Experimental Study on the Partial Discharge Characteristics of Palm Oil and Coconut Oil Based Al2O3 Nanofluids in the Presence of Sodium Dodecyl Sulfate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanofluids
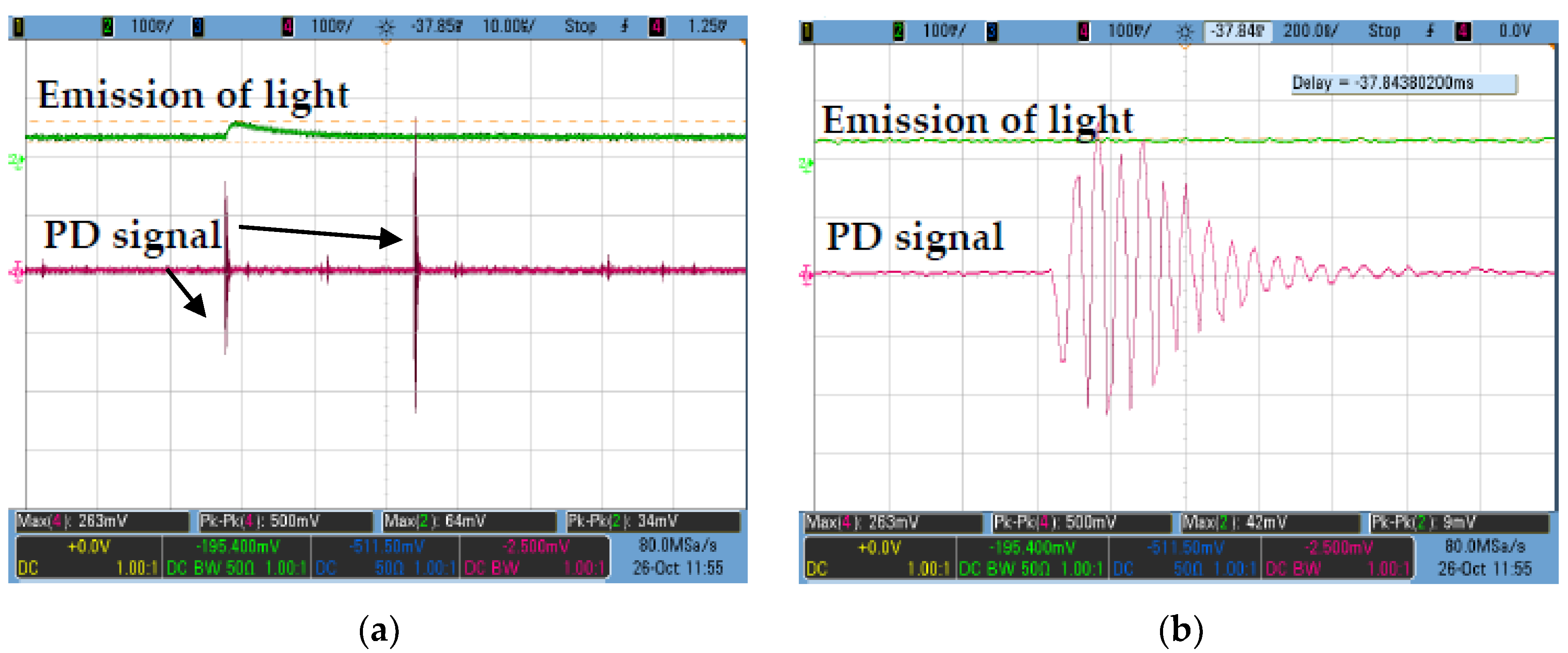
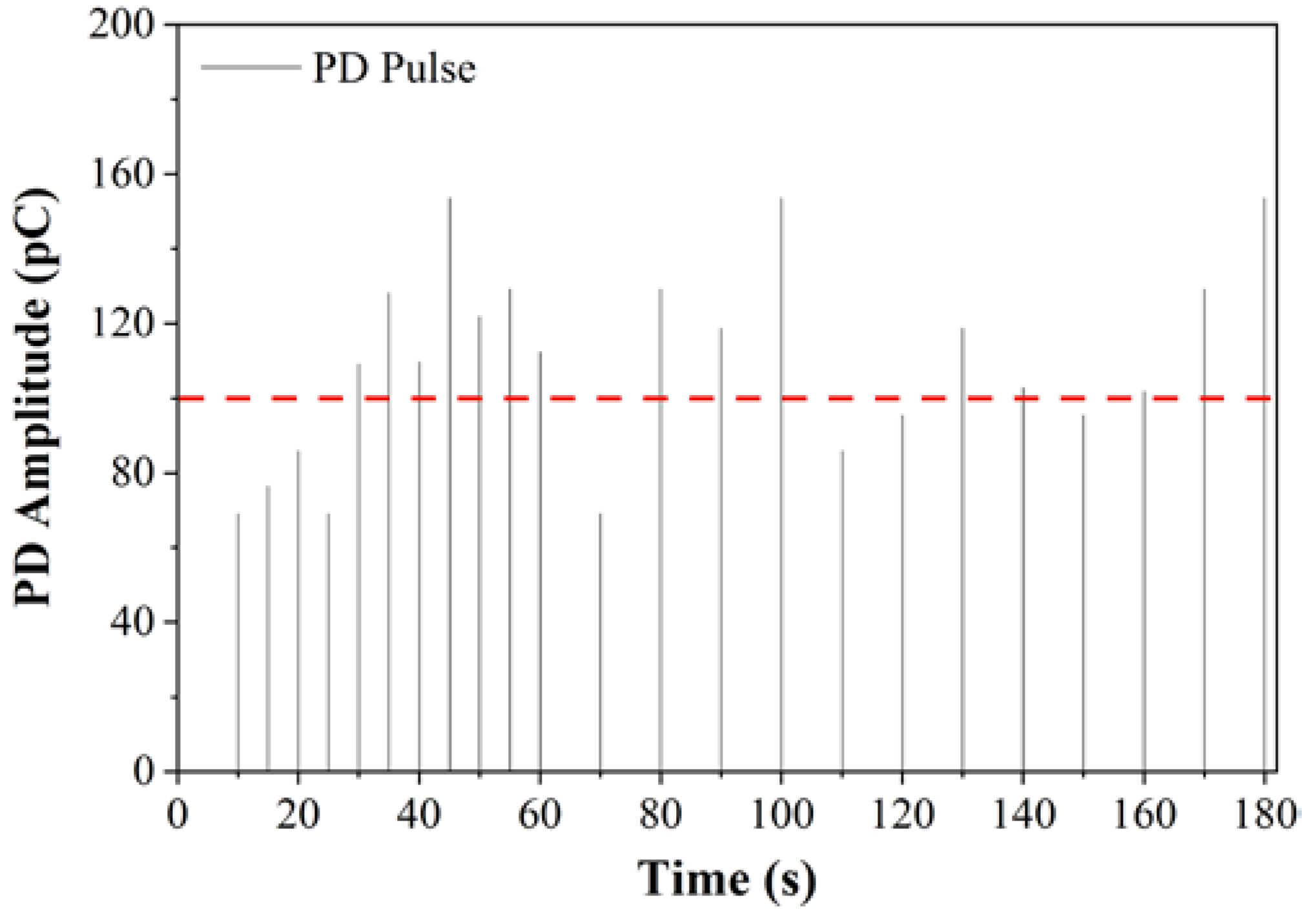
2.3. Partial Discharge
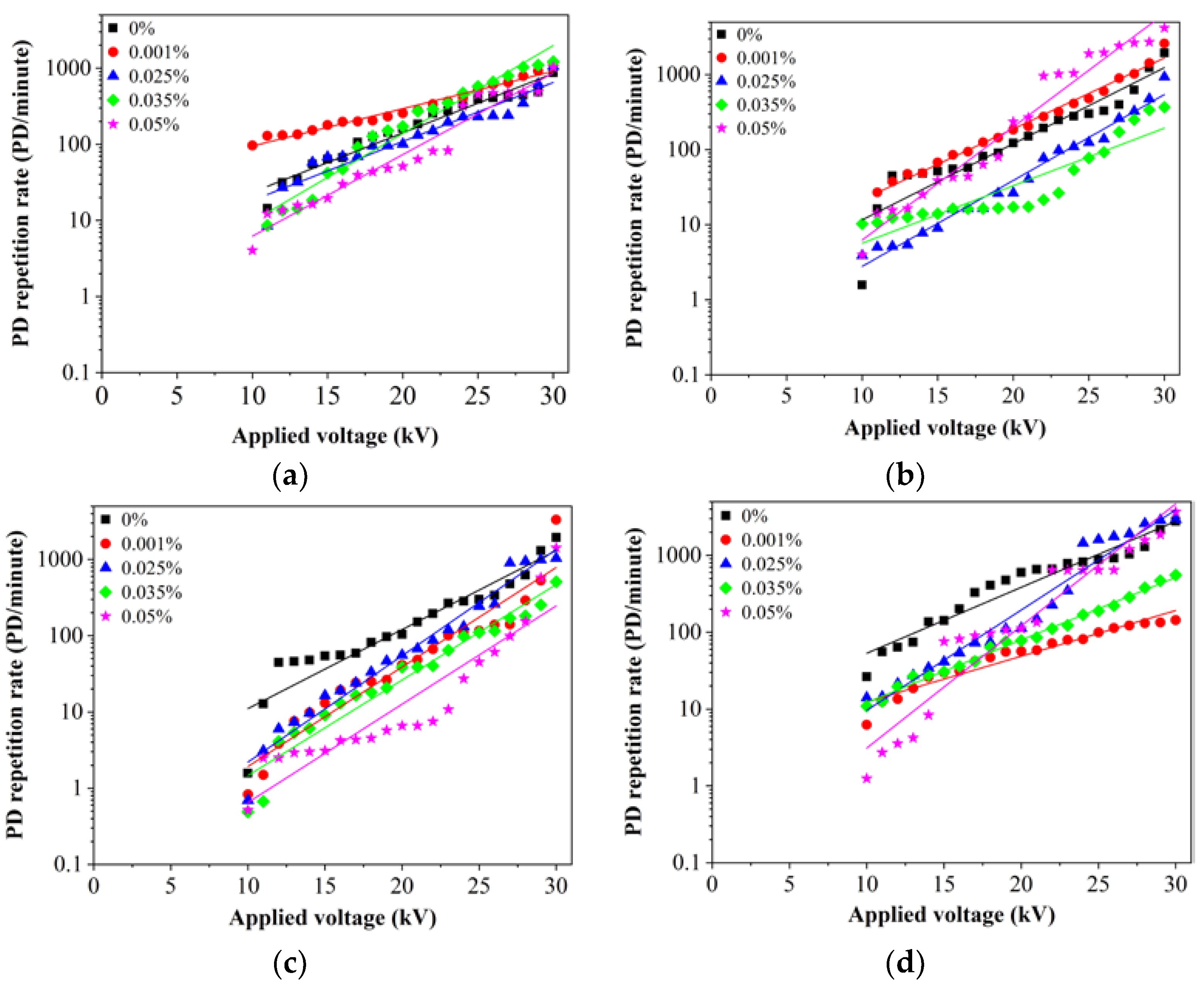
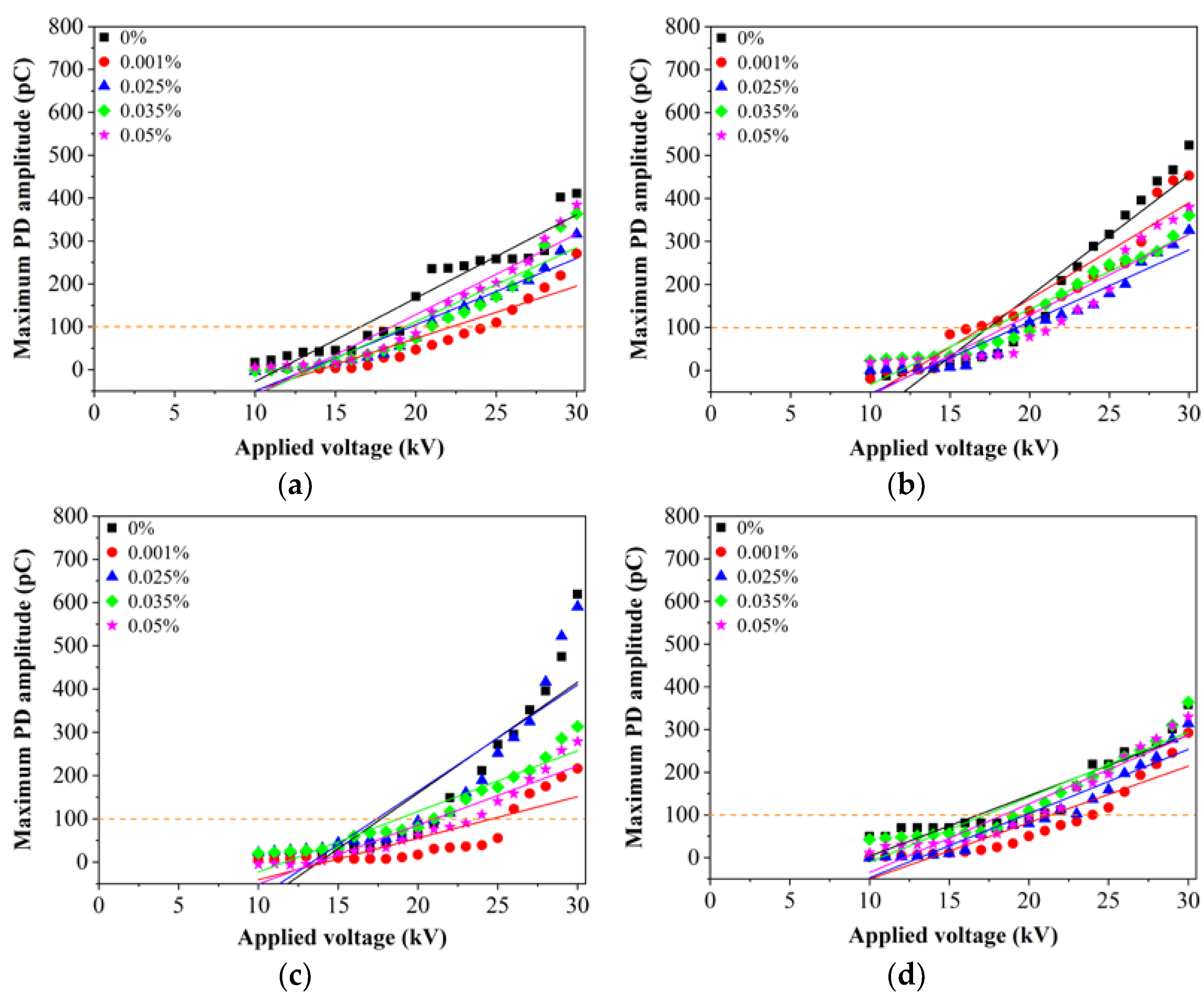
3. Results
3.1. RBDPO and CO Based Al2O3 Nanofluids without SDS
3.2. RBDPO, CO and MO Based Al2O3 Nanofluids with SDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X. Partial Discharge Behaviours and Breakdown Mechanisms of Ester Transformer Liquids under AC Stress. Ph.D. Thesis, University of Manchester, Manchester, UK, 2011. [Google Scholar]
- Azmi, K.; Ishak, D.; Kamarol, M.; Zuhairi, A. Comparison of Partial Discharge Behavior in Mineral Oil and PFAE under Influence of Spherical Metal Particle. In Proceedings of the International Conference on High Voltage Engineering and Power System, Bali, Indonesia, 2–5 October 2017; pp. 519–523. [Google Scholar]
- Lesaint, O. Streamers in Liquids: Relation with Practical High Voltage Insulation and Testing of Liquids. In Proceedings of the IEEE International Conference Dielectirc Liquids, Futuroscope-Chasseneuil, France, 30 June–3 July 2008; pp. 1–6. [Google Scholar]
- Natrras, D.A. Partial Discharge Measurement and Interpretation. IEEE Electr. Insul. Mag. 1988, 4, 10–23. [Google Scholar] [CrossRef]
- Forster, E.O. Partial Discharge and Streamers in Liquid Dielectrics—The Significance of the Inception Voltage. IEEE Trans. Dielectr. Electr. Insul. 1993, 28, 941–946. [Google Scholar] [CrossRef]
