all-solid-state symmetric capacitors. J. Alloys Compd. 2018, 748, 134–144. [Google Scholar] [CrossRef]
- Van Byren, F.R.; Broers, G.H.J.; Bouman, A.J.; Boesveld, C. The electrochemical determination of oxygen ion diffusion coefficients in La0.5OSr0.5CoO3-y; Experimental results and related properties. J. Electroanal. Chem. Interface 1978, 88, 353–361. [Google Scholar] [CrossRef]
- Oloore, E.O.; Mohammed, A.G.; Idris, K.P.; Abdul, J.P. CdS Quantum dots-organometallic halide perovskites bilayer electrodes structures for supercapacitor applications. Chem. Electrochim. Chem. 2019. [Google Scholar] [CrossRef]
- Muhammed Shafi, P.; Chandrabose, A.; Vinu, A. LaMnO3/RGO/PANI ternary nanocomposites for supercapacitor electrode application and their outstanding performance in all-solid state asymmetrical device design. ACS Appl. Energy Mater. 2018, 1, 2802–2812. [Google Scholar] [CrossRef]
- Hu, Q.; Yue, B.; Yang, F.; Shao, H.; Wang, J.; Ji, L.; Jia, Y.; Wang, Y.; Liu, J. Facile synthesis and electrochemical properties of perovskite-type CeMnO3 nanofiber. Chem. Sel. 2019, 4, 11903–11912. [Google Scholar]
- Rafique, M.; Hajra, S.; Iqbal, M.Z.; Nabi, G.; Gillani, S.S.A.; Tahir, M.B. Fabrication of novel perovskite oxide BaxMn1-xO3 electrode for supercapacitors. Int. J. Energy Res. 2021, 45, 4145–4154. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Qiu, M.; Tong, Y.; Mai, W. Insitu monitoring small energy storage change of electrochromic supercapacitors via perovskite photodetectros. Small Methods 2019, 2019, 1900731. [Google Scholar]
- Fan, H.; Zhang, X.; Wang, Y.; Lang, J.; Goa, R. Highly conductive KNiF3@carbon nanotubes composite materials with cross-linked structure for high performance supercapacitor. J. Power Sources 2020, 474, 228603. [Google Scholar] [CrossRef]
- Lang, X.; Mo, H.; Hu, X.; Tian, H. Supercapacitor peroformance of perovskite La1-xSrxMnO3. Dalton Trans. 2017, 46, 13720. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Choudhary, R.J.; Phase, D.M.; Devan, R.S. Structural correlation of a nanoparticle-embedded mesoporous CoTiO3 perovskite for an efficient electrochemical supercapacitors. RSC Adv. 2020, 10, 23446–23456. [Google Scholar] [CrossRef]
- Elsiddig, Z.A.; Xu, H.; Wang, D.; Zhang, W.; Guo, X.; Zhang, Y.; Sun, Z.; Chen, J. Modulating Mn4+ ions and oxygen vacancies in non stoichiometric LaMnO3 perovskite by a facile sol-gel method as high-perrformance supercapacitor electrodes. Electr. Electrochim. Acta 2017, 253, 422–429. [Google Scholar] [CrossRef]
- George, G.; Jackson, S.L.; Luo, C.Q.; Fang, D.; Luo, D.; Hu, D.; Luo, Z. Effect of doping on the performance of high-crystalline SrMnO3 perovskite nano fibers as a supercapacitor electrode. Ceram. Int. 2018, 44, 21982–21992. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Hu, Y.; Li, F.; Huang, P.; Wang, Z.; Jiang, J.; Wang, C. Three-dimensional coral-like Ni2P-ACC nanostructure as binder-free electrode for greatly improved supercapacitor. Electrochim. Acta 2020, 349, 136259. [Google Scholar] [CrossRef]
- Hu, Q.; Yue, B.; Shao, H.; Yang, F.; Wang, J.; Wang, Y.; Liu, J. Facile synthesis of cerium-based CeMO3 (M = Co, Ni, Cu) perovskite nanomaterials for high performance supercapacitor electrodes. J. Mater. Sci. 2020, 55, 8421–8434. [Google Scholar] [CrossRef]
- Minkim, B.; Kim, H.Y.; Ju, Y.W.; Shin, J. Influence of the perovskite La0.8Sr0.2Mn0.5Co0.5O3-δ on the electrochemical performance of the graphene-based supercapacitor. Energies 2020, 13, 3030. [Google Scholar]
- Soam, A.; Kumar, R.; Mahender, C.; Singh, M.; Thatoi, D.; Dusane, R.O. Development of paper based flexible supercapacitor; bismuth ferrite/graphene nanocomposite as an active electrode materials. J. Alloys Compd. 2020, 813, 152145. [Google Scholar] [CrossRef]
- Long, X.; Sun, X.; Liu, Z.; Nan, H.; Li, C.; Hu, X.; Tian, H. Ag nanoparticles decorated perovskite La0.85Sr0.15MnO3 as electrode materials for supercapacitors. Mater. Lett. 2019, 243, 34–37. [Google Scholar] [CrossRef]
- Gonzalez, P.; Cherepanov, S.; Gonzalaez, V.R.; Enriquez, A.I.E.; Padmasree, K.P.; Zakhidov, A.; Oliva, J. Using Ca2.9Nd0.1Co4O9+δ perovskites to convert a flexible carbon nanotube based supercapacitor to a battery like device. Electrochim. Acta 2020, 355, 136768. [Google Scholar] [CrossRef]
- Rezanezhad, A.; Rezaie, E.; Ghadimi, L.S.; Hajalilou, A.; Lotf, E.A.; Arsalani, N. Outstanding supercapacitor performance of Nd-MnCo-doped perovskite LaFeO3@nitrogen-doped graphene oxide nanocomposites. Electrochim. Acta 2020, 355, 135699. [Google Scholar] [CrossRef]
- Saranya, P.E.; Selladurai, S. Facile synthesis of NiSnO3/graphene nanocompoiste for high performance electrode towards asymmetric supercapacitor device. J. Mater. Sci. 2018, 53, 16022–16046. [Google Scholar] [CrossRef]
- Nayak, S.; Soam, A.; Nanda, J.; Mahender, C.; Singh, M.; Mohapatra, D.; Kumar, R. Sol-gel synthesized BiFeO3–Graphene nanocomposite as efficient electrode for supercapacitor application. J. Mater. Sci. Mater. Electron. 2018, 29, 9361–9368. [Google Scholar] [CrossRef]
- Moitra, D.; Anand, C.; Ghosh, B.K.; Chandel, M.; Ghosh, N.N. One-dimensional BiFeO3 nanowire-reduced graphene oxide nanocomposite as excellent supercapacitor electrode materials. ACS Appl. Energy Mater. 2018, 1, 464–474. [Google Scholar] [CrossRef]
- Liu, P.; Weng, X.; Liu, Z.; Zhang, Y.; Qiu, Q.; Wang, W.; Zhou, M.; Cai, W.; Ni, M.; Li, M.; et al. High-performance quasi-solid state supercapacitor based on CuO nanoparticles with commercial-level mass loading on ceramic materials La1-xSrxCoO3-δ as cathode. ACS Appl. Energy Mater. 2019, 2, 1480–1488. [Google Scholar] [CrossRef]
- Hooshyari, K.; Moradi, M.; Salarizadeh, P. Novel composite membranes based on PBI and doped-perovskite nanoparticles as a strategy for improving PEMFC performance at high temperatures. Int. J. Energy Res. 2020, 44, 2617–2633. [Google Scholar] [CrossRef]
- Akhtar, S.; Ali, A.; Ahmad, M.A.; Aslam, M.N.; Shakir, I.; Javed, M.S.; Qureshi, M.K.; Raza, R. Quantum mechanical interpretations and analysis of perovskite material based single layer fuel cells (SLFCs). Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, J.; Zou, M.; Mu, S.; Huang, H.; Meng, Y.; He, K.; Brinkman, K.S.; Tong, J. Novel twin-perovskite nanocomposite of Ba-Ce-Fe-Co-O as a promising triple conductivity cathode material for protonic ceramic fuel cells. J. Power Sources 2020, 450, 227609. [Google Scholar] [CrossRef]
- Tang, W.; Ding, H.; Bian, W.; Wu, W.; Li, W.; Liu, X.; Gomez, J.Y.; Vera, C.Y.R.; Zhou, M.; Ding, D. Understanding of A-site deficiency in layered perovskites; Promotion of dual reaction kinetics for water oxidation and oxygen reduction in proton ceramic electrochemical cells. J. Mater. Chem. A 2020. [Google Scholar] [CrossRef]
- Mahimai, B.M.; Kulasekaran, P.; Sivasubramanian, G.; Deivanayagam, P. Sulfonated poly(ether ether ketone)/barium strontium titanium oxide polymer nanocomposite membranes for fuel cell applications. Polym. Plast. Technol. Mater. 2020. [Google Scholar] [CrossRef]
- Raja, K.; Pugalenthi, R.M.; Prabhu, R.M. Investigation on SPEEK/PAI/SrTiO3-based nanocomposite membrane for high temperature proton exchange membrane fuel cells. Ionics 2019, 25, 5177–5188. [Google Scholar] [CrossRef]
- Yavari, Z.; Noroozifar, M.; Motlagh, M.K. Presentation of anodic electrocatalyst for polymeric fuel cells; Pt nanoparticles immobilized on NdFeO3 nanocrystals and carbon nanotubes. Indian J. Chem. Technol. 2019, 26, 9–22. [Google Scholar]
- He, F.; Liang, M.; Wang, W.; Ran, R.; Yang, G.; Zhu, W.; Shao, Z. High-performance proton-conducting fuel cell with B-site-deficient perovsite for all cell compounds. Energy Fuels 2020, 34, 11464–11471. [Google Scholar] [CrossRef]
- Shao, K.; Li, F.; Zhang, G.; Zhang, Q.; Maliutina, K.; Fan, L. Approaching durable single-layer fuel cells; Promotion of electroactivity and charge separation via nano alloy redox exsolution. ACS Appl. Mater. Interfaces 2019, 11, 27924–27933. [Google Scholar] [CrossRef]
- Asghar, M.I.; Heikkila, M.; Lund, P.D. Advanced lo-temperature ceramic nanocomposite fuel cells using ultrahigh ionic conductivity electrolytes synthesized through freeze-dried method and solid route. Mater. Today Energy 2017, 5, 338–346. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, B.; Deng, H.; Luo, Y.; Sun, W.; Liu, H.; Zhang, W.; Wang, X.; Qian, Y.; Hu, X.; et al. Advanced fuel cell based on perovskite La-SrTiO3 semiconductor as the electrolyte with superoxide-ion conduction. ACS Appl. Mater. Interfaces 2018, 10, 33179–33186. [Google Scholar] [CrossRef]