- Cavallini, A.; Montanari, G.C.; Ciani, F. Analysis of Partial Discharge Phenomena in Paper-oil Insulation Systems as a Basis for Risk Assessment Evaluation. In Proceedings of the IEEE International Conference on Dielectric Liquids, Coimbra, Portugal, 26 June–1 July 2005; pp. 241–244. [Google Scholar]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanism and Applications. J. Nanomater. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced Thermal Conductivity of TiO2—Water based Nanofluids. Inter. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Daungthongsuk, W.; Wongwises, S. A Critical Review of Convective Heat Transfer of Nanofluids. Renew. Sustain. Energy Rev. 2007, 11, 797–817. [Google Scholar] [CrossRef]
- Utomo, A.; Poth, H.; Robbins, P.; Pacek, A. Experimental and Theoretical Studies of Thermal Conductivity, Viscosity and Heat Transfer Coefficient of Titania and Alumina Nanofluids. Int. J. Heat Mass Transf. 2012, 55, 7772–7781. [Google Scholar] [CrossRef]
- Gkountas, A.A.; Benos, T.L.; Sofiadis, N.G.; Sarris, E.I. A Printed-Circuit Heat Exchanger Consideration by Exploiting an Al2O3-water Nanofluid: Effect of the Nanoparticles Interfacial Layer on Heat Transfer. Therm. Sci. Eng. Prog. 2021, 22, 1–10. [Google Scholar]
- Attalla, M.; Maghrabie, H.M. An Experimental Study on Heat Transfer and Fluid Flow of Rough Plate Heat Exchanger using Al2O3/Water Nanofluid. J. Therm. Energy Gener. Transp. Storage Convers. 2019, 33, 261–281. [Google Scholar] [CrossRef]
- Gkountas, A.A.; Benos, T.L.; Nikas, K.S.; Sarris, I.E. Heat Transfer Improvement by an Al2O3-water nanofluid coolant in Printed-Circuit Heat Exchangers of Supercritical CO2 Brayton Cycle. Therm. Sci. Eng. Prog. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Benos, L.; Karvelas, E.G.; Sarris, I.E. A Theoretical Model for the Magnetohydrodynamic Natural Convection of A CNT-Water Nanofluid Incorporating a Renovated Hamilton-Crosser Model. Int. J. Heat Mass Transf. 2019, 135, 548–560. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Isfahani, A.H.M.; Hojaji, M. Experimental Investigation of Heat Transfer and Friction Coefficient of the Water/Graphene Oxide Nanofluid in a Pipe Containing Twisted Tape Inserts Under Air Cross-Flow. J. Therm. Energy Gener. Transp. Storage Convers. 2018, 31, 373–390. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat Transfer Enhancement of Nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.Z.; Li, C. A Review on Properties, Opportunities and Challenges of Transformer Oil-based Nanofluids. J. Nanomater. 2016, 2016, 1–23. [Google Scholar] [CrossRef]
- Jin, H.F.; Morshuis, P.; Rodrigo Mor, A.; Smit, J.J.; Andritsh, T. Partial Discharge Behavior of Mineral Oil based Nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2747–2753. [Google Scholar] [CrossRef]
- Du, Y.F.; Lv, Y.Z.; Li, C.; Chen, M.; Zhong, Y.; Zhou, J.; Li, X.X.; Zhou, Y. Effect of Semiconductive Nanoparticles on Insulating Performances of Transformer Oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770–776. [Google Scholar]
- Makmud, M.Z.H.; Alias, H.A.; Chee, C.Y.; Dabbak, S.Z.A. Partial Discharge in Nanofluid Insulation Material with Conductive and Semiconductive Nanoaparticles. Materials 2019, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lv, Y.; Li, C.; Du, Y.; Chen, M.; Zhang, S.; Zhou, Y.; Chen, L. Insulating Properties and Charge Characteristics of Natural Ester Fluid Modified by TiO2 Semiconductive Nanoparticles. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 135–140. [Google Scholar] [CrossRef]
- Prasad, D.; Chandrasekar, S. Effect of Nano-SiO2 Particles on Partial Discharge Signal Characteristics of FR3 Transformer Oil. J. Adv. Chem. 2017, 13, 1–10. [Google Scholar] [CrossRef]
- Lv, Y.Z.; Du, Y.F.; Zhou, J.Q.; Li, X.X.; Chen, M.T.; Li, C.R.; Wang, G.L. Nanoparticle Effect on Electrical Properties of Aged Mineral Oil based Nanofluids. CIGRE 2012, D1-106, 1–6. [Google Scholar]