- Ma, M.; Qiao, J.; Yang, X.; Ren, R.; Sun, W.; Sun, K. Enhanced stability and catalytic activity on layered perovskite anode for high performance hybrid direct carbon fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 12938–12948. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Li, L.; Wei, J.; Li, J.; Yang, X.; Yan, C.; Zhou, C.; Zhu, B. Proton conduction and fuel cell using the CuFe-oxide mineral composite based on CuFeO2 structure. ACS Appl. Energy. Mater. 2018, 1, 580–588. [Google Scholar] [CrossRef]
- Attaran, A.A.M.; Javanbakht, M.; Hooshyari, K.; Enhessari, M. New proton conducting nanocomposite membrane based on polyvinyl alcohol/polyvinyl pyrolidone/BaZrO3 for proton exchange membrane fuel cells. Solid State Ion. 2015, 269, 98–105. [Google Scholar] [CrossRef]
- Muthuraja, P.; Prakash, P.; Shanmugam, V.M.; Radhakrishnan, S.; Manisankar, P. Novel perovskite structured calcium titanate-PBI composite membrane for high-temperature PEM fuel cells: Synthesis and characterizations. Int. J. Hydrog. Energy 2017, 43, 4763–4772. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, F.; Wang, X.; Zhou, Q.; Liu, J.; Liu, M. Direct operation of Ag-based anode solid oxide fuel cells on propane. J. Power Sources 2017, 366, 56–64. [Google Scholar] [CrossRef]
- Yi, Y.; Rao, A.D.; Brouwer, J.; Samuelsen, G.S. Fuel flexibility study of an integrated25kW SOFC reformer system. J. Power Sources 2005, 144, 67–76. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Singhal, B. Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb. Chem. High Throughput Screen 2011, 14, 284–302. [Google Scholar] [CrossRef]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Vohs, J.M.; Gorte, R.J. High-performance SOFC cathodes prepared by infiltration. Adv. Mater. 2009, 21, 943–956. [Google Scholar] [CrossRef]
- Gross, M.D.; Vohs, J.M.; Gorte, R.J. Recent progress in SOFC anodes for direct utilization of hydrocarbons. J. Mater. Chem. 2007, 17, 3071–3077. [Google Scholar] [CrossRef]
- Li, H.P.; Song, Y.; Xu, M.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Exsolved alloy nanoparticles decorated ruddlesden−popper perovskite as sulfur-tolerant anodes for solid oxide fuel cells. Energy Fuels 2020, 34, 11449–11457. [Google Scholar] [CrossRef]
- Kim, Y.D.; Yang, J.Y.; Saqib, M.; Park, S.J.; Shin, J.S.; Jo, M.; Park, K.M.; Lim, H.T.; Song, S.J.; Park, J.Y. Cobalt-free perovskite Ba1-xNdxFeO3-δ air electrode materials for reversible solid oxide cells. Ceram. Int. 2021, 47, 7985–7993. [Google Scholar] [CrossRef]
- Blennow, P.; Hansen, K.K.; Wallenberg, L.R.; Mogensen, M. Electrochemical characterization and redox behavior of Nb-doped SrTiO3. Solid State Ion. 2009, 180, 63–70. [Google Scholar] [CrossRef]
- Kim, J.H.; Cassidy, M.; Irvine, J.T.S.; Bae, J. Electrochemical investigation of composite cathodes with SmBa0.5Sr0.5Co2O5+δ cathodes for intermediate temperature-operating solid oxide fuel cell. Chem. Mater. 2010, 22, 883–892. [Google Scholar] [CrossRef]
- Huang, Y.H.; Liang, G.; Croft, M.; Lehtimaki, M.; Karppinen, M.; Goodenough, J.B. Double-perovskite anode materials Sr2MMoO6 (M = Co, Ni) for solid oxide fuel cells. Chem. Mater. 2009, 21, 2319–2326. [Google Scholar] [CrossRef]
- Adijanto, L.; Sampath, A.; Yu, A.S.; Cargnello, M.; Fornasiero, P.; Gorte, R.J.; Vohs, J.M. Synthesis and stability of Pd@CeO2 core−shell catalyst films in solid oxide fuel cell anodes. ACS Catal. 2013, 3, 1801–1809. [Google Scholar] [CrossRef]
- Song, Y.; Wang, W.; Qu, J.; Zhong, Y.; Yang, G.; Zhou, W.; Shao, Z. Rational design of perovskite-based anode with decent activity for hydrogen electro-oxidation and beneficial effect of sulfur for promoting power generation in solid oxide fuel cells. ACS. Appl. Mater. Interfaces 2018, 10, 41257–41267. [Google Scholar] [CrossRef]
- Hibino, T.; Hashimoto, A.; Inoue, T.; Tokuno, J.; Yoshida, S.; Sano, M. A low-operating-temperature solid oxide fuel cell in hydrocarbon-air mixtures. Science 2000, 288, 2031–2033. [Google Scholar] [CrossRef]
- Hui, S.R.; Petric, A. Evaluation of Yttrium-doped SrTiO3 as an anode for solid oxide fuel cells. J. Eur. Ceram. 2002, 22, 1673–1681. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Du, Z.; Chen, T.; Chen, N.; Liu, X.; Skinner, S.J. Effects of Co doping on the electrochemical performance of double perovskite oxide Sr2MgMoO6−δ as an anode material for solid oxide fuel cells. J. Phys. Chem. C 2012, 116, 9734–9743. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Perovskite as a cathode material: A review of its role in solid-oxide fuel cell technology. ChemElectroChem 2016, 3, 511–530. [Google Scholar] [CrossRef]
- Liu, Q.; Xiao, Z.; Xie, H.; Gao, J.; Yuan, M.; Dong, W. Multifunctional layer-perovskite oxide La2-xCexCuO4 for solid oxide fuel cell applications. Int. J. Hydrog. Energy 2020, 46, 9818–9825. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, B.Y.; Wu, Y.J.; Sun, G.; Chen, G.; Hu, K.A. Effect of B-site doping on Sm0.5Sr0.5MxCo1-xO3-δ properties for IT-SOFC cathode material (M = Fe, Mn). Mater. Res. Bull. 2007, 42, 1999–2012. [Google Scholar] [CrossRef]
- Marina, O.A.; Canfield, N.L.; Stevenson, J.W. Thermal, electrical, and electrocatalytical properties of lanthanum-doped strontium titanate. Solid State Ion. 2002, 149, 21–28. [Google Scholar] [CrossRef]
- Niu, Y.; Sunarso, J.; Liang, F.; Zhou, W.; Zhu, Z.; Shao, Z. A comparative study of oxygen reduction reaction on Bi- and La-doped SrFeO3-δ perovskite cathodes. J. Electrochem. Soc. 2011, 158, B132–B138. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, Z.; Wang, X.; Tu, B.; Cheng, M. Self-assembled cubic-hexagonal perovskite nanocomposite as intermediate temperature solid oxide fuel cell cathode. Ceram. Int. 2020, 46, 22282–22289. [Google Scholar] [CrossRef]
- Sengodan, S.; Choi, S.; Jun, A.; Shin, T.H.; Ju, Y.W.; Jeong, H.Y.; Shin, J.; Irvine, J.T.S.; Kim, G. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 2014, 14, 205–209. [Google Scholar] [CrossRef]
- Kausar Shaheen, K.; Suo, H.; Shah, Z.; Hanif, M.B.; Hussain, Z.; Ali, S.; Liu, M.; Ma, L.; Cui, J.; Jia, Y.T.; et al. Electrochemical performance of multi-fuel based nanocomposite for solid oxide fuel cell. Ceram. Int. 2019, 46, 8832–8838. [Google Scholar] [CrossRef]
- Godickemeier, M.; Sasaki, K.; Gauckler, L.J.; Riess, I. Perovskite cathodes for solid oxide fuel cells based on ceria electrolytes. Solid State Ion. 1996, 86–88, 691–701. [Google Scholar] [CrossRef]
- Xia, C.; Liu, M. Novel cathodes for low-temperature solid oxide fuel cells. Adv. Mater. 2002, 14, 521–523. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, W.; Sunarso, J.; Ge, L.; Zhu, Z.; Shao, Z. High performance cobalt-free perovskite cathode for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 9619–9622. [Google Scholar] [CrossRef]
- Jhuang, J.W.; Lee, K.R.; Lee, S.W.; Wang, B.; Xia, C.; Hung, I.M.; Tseng, C.J. A triple (e−/O2−/H+) conducting perovskite BaCo0.4Fe0.4Zr0.1Y0.1O3-δ for low temperature solid oxide fuel cell. Int. J. Hydrog. Energy 2020, 46, 9767–9774. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in tubular solid oxide fuel cell technology. Proceedings of the electrochemical society. ECS Proc. Vol. 1995, 1995, 195–207. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Vernardou, D.; Makri, D.; Brintakis, K.; Savva, K.; Stratakis, E. Highly stable metal halide perovskite microcube anodes for lithium-air batteries. J. Power Sources Adv. 2020, 3, 100015. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, W.; Wu, S.; Yang, X.; Xia, W.; Shen, Y.; Huang, Y.; Cao, A.; Yuan, Q. Perovskite-Type LaSrMnO Electrocatalyst with Uniform Porous Structure for an Efficient Li−O2 Battery Cathode. ACS Nano 2016, 10, 1240–1248. [Google Scholar] [CrossRef]
- Xia, T.; Li, Y.; Huang, L.; Ji, W.; Yang, M.; Zhao, X. Room-temperature stable inorganic halide perovskite as potential solid electrolyte for chloride ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 18634–18641. [Google Scholar] [CrossRef]
- Deng, Z.; Radhakrishnan, B.; Ong, S.P. Rational composition optimization of the lithium-rich Li3OCl1−xBrx anti-perovskite superionic conductors. Chem. Mater. 2017, 27, 3749–3755. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.; Xia, L.; Wang, S.; Ding, L.X.; Li, D.; Xiao, K.; Dai, S.; Wang, H. Hierarchical mesoporous/macroporous perovskite La0.5Sr0.5CoO3−x nanotubes: A bi-functional catalyst with enhanced activity and cycle stability for rechargeable lithium oxygen batteries. ACS. Appl. Mater. Interfaces 2015, 7, 22478–22486. [Google Scholar] [CrossRef]