- Kurimsky, J.; Rajnak, M.; Cimbala, R.; Paulovicova, K.; Rozynek, Z.; Kopcansky, P.; Timko, M. Electrical Discharges in Ferrofluids based on Mineral Oil and Novel Gas-to-Liquid Oil. J. Mol. Liq. 2021, 325, 1–9. [Google Scholar] [CrossRef]
- Swati, K.; Sarathi, R.; Yadav, K.S.; Taylor, N.; Edin, H. Corona Discharge Activity in Nanoparticle Dispersed Transformer Oil under Composite Voltages. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1731–1737. [Google Scholar] [CrossRef]
- Swati, K.; Yadav, K.S.; Sarathi, R.; Vinu, R.; Danikas, M.G. Understanding corona discharge activity in titania nanoparticles dispersed in transformer oil under ac and dc voltages. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2325–2336. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhou, J.; Li, X.; Zhou, Y.; Tu, Y. Effect of Electron Shallow Trap on Breakdown Performance of Transformer Oil-based Nanofluids. J. Appl. Phys. 2011, 110, 104104. [Google Scholar] [CrossRef]
- Cavallini, A.; Karthik, R.; Negri, F. The Effect of Magnetite, Graphene Oxide and Silicone Oxide Nanoparticles on Dielectric Withstand Characteristics of Mineral Oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2592–2600. [Google Scholar] [CrossRef]
- Peppas, G.D.; Charalampakos, V.P.; Pyrgioti, E.C.; Tsovilis, T.; Politis, Z.; Gonos, I.F. Partial Discharge Study of Ultrastable Colloidal Nanofluid Impregnated Paper. In Proceedings of the 20th International Symposium on High Voltage Engineering, Buenos Aires, Argentina, 28 August–1 September 2017; pp. 1–6. [Google Scholar]
- Mohamad, N.A.; Azis, N.; Jasni, J.; Ab Kadir, M.Z.A.; Yunus, R.; Yaakub, Z. Effect of Surfactants on the Lightning Breakdown Voltage of Palm Oil and Coconut Oil based Al2O3 Nanofluids. Nanotechnology 2020, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.A.; Azis, N.; Jasni, J.; Ab Kadir, M.Z.A.; Yunus, R.; Yaakub, Z. Impact of Fe3O4, CuO and Al2O3 on the AC Breakdown Voltage of Palm Oil and Coconut Oil in the Presence of CTAB. Energies 2019, 12, 1605. [Google Scholar] [CrossRef]
- ISO 5508:1990. Animal and Vegetable Fats and Oils-Analysis by Gas Chromatography (GC) of Methyl-Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 1990. [Google Scholar]
- Mohamad, N.A.; Azis, N.; Jasni, J.; Ab Kadir, M.Z.A.; Yunus, R.; Yaakub, Z. Effects of Different Types of Surfactants on AC Breakdown Voltage of Refined, Bleached and Deodorized Palm Oil based CuO Nanofluids. In Proceedings of the IEEE PES Asia-Pacific Power and Energy Engineering Conference (APPEEC), Kota Kinabalu, Malaysia, 7–10 October 2018; pp. 768–771. [Google Scholar]
- IEC 60270. High-Voltage Test Techniques—Partial Discharge Measurements; International Electrotechnical Commission: Geneva, Switzerland, 2000. [Google Scholar]
- IEC 61294. Insulating Liquids—Determination of the Partial Discharge Inception Voltage (PDIV)—Test Procedure; International Electrotechnical Commission: Geneva, Switzerland, 1993. [Google Scholar]
- Zawawi, W.M.W.A.I.; Makmud, M.Z.H.; Arief, Y.Z. A Study on the Performance of Impedance Matching Circuit in Partial Discharge Measuring System. Borneo Sci. 2012, 30, 24–31. [Google Scholar]
- Azmi, K.; Zuhairi, A.; Ishak, D.; Muhamad, N.A.; Kamarol, M. Partial Discharge Characteristics of Spherical Metal Particle in Mineral Oil and PFAE under AC Voltage. In Proceedings of the International Symposium Electrical Insulating Materials (ISEIM), Penang, Malaysia, 5–8 November 2017; pp. 576–579. [Google Scholar]
- Abrie, P.L.D. The Design of Impedance-Matching Networks for Radio-Frequency and Microwave Amplifiers, 1st ed.; Artech House Inc. Publisher: Norwood, MA, USA, 1985; p. 377. [Google Scholar]