- Yan, S.; Xue, Y.; Li, S.; Shao, G.; Liu, Z. Enhanced bi-functional catalytic activity of manganese oxide/perovskite hierarchical core-shell materials by adjusting the interface for metal–air batteries. ACS Appl. Mater. Interfaces 2019, 11, 25870–25881. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A highly efficient and robust cation ordered perovskite oxide as a bi-functional catalyst for rechargeable zinc-air batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Park, M.G.; Ismayilov, V.; Chen, Z. Synergistic bi-functional catalyst design based on perovskite oxide nanoparticles and intertwined carbon nanotubes for rechargeable zinc-air battery applications. ACS Appl. Mater. Interfaces 2015, 7, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Hu, Q.; Ji, L.; Wang, Y.; Liu, J. Facile synthesis of perovskite CeMnO3 nanofibers as an anode material for high performance lithium ion batteries. RSC Adv. 2019, 9, 38271–38279. [Google Scholar] [CrossRef]
- Tong, W.; Yoon, W.S.; Hagh, N.M.; Amatucci, G.G. Novel silver molybdenum oxy-fluoride perovskite as a cathode material for lithium batteries. Chem. Mater. 2009, 21, 2139–2148. [Google Scholar] [CrossRef]
- Weidenkaff, A.; Ebbinghaus, S.G.; Lippert, T. Ln1-xAxCoO3 (Ln) Er, La; A (Ca, Sr)/carbon nanotube composite materials applied for rechargeable Zn/Air batteries. Chem. Mater. 2002, 14, 1797–1805. [Google Scholar] [CrossRef]
- Wang, X.; Ge, L.; Lu, Q.; Dai, J.; Guan, D.; Ran, R.; Weng, S.C.; Hu, Z.; Zhou, W.; Shao, Z. High performance metal-organic frame-work perovskite hybrid as an important component of the air-electrode for rechargeable Zn-air battery. J. Power Sources 2020, 468, 228377. [Google Scholar] [CrossRef]
- Hardin, W.G.; Slanac, D.A.; Wang, X.; Dai, S.; Johnson, K.P.; Stevenson, K.J. Highly active, nonprecious metal perovskite electrocatalysts for bi-functional metal-air battery electrodes. J. Phys. Chem. Lett. 2013, 4, 1254–1259. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, G.H.; Sung, M.C.; Kim, D.W. Orthorhombically distorted perovskite SeZnO3 nanosheets as an electrocatalyst for lithium-oxygen batteries. Chem. Eng. Technol. 2021, 406, 126896. [Google Scholar] [CrossRef]
- Lua, C.H.; Naresha, N.; Senthil Kumara, P.; Soma, S. Microwave-assisted solvothermal synthesis and electrochemical characterizations of ternary perovskite NiTiO3 anode materials for lithium ion batteries. Ceram. Int. 2019, 45, 19517–19521. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Z.; Yu, Y.; Yin, J.; Song, K.; Yang, B.; Yuan, L.; Hu, X. Copper/cobalt-doped LaMnO3 perovskite oxide as a bifunctional catalyst for rechargeable Li-O2 batteries. J. Alloy. Compd. 2019, 801, 19–26. [Google Scholar] [CrossRef]
- Miao, H.; Wu, X.; Chen, B.; Wang, Q.; Wang, F.; Wang, J.; Zhang, C.; Zhang, H.; Yuan, J.; Zhang, Q. A-site deficient/excessive effects of LaMnO3 perovskite as bi-functional oxygen catalyst for zinc-air batteries. Electrochim. Acta 2020, 333, 135566. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Dai, L. Efficiently photo-charging lithium-ion battery by perovskite solar cell. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Tian, G.; Zhang, Q.; Knapp, M.; Ehrenberg, H.; Chen, G.; Shen, Z.; Yang, G.; Gu, L.; et al. Lithium lanthanum titanate perovskite as an anode for lithium ion batteries. Nat. Commun. 2020, 11, 3490. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Liu, H.; Yu, Z.; Zheng, Z.; Shao, C. The electrochemical performance of La0.6Sr0.4Co1-xNixO3 perovskite catalysts for Li-O2 batteries. Ionics 2016, 22, 869–876. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, X.; Li, Z.; Xu, M.; Lu, Y.; Liu, S.; Zhang, Y.; Sun, C. Perovskite Sr0.9Y0.1CoO3-# nano rods modified with CoO nanoparticles as a bi-functional catalyst for rechargeable Li-O2 batteries. ACS Appl. Energy Mater. 2018, 1, 5557–5566. [Google Scholar]
- Wang, S.; Cui, B.; Zhuang, Q.; Shi, Y.; Zheng, H. Scalable synthesis nano-perovskite K(Mn0.95Ni0.05)F3 cathode by homogeneous precipitation method for potassium-ion Batteries. Nanoscale Res. Lett. 2019, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Xu, D.; Wang, Z.L.; Wang, H.G.; Zhang, L.L.; Zhang, X.B. Synthesis of perovskite-based porous La0.75Sr0.25MnO3 nanotubes as a highly efficient electrocatalyst for rechargeable lithium-oxygen batteries. Angew. Chem. Int. Ed. 2013, 52, 3887–3890. [Google Scholar] [CrossRef]
- Xu, Q.; Song, S.; Zhang, Y.; Wang, Y.; Zhang, J.; Ruana, Y.; Han, M. Ba0.9Co0.7Fe0.2Nb0.1O3-d perovskite as oxygen electrode catalyst for rechargeable Li-oxygen batteries. Electrochim. Acta 2016, 191, 577–585. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, H.; Sun, S.; Wang, Q.; Li, S.; Liu, Z. (La1-xSrx)0.98 MnO3 perovskite with A-site deficiencies toward oxygen reduction reaction in aluminium-air batteries. J. Power Sources 2017, 342, 192–201. [Google Scholar] [CrossRef]
- Manders, J.R.; Tsang, S.W.; Hartel, M.J. Solution-processed nickel oxide hole transport layers in high efficiently polymer photovoltaic cells. Adv. Funct. Mater. 2013, 23, 2993–3001. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, Y.; Liu, Y.; Gao, H.; Mao, Y. An inorganic hole-transport materials of CuInSe2 for stable and efficient perovskite solar cells. Org. Electron. 2019, 67, 168–174. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, B.; Cao, C.; Wu, R.; Huang, Y.; Sun, J.; Zhang, J.; Liu, C.; Tao, S.; Gao, Y.; et al. Interface degradation of perovskite solar cells and its modification using an annealing-free TiO2 NPs layer. Org. Electron. 2016, 30, 30–35. [Google Scholar] [CrossRef]
- Lv, M.; Zhu, J.; Huang, Y.; Shao, Z.; Dai, S. Colloidal CuInS2 quantum dots as inorganic hole-transporting materials in perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 17482–17488. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Ball, J.M.; Barea, E.M.; Abate, A.; Webber, J.A.A.; Huang, J.; Saliba, M.; Sero, I.M.; Bisquert, J.; Snaith, H.J.; et al. Low-temperature processed electron collection layers of graphene/TiO2 nanocomposites in thin film perovskite solar cells. Nano Lett. 2014, 14, 724–730. [Google Scholar] [CrossRef]
- Yang, H.; Kwon, H.C.; Ma, S.; Kim, K.; Yun, S.C.; Jang, G.; Park, J.; Lee, H.; Goh, S.; Moon, J. Energy level graded Al-doped ZnO protection layers of for copper nanowire-based window electodes for efficient flexible perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 13824–13835. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Y.; Shen, H.Q.; Luo, Q.; Zhao, X.; Li, J.; Lin, H. Working from sides; composite metallic semitransparent top electrode for high performance perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 4523–4531. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hsu, F.C.; Huang, J.Y.; Wang, L.; Chen, Y.F. Bi-functional polymer nanocomposites as hole transport layers for efficient light harvesting; Application to perovskite solar cells. ACS Appl. Mater. Interfaces 2015. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Baker, R.H.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sentized solar cells. Nature 2013, 499, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.J.; Ramirez, M.O.; Nazeeruddin, M.; Gratzel, M.; Elipe, A.R.G.; Ahmad, S. Nanocolumnar 1-dimensional TiO2 photoanodes deposited by PVD-OAD for perovskite solar cell fabrication. J. Mater. Chem. A 2015, 3, 13291–13298. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.W.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.J.; Jutteau, S.; Posada, J.; Bercegol, A.; Rebai, A.; Guillemot, T.; Bodeux, R.; Schneider, N.; Loones, N.; Ory, D.; et al. Highly efficient MoOx-free semitransparent perovskite cell for 4T tandem application improving the efficiency of commercially available Al-BSF silicon. Sci. Rep. 2018, 8, 16139. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Zhang, Z.; Chao, C.; An, Q.; Paulus, F.; Krole, M.; Loffler, M.; Nehm, F.; Rellinghous, B.; Leo, K.; et al. Thermally evaporated methylammonium-free pervoskite solar cells. J. Mater. Chem. C 2020, 8, 7725–7733. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Chin, X.Y.; Dewi, H.A.; Vergeer, K.; Goh, T.W.; Lim, H.W.M.; Lew, J.H.; Loh, K.P.; Soci, C.; et al. Highly efficient thermally co-evaporated perovskite solar cells and mini-modules. Jule 2020, 4, 1035–1053. [Google Scholar] [CrossRef]
- Longo, G.; Escrig, L.G.; Degen, M.J.; Sessolo, M.; Bolink, H.J. Perovskite solar cells prepared by flash evaporation. Chem. Commun. 2015, 5, 7376–7378. [Google Scholar] [CrossRef]
- Li, X.; Bi, D.; Yi, C.; Decoppet, J.D.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Gratzel, M. A vacuum flash-assisted solution process for high efficiency large-area perovskite solar cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef]
- Jones, T.W.; Duffy, N.W.; Wilson, G.J. Efficient all-printable solid-state dye-sensitized solar cells based on a low resistivity carbon composite counter electrode and highly doped hole transport materials. J. Phy. Chem. C 2015, 119, 11410–11418. [Google Scholar] [CrossRef]