- Mazzetti, C.; Pompili, M.; Forster, E.O. A Study of Partial Discharge Measurements in Dielectric Liquids. IEEE Trans. Dielectr. Electr. Insul. 1992, 27, 445–450. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.D. Secondary Reverse Streamer Observed in an Ester Insulating Liquid under Negative Impulse Voltage. J. Phys. D Appl. Phys. 2011, 44, 1–10. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.D. Streamer Characteristic and Breakdown in Synthetic and Natural Ester Transformer Liquids with Pressboard Interface under Lightning Impulse Voltage. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1908–1917. [Google Scholar] [CrossRef]
- Eman, G.A.; Mansour, D.E.A.; Izzularab, M.A. Partial Discharge Development in Oil-based Nanofluids: Inception, Propagation and Time Transition. IEEE Access 2020, 8, 28–35. [Google Scholar]
- Kurimsky, J.; Rajnak, M.; Cimbala, R.; Jakub Rajnic, M.T.; Kopcansky, P. Effect of Magnetic Nanoparticles on Partial Discharges in Transformer Oil. J. Magn. Magn. Mater. 2020, 496, 1–5. [Google Scholar] [CrossRef]
- Wang, Q.; Rafiq, M.; Lv, Y.; Li, C.; Yi, K. Preparation of Three Types of Transformer Oil-based Nanofluids and Comparative Study on the Effect of Nanoparticle Concentrations on Insulating Property of Transformer Oil. J. Nanotech. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Holtzhausen, J.P.; Vosloo, W.L. High Voltage Engineering: Practice and Theory; Stellenbosch University: Stellenbosch, South Africa, 2008. [Google Scholar]
- Ali, N.; Teixeira, J.A.; Addali, A. A Review on Nanofluids: Fabrication, Stability, and Thermophysical Properties. J. Nanomater. 2018, 2018, 1–33. [Google Scholar] [CrossRef]