- Hu, Z.; Martin, J.M.G.; Li, Y.; Billot, L.; Sun, B.; Fresno, F.; Martin, A.G.; Gonzalez, M.U.; Aigouy, L.; Chen, Z. TiO2 nanocolumn array for more efficient and stable perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 5979–5989. [Google Scholar] [CrossRef]
- Hvojnik, M.; Popovicova, A.M.; Pavlickova, M.; Hatala, M.; Gemeiner, P.; Mullerova, J.; Tomanova, K.; Mikula, M. Solution-processed TiO2 blocking layers in printed carbon-based perovskite solar cells. Appl. Surf. Sci. 2021, 536, 147888. [Google Scholar] [CrossRef]
- Dadashbeik, M.; Fathi, D.; Eskandari, M. Design and simulation of perovskite solar cell based on graphene and TiO2/graphene nanocomposite as electron transport layer. Sol. Energy 2020, 207, 917–924. [Google Scholar] [CrossRef]
- Song, J.; Hu, W.; Li, Z.; Wang, X.F.; Tian, W. A double hole-transport layer strategy toward efficient mixed tin-lead iodide perovskite solar cells. Sol. Energy Mater. Sol. Cells 2020, 207, 110351. [Google Scholar] [CrossRef]
- Amini, A.; Abdizadeh, H.; Golobostanfaard, M.R. Hybrid 1D/2D carbon nanostrucuture incorporated titania photoanode of perovskite solar cells. ACS Appl. Energy Mater. 2020, 3, 6195–6204. [Google Scholar] [CrossRef]
- Shin, D.H.; Ko, J.S.; Kang, S.K.; Choi, S.H. Enhanced flexibility and stability in perovskite photodiode-solar cell nano system using MoS2 electron-transport layer. ACS Appl. Mater. Interfaces 2020, 12, 4586–4593. [Google Scholar] [CrossRef]
- Chen, J.; Dong, H.; Zhang, L.; Li, J.; Jia, F.; Jiao, B.; Xu, J.; Hou, X.; Liu, J.; Wu, Z. Graphitic carbon nitride doped SnO2 enabling efficient perovskite solar cells with PCEs exceeding 22%. J. Mater. Chem. A 2020, 8, 2644–2653. [Google Scholar] [CrossRef]
- Han, G.S.; Song, Y.H.; Jin, Y.U.; Lee, J.W.; Park, N.G.; Kang, B.K.; Lee, J.K.; Cho, I.S.; Yoon, D.H.; Jung, H.S. Reduced graphene oxide/mesoporous TiO2 nanocomposite based perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 23521–23526. [Google Scholar] [CrossRef] [PubMed]
- Belchi, R.; Habert, A.; Foy, E.; Gheno, A.; Vedraine, S.; Antony, R.; Ratier, B.; Boucle, J.; Boime, N.H. One-step synthesis of TiO2/graphene nanocomposite by laser pyrolysis with well-controlled properties and application in perovskite solar cells. ACS Omega 2019, 4, 11906–11913. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Marry, A.J.C.; Bose, A.C. Electrochemical performance of ANiO3 (A=La, Ce) perovskite oxide materials and its device performance for supercapacitor application. Electrochim. Acta 2020, 362, 137095. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Kumar, A. Energy storage properties of double perovskites Gd2NiMnO6 for electrochemical supercapacitor applications. Solid State Sci. 2020, 105, 106252. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Ding, Y. Li-Ion conductivity and electrochemical stability of A-site deficient perovskite structured Li3x-yLa1-yAl1-yTiyO3 electrolytes. Mater. Res. Bull. 2021, 133, 111019. [Google Scholar] [CrossRef]
- Kang, K.N.; Lee, H.; Kim, J.; Kwak, M.J.; Jeong, H.Y.; Kim, G.; Jang, J.H. Co3O4 exsolved defective layered perovskite oxide for energy storage system. ACS Energy Lett. 2020, 5, 3828–3836. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Ma, Y.R.; Shirage, P.M.; Devan, R.S. Mesorporous perovskite of interlocked nickel titanate nanoparticles for efficient electrochemical supercapacitor electrode. J. Alloys Compd. 2020, 833, 155134. [Google Scholar] [CrossRef]
- Pious, J.K.; Lekshmi, M.L.; Muthu, C.; Rakhi, R.R.; Nair, V.C. Zero-dimensional methylammonium bismuth iodide-based lead-free perovskite capacitor. ACS Omega 2017, 2, 5798–5802. [Google Scholar] [CrossRef] [PubMed]


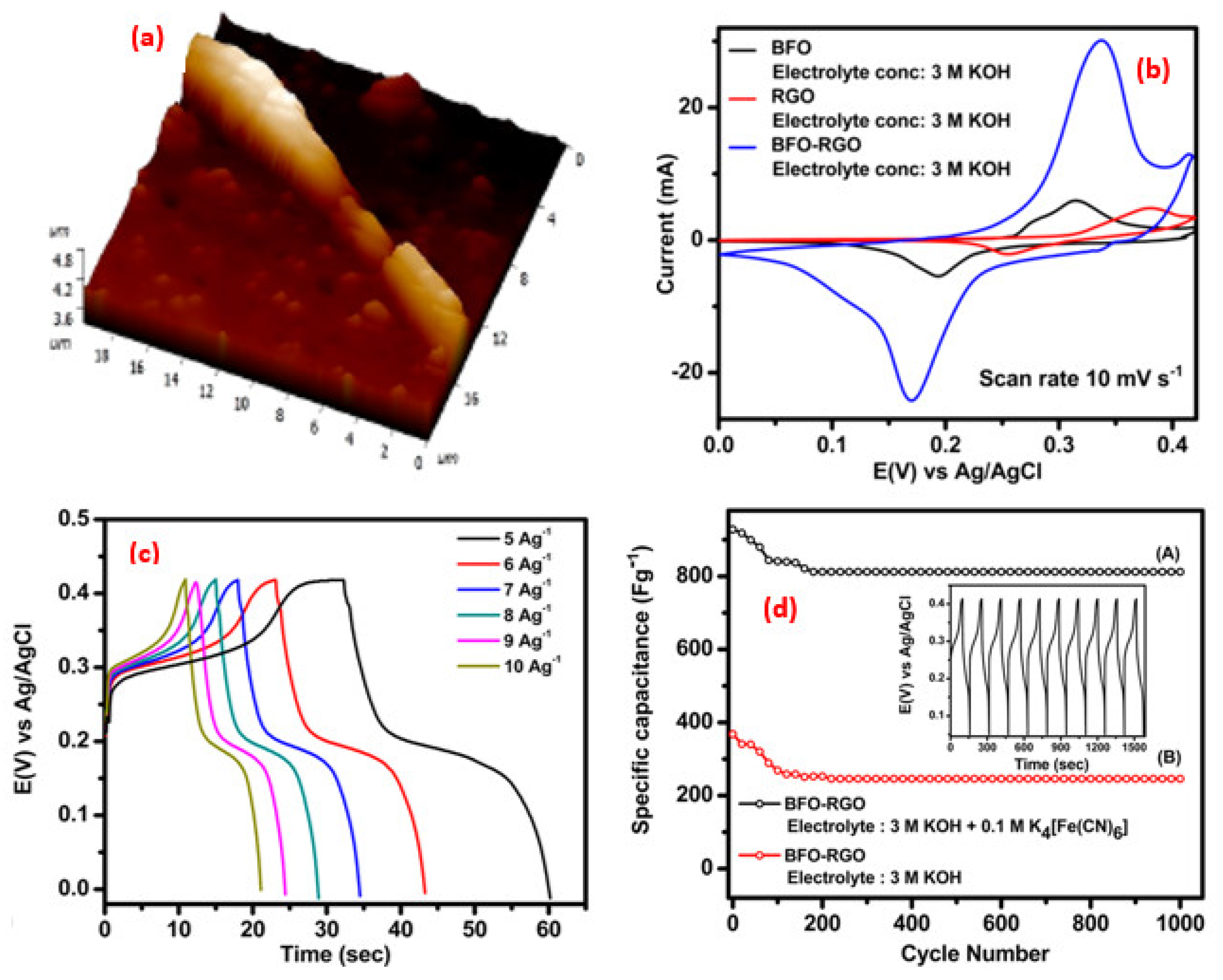
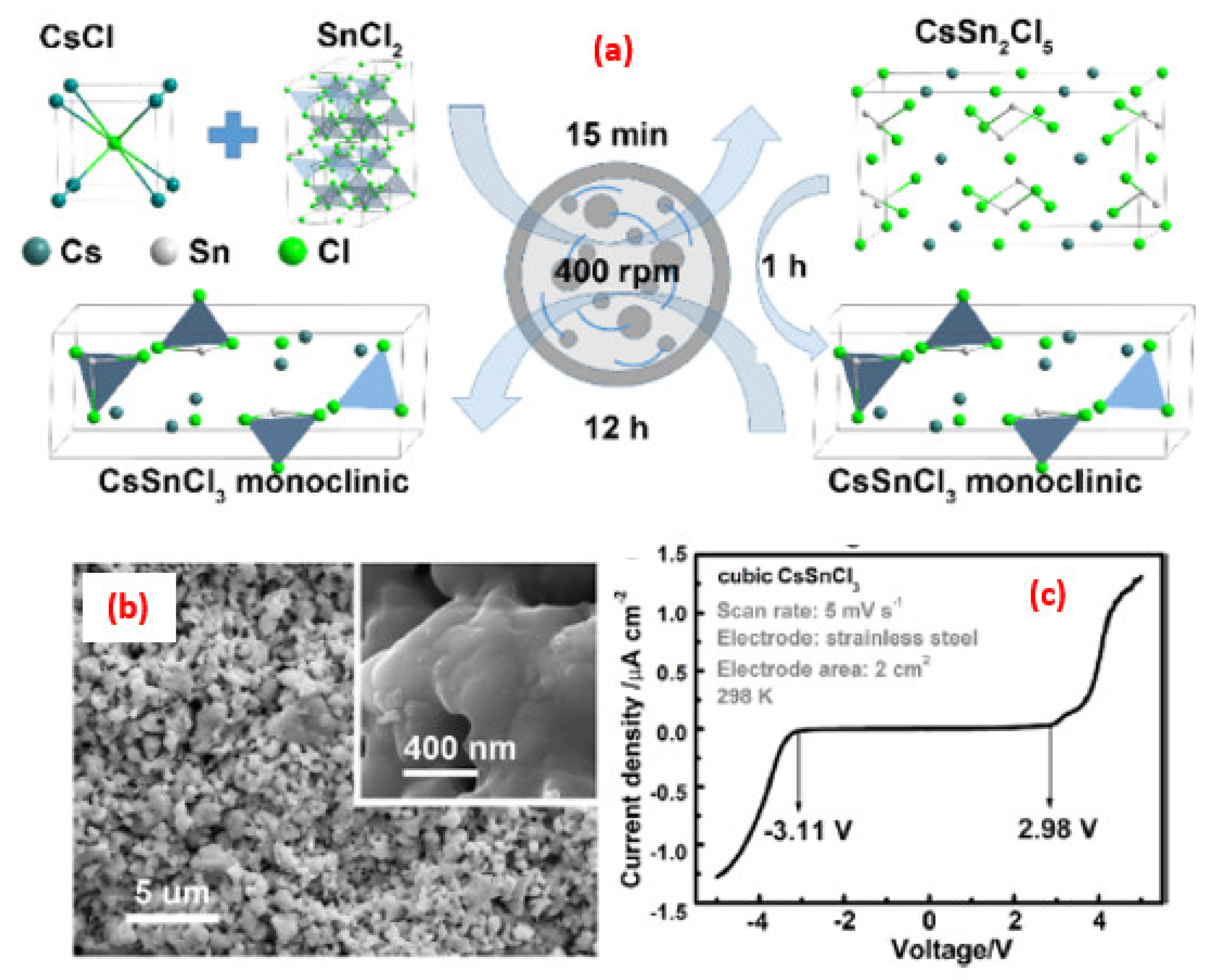
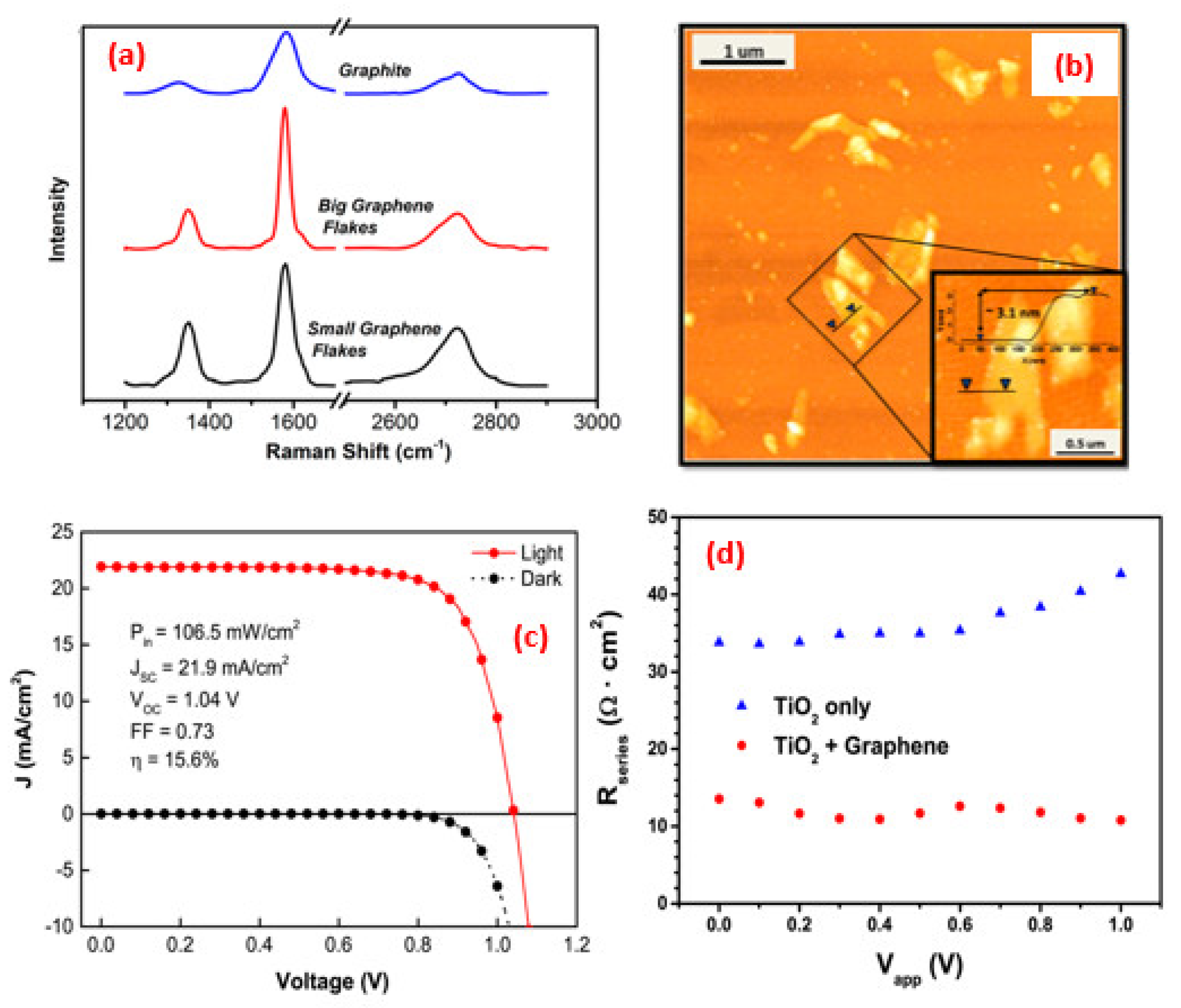
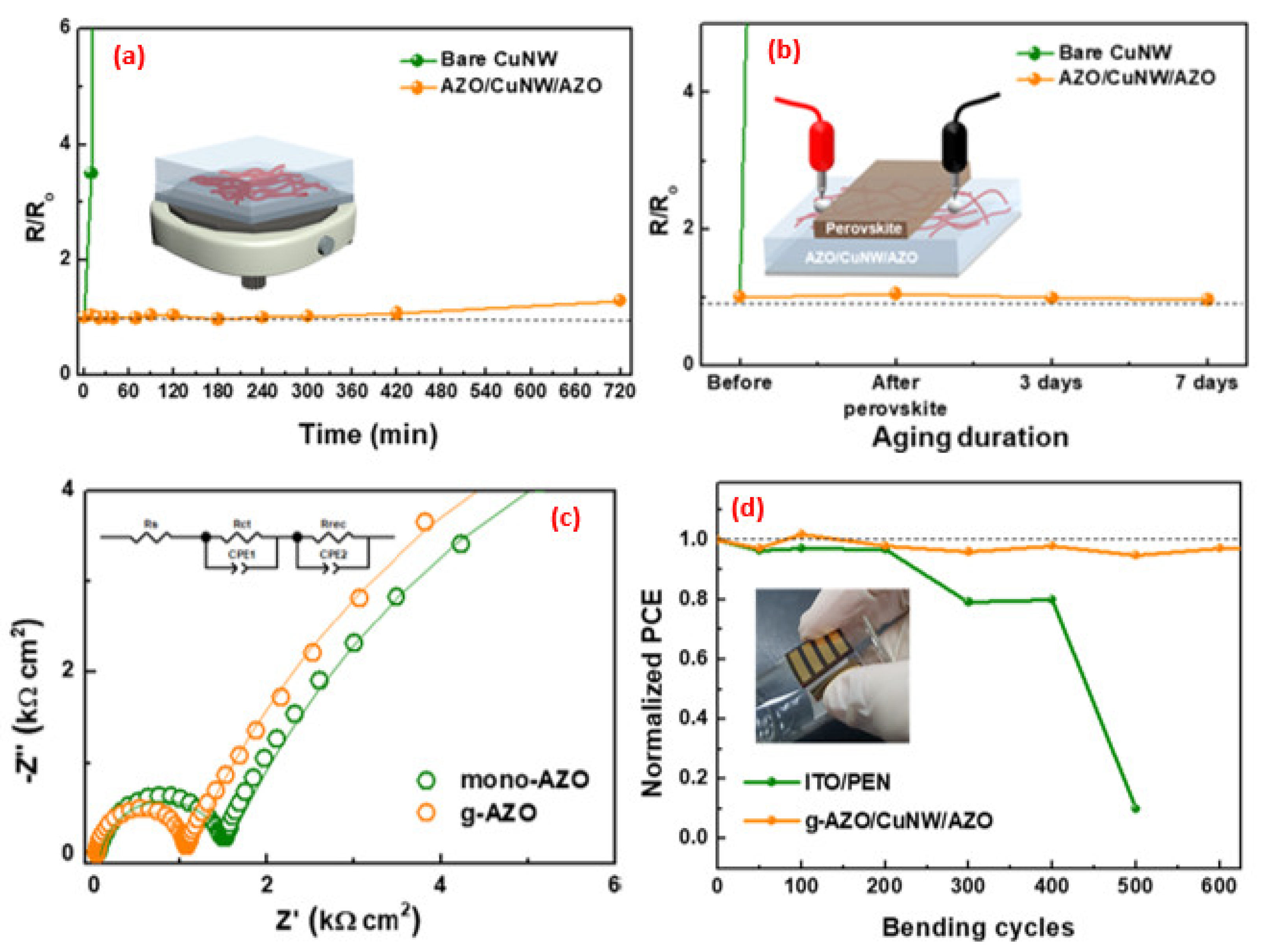
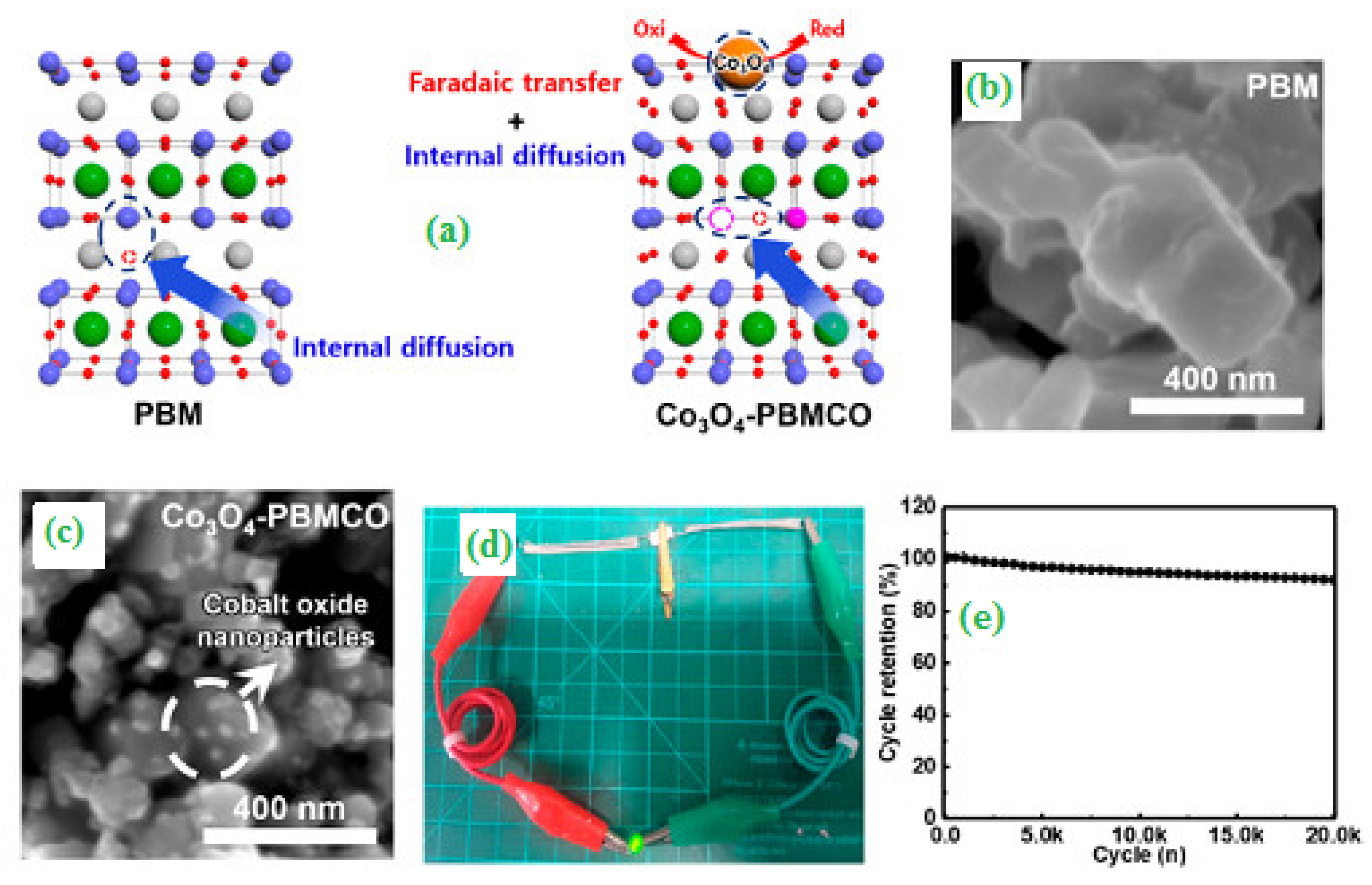
| Synthesis Strategies | Materials | Typical Morphology | Specific Capacitance (F g−1) | Power Density (W kg−1) | Energy Density (Wh kg−1) | Cycles | Ref. |
|---|---|---|---|---|---|---|---|
| Coprecipitation | La1−xSrxBO3−δ | - | 492 | 31 | 10,000 | - | [41] |
| Sol-gel | SrMnO3 | Nanofiber | 446.8 | 37.3 | 400 | 5000 | [53] |
| Sol-gel | NiTiO4 | Rod | 542.26 | 8.06 | 4320 | 2100 | [51] |
| Hydrothermal | BFO-RGO | Nanowire | 928.43 | 18.62 | 950 | 1000 | [63] |
| Solid-state | CuO/LSC73 | Porous | 545 | 4.92 | - | 3500 | [39] |
| Synthesis Strategies | Anode Materials | Typical Morphology | Open Circuit Potential (V) | Pmax (mW cm−2) | Stability | Ref. |
|---|---|---|---|---|---|---|
| Solution-casting | La0.9Sr0.1CrO3-δ | Nanoparticles | 0.5 | 62 | 180 °C | [65] |
| One-pot Pechini method | Ba-Ce-Fe-Co-O | Grains | 1.036 | 335 | 700 °C | [67] |
| Sol-gel | La0.25Sr0.75TiO3 | Core-shell | 1.11 | 908.2 | 550 °C | [75] |
| Pechini process | (PrBa)0.95Fe1.8−xCuxNb0.2O5+δ | Porous | 1.09 | 431 | 800 °C | [76] |
| Solid phase blending method | CuFeO2-LZSDC | Pore | 1.1 | 672 | 550 °C | [77] |
| Synthesis Strategies | Anode Materials | Typical Morphology | Pmax (mW cm−2) | Stability | Ref. |
|---|---|---|---|---|---|
| In situ method | La0.6Sr0.4Mn0.2Fe0.6O3−δ | Nanoparticles | 632 | 800 °C | [86] |
| In situ exsolution method | La0.35Ca0.50TiO3−δ(LCT)/Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) | Core-shell | 460 | 800 °C | [92] |
| Ball milling | SmBa0.5Sr0.5Co2O5+δ | - | 131 | 800 °C | [89] |
| Tape-casting method | Pd@CeO2 | Core-shell | 30 | 800 °C | [91] |
| Solid-state method | Sr0.88Y0.08TiO3−δ | Grains | 58 | 900 °C | [94] |
| Synthesis Strategies | Materials | Typical Morphology | Specific Capacity (mAh g−1) | Stability (Cycles) | Ref |
|---|---|---|---|---|---|
| Hummers | G/Meso-LaSrMnO | Nanosheets | 6515 | 50 | [110] |
| Electrospinning | La0.5Sr0.5CoO3−x | Nanotubular | 500 | 50 | [113] |
| Electrospinning | CeMnO3 | Nanofiber | 2159 | 60 | [117] |
| Mechano-chemical | SMOF | - | 153 | - | [118] |
| Simple wet chemistry | SeZnO3 | Nanosheets | 13,200 | 140 | [122] |
| Synthesis Strategies | Architectures | Typical Morphology | Power Conversion Efficiency (PCE) (%) | VOC (V) | Fill Factor (%) | Ref. |
|---|---|---|---|---|---|---|
| Spin-coating | TiO2/CH3NH3PbI3/HTM/Au | Core-shell | 8.38 | 0.924 | 48.7 | [138] |
| Spin-coating | Graphene/TiO2 | Nanoflakes | 15.6 | 1.04 | 0.73 | [139] |
| Spin-coating | g-AZO/CuNW/AZO | Nanowire | 14.18 | 1.08 | 70.7 | [140] |
| Spin-coating | AgNWs | Nanowire | 11.0 | 0.96 | 0.23 | [141] |
| Spin-coating | P3HT:AuNPs | Nanoparticle | 10.71 | 0.75 | 64.79 | [142] |
| Spray-pyrolysis | TiO2/PbI2 | Mesoporous | 15.00 | 0.993 | 0.73 | [143] |
| Physical vapor deposition (PVD) | Nanocolumnan 1D TiO2 | Nanowire | 10.52 | 0.949 | 0.64 | [144] |
| Spray-pyrolysis | mp-TiO2 | Mesoporous | 16.2 | 1.06 | 0.75 | [145] |
| Spray-pyrolysis | Al-BSF-Si wafer | Hollow squares | 19.52 | 0.693 | 79.4 | [146] |
| - | Cs0.1FAxPbI2+x | - | 16.6 | 1.07 | 80 | [147] |
| Spin-coating | MAPbI3 | Mesoporous | 20.28 | 1.12 | 77.7 | [148] |
| Flash evaporation | CH3NH3PbI3 | - | 12.2 | 1.07 | 68 | [149] |
| Vacuum-flash-assisted solution processing | FA0.81MA0.15PbI2.51Br0.45 | Mesoporous | 19.6 | 1.143 | 0.76 | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-W.; Ramachandran, R.; Chen, S.-M.; Anushya, G.; Divya Rani, S.; Mariyappan, V.; Elumalai, P.; Vasimalai, N. High-Performance-Based Perovskite-Supported Nanocomposite for the Development of Green Energy Device Applications: An Overview. Nanomaterials 2021, 11, 1006. https://doi.org/10.3390/nano11041006
Chen T-W, Ramachandran R, Chen S-M, Anushya G, Divya Rani S, Mariyappan V, Elumalai P, Vasimalai N. High-Performance-Based Perovskite-Supported Nanocomposite for the Development of Green Energy Device Applications: An Overview. Nanomaterials. 2021; 11(4):1006. https://doi.org/10.3390/nano11041006
Chicago/Turabian StyleChen, Tse-Wei, Rasu Ramachandran, Shen-Ming Chen, Ganesan Anushya, Selvarajan Divya Rani, Vinitha Mariyappan, Perumal Elumalai, and Nagamalai Vasimalai. 2021. "High-Performance-Based Perovskite-Supported Nanocomposite for the Development of Green Energy Device Applications: An Overview" Nanomaterials 11, no. 4: 1006. https://doi.org/10.3390/nano11041006
APA StyleChen, T.-W., Ramachandran, R., Chen, S.-M., Anushya, G., Divya Rani, S., Mariyappan, V., Elumalai, P., & Vasimalai, N. (2021). High-Performance-Based Perovskite-Supported Nanocomposite for the Development of Green Energy Device Applications: An Overview. Nanomaterials, 11(4), 1006. https://doi.org/10.3390/nano11041006