- Simpson, S.; Schelfhout, A.; Golden, C.; Vafaei, S. Nanofluid Thermal Conductivity and Effective Parameters. Appl. Sci. 2018, 9, 87. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Wang, Z.D.; Jarman, P.; Krause, C.H.; Smith, P.W.R.; Gyore, A. Partial Discharge Behaviour of Transformer Liquids and the Influence of Moisture Content. In Proceedings of the IEEE International Conference Liquids Dielectric, Bled, Slovenia, 29 June–3 July 2014; pp. 1–4. [Google Scholar]
- Lesaint, O.; Massala, G. Positive Streamer Propagation in Large Oil Gaps: Experimental Characterization of Propagation Modes. IEEE Trans. Dielectr. Electr. Insul. 1998, 5, 360–370. [Google Scholar] [CrossRef]
- Demissie, H.; Duraisamy, R. Effects of Electrolytes on the Surface and Micellar Characteristics of Sodium Dodecyl Sulphate Surfactant Solution. J. Sci. Innov. Res. 2016, 5, 208–214. [Google Scholar]
- Cieśla, J.; Koczańska, M.; Narkiewicz-Michałek, J.; Szymula, M.; Bieganowski, A. The Physicochemical Properties of CTAB Solutions in the Presence of α-tocopherol. J. Mol. Liq. 2016, 222, 463–470. [Google Scholar] [CrossRef]





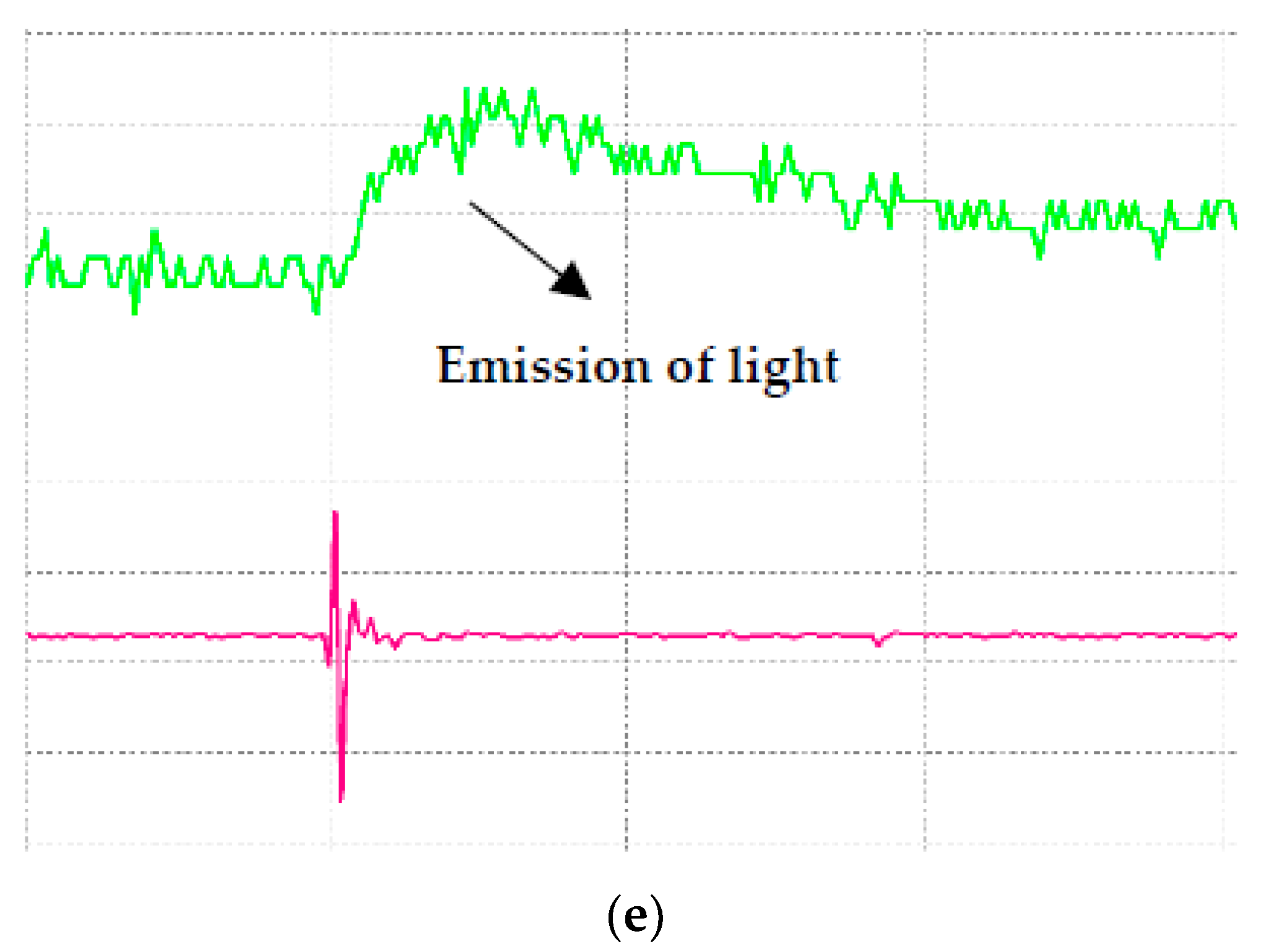

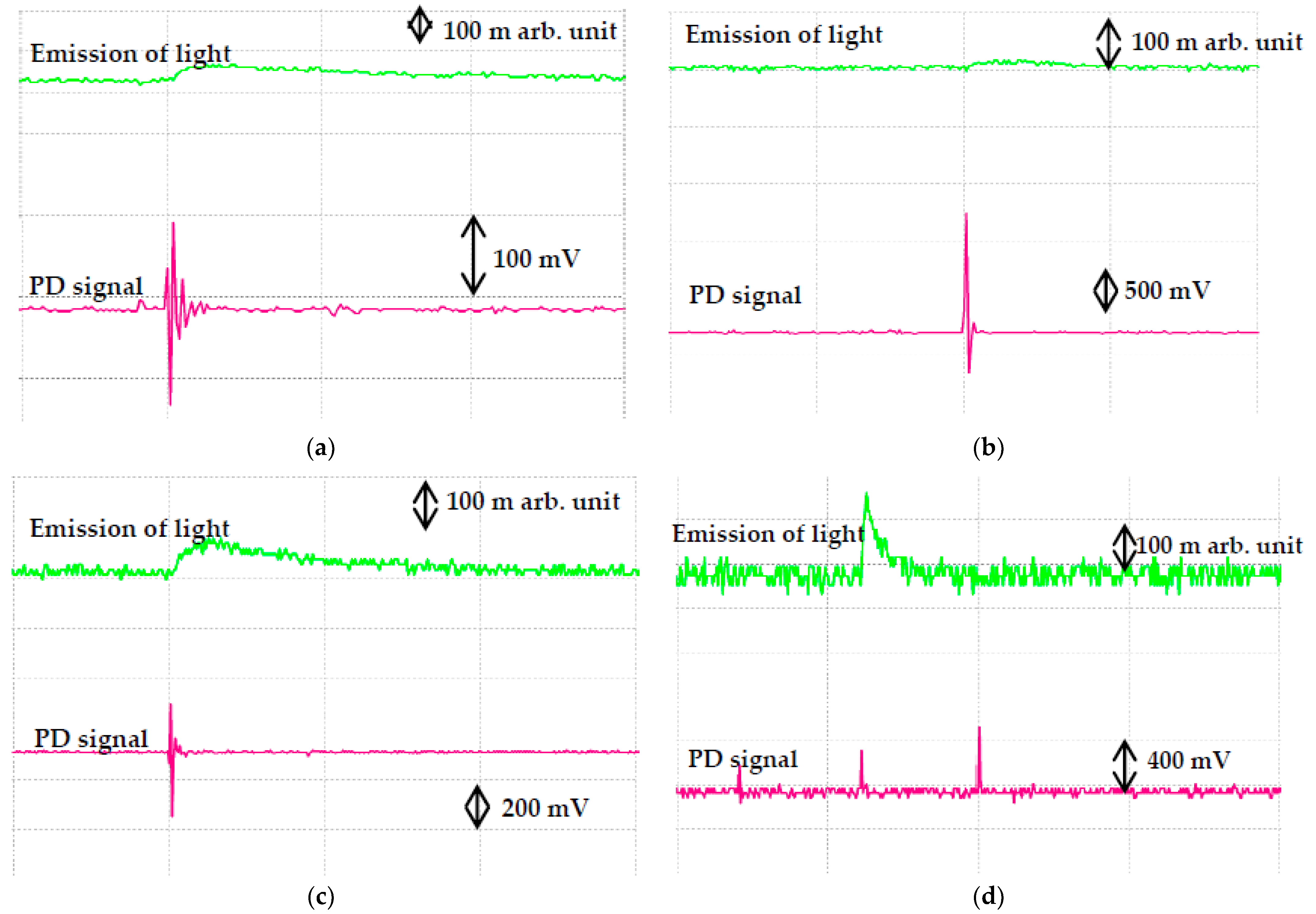

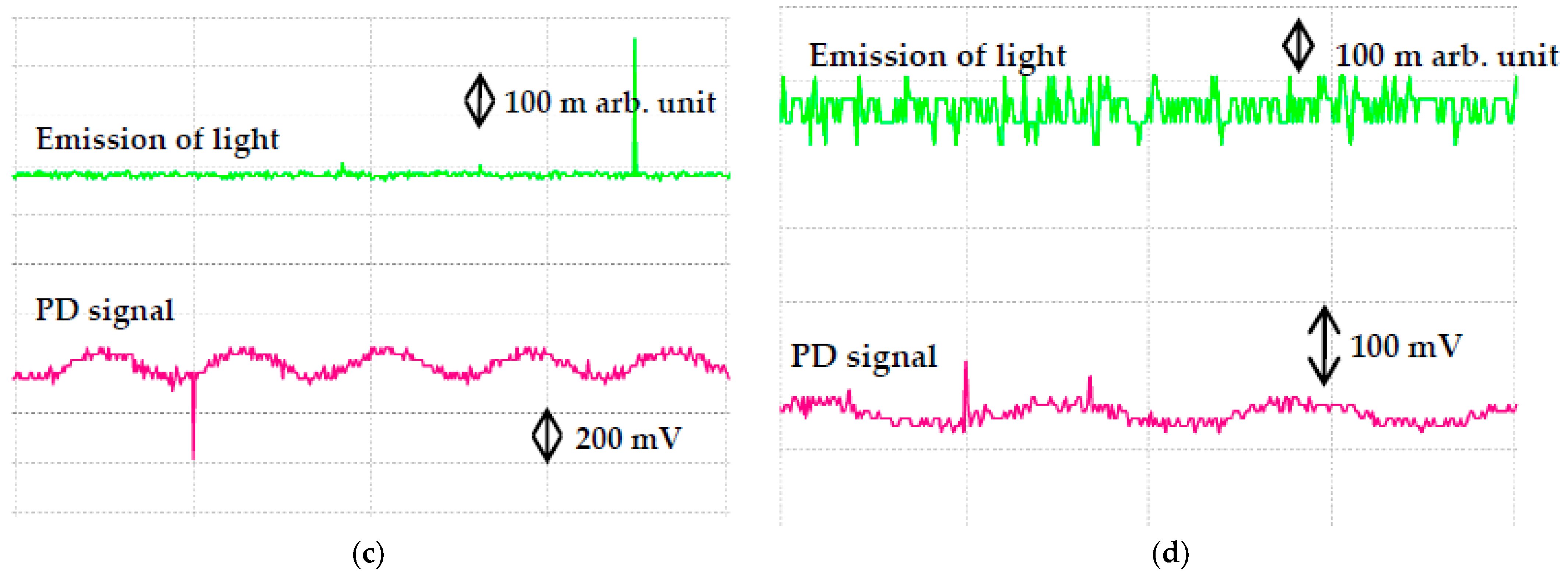
| Samples | Volume of Concentration (%) | Percentage Increment or Decrement (%) | |
|---|---|---|---|
| Without SDS | With SDS | ||
| RBDPOA | 0.001 | +15.6 | +34 |
| 0.025 | +23.9 | +18.8 | |
| 0.035 | +7.4 | +16.4 | |
| 0.05 | −1.8 | +11.6 | |
| RBDPOB | 0.001 | +7.5 | −2.2 |
| 0.025 | +3.7 | +10.2 | |
| 0.035 | −1.8 | +0.8 | |
| 0.05 | −15.8 | +5.9 | |
| CO | 0.001 | −3.2 | +39.3 |
| 0.025 | +2 | −1.6 | |
| 0.035 | +9.7 | +6.2 | |
| 0.05 | +27.7 | +19 | |
| MO | 0.001 | +10.6 | +27 |
| 0.025 | −16.4 | −17.6 | |
| 0.035 | +24.2 | +2.6 | |
| 0.05 | −10.8 | +9.4 | |
| Samples | Volume of Concentration (%) | Average Emission of Light Signals (m arb. Unit) | |
|---|---|---|---|
| Without SDS | With SDS | ||
| RBDPOA | 0 | 60 | 60 |
| 0.001 | 77.6 | 109.9 | |
| 0.025 | 52.3 | 154.1 | |
| 0.035 | 53.6 | 111.6 | |
| 0.05 | 44.6 | 219.4 | |
| RBDPOB | 0 | 96 | 96 |
| 0.001 | 82.2 | 49 | |
| 0.025 | 24.1 | 172 | |
| 0.035 | 24 | 49.7 | |
| 0.05 | 45.6 | 152.2 | |
| CO | 0 | 105.4 | 105.4 |
| 0.001 | 61.3 | 78.5 | |
| 0.025 | 71 | 140.8 | |
| 0.035 | 36.8 | 163.5 | |
| 0.05 | 264.2 | 280.3 | |
| MO | 0 | 56.7 | 56.7 |
| 0.001 | 150.2 | 120.8 | |
| 0.025 | 328.8 | 128.9 | |
| 0.035 | 224.8 | 348.4 | |
| 0.05 | 102.4 | 428 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad, N.A.; Azis, N.; Jasni, J.; Kadir, M.Z.A.A.; Yunus, R.; Yaakub, Z. Experimental Study on the Partial Discharge Characteristics of Palm Oil and Coconut Oil Based Al2O3 Nanofluids in the Presence of Sodium Dodecyl Sulfate. Nanomaterials 2021, 11, 786. https://doi.org/10.3390/nano11030786
Mohamad NA, Azis N, Jasni J, Kadir MZAA, Yunus R, Yaakub Z. Experimental Study on the Partial Discharge Characteristics of Palm Oil and Coconut Oil Based Al2O3 Nanofluids in the Presence of Sodium Dodecyl Sulfate. Nanomaterials. 2021; 11(3):786. https://doi.org/10.3390/nano11030786
Chicago/Turabian StyleMohamad, Nur Aqilah, Norhafiz Azis, Jasronita Jasni, Mohd Zainal Abidin Ab. Kadir, Robiah Yunus, and Zaini Yaakub. 2021. "Experimental Study on the Partial Discharge Characteristics of Palm Oil and Coconut Oil Based Al2O3 Nanofluids in the Presence of Sodium Dodecyl Sulfate" Nanomaterials 11, no. 3: 786. https://doi.org/10.3390/nano11030786
APA StyleMohamad, N. A., Azis, N., Jasni, J., Kadir, M. Z. A. A., Yunus, R., & Yaakub, Z. (2021). Experimental Study on the Partial Discharge Characteristics of Palm Oil and Coconut Oil Based Al2O3 Nanofluids in the Presence of Sodium Dodecyl Sulfate. Nanomaterials, 11(3), 786. https://doi.org/10.3390/nano11030786





